- Department of Cardiology, Fribourg University and Hospital, Fribourg, Switzerland
Background: Bioresorbable vascular scaffolds (BVSs) have been developed as a potential solution to mitigate late complications associated with drug-eluting metallic stents (DESs) in percutaneous coronary intervention for coronary artery disease. While numerous studies have compared BVSs to DESs, none have assessed clinical outcomes beyond 5 years.
Objectives: This study aimed to compare the 10-year clinical outcomes of patients treated with BVSs vs. DESs.
Methods: The EverBio-2 trial (Comparison of Everolimus- and Biolimus-Eluting Coronary Stents with Everolimus-Eluting Bioresorbable Vascular Scaffold) is a single-center, assessor-blinded, randomized controlled trial that enrolled 240 patients allocated in a 1:1:1 ratio to receive BVSs, everolimus-eluting stents, or biolimus-eluting stents (BESs). Clinical follow-up was scheduled for 10 years.
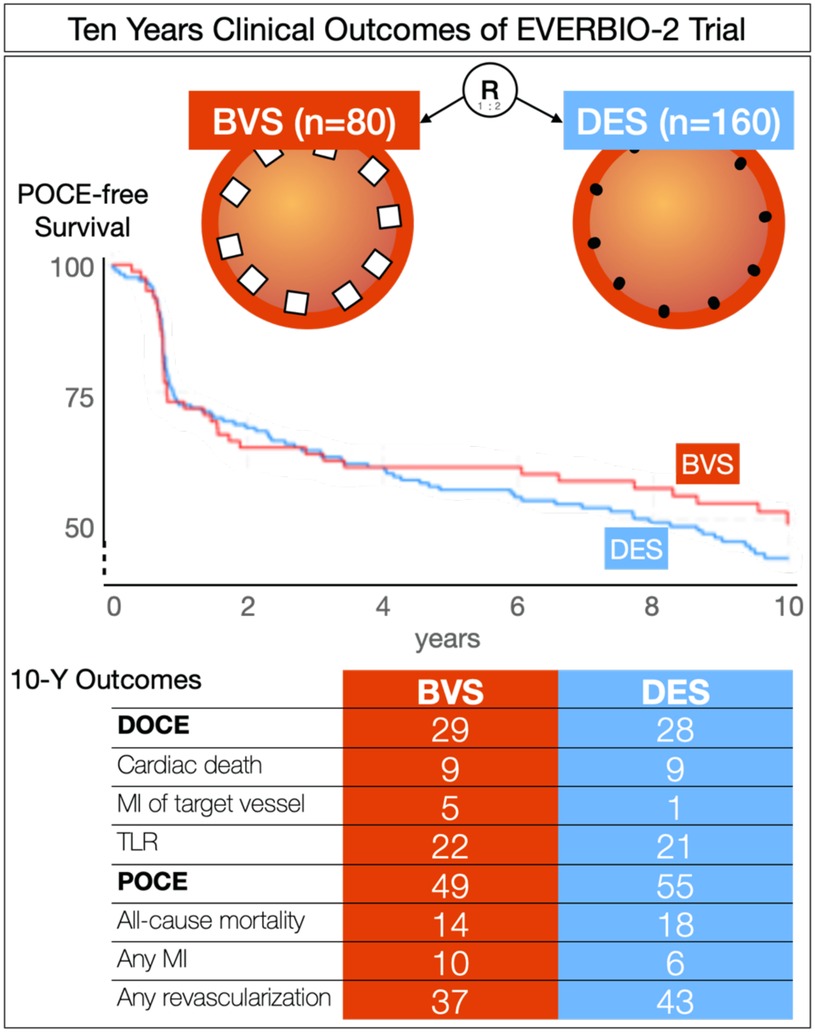
Results: Clinical follow-up was completed in 222 patients (93%) at the 10-year mark. The rate of device-oriented composite events (DOCE) was 28% in the DES group and 29% in the BVS group (p = 0.72) at 10 years. Similarly, the rate of patient-oriented composite events (POCE) was 55% in the DES group and 49% in the BVS group (p = 0.43) at 10 years. Notably, the rate of myocardial infarction (MI) within the target vessel was 5% in the BVS group and 0% in the BES group (p = 0.04), while the rate of any MI was 10% in the BVS group and 2% in the BES group (p = 0.04). In addition, the rate of Academic Research Consortium (ARC) possible stent thrombosis was 3% in the BVS group and 0% in the DES group (p = 0.04).
Conclusions: Over 10 years, the rates of clinical DOCE and POCE were similar between the BVS and DES groups but individual outcomes of stent thrombosis were higher (3%) in the BVS group compared to the DES group.
Clinical Trial Registration: ClinicalTrials.gov, identifier (NCT01711931).
Introduction
In interventional cardiology, bioresorbable vascular scaffolds (BVSs) have gained attention as an alternative to drug-eluting stents (DESs) since the first fully bioresorbable scaffold was described by Igaki-Tamai in 1998. The Absorb BVS was the first bioresorbable scaffold with drug-elution in 2006 (1). While DESs are the standard for percutaneous coronary intervention (PCI), BVSs were aimed to reduce late-occurring complications such as neoatherosclerosis and late stent thrombosis (2). Initially deemed safe for simple lesions (3), subsequent RCTs found the Absorb BVS comparable to DES at 1 year but divergent outcomes at 3 years (4–7), leading to early termination of one study due to higher device thrombosis rates at 2 years (8). Two meta-analyses confirmed increased scaffold thrombosis (9, 10). In early 2017, the European Society of Cardiology - European Association of Percutaneous Cardiovascular Interventions task force advised against routine BVS use, limiting it primarily to research (11). The most extensively studied BVS was withdrawn in September 2017, followed by an FDA warning (12). Ongoing monitoring of patients who received this BVS confirmed increased complications, while research has explored tailored stenting techniques, such as the “Predilatation, Sizing and Postdilatation” technique, in high-risk restenosis patients. These studies have yielded mixed results, from BVS non-inferiority to higher device thrombosis risks (13–17). Although the use of BVSs has been completely discontinued, over a million patients have been treated, and follow-up of these patients is necessary. In this regard, the current study presents the 10-year clinical follow-up of patients included in the EverBio-2 study.
Methods
The EverBio-2 trial (Comparison of Everolimus- and Biolimus-Eluting Coronary Stents with Everolimus-Eluting Bioresorbable Vascular Scaffold) is a single-center, assessor-blinded, randomized controlled trial in which 240 patients were randomly allocated (1:1:1) to the BVS, everolimus-eluting stent (EES), or biolimus-eluting stent (BES) groups and was conducted at University & Hospital Fribourg. The methods of the trial have previously been reported (18–21). Clinical follow-up was initially planned at 3, 6, 9, and 12 months and at 2, 5, and 10 years. Angiographic follow-up was scheduled for 9 months and 5 years. Clinical follow-up was conducted through clinic visits, phone calls, or correspondence. The primary endpoint was late lumen loss at 9 months. Clinical endpoints included Academic Research Consortium (ARC)-defined composites: device-oriented clinical events (DOCEs), which is a composite of cardiac death, target-vessel myocardial infarction (TV-MI), and target lesion revascularization (TLR); and patient-oriented clinical events (POCEs), which is a composite of all-cause death, any MI, and any revascularization. In addition, device thrombosis and target-vessel revascularization (TVR) were also assessed (22). Blinded assessors adjudicated angiographic and clinical outcomes. A complete list of the endpoints can be found elsewhere. All patients provided written, informed consent for participation and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The trial is registered in ClinicalTrials.gov, number (NCT01711931).
Studied devices
The Absorb BVS (Abbott Vascular) has a poly-dl-lactide coating that releases everolimus and is completely degraded via the Krebs cycle. The scaffold has 150-μm struts. The Promus Element stent (Boston Scientific, Marlborough, USA) is a platinum chromium alloy with everolimus (100 μg/cm2) applied in a durable polymer. The Biomatrix Flex stent (Biosensors Europe SA, Morges, Switzerland) is stainless steel (strut thickness of 112 μm) with an abluminal biodegradable polymer layer (20 μm) that elutes biolimus.
Statistical analysis
Categorical variables are reported as counts and percentages and continuous variables are reported as means and standard deviations. Categorical variables were compared using Fisher's exact test. Continuous variables were analyzed using Student’s t-test or the Wilcoxon rank-sum test according to their distribution. Survival analysis was performed using Kaplan–Meier curves and the log-rank test. Patients who received metallic stents were compared to patients treated with BVSs. To fully disclose the results, post hoc inferential statistics were performed comparing the distinct metallic DES individually to the BVS. All statistical analyses were performed using dedicated software (Stata version 18, StataCorp LP, College Station, TX, USA) at a two-tailed significance level of α = 0.05.
Results
Baseline patient characteristics
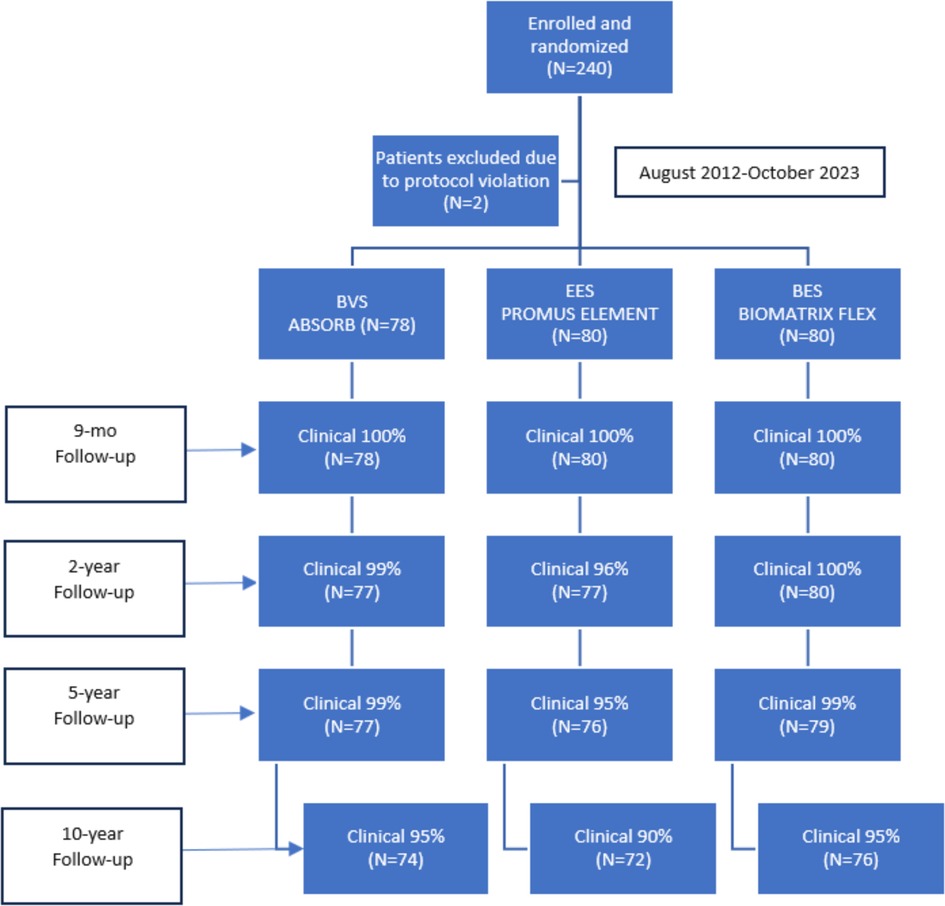
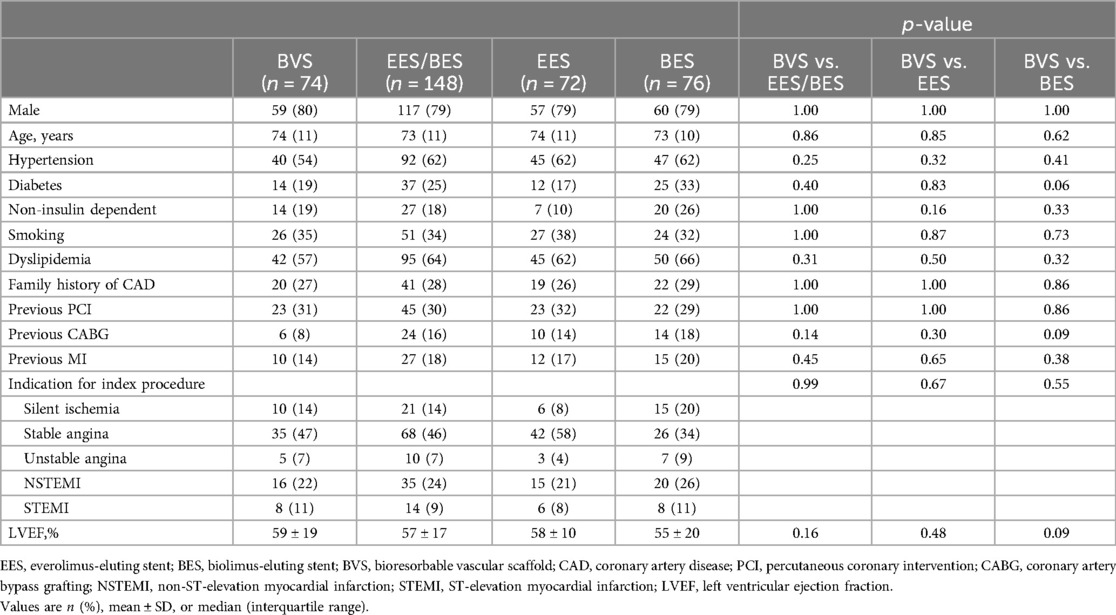
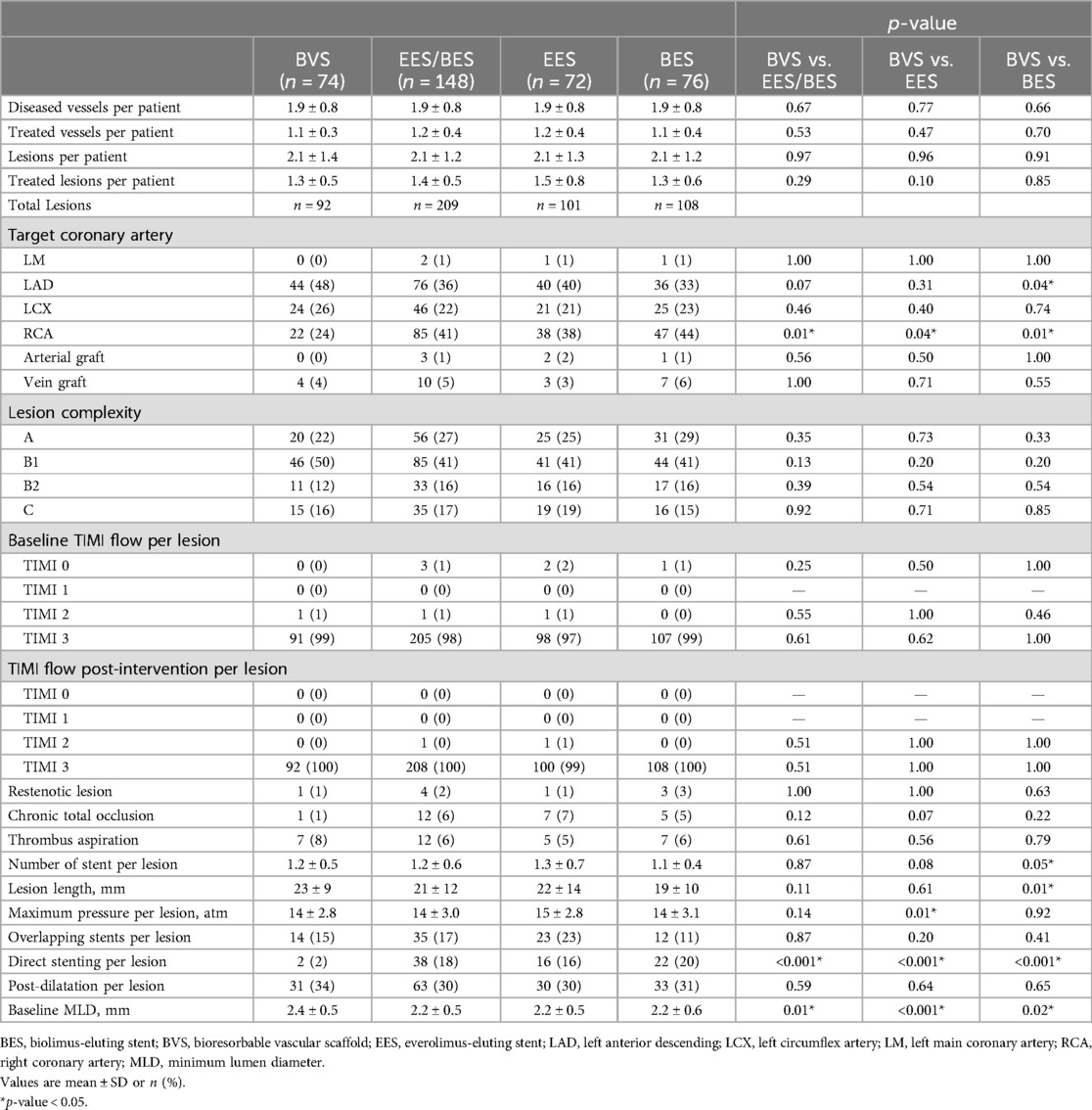
The flowchart up to 10 years follow-up is depicted in Figure 1. Baseline patient (Table 1) and angiographic and procedural (Table 2) characteristics were well balanced between the treatment arms. From the initial cohort of 240 patients enrolled in the study in 2012, 222 patients (93%) were available for clinical follow-up at 10 years. This represented 95% (n = 76) of patients in the EES group, 90% (n = 72) in the BES group, and 95% (n = 74) in the BVS group.
Clinical outcomes at 10 years
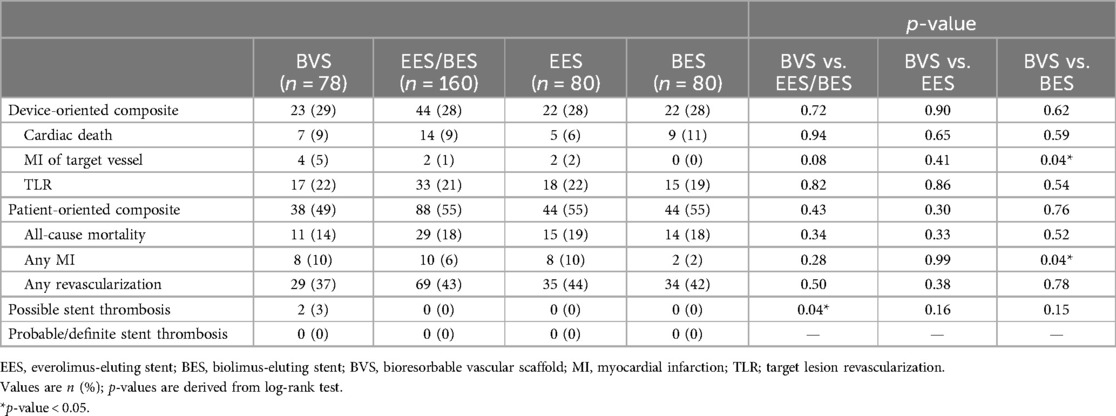
Clinical adverse events are detailed in Table 3.
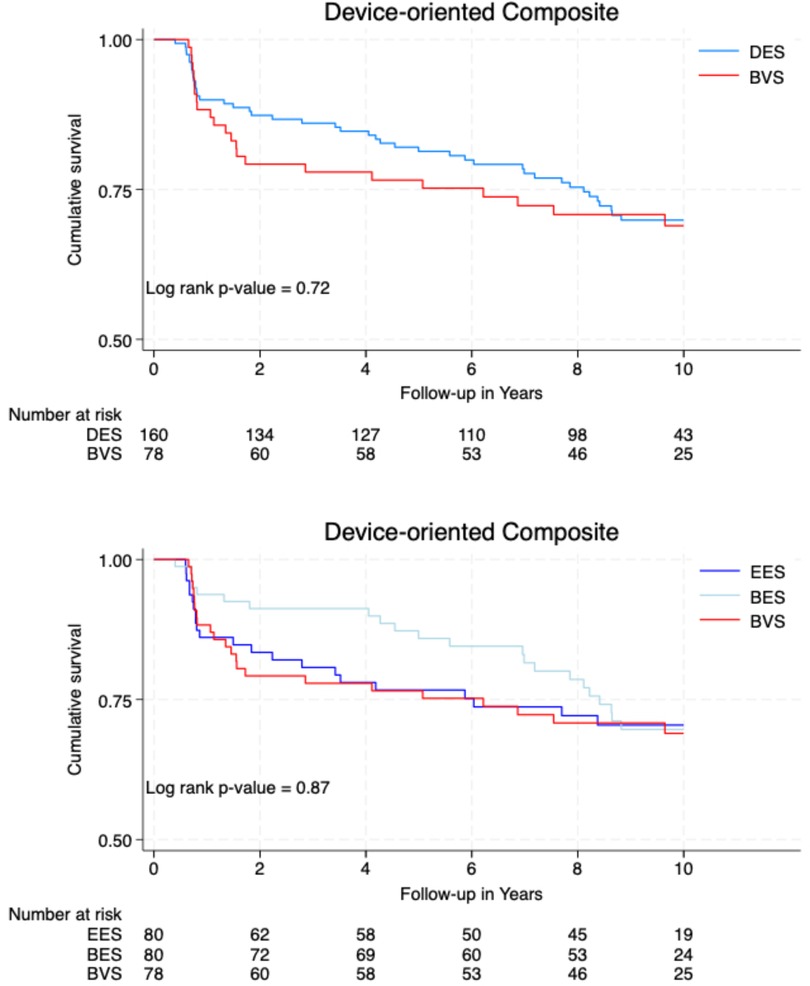
ARC-defined DOCEs occurred in 23 (29%) patients treated with a BVS, 22 (28%) patients in the EES group, and 22 (28%) patients in the BES group. There was no significant difference in the DOCE rates between the metallic DES (n = 44, 28%) and a BVS (n = 23, 29%; p = 0.72). Regarding individual endpoints, the rate of MI within the target vessel was significantly higher in the BVS group (n = 4, 5%) compared to the BES group (0, 0%; p = 0.04), with a trend toward significance when compared to the DES group (2, 1%; p = 0.08).
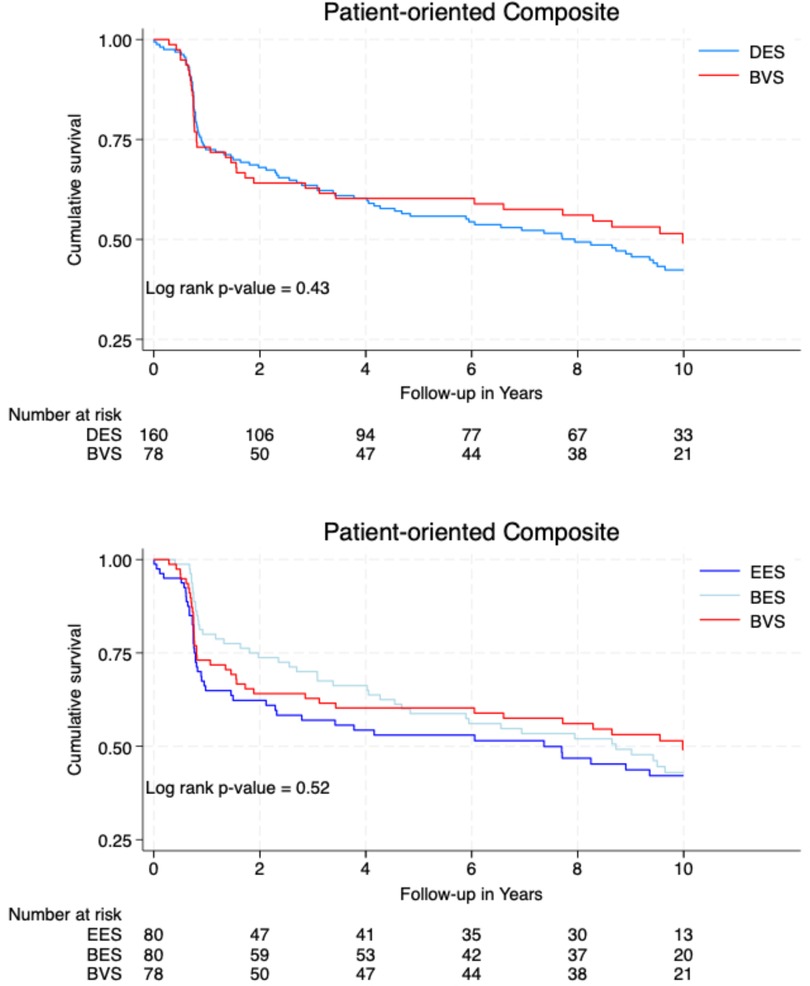
There was no significant difference in the POCEs between the DES (n = 88, 55%) and BVS groups (n = 38, 49%; p = 0.43). However, the rate of MI was significantly higher in the BVS group (n = 8, 10%) compared to the BES group (2, 2%; p = 0.04).
Possible stent thrombosis according to ARC definitions (22) occurred significantly more frequently in the BVS group (n = 2, 3%) compared to the DES group (0, 0%; p = 0.04). Neither probable nor definite stent thrombosis was observed.
Figure 2 illustrates the cumulative survival free from the occurrence of DOCEs and POCEs according to the type of implanted stent/scaffold.
Discussion
In the EverBio-2 trial, we compared a bioresorbable vascular scaffold with everolimus- and biolimus-eluting metallic stents in routine PCI. Initial analysis revealed comparable clinical outcomes for DESs and BVSs at 9 months (19), followed by subsequent studies showing no difference in major clinical outcomes at 2 (20) and 5 years (21) after inclusion in the same population. To our knowledge, this study represents the first examination of clinical outcomes in a head-to-head comparison of BVSs and DESs beyond 5 years.
Composite endpoints (DOCE and POCE)
This 10-year analysis comparing the Absorb BVS to DESs (EESs and BESs) did not show a significant difference in the major clinical outcomes, DOCEs and POCEs. DOCE occurrences were well balanced in the different groups with 28% in the DES group and 29% in the BVS group. POCE occurrences were insignificantly lower with a BVS (49%) than a DES (55%) (p = 0.43). Comparatively, in the study of the same population at 5 years, DOCEs were insignificantly more frequent in the BVS group (22%) than in the BES group (14%), but quite similar compared to the DES group (18%). POCE occurrences were insignificantly lower with a BVS (40%) than with a DES (43%). In major BVS studies [ABSORB-JAPAN (23), ABSORB-III (24), ABSORB-IV (25)], at 5 years, DOCEs or target vessel failure (TVF) in the BVS groups ranged from 16.1% to 23.2%, while POCEs ranged from 25% to 29.9%. The number of DOCEs and POCEs in our study showed a certain progression explained by the aging of a polymorbid population.
In the Reset study (26), a randomized trial comparing a new-generation everolimus-eluting stent to a first-generation sirolimus-eluting stent by Shiomi et al., at 10 years, among the 1,446 patients who received an everolimus-eluting stent, 26.7% experienced a TVF with 19.6% target lesion failure (TLF). These results align well with what we observed as investigators.
It is important to note that the significant increase in DOCEs and POCEs observed in our study is likely influenced by the high rate of revascularization resulting from the two serial angiographic follow-ups at 9 months and 5 years. In addition, it appears that a substantial number of events occurred around 8 years, coinciding with the COVID-19 pandemic (27), which has been associated with chest pain and elevated troponin levels, leading to more unplanned, repeat coronary angiograms. Moreover, multiple deaths of unknown cause, classified as cardiac deaths according to the standardized definitions for clinical endpoints in coronary stent trials, may have further contributed to the increase in DOCEs.
Risk of myocardial infarction
Although representing a low number of events, the individual endpoint of MI of the target vessel was significantly higher in the BVS group compared to the BES group, as was all-cause MI. This confirms what we had already described at the 5-year follow-up and is consistent with the 5-year results of the ABSORB-JAPAN, ABSORB-III, and ABSORB-IV studies. In the Reset study, at 10 years, among the 1,446 patients who received an everolimus-eluting stent, MI from any vessel was experienced by 8.6% of the patients which is similar to our findings.
Stent thrombosis
At 10 years, a possible stent thrombosis occurred only in the BVS group (n = 2, 3%), representing a significant difference compared to the DES group (n = 0, 0%; p = 0.04). In the same population at 5 years, there was one occurrence of possible stent thrombosis (1%) in the BVS group, with none in the DES group, which did not represent a significant difference. In major BVS studies (ABSORB-JAPAN, ABSORB-III, and ABSORB-IV), the 5-year stent thrombosis rate ranged from 1% to 3.8% in the BVS groups and this was significantly higher than in those with an EES only in ABSORB-III (BVS = 2.5%, EES = 1.1%; p = 0.03).
In the Reset study, at 10 years, among the 1,446 patients who received an everolimus-eluting stent, stent thrombosis was probable or definite in 1.3%.
Limitations
The present study has some limitations. First, with 240 patients and a follow-up loss of 16 individuals (7%), the sample size is insufficiently powered to detect small differences in clinical outcomes and does not allow for adjustment for multiple comparisons, which could lead to type 1 errors. Second, at the time of inclusion, there was no BVS-specific implantation protocol, resulting in procedural heterogeneity and lower rates of intracoronary imaging, pre-dilatation, sizing, and post-dilatation. This variability may have influenced clinical outcomes differently compared to more recent large-scale trials with dedicated implantation protocols. Finally, this was a single-center trial with uniform procedural strategies among operators, which may limit the generalizability of the findings to other centers.
Conclusions
In this 10-year clinical comparison of BVSs to DESs, we showed no difference in the major clinical outcomes of device-oriented composite or patient-oriented composite endpoints. We found increased rates of target-vessel MI (5%) and all-cause MI (10%) in the BVS group in comparison to BES but not in comparison to DES. The scaffold thrombosis rate was increased in the BVS group (3%) in comparison to the DES group despite low event occurrences (Figure 3).
As the first study available assessing the 10-year clinical evolution of patients who received a bioresorbable drug-eluting vascular scaffold, our clinical investigation shows relative stability in the occurrence of DOCEs and POCEs beyond 5 years without worrying signs indicating a high incidence increase of late stent complications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by local ethics committees of Fribourgand Vaud (043/12-CER-FR, PB_2017-00237). ClinicalTrials.gov, number NCT01711931. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SB: Writing – original draft, Writing – review & editing, Data curation, Formal Analysis, Methodology. MC: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing, Investigation. SL: Investigation, Writing – review & editing, Methodology, Project administration. DA: Writing – review & editing, Supervision, Validation. MT: Supervision, Writing – review & editing, Data curation, Investigation, Resources. SP: Data curation, Investigation, Writing – review & editing, Conceptualization, Formal Analysis, Methodology, Validation. SC: Conceptualization, Investigation, Validation, Writing – review & editing, Funding acquisition, Supervision, Writing – original draft.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. The Fonds Scientifique Cardiovasculaire, Fribourg, Switzerland, funded the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ormiston JA, Webster MW, Armstrong G. First-in-human implantation of a fully bioabsorbable drug-eluting stent: the BVS poly-L-lactic acid everolimus-eluting coronary stent. Catheter Cardiovasc Interv. (2007) 69:128–31. doi: 10.1002/ccd.20895
2. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. (2006) 48:193–202. doi: 10.1016/j.jacc.2006.03.042
3. Onuma Y, Serruys PW, Ormiston JA, Regar E, Webster M, Thuesen L, et al. Three-year results of clinical follow-up after a bioresorbable everolimus-eluting scaffold in patients with de novo coronary artery disease: the ABSORB trial. EuroIntervention. (2010) 6:447–53. doi: 10.4244/EIJ30V6I4A76
4. Serruys PW, Chevalier B, Sotomi Y, Cequier A, Carrié D, Piek JJ, et al. Comparison of an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent for the treatment of coronary artery stenosis (ABSORB II): a 3 year, randomised, controlled, single-blind, multicentre clinical trial. Lancet. (2016) 388:2479–91. doi: 10.1016/S0140-6736(16)32050-5
5. Onuma Y, Sotomi Y, Shiomi H, Ozaki Y, Namiki A, Yasuda S, et al. Two-year clinical, angiographic, and serial optical coherence tomographic follow-up after implantation of an Everolimus-eluting bioresorbable scaffold and an everolimus-eluting metallic stent: insights from the randomised ABSORB Japan trial. EuroIntervention. (2016) 12:1090–101. doi: 10.4244/EIJY16M09_01
6. Xu B, Yang Y, Han Y, Huo Y, Wang L, Qi X, et al. Comparison of everolimus-eluting bioresorbable vascular scaffolds and metallic stents: three-year clinical outcomes from the ABSORB China randomised trial. EuroIntervention. (2018) 14:e554–61. doi: 10.4244/EIJ-D-17-00796
7. Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS, et al. 3-year clinical outcomes with everolimus-eluting bioresorbable coronary scaffolds: the ABSORB III trial. J Am Coll Cardiol. (2017) 70:2852–62. doi: 10.1016/j.jacc.2017.10.010
8. Wykrzykowska JJ, Kraak RP, Hofma SH, van der Schaaf RJ, Arkenbout EK, IJsselmuiden AJ, et al. Bioresorbable scaffolds versus metallic stents in routine PCI. N Engl J Med. (2017) 376:2319–28. doi: 10.1056/NEJMoa1614954
9. Collet C, Asano T, Sotomi Y, Cavalcante R, Miyazaki Y, Zeng Y, et al. Early, late and very late incidence of bioresorbable scaffold thrombosis: a systematic review and meta-analysis of randomized clinical trials and observational studies. Minerva Cardioangiol. (2017) 65:32–51. doi: 10.23736/S0026-4725.16.04238-9
10. Ali ZA, Gao R, Kimura T, Onuma Y, Kereiakes DJ, Ellis SG, et al. Three-year outcomes with the absorb bioresorbable scaffold: individual-patient-data meta-analysis from the ABSORB randomized trials. Circulation. (2018) 137:464–79. doi: 10.1161/CIRCULATIONAHA.117.031843
11. Byrne RA, Stefanini GG, Capodanno D, Onuma Y, Baumbach A, Escaned J, et al. Report of an ESC-EAPCI task force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention: executive summary. Eur Heart J. (2018) 39:1591–601. doi: 10.1093/eurheartj/ehx488
12. FDA. FDA investigating increased rate of major adverse cardiac events observed in patients receiving Abbott Vascular’s Absorb GT1 Bioresorbable Vascular Scaffold (BVS)—letter to health care providers. (2017). Available online at: https://www.fda.gov/medical-devices/letters-health-care-providers. (Accessed March 15, 2024).
13. Kereiakes DJ, Onuma Y, Serruys PW, Stone GW. Bioresorbable vascular scaffolds for coronary revascularization. Circulation. (2016) 134:168–82. doi: 10.1161/CIRCULATIONAHA.116.021539
14. Bangalore S, Bezerra HG, Rizik DG, Armstrong EJ, Samuels B, Naidu SS, et al. The state of the absorb bioresorbable scaffold: consensus from an expert panel. JACC Cardiovasc Interv. (2017) 10:2349–59. doi: 10.1016/j.jcin.2017.09.041
15. Stone GW, Abizaid A, Onuma Y, Seth A, Gao R, Ormiston J, et al. Effect of technique on outcomes following bioresorbable vascular scaffold implantation: analysis from the ABSORB trials. J Am Coll Cardiol. (2017) 70:2863–74. doi: 10.1016/j.jacc.2017.09.1106
16. Smits PC, Chang CC, Chevalier B, West NEJ, Gori T, Barbato E, et al. Bioresorbable vascular scaffold versus metallic drug-eluting stent in patients at high risk of restenosis: the COMPARE-ABSORB randomised clinical trial. EuroIntervention. (2020) 16:645–53. doi: 10.4244/EIJ-D-19-01079
17. Stone GW, Ellis SG, Gori T, Metzger DC, Stein B, Erickson M, et al. Blinded outcomes and angina assessment of coronary bioresorbable scaffolds: 30-day and 1-year results from the ABSORB IV randomised trial. Lancet. (2018) 392:1530–40. doi: 10.1016/S0140-6736(18)32283-9
18. Arroyo D, Togni M, Puricel S, Baeriswyl G, Lehmann S, Corpataux N, et al. Comparison of everolimus-eluting and biolimus-eluting coronary stents with everolimus-eluting bioresorbable scaffold: study protocol of the randomized controlled EVERBIO II trial. Trials. (2014) 15:9. doi: 10.1186/1745-6215-15-9
19. Puricel S, Arroyo D, Corpataux N, Baeriswyl G, Lehmann S, Kallinikou Z, et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol. (2015) 65:791–801. doi: 10.1016/j.jacc.2014.12.017
20. Arroyo D, Gendre G, Schukraft S, Kallinikou Z, Müller O, Baeriswyl G, et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds: two-year clinical outcomes of the EVERBIO II trial. Int J Cardiol. (2017) 243:121–5. doi: 10.1016/j.ijcard.2017.05.053
21. Schukraft S, Arroyo D, Togni M, Goy JJ, Wenaweser P, Stadelmann M, et al. Five-year angiographic, OCT and clinical outcomes of a randomized comparison of everolimus and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. Catheter Cardiovasc Interv. (2022) 99:523–32. doi: 10.1002/ccd.29837
22. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313
23. Kozuma K, Tanabe K, Hamazaki Y, Okamura T, Ando J, Ikari Y, et al. Long-term outcomes of absorb bioresorbable vascular scaffold vs. everolimus-eluting metallic stent—a randomized comparison through 5 years in Japan. Circ J. (2020) 84:733–41. doi: 10.1253/circj.CJ-19-1184
24. Kereiakes DJ, Ellis SG, Metzger DC, Caputo RP, Rizik DG, Teirstein PS, et al. Clinical outcomes before and after complete everolimus-eluting bioresorbable scaffold resorption: five-year follow-up from the ABSORB III trial. Circulation. (2019) 140:1895–903. doi: 10.1161/CIRCULATIONAHA.119.042584
25. Stone GW, Kereiakes DJ, Gori T, Metzger DC, Stein B, Erickson M, et al. 5-year outcomes after bioresorbable coronary scaffolds implanted with improved technique. J Am Coll Cardiol. (2023) 82:183–95. doi: 10.1016/j.jacc.2023.05.003
26. Shiomi H, Kozuma K, Morimoto T, Kadota K, Tanabe K, Morino Y, et al. Ten-year clinical outcomes from a randomized trial comparing new-generation everolimus-eluting stent versus first-generation sirolimus-eluting stent: results from the RESET extended study. Catheter Cardiovasc Interv. (2023) 102:594–607. doi: 10.1002/ccd.30791
Keywords: percutaneous coronary intervention (PCI), drug-eluting stent (DES), bioresorbable vascular scaffold (BVS), randomized clinical trial, coronary artery disease
Citation: Bengueddache S, Cook M, Lehmann S, Arroyo D, Togni M, Puricel S and Cook S (2024) Ten-year clinical outcomes of everolimus- and biolimus-eluting coronary stents vs. everolimus-eluting bioresorbable vascular scaffolds—insights from the EVERBIO-2 trial. Front. Cardiovasc. Med. 11:1426348. doi: 10.3389/fcvm.2024.1426348
Received: 1 May 2024; Accepted: 22 August 2024;
Published: 10 September 2024.
Edited by:
Chun Chin Chang, Taipei Veterans General Hospital, TaiwanReviewed by:
Yuki Katagiri, Sapporo Higashi Tokushukai Hospital, JapanJiang Ming Fam, National Heart Centre Singapore, Singapore
Copyright: © 2024 Bengueddache, Cook, Lehmann, Arroyo, Togni, Puricel and Cook. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephane Cook, c3RlcGhhbmUuY29va0B1bmlmci5jaA==
†These authors have contributed equally to this work
 Samir Bengueddache
Samir Bengueddache Malica Cook†
Malica Cook† Diego Arroyo
Diego Arroyo Serban Puricel
Serban Puricel Stephane Cook
Stephane Cook