- 1Division of Cardiovascular Medicine, Department of Medicine, Stanford University School of Medicine, Stanford, CA, United States
- 2Stanford Center for Inherited Cardiovascular Disease, Stanford University School of Medicine, Stanford, CA, United States
- 3Wu Tsai Human Performance Alliance, Stanford University School of Medicine, Stanford, CA, United States
- 4Center for Digital Health, Stanford University School of Medicine, Stanford, CA, United States
- 5Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, United States
- 6Stanford Diabetes Research Center, Stanford University School of Medicine, Stanford, CA, United States
- 7Department of Genetics, Stanford University School of Medicine, Stanford, CA, United States
- 8Department of Biomedical Data Science, Stanford University School of Medicine, Stanford, CA, United States
Mavacamten is a first-in-class cardiac myosin ATPase inhibitor, approved by the United States Food and Drug Administration for the treatment of hypertrophic cardiomyopathy with obstructive physiology (oHCM). Here, we present the real-world use of mavacamten in 50 patients with oHCM at a tertiary care referral center. In both our highlighted case and in our aggregate data, we report significant improvement in wall thickness, mitral regurgitation, left ventricular outflow tract obstruction and New York Heart Association symptom class. Moreover, in our center's experience, neither arrhythmia burden, nor contractility have worsened in the vast majority of patients: we note a clinically insignificant mean decrease in left ventricular ejection fraction (LVEF), with only two patients requiring temporary mavacamten discontinuance for LVEF < 50%. Adverse events were rare, unrelated to mavacamten itself, and seen solely in patients with disease too advanced to have been represented in clinical trials. Moreover, our multidisciplinary pathway enabled us to provide a large number of patients with a novel closely-monitored therapeutic within just a few months of commercial availability. These data lead us to conclude that mavacamten, as a first-in-class cardiac myosin inhibitor, is safe and efficacious in real-world settings.
Introduction
Hypertrophic cardiomyopathy is a genetic disease associated with variants in the genes of the cardiac sarcomere and characterized by enhanced cardiac actin-myosin crossbridge cycling leading to hyperdynamic contractility, cardiac hypertrophy, and diastolic dysfunction (1). Obstructive hypertrophic cardiomyopathy (oHCM) occurs when hypercontractility, hypertrophy, and mitral valve morphology contribute to outflow tract obstruction (1). Mavacamten is a first-in-class, small molecule inhibitor of cardiac myosin ATPase approved by the US Food and Drug Administration (FDA) in April 2022 for use in patients with symptomatic oHCM. Mavacamten reduces contractile force through decreased myosin head availability (2). We now have more than 18 months of experience with commercial mavacamten following the Risk Evaluation and Mitigation Strategies (REMS) pathway, and there are few published reports of its longer term, real-world effects (3). Here, we report a case series of 50 patients with oHCM treated with mavacamten and closely monitored for an average of 36 weeks through a multidisciplinary program at a single center.
Methods
This was a retrospective observational study of the first 50 adult (age > 18 years old) patients who were evaluated at Stanford University and initiated on mavacamten for symptomatic oHCM. All patients provided informed, written consent and were enrolled in the FDA-mandated Risk Evaluation and Mitigation Strategies (REMS) pathway (4). Electronic medical records were reviewed for baseline and post-mavacamten clinical and echocardiographic data. Appropriate institutional review board approval was obtained from Stanford University.
Echocardiography
Prior to initiation of mavacamten, all patients underwent comprehensive echocardiogram evaluation to evaluate left ventricular ejection fraction (LVEF), left ventricular outflow tract (LVOT) obstruction gradient, mitral regurgitation severity, maximal thickness of the interventricular septum, right ventricular systolic pressure (RVSP) estimates, and measures of diastolic function. Of note, only the LVEF and LVOT measurements were required for initiation of mavacamten. Per the REMS pathway, patients had follow-up echocardiograms performed at 4, 8, and 12 weeks following mavacamten initiation, with subsequent follow-up every 3 months afterward (4). As echocardiograms could be performed locally due to a patients’ preference, not all the aforementioned measurements were obtained at follow-up. Each echocardiogram was reviewed by the prescribing Stanford physician to confirm LVEF and LVOT gradient measurements prior to the next dispensing of mavacamten.
Outcomes
Manual chart review and data extraction was performed to obtain baseline and follow-up clinical data. New York Heart Association (NYHA) symptom class was recorded at 4, 8, 12 weeks and at latest follow-up. Adverse events, such as death, hospitalization, temporary/permanent discontinuation of mavacamten, and arrhythmia development (both atrial fibrillation and ventricular tachycardia) were recorded.
Statistical analysis
All analyses were performed in R (https://www.r-project.org). Continuous variables are presented as mean and standard deviation. Categorical variables are presented as number and percentage. As each patient served as their own pre- and post- comparison, a paired t-test was used to test for significant difference across the cohort for continuous variables. Similarly, a McNemar-Bowker test was used to test for significant differences across categorical variables. P < 0.05 was considered significant.
Results
Case report
A 26-year-old man presented with severely reduced exercise capacity (VO2max 67% of predicted) due to obstructive HCM characterized by severe septal hypertrophy (2.8 cm) with associated late gadolinium enhancement on cardiac magnetic resonance imaging. He had normal-to-hyperdynamic left ventricular ejection fraction (LVEF, 63%) and a resting LVOT gradient of 90 mmHg with systolic anterior motion (SAM) of the mitral valve causing moderate mitral regurgitation. Genetic testing was unavailable in the proband due to lack of insurance coverage, but his sister (who was also seen in our clinic) underwent testing that revealed a likely pathogenic variant in MYH7 (c.1051A>G, p.Lys351Glu).
Over years of follow-up, the patient developed decreasing exercise tolerance, with increasing left ventricular septal hypertrophy to 3.1 cm (Figure 1A) and LVOT gradient of 112 mmHg at rest, with possible left ventricular mid-cavitary gradient that was not formally measured at the time of imaging (Figure 1B). His SAM-associated moderate mitral regurgitation worsened (Figure 1C). He had echocardiographic evidence of diastolic dysfunction, with reduced lateral e’ of 3.9 cm/s (Figure 1D). He deferred surgical myectomy out of concern for operative risk.
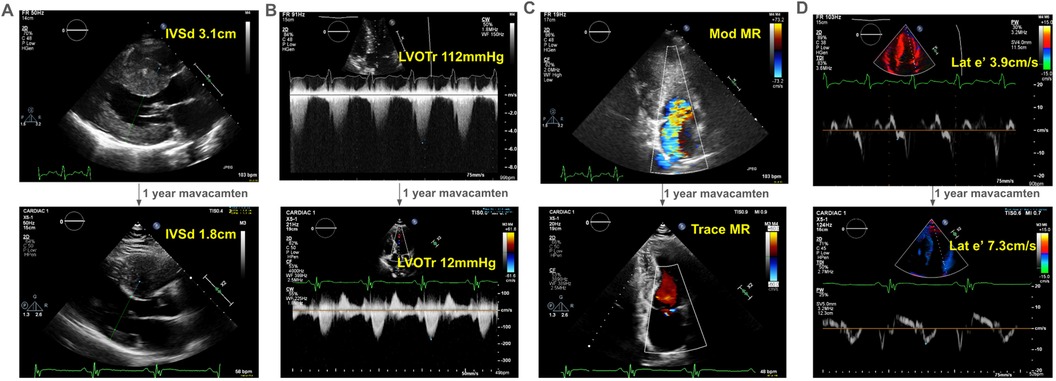
Figure 1. Echocardiographic changes after one-year of mavacamten in a 41-year old man with obstructive hypertrophic cardiomyopathy. With one-year of treatment with mavacamten, our proband experienced improved symptoms, which correlated echocardiographically (see Supplementary Video S1) to (A) a decrease in interventricular septum thickness on parasternal long axis view, (B) his left ventricular outflow tract gradient at rest, (C) the degree of mitral regurgitation, and (D) his diastolic function as represented by his lateral e’. IVSd, interventricular septum thickness in diastole in cm; LVOTr, left ventricular outflow tract gradient at rest in mmHg; MR, mitral regurgitation; lat e’, lateral e’ in cm/s, a spectral Doppler measurement reflecting diastolic function.
He started mavacamten at 40 years of age. After one year, there was dramatic improvement in his symptoms with a reduction in his interventricular septal thickness from 3.1 cm to 1.8 cm (Figure 1A) and resting LVOT gradient from 112 mmHg to 12 mmHg (Figure 1B). Mitral regurgitation reduced to trace (Figure 1C). Finally, he had improvement in his lateral e’ velocity from 3.9 cm/s to 7.3 cm/s (Figure 1D, Supplementary Video S1).
One year experience of real world mavacamten therapy
We have observed similar, though typically less dramatic trends, across patients on mavacamten. In our cohort, 64% (32) were women with an average age of 63.5 ± 13.5 years (standard deviation, SD) and body-mass index of 28.5 ± 5.4 kg/m2. 41 patients (82%) were on either beta-blocker or calcium-channel blocker therapies, with 68% (N = 34), 16% (8), and 2% (1) on beta-blocker therapy, calcium channel blocker therapy, or both medication classes, respectively. All patients were closely monitored according to the FDA-mandated REMS pathway (4), with an average 36 weeks follow up. Mavacamten was temporarily held in 5 patients (10%) due to Valsalva LVOT gradient <20 mmHg, and in 2 patients (4%) for LVEF <50%, in both cases for 4-weeks before re-initiation of mavacamten therapy at a lower dose than prior4. We note that holding mavacamten for an LVOT gradient <20 mmHg is mandated by the FDA REMS pathway algorithm4, despite such a reduction being the desired physiologic effect. Mavacamten was stopped in 3 patients (6%) for fatigue/malaise (n = 2), and loss of insurance coverage (n = 1). We noted minimal atrial fibrillation or non-sustained ventricular tachycardia on follow-up monitoring. Mavacamten doses on most recent follow-up, adjusted per the FDA REMS pathway algorithm4, were: 2.5 mg (9, 20%), 5 mg (17, 38%), 10 mg (13, 29%), and 15 mg (6, 13%, Table 1).
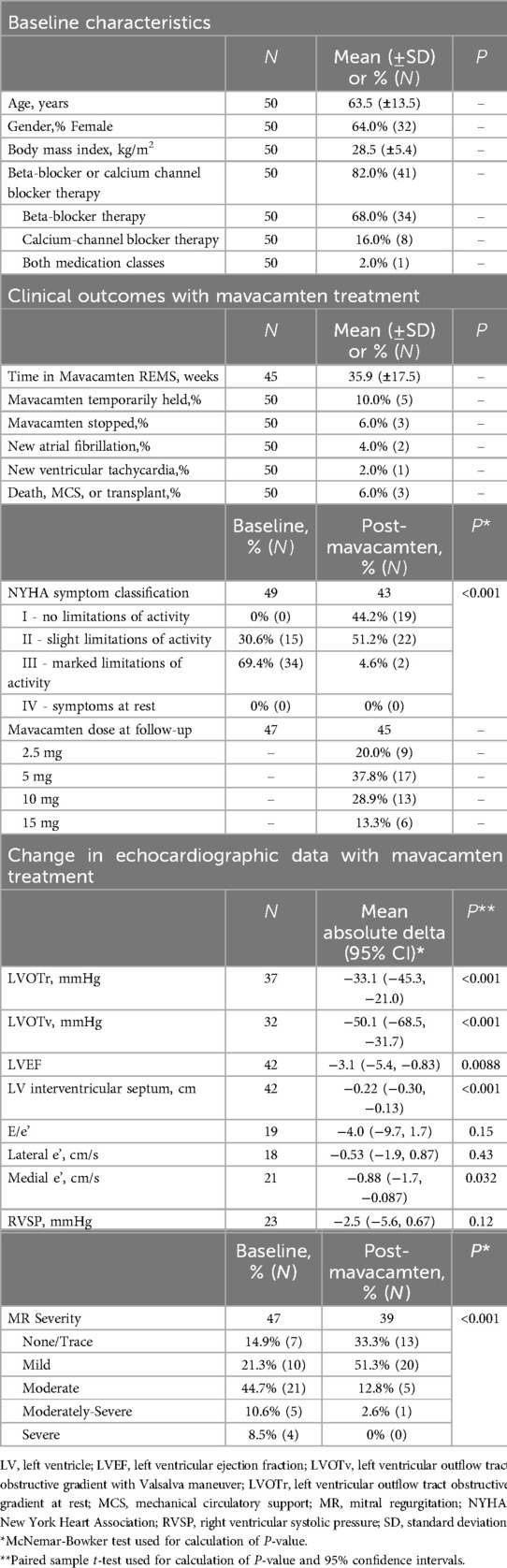
Table 1. Baseline characteristics, clinical outcomes, and changes to echocardiographic data with mavacamten treatment in 50 patients with obstructive hypertrophic cardiomyopathy.
We observed significant changes in echocardiographic measurements of mavacamten-treated patients at most recent follow-up compared to baseline. In line with trial data, there was a dramatic mean decrease in LVOT gradient at rest and with Valsalva with reductions of −33 mmHg [−45, −21] (95% CI) in resting LVOT gradient, and −51 mmHg [−69, −32] reduction in Valsalva LVOT gradient (P < 0.001, pairwise T-test.) There was significant improvement in the proportion of patients with moderate or severe mitral regurgitation (P < 0.001, McNemar-Bowker, Table 1). Accompanying this change in LVOT gradient, we noted a significant decrease in diastolic interventricular septal thickness [−0.2 (−0.30, −0.13) cm, P < 0.001, Table 1]. We observed a clinically insignificant reduction in mean LVEF from 67% to 64% [−3.1 (−5.4, −0.8)%, P = 0.008] without change in right ventricular systolic pressure (P = 0.12, Table 1). There was a significant decrease in medial e’ [−0.9 cm/s (−1.7, −0.09), P = 0.03], but no significant change in E/e’ or lateral e’ (Table 1). We note that the changes in measures diastolic function may reflect mavacamten-specific improvements in diastolic stiffness likely owing to stabilization of the super-relaxed state of myosin (5). These positive changes in echocardiographic measurements were mirrored by New York Heart Association (NYHA) symptom class, which improved dramatically with mavacamten treatment (McNemar-Bowker test, P < 0.001).
To compare the findings of our patients who started on mavacamten, we considered a subset of patients (N = 5) with symptomatic oHCM (NYHA symptom classification ≥ II), referred for mavacamten but who ultimately did not start the medication (Table 2). These patients had an average age of 70.6 years, 60% (3) were female, had an average BMI of 32.2, and 80% of the participants (4) on beta-blockers; overall similar in demographics and baseline clinical characteristics to the subset who received mavacamten. In contrast to those started on mavacamten, there was slight worsening in NYHA symptom classification in follow-up without mavacamten, with two patients previously NYHA II progressing to NYHA III symptoms. In addition, LVOT gradients increased by 19.2 mmHg and 13.2 mmHg during the follow-up period for gradients at rest and with Valsalva. These physiologic changes were accompanied by an increase in E/e’ of 2.9 and slight decreases of 0.6 cm/s and 0.8 cm/s in lateral and medial e’, respectively. There were small changes in LVEF (+2%) and interventricular septum thickness (−0.04 cm). One patient improved their mitral regurgitation classification from moderate to mild.
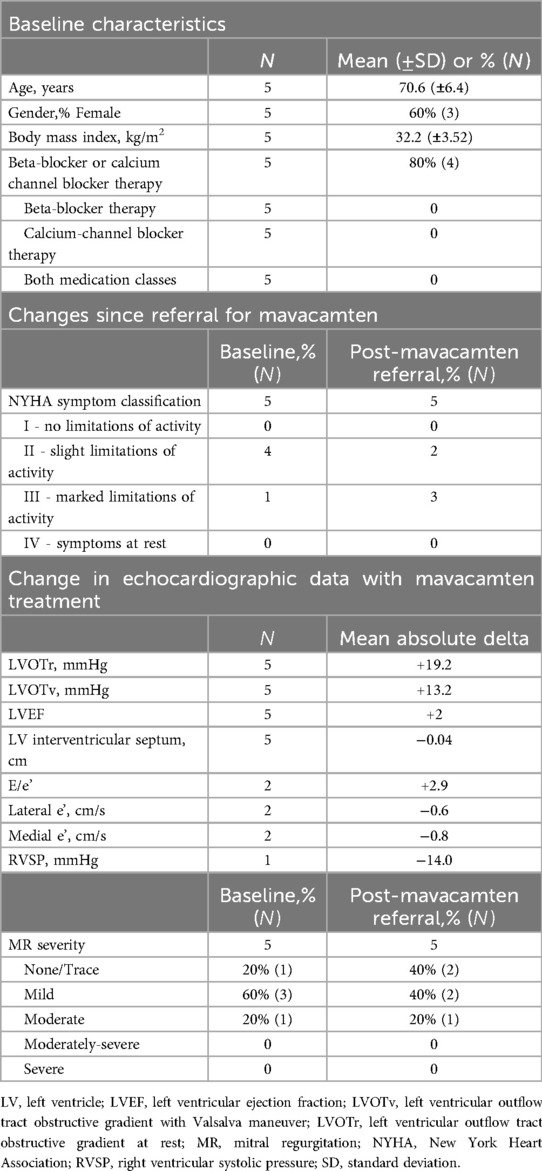
Table 2. Clinical characteristics of a subset of patients referred for mavacamten, who ultimately did not start the medication.
Two elderly patients with severe concomitant disease unrelated to oHCM (4%) died during follow up: One had pre-existing hypoxemic pulmonary hypertension that progressed. The second died from unrelated septic shock. One patient (2%) developed septic shock due to tricuspid valve endocarditis during which mavacamten was discontinued, and ultimately required ECMO and complex cardiac surgery. Only a limited subset of our cohort had pre- and post-mavacamten cardiopulmonary exercise stress testing (n = 7), precluding meaningful statistical analysis.
To enable timely and optimally monitored access to cardiac myosin inhibitor therapy for our patients, our center developed a nurse-led training course for the FDA REMS program (4). This training focuses on appropriate patient selection, prescription, authorization, triage and surveillance, and provides education on common clinical problems that can arise during therapy with an emphasis on interdisciplinary care. Particular emphasis was placed on REMS monitoring and dose adjustment based on echocardiographic parameters.
Discussion
Limitations of our real-world data include the lack of a single center performing all laboratories and echocardiograms – as a result, not all echocardiographic parameters were reported on follow-up imaging performed at local centers (per patient preferences) and an inability to track changes in laboratories (e.g., nt-proBNP and troponin levels) longitudinally. In addition, while we have collected one of the largest single-center collection of patients with oHCM on mavacamten, our sample size of 50 patients may be prone to regional bias of the population of patients served by our center. These limitations combine to potentially limit the generalizability of our findings. As a result of these shortcomings, we are actively collaborating in the HCM SHaRe registry to pool data with other centers to ensure that future analyses are representative of the larger United States population and sufficiently statistically powered to draw inferences from clinical, laboratory, and echocardiographic data (6).
Our novel data is one of the first real-world reports of mavacamten use in oHCM patients (3) and the resultant physiologic and clinical responses due to cardiac myosin inhibition (7, 8). Specifically, we highlight that mavacamten results in significant improvement in wall thickness, mitral regurgitation, LVOT obstruction (both at rest and with Valsalva), and symptomatic improvement in NYHA class outside of the clinical trial setting. Moreover, we did not find worsened arrhythmia burden or reductions in contractility in the vast majority of patients (two patients required temporary discontinuance for LVEF < 50%, as mandated by the FDA REMS monitoring (4). Adverse events were rare, unrelated to mavacamten itself, and seen solely in patients with disease too advanced to have been represented in clinical trials. These data lead us to conclude that mavacamten, as a first-in-class cardiac myosin inhibitor (7, 8), is safe and efficacious in real-world settings and can be broadly used in clinical practice for treatment of symptomatic oHCM.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Stanford University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DK: Conceptualization, Formal Analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. EC: Data curation, Writing – review & editing. EK-M: Data curation, Writing – review & editing. IP: Data curation, Writing – review & editing. WT: Data curation, Writing – review & editing. JO: Data curation, Writing – review & editing. YD: Data curation, Writing – review & editing. ML: Data curation, Writing – review & editing. EM: Data curation, Writing – review & editing. KH: Data curation, Writing – review & editing. IC: Data curation, Writing – review & editing. BK: Data curation, Writing – review & editing. EB: Data curation, Writing – review & editing. CB: Data curation, Writing – review & editing. AL: Data curation, Writing – review & editing. KL: Data curation, Writing – review & editing. NR: Data curation, Writing – review & editing. CL: Data curation, Writing – review & editing. AS: Data curation, Writing – review & editing. JK: Supervision, Writing – review & editing. MP: Supervision, Writing – review & editing. MK: Supervision, Writing – review & editing. KS: Supervision, Writing – review & editing. CW: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. MW: Supervision, Writing – review & editing. VP: Conceptualization, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing. HS: Writing – review & editing. EA: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
Stanford center for inherited cardiovascular disease author masthead
Karim I Sallam, Masataka Kawana, Chad S Weldy, Marco Perez, Joshua W Knowles, Jason Tso, Cindy Lamendola, Allysonne Smith, Nancy Robles, Colleen Bonnett, Ellen Bacolor, Kimberly Hecker, Isabella Cuenco, Beth Kao, Elise Munsey, Andrea Linder, Kathleen Lacar, Julia Platt, Chloe Reuter, Tia Moscarello, Ryan Murtha, Jennifer Kohler, Hannah Ison, Mitchel Pariani, Anusha Klinder, Priya Nair, Jennifer Marino, Andrea Linder, Ruchi Patel, Matthew T Wheeler, Euan A Ashley, Victoria N Parikh.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. VP has consulting and advisory relationships with BioMarin, Lexeo Therapeutics and Viz.ai and receives funding from BioMarin, the John Taylor Babbitt Foundation, the Sarnoff Cardiovascular Research Foundation, and NHLBI R01HL168059 and K08HL143185. MW reports research grant and in kind support from Bristol Myers Squibb, consulting for Leal Therapeutics, outside the submitted work. EA reports advisory board fees from Apple and Foresite Labs. EA has ownership interest in SVEXA, Nuevocor, DeepCell, and Personalis, outside the submitted work. EA is a board member of AstraZeneca. DK is supported by the Wu-Tsai Human Performance Alliance as a Clinician-Scientist Fellow, the Stanford Center for Digital Health as a Digital Health Scholar, and NIH 1L30HL170306. CW reports consultancy fees from AiRNA Bio and Avidity Biosciences. CW is supported by NIH grants K08HL167699, L30HL159413, F32HL160067 and American Heart Association grant 23CDA1042900.
Conflict of interest
VP has consulting and advisory relationships with BioMarin, Lexeo Therapeutics and Viz.ai and receives funding from BioMarin, the John Taylor Babbitt Foundation. MW reports research grant and in kind support from Bristol Myers Squibb, consulting for Leal Therapeutics, outside the submitted work. EA reports advisory board fees from Apple and Foresite Labs. EA has ownership interest in SVEXA, Nuevocor, DeepCell, and Personalis, outside the submitted work. EA is a board member of AstraZeneca. CW reports consultancy fees from AiRNA Bio and Avidity Biosciences.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1429230/full#supplementary-material
References
1. Members WC, Ommen SR, Ho CY, Asif IM, Balaji S, Burke MA, et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy a report of the American Heart Association/American College of Cardiology Joint Committee on clinical practice guidelines. J Am Coll Cardiol. (2024) 83(3):2324–405. doi: 10.1016/j.jacc.2024.02.014
2. Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC, et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. (2016) 351:617–21. doi: 10.1126/science.aad3456
3. Desai MY, Hajj-Ali A, Rutkowski K, Ospina S, Gaballa A, Emery M, et al. Real-world experience with mavacamten in obstructive hypertrophic cardiomyopathy: observations from a tertiary care center. Prog Cardiovasc Dis. (2024):S0033-0620(24)00022-7. doi: 10.1016/j.pcad.2024.02.001
4. Squibb B-M. CAMZYOS (mavacamten) REMS Program. Availability online at: https://www.camzyosrems.com (accessed November 14, 2023).
5. Sewanan LR, Shen S, Campbell SG. Mavacamten preserves length-dependent contractility and improves diastolic function in human engineered heart tissue. Am J Physiol-Hear Circ Physiol. (2021) 320:H1112–23. doi: 10.1152/ajpheart.00325.2020
6. Alaiwi SA, Roston TM, Marstrand P, Claggett BL, Parikh VN, Helms AS, et al. Left ventricular systolic dysfunction in patients diagnosed with hypertrophic cardiomyopathy during childhood: insights from the SHaRe registry. Circulation. (2023) 148:394–404. doi: 10.1161/circulationaha.122.062517
7. Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2020) 396:759–69. doi: 10.1016/s0140-6736(20)31792-x
Keywords: mavacamten, MYK-461, hypertrophic cardiomyopathy, cardiac myosin inhibitor, hypertrophic cardiomyopathy with obstruction (oHCM), hypertrophic obstructive cardiomyopathy (HOCM), left ventricular outflow tract obstruction
Citation: Kim DS, Chu EL, Keamy-Minor EE, Paranjpe ID, Tang WL, O’Sullivan JW, Desai YB, Liu MB, Munsey E, Hecker K, Cuenco I, Kao B, Bacolor E, Bonnett C, Linder A, Lacar K, Robles N, Lamendola C, Smith A, Knowles JW, Perez MV, Kawana M, Sallam KI, Weldy CS, Wheeler MT, Parikh VN, Salisbury H, Ashley EA and the Stanford Center for Inherited Cardiovascular Disease (2024) One-year real-world experience with mavacamten and its physiologic effects on obstructive hypertrophic cardiomyopathy. Front. Cardiovasc. Med. 11:1429230. doi: 10.3389/fcvm.2024.1429230
Received: 7 May 2024; Accepted: 13 August 2024;
Published: 30 August 2024.
Edited by:
Qianman Peng, Harvard Medical School, United StatesReviewed by:
Bruno Pinamonti, University Health Organization Giuliano Isontina (ASU GI), ItalyHector A. Cabrera-Fuentes, University of Giessen, Germany
Copyright: © 2024 Kim, Chu, Keamy-Minor, Paranjpe, Tang, O'Sullivan, Desai, Liu, Munsey, Hecker, Cuenco, Kao, Bacolor, Bonnett, Linder, Lacar, Robles, Lamendola, Smith, Knowles, Perez, Kawana, Sallam, Weldy, Wheeler, Parikh, Salisbury, Ashley and the Stanford Center for Inherited Cardiovascular Disease. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Euan A. Ashley, ZXVhbkBzdGFuZm9yZC5lZHU=; Heidi Salisbury, aHNhbGlzYnVyeUBzdGFuZm9yZGhlYWx0aGNhcmUub3Jn
†These authors have contributed equally to this work
‡These authors share senior authorship
 Daniel Seung Kim
Daniel Seung Kim Emily L. Chu1,†
Emily L. Chu1,† Wilson L. Tang
Wilson L. Tang Andrea Linder
Andrea Linder Joshua W. Knowles
Joshua W. Knowles Masataka Kawana
Masataka Kawana Karim I. Sallam
Karim I. Sallam Chad S. Weldy
Chad S. Weldy Matthew T. Wheeler
Matthew T. Wheeler