- 1Institute of Sports Medicine and Health, Chengdu Sport University, Chengdu, China
- 2School of Sport Human Science, Beijing Sport University, Beijing, China
- 3College of Physical Education and Health, Guangxi Normal University, Guilin, China
- 4College of Physical Education and Health, Longyan University, Longyan, China
- 5School of Physical Education, Jining University, Jining, China
Objective: Hypertension is a risk factor of cardiovascular disease. Dance, a type of aerobic exercise, is beneficial as a therapy in reducing blood pressure. This study aimed to systematically review the therapeutic effectiveness of dance therapy (DT) on blood pressure and blood lipid of patients with hypertension.
Methods: Searching CNKI, VIP, Wan Fang Databases, CBM, PubMed, EBSCO (MEDLINE), Cochrane Library, and Web of Science to collect randomized controlled trials (RCTs) about dance therapy in the treatment of patients with hypertension according to the inclusion and exclusion criteria, with the search time ranged from the date of database construction to January 2024. The Cochrane risk-of-bias tool and PEDro were used to evaluate the risk of included trials. The meta-analysis was implemented by using RevMan 5.4 and Stata 12.0 software.
Results: A total of 983 patients were included in 11 randomized controlled trials. According to the meta-analysis, compared with the control group, Dance Therapy effectively reduced systolic blood pressure (SBP) [MD = −7.45, 95% CI (−8.50, −6.39), p < 0.0001] and diastolic blood pressure (DBP) [MD = −2.95, 95% CI (−3.78, −2.13), p < 0.0001], and it increased high-density lipoprotein cholesterol (HDL-C) [MD = 0.20, 95% CI (−0.02, 0.42), p < 0.0001]. The subgroup analysis results showed that the treatment efficacy was more excellent with the frequency more than 3 times per week, the cycle less than 12 weeks, and the duration less than 60 min every time.
Conclusion: The results indicates that SBP, DBP, and HDL-C in hypertensive patients have been effectively improved after dance therapy intervention. In addition, it is recommended to implement dance therapy for hypertensive patients with a treatment cycle of 12 weeks, and treat at least 3 times a week, with each treatment duration controlled within 60 min.
Systematic Review Registration: [http://www.crd.york.ac.uk/PROSPERO], identifier [CRD42024500807].
1 Introduction
Hypertension is the most prevalent risk factor of cardiovascular disease. About 9.4 million individuals worldwide succumb to cardiovascular complications or other complications caused by hypertension every year, accounting for about one fifth of the total deaths worldwide (1). Globally, over 1.3 billion individuals suffer from hypertension (2, 3). It is estimated that the proportion of patients with hypertension will rise to 29%, about 1.5 billion people, in 2025 (4). It is a very serious challenge for the global healthcare system. Drug therapy to reduce blood pressure entail higher costs, increased side effects and poor patient compliance (5). In 2020, the World Health Organization proposed that physical activity in patients with hypertension can improve the incidence of cardiovascular disease, reduce mortality, and improve the quality of life (6). Effective physical exercise and a healthy lifestyle constitute pivotal elements in cardiovascular disease prevention and blood pressure management. Aerobic physical exercises, such as dancing, walking, jogging and swimming, no less than 5 days per week and no less than 30 min per day are conducive to reducing blood pressure (7–9). Low salt diet and reducing the intake of red meat, sugar and trans-fat are also conducive to blood pressure management (10, 11).
Dance, as a long-term, strong dynamic, large muscle group participation and communal aerobic exercise, plays a positive role in reducing blood pressure, improving cardiopulmonary function, enhancing muscle strength and physical flexibility, alleviating stress and anxiety, and promoting healthy lifestyle habits (12). Dance therapy (DT), which is derived from dance, is a therapeutic method that combines personal emotion, cognition, physicality, and social interaction through dance to regulate emotions, manage illnesses, and establish physical and mental balance (13).
Different studies employed different exercise methods, durations, frequencies, and cycles of DT interventions, leading to different results in blood pressure improvement among patients with hypertension. In this systematic review and meta-analysis, we integrated the results of recent randomized controlled studies (RCTs) on dance therapy interventions for hypertension, and conducted a systematic, objective and quantitative statistical analysis. This study aims to investigate the potential benefits of DT on blood pressure and blood lipid levels in patients with hypertension, and to offer the latest evidence-based guidance for the clinical practice of DT.
2 Materials and methods
2.1 Retrieval strategy
The systematic review and meta-analysis were planned and implemented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (14). The review project was registered on the international prospective register of systematic reviews (http://www.crd.york.ac.uk/PROSPERO) with a registration number CRD42024500807.
We searched multiple databases including CNKI, VIP, Wanfang Data, China Biology Medicine disc (CBM), PubMed, EBSCO (MEDLINE), Cochrane Library and Web of Science. We limited the search period from the dates of databases established to January 2024. The final search was conducted on January 10, 2024. The search terms comprised “dance”, “dancing exercises”, “dance training”, “aerobic dance”, “hypertension”, “blood pressure”, “high blood pressure” and “hypertension”. In order to obtain the most complete RCTs related to the treatment of DT in hypertensive patients, we also traced the references of the included literature to supplement the retrieval of relevant literature. The complete search strategies for each database are provided in Supplementary Table S1.
2.2 Literature inclusion, exclusion criteria and outcome indicator
Inclusion criteria: ① The study design should be a randomized controlled trial about DT in the treatment of patients with hypertension. ② participants included in the study were patients with primary hypertension (regardless of gender, age, race and nationality). Diagnosis of hypertension adhered to international diagnostic criteria, such as systolic blood pressure (SBP) ≥ 140 mmHg (1 mmHg = 0.133 kPa) and diastolic blood pressure (DBP) ≥ 90 mmHg. Studies involving participants with secondary hypertension or other cardiovascular diseases were excluded. ③ The main intervention method in the included studies should be dance training alone or in combination with other interventions. The intervention method of the controls should be any other treatment method, including conventional medication treatment, usual care, health education, other exercise therapy or no treatment. ④ The included studies should have complete original data for direct or indirect extraction for analysis. ⑤ The included should be published in either Chinese or English.
Exclusion criteria: Non-Chinese and English literature, repeatedly published literature, literature from which data extraction and retrieval of the original text were not effectively feasible, non-journal-published research, animal studies, cross-sectional studies, non-clinical experimental research, and experimental research without intervention design were excluded.
Outcome indicators included systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), Body Mass Index(BMI) and Resting heart rate (RHR).
2.3 Literature screening and information extraction
Literature screening and data extraction were conducted by two independent reviewers (Jialin Wang and Yongsheng Liu). Any disagreements encountered during cross-checking will be resolved through discussion or negotiation with the third reviewer (Qihan Lin). During literature screening, we reviewed the article titles first. Subsequently, we excluded obviously irrelevant literature and proceeded to review the abstracts and full texts to determine inclusion. If necessary, we will contact the original study author via email or phone to obtain the undetermined information.
Data extraction encompassed the following aspects: ① Basic information of included studies, including the first author, the publication year, the sample size, the intervention measure, the intervention frequency, the intervention duration and the evaluation indicator; ②Specific intervention details and the follow-up duration; ③ Key elements of bias risk assessment; ④ Outcome indicators and measurement data of interest.
2.4 Assessment of risk of bias and study quality
Two independent reviewers (Jialin Wang and Yikun Yin). conducted the assessment of risk of bias and study quality. If the evaluation results of the two reviewers are inconsistent, consultation with the third independent reviewer (Zhengze Yu) is required. Risk of bias was assessed using Cochrane Collaboration Tool 5.1.0 for randomized controlled trials. Risk of bias figures were generated using RevMan5.4. The Physiotherapy Evidence Database (PEDro) scale was used to assess the risk of bias and methodological quality of included studies (15). The PEDro scale scores study on a scale of 0–10. The evaluation criteria state that 1 point is awarded for each criterion met from items 2–11, with a maximum score of 10 points. Studies scoring ≥6 are considered high quality, those scoring 4–5 are considered moderate quality, and those scoring ≤3 are considered low quality (16).
2.5 Statistical analysis
Statistical analysis of data extracted from the included literature was performed using RevMan5.4, a meta-analysis software provided by the Cochrane Collaboration. Continuous variable outcome indicators from the included literature were statistically analyzed using mean difference (MD) and 95% confidence interval (CI). If the same outcome indicator is extracted with identical measurement methods and units, the weighted mean difference (WMD), the weighted mean difference (WMD) is selected as the effect size indices. Heterogeneity among the results of the included studies was assessed using p-value and I2 quantification. No heterogeneity was observed among studies when p ≥ 0.10, whereas heterogeneity existed when p < 0.10. I2 represents the level of heterogeneity between studies. If I2 < 50%, it indicates that there was slight heterogeneity between the studies, and the fixed effect model was used for analysis. If I2 ≥ 50%, there was heterogeneity in the study, and the random effect model was used for analysis (17). The α value was set at 0.05.Additionally, Stata 12.0 software was employed to perform publication bias analysis and sensitivity analysis using Begg's test for studies with more than 5 included outcome indicators. The threshold for statistical significance was set at p < 0.05 (18).
3 Result
3.1 Study selection
461 studies were retrieved from the databases, and 3 studies were found through reference tracing. Endnote X9 was used to manage retrieved literature and remove duplicates, resulting in 268 studies remaining. After reading the title and abstract, 29 studies were selected. After reading the full texts, 18 studies were excluded due to not meeting the inclusion and exclusion criteria. Finally, 11 studies were included (7, 12, 19–27). The process of study inclusion is shown in Figure 1.
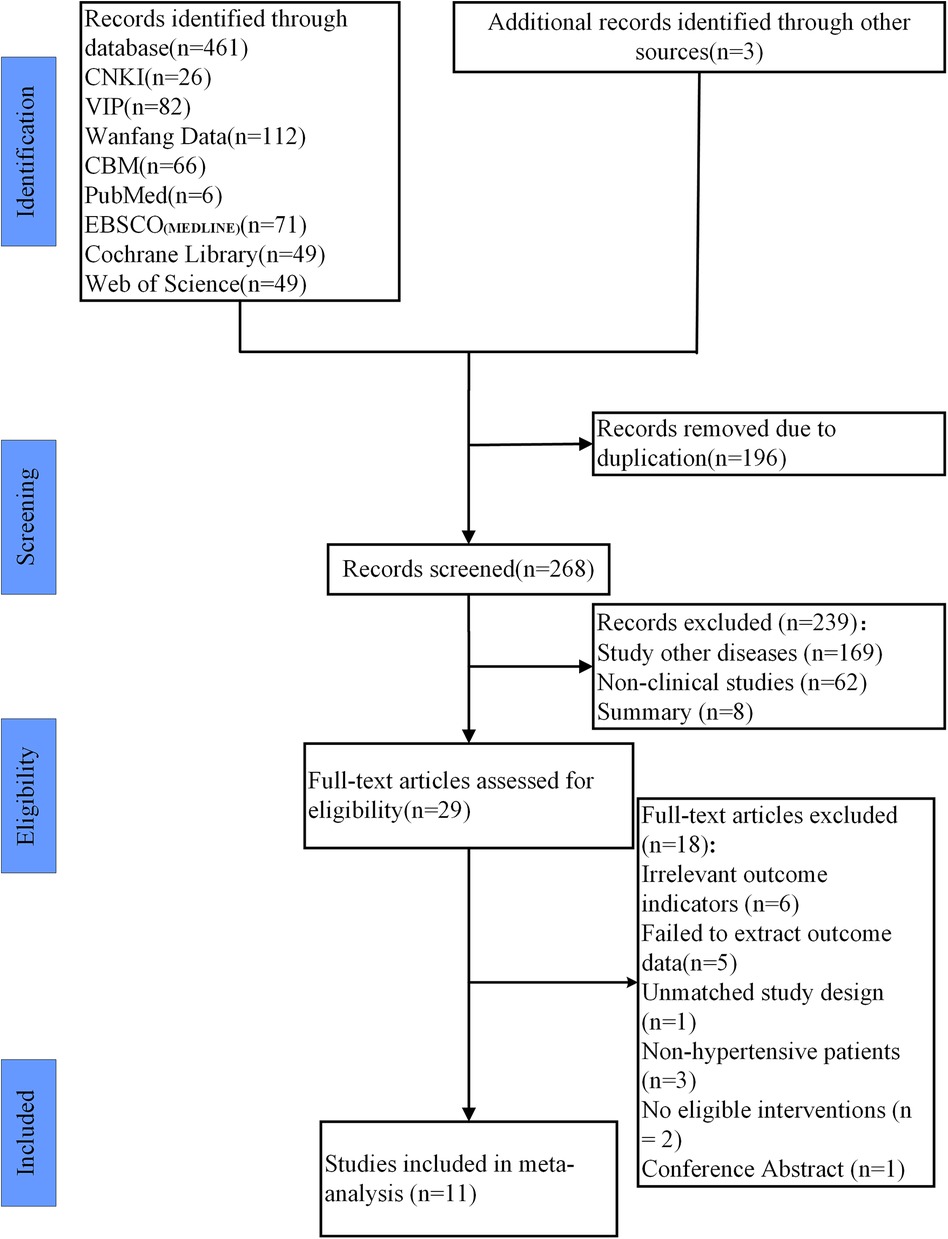
Figure 1. Study selection represented by PRISMA flowchart. Note: After screening the literature, we reviewed the references and found an additional 3 studies.
3.2 Study characteristics
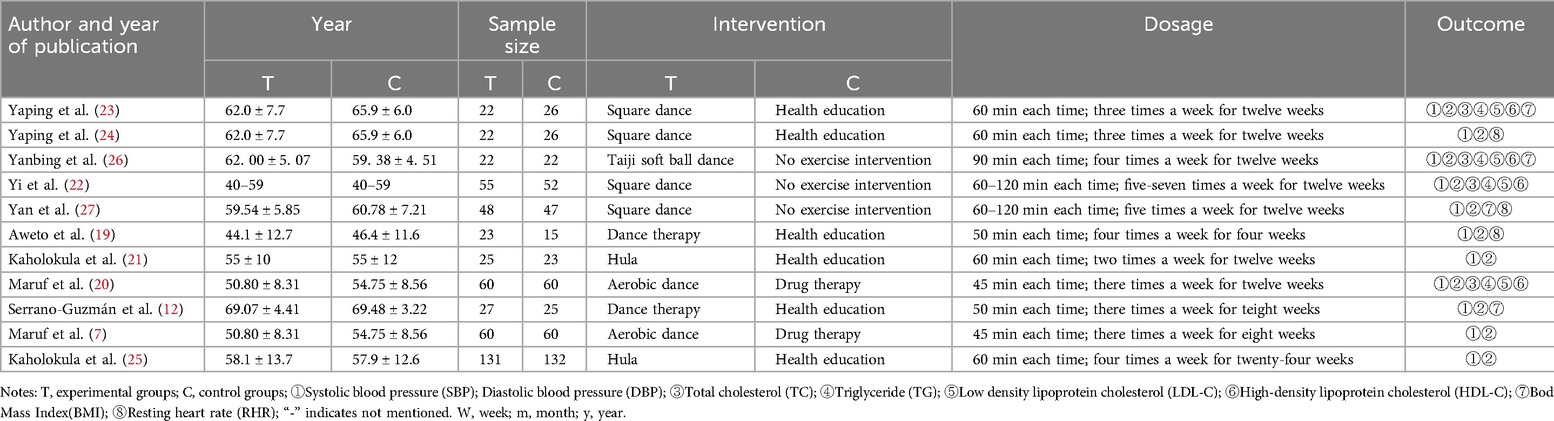
11 included studies involved a total of 983 participants (7, 12, 19–27), with intervention durations ranging from 4 to 24 weeks. Participants’ average age ranged from 31.4 to 72.7 years, and sample sizes ranged from 44 to 120 participants. 9 of the included studies, accounting for 90%, were published in the past decade (2014–2023). Among the included studies, 4 studies utilized square dance as the intervention for the experimental group (22–24, 27), 2 studies used aerobic dance (7, 20), and 6 studies employed health education as the intervention for the control group (12, 19, 21, 23–25). Additionally, 3 studies had no exercise intervention (22, 26, 27), while 2 studies conducted drug therapy (7, 20). Among the included studies, 7 had a 12-week exercise cycle, 3 had a cycle of less than 12 weeks, with an average cycle duration of 10.4 weeks. The average exercise frequency was 3.5 times per week. 6 studies had exercise durations of 60 min or more, while 4 had durations less than 60 min. The details of study characteristics are shown in Table 1 and Supplementary Table S2.
3.3 Study quality
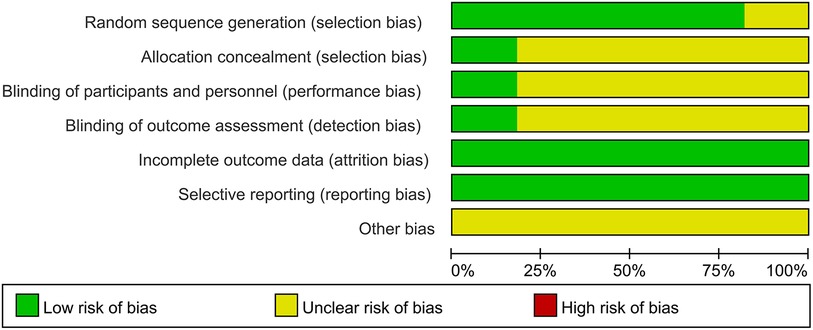
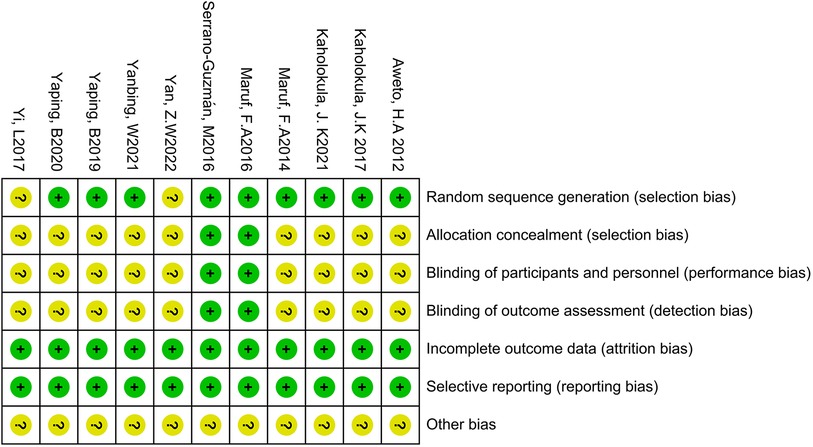
The quality of the 11 included studies was assessed using the Cochrane Collaboration Tool. The results are presented in Figures 2, 3. All studies underwent randomization grouping. According to the PEDro score, all included studies were of high quality, with an average score of 6.36 points. The included studies generally met high quality standards. Supplementary Table S3 presents a detailed summary of the methodological quality assessment, including individual PEDro scores for each study.
3.4 Meta-analysis results
3.4.1 Effects of DT on SBP
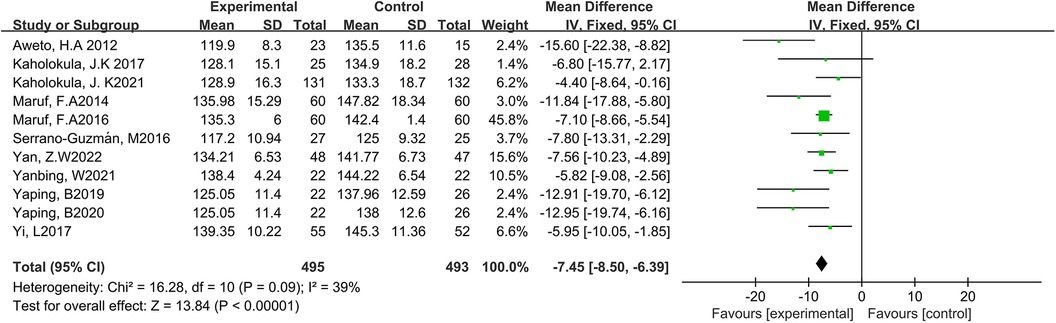
Eleven studies reported the effect of DT on SBP (7, 12, 19–27). Due to the low heterogeneity among the included studies (p = 0.09, I2 = 39%), the fixed-effect model was employed for analysis. The results showed a statistically significant reduction in SBP through DT intervention [MD = −7.45, 95% CI (−8.50, −6.39), p < 0.0001]. As shown in Figure 4.
3.4.2 Effects of DT on DBP
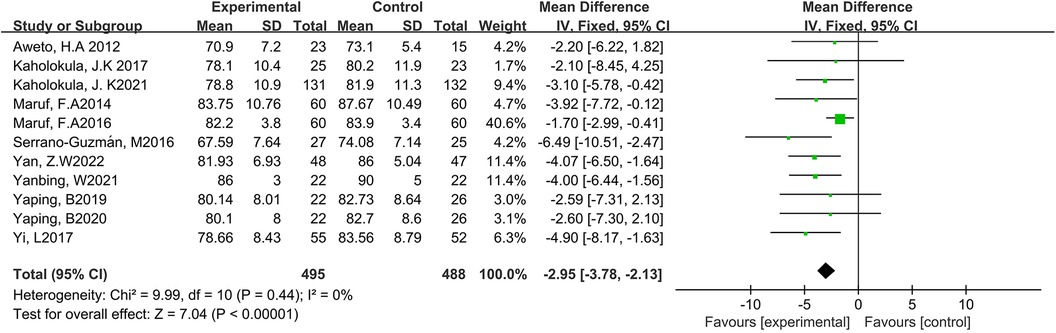
Eleven studies reported the effect of DT on DBP (7, 12, 19–27). Due to the low heterogeneity among the included studies (p = 0.44, I2 = 10%), the fixed-effect model was employed for analysis. The results showed a statistically significant reduction in DBP through DT intervention [MD = −2.95, 95% CI (−3.78, −2.13), p < 0.0001]. As shown in Figure 5.
3.4.3 Effects of DT on TC, TG, HDL-C and LDL-C
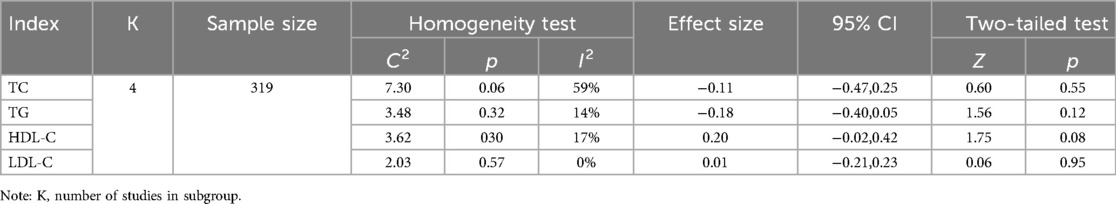
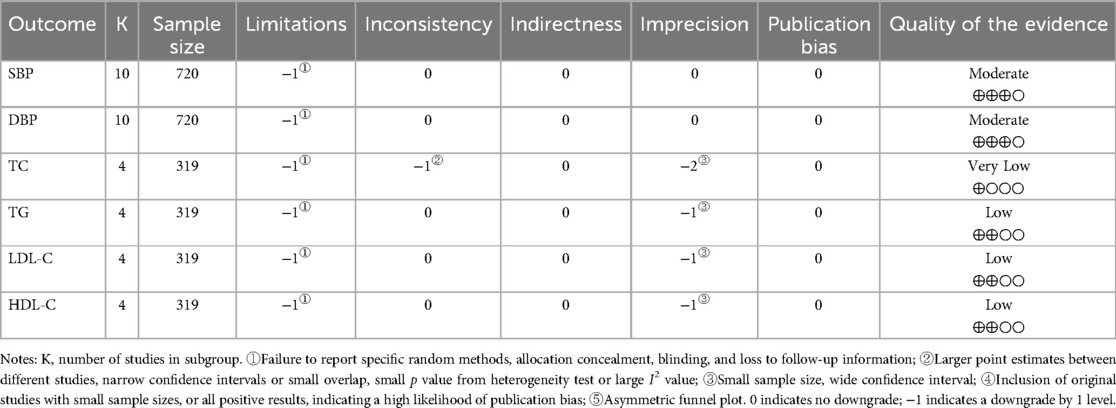
Four studies reported the effect of DT on TC, TG, HDL-C and LDL-C (20, 22, 23, 26). The heterogeneity among the included studies respectively was TC (p = 0.06, I2 = 59%), TG (p = 0.32, I2 = 14%), HDL-C (p = 0.30, I2 = 17%) and LDL-C (p = 0.57, I2 = 0%). Due to the high heterogeneity among the included studies for TC, the random-effect model was employed for analysis. The results indicated that DT did not significantly reduce TC levels [MD = −0.11, 95% CI (−0.47, 0.25), p = 0.55]. Due to the low heterogeneity among the included studies for TG, HDL-C and LDL-C, the fixed-effect model was employed for analysis. The results revealed that DT significantly improved HDL-C levels in hypertensive patients [MD = 0.20, 95% CI (−0.02, 0.42), p < 0.0001]. However, DT did not have a significant effect on TG and LDL-C levels in hypertensive patients. As shown in Table 2.
3.4.4 Effects of DT on BMI and RHR
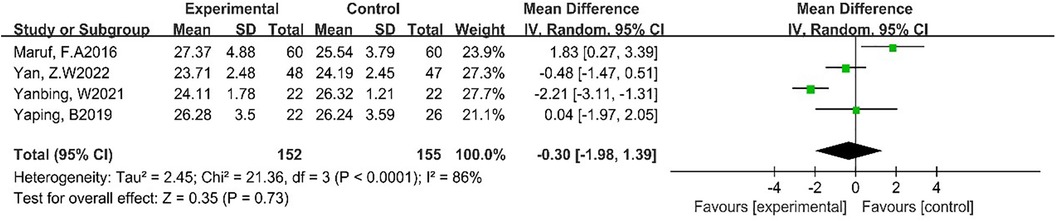
Four studies reported the effect of DT on BMI (7, 23, 26, 27). Due to the high heterogeneity among the included studies (p < 0.0001, I2 = 86%), the random-effect model was employed for analysis. The results showed that DT did not significantly reduce BMI [MD = −0.30, 95% CI (−1.98, 1.39), p = 0.73]. As shown in Figure 6.
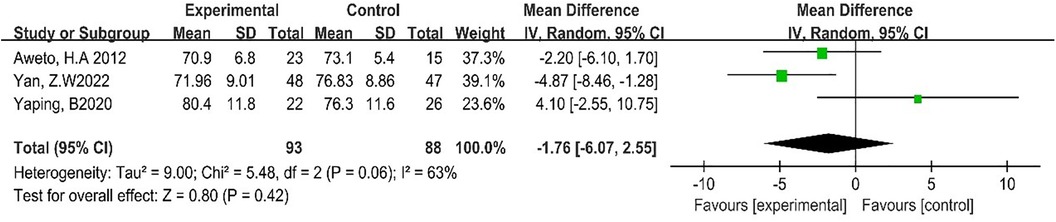
Three studies reported the effect of DT on RHR (19, 24, 27). Due to the high heterogeneity among the included studies (p = 0.06, I2 = 63%), the random-effect model was employed for analysis. The results showed that DT did not significantly improve RHR [MD = −1.76, 95% CI (−6.07, 10.75), p = 0.42]. As shown in Figure 7.
3.4.5 Subgroup analysis
Considering that exercise cycle, frequency and duration may impact blood pressure improvement in hypertensive patients, we conducted a subgroup analysis. The subgroup analysis results indicated that DT significantly reduced SBP and DBP levels in hypertensive patients when the exercise cycle was less than 12 weeks, the frequency was more than 3 times per week, and each session lasted less than 60 min. As shown in Supplementary Table S4.
3.4.6 Assessment of publication bias
Egger's test was employed to assess publication bias in SBP and DBP outcome indicators. The results showed that both SBP (t = −1.76, p = 0.112) and DBP (t = −1.90, p = 0.089) had p-values greater than 0.05, suggesting no significant publication bias. As shown in Supplementary Figures S1, S2.
3.4.7 GRADE quality evaluation
We conducted GRADE quality evaluation for the key outcome indicators of the included studies, included SBP, DBP, TC, TG, LDL-C, and HDL-C. GRADE system categorizes evidence quality into four levels: high, medium, low, and very low. It evaluates evidence based on five factors: risk of bias, inconsistency, imprecision, indirectness, and publication bias. The results are presented in Table 3.
4 Discussion
4.1 Effects of DT on blood pressure in patients with hypertension
Continuous elevation of systolic and diastolic blood pressure in patients with hypertension leads to chronic strain on the heart and blood vessels, causing the blood vessels gradual loss of elasticity and becoming stiffness, and increasing susceptibility to cardiovascular and cerebrovascular diseases, such as coronary heart disease, myocardial infarction, and stroke (28, 29). Research has shown that aerobic exercise may be highly effective in lowering blood pressure in hypertensive patients (30). Dance involves prolonged activity, engages large muscle groups, and fosters strong motivation and social cohesion. Dance exercise of appropriate intensity positively impacts cardiovascular blood circulation, enhancing myocardial contractility and output while alleviating cardiac strain, leading to reducing the burden of heart, preventing and improving cardiovascular disease (27, 31). Previous meta-analysis suggested that effective aerobic physical activity in hypertensive patients could reduce SBP by 5–10 mmHg and DBP by 1–6 mmHg (32). This meta-analysis showed a reduction in SBP by 7.65 mmHg and DBP by 2.94 mmHg following DT intervention consistent with previous research findings (32). The reduction of blood pressure plays a crucial role in the prevention of cardiovascular and cerebrovascular diseases, as a 5 mmHg decrease in SBP correlates with a 13% decrease in stroke risk (33, 34).
4.2 Effects of DT on blood lipid in patients with hypertension
Hypertension is frequently associated with dyslipidemia (35). Dyslipidemia impacts cell membrane lipid composition, leading to disrupting Ca2+ transport, damaging the vascular endothelium, promoting vascular smooth muscle cell hypertrophy, and inducing arterial structural alterations (36, 37). Arterial structural abnormalities and stiffening impede blood flow, exacerbating dyslipidemia and initiating a vicious cycle of elevated blood pressure and aberrant lipid metabolism (38, 39). Therefore, controlling lipid levels is crucial for managing blood pressure in hypertensive patients. This meta-analysis revealed improvements in TC, TG, and HDL-C following DT intervention compared to the controls, with the most significant improvement seen in HDL-C, but not in LDL-C. Long-term aerobic exercise promotes fat utilization for energy, leading to gradual normalization of lipid metabolism and blood sugar levels (40). HDL-C, an anti-atherosclerotic lipoprotein, acts as a protective factor against coronary atherosclerotic heart disease, facilitating cholesterol clearance and prevention of atherosclerosis and coronary heart disease (41). Moreover, HDL-C possesses anti-inflammatory and antioxidant properties, safeguarding vascular endothelial cells from damage, so HDL-C levels are important for lipid metabolism in hypertensive patients (42). Regular exercise can enhance lipoprotein lipase activity and the skeletal muscle's ability to utilize fatty acids for energy, thereby promoting lipid metabolism (43). Exercise may also regulate the synthesis, transport, and catabolism of lipoproteins by altering the activities of lecithin-cholesterol acyltransferase (LCAT), lipoprotein lipase (LPL), and hepatic triglyceride lipase (HTGL) (44). Additionally, exercise can induce lipolysis of lipids in adipose tissue and muscle, transporting fatty acids to muscles and regulating transmembrane transport and mitochondrial metabolism of fatty acids in muscle cells, ultimately improving lipid metabolism and lowering TC, TG, and LDL-C levels in patients with hypertension (45).
4.3 Effects of DT on BMI and RHR in patients with hypertension
With the improvement of living standards, the prevalence of obesity has been increasing annually (46). Excess body fat significantly influences the occurrence and development of cardiovascular diseases such as hypertension (47, 48). obesity can lead to metabolic disorders and increase the risk of cardiovascular diseases, thus increasing the incidence and mortality of hypertension (49). Heart rate, a clinical indicator for assessing sympathetic nervous system activity, is frequently employed to predict the prognosis of cardiovascular diseases such as hypertension (50). Patients with hypertension generally exhibit heightened sympathetic nervous system activity, contributing to elevated heart rates. Long-term maintenance of this state will lead to increased blood vessel pressure, aggravate hypertension, and precipitate severe conditions such as myocardial infarction, posing a life-threatening risk to patients (51, 52). This meta-analysis showed that BMI and RHR improved after the dance therapy intervention, however, the improvements were not statistically significant relative to the control group (p > 0.05). Dance can control the body mass of hypertensive patients, enhance cardiac contractility and muscle endurance, modulate vagal tone and diminish sympathetic nervous system activity (53). This enhances the efficiency of blood pumping by the heart, thereby reducing the heart rate (54).
5 Conclusion
Dance, as a group activity, fosters integration within hypertensive patients’ communities, rebuilds social relationships and preserves youthful vitality. In addition, dance exercise can also relieve stress, alleviate anxiety and promote physical and mental relaxation (55). Through enhancing the function of the human endocrine system, dance can regulate emotions and psychological states, thereby releasing personal pressure (56). It significantly benefits the physical and mental health of hypertensive patients and improves the treatment effects of susceptible diseases in middle-aged and elderly people (24).
This systematic review and meta-analysis provided evidence indicating a significant reduction in SBP and DBP following dance therapy. When the exercise cycle was less than 12 weeks, the exercise frequency was more than 3 times per week, and the duration of each exercise was less than 60 min, the levels of SBP and DBP decreased greatly. Therefore, it is recommended that patients with hypertension perform DT for 12 weeks as an exercise cycle, with sessions exceeding 3 times weekly, each lasting within 60 min. At present, there lacks a standardized exercise prescription for dance therapy. It is necessary for further research to provide additional clinical evidence for the application and promotion of dance therapy.
6 Limitation
(1) This systematic review and meta-analysis included only 11 studies, predominantly from Chinese sources, and the difference in studies quality might influence the results.
(2) None of the studies we included provided post-intervention follow-up.
(3) High heterogeneity among some included studies may affect the reliability of the results of meta-analysis.
(4) Differences in exercise prescription designs for dance therapy within the included studies may influence the intervention effect to some extent and may affect the reliability of the meta-analysis results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JW: Writing – original draft, Data curation, Conceptualization. YY: Writing – review & editing, Writing – original draft. ZY: Software, Writing – review & editing. QL: Methodology, Software, Supervision, Writing – review & editing. YL: Writing – review & editing, Visualization, Data curation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1421124/full#supplementary-material
References
1. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380(9859):2224–60. doi: 10.1016/s0140-6736(12)61766-8
2. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. (2020) 396(10258):1223–49. doi: 10.1016/S0140-6736(20)30752-2
3. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16(4):223–37. doi: 10.1038/s41581-019-0244-2
4. Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. (2013) 31(7):1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc
5. Yin Y, Yu Z, Wang J, Sun J. Effects of the different Tai Chi exercise cycles on patients with essential hypertension: a systematic review and meta-analysis. Front Cardiovasc Med. (2023) 10:1016629. doi: 10.3389/fcvm.2023.1016629
6. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. (2020) 54(24):1451–62. doi: 10.1136/bjsports-2020-102955
7. Maruf FA, Akinpelu AO, Salako BL, Akinyemi JO. Effects of aerobic dance training on blood pressure in individuals with uncontrolled hypertension on two antihypertensive drugs: a randomized clinical trial. J Am Soc Hypertens. (2016) 10(4):336–45. doi: 10.1016/j.jash.2016.02.002
8. Lee LL, Mulvaney CA, Wong YKY, Chan ES, Watson MC, Lin HH. Walking for hypertension. Cochrane Database Syst Rev. (2021) 2(2):Cd008823. doi: 10.1002/14651858.CD008823.pub2
9. Valenzuela PL, Carrera-Bastos P, Gálvez BG, Ruiz-Hurtado G, Ordovas JM, Ruilope LM, et al. Lifestyle interventions for the prevention and treatment of hypertension. Nat Rev Cardiol. (2021) 18(4):251–75. doi: 10.1038/s41569-020-00437-9
10. Strilchuk L, Cincione RI, Fogacci F, Cicero AFG. Dietary interventions in blood pressure lowering: current evidence in 2020. Kardiol Pol. (2020) 78(7-8):659–66. doi: 10.33963/kp.15468
11. Cicero AFG, Veronesi M, Fogacci F. Dietary intervention to improve blood pressure control: beyond salt restriction. High Blood Press Cardiovasc Prev. (2021) 28(6):547–53. doi: 10.1007/s40292-021-00474-6
12. Serrano-Guzmán M, Aguilar-Ferrándiz ME, Valenza CM, Ocaña-Peinado FM, Valenza-Demet G, Villaverde-Gutiérrez C. Effectiveness of a flamenco and sevillanas program to enhance mobility, balance, physical activity, blood pressure, body mass, and quality of life in postmenopausal women living in the community in Spain: a randomized clinical trial. Menopause. (2016) 23(9):965–73. doi: 10.1097/gme.0000000000000652
13. Koch SC, Riege RFF, Tisborn K, Biondo J, Martin L, Beelmann A. Effects of dance movement therapy and dance on health-related psychological outcomes. A meta-analysis update. Front Psychol. (2019) 10:1806. doi: 10.3389/fpsyg.2019.01806
14. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4(1):1. doi: 10.1186/2046-4053-4-1
15. Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. (2003) 83(8):713–21. doi: 10.1093/ptj/83.8.713
16. de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. (2009) 55(2):129–33. doi: 10.1016/s0004-9514(09)70043-1
17. Cochrane M, Mitchell E, Hollingworth W, Crawley E, Trépel D. Cost-effectiveness of interventions for chronic fatigue syndrome or myalgic encephalomyelitis: a systematic review of economic evaluations. Appl Health Econ Health Policy. (2021) 19(4):473–86. doi: 10.1007/s40258-021-00635-7
18. Irwig L, Macaskill P, Berry G, Glasziou P. Bias in meta-analysis detected by a simple, graphical test. Graphical test is itself biased. Br Med J. (1998) 316(7129):470. author reply 470-471. doi: 10.1136/bmj.315.7109.629
19. Aweto HA, Owoeye OB, Akinbo SR, Onabajo AA. Effects of dance movement therapy on selected cardiovascular parameters and estimated maximum oxygen consumption in hypertensive patients. Nig Q J Hosp Med. (2012) 22(2):125–9.23175912
20. Maruf FA, Akinpelu AO, Salako BL. A randomized controlled trial of the effects of aerobic dance training on blood lipids among individuals with hypertension on a thiazide. High Blood Press Cardiovasc Prev. (2014) 21(4):275–83. doi: 10.1007/s40292-014-0063-2
21. Kaholokula JK, Look M, Mabellos T, Zhang G, de Silva M, Yoshimura S, et al. Cultural dance program improves hypertension management for native hawaiians and pacific islanders: a pilot randomized trial. J Racial Ethn Health Disparities. (2017) 4(1):35–46. doi: 10.1007/s40615-015-0198-4
22. Yi L, Weiwei Z, Dongyun Z, Lili W, Liu M, Zhongzheng Y. Improvement of indexes about ambulatory blood pressure, biology and psychosomatic symptoms by square dance activity in community perimenopausal women with hypertension. Chin J Rehabil. (2017) 32(06):496–9. doi: 10.3870/zgkf.2017.06.016
23. Yaping B, Ying L, Dongmei W, Cuihong Z. Effect of community square dance on blood pressure and blood lipid levels in middle-aged and elderly patients with hypertension. Chin J Hypertens. (2019) 27(05):474–8. doi: 10.16439/j.cnki.1673-7245.2019.05.021
24. Yaping B, Ying L, Dongmei W, Cuihong Z. Effect of community square dance on sleep quality in middle-aged and elderly patients with hypertension. Chin J Hypertens. (2020) 28(04):367–73. doi: 10.16439/j.cnki.1673-7245.2020.04.017
25. Kaholokula JK, Look M, Mabellos T, Ahn HJ, Choi SY, Sinclair KA, et al. A cultural dance program improves hypertension control and cardiovascular disease risk in native hawaiians: a randomized controlled trial. Ann Behav Med. (2021) 55(10):1006–18. doi: 10.1093/abm/kaaa127
26. Yanbing W, Yuxiu H, Dapeng W. Effect of tai Chi soft ball dance and apparatus exercise on blood lipids and hypersensitive C-reactive protein in obese hypertensive middle-aged and elderly women. Chin J Gerontol. (2021) 41(01):83–5. doi: 10.3969/j.issn.1005-9202.2021.01.025
27. Yan ZW, Yang Z, Yang JH, Song CL, Zhao Z, Gao Y. Comparison between Tai Chi and square dance on the antihypertensive effect and cardiovascular disease risk factors in patients with essential hypertension: a 12-week randomized controlled trial. J Sports Med Phys Fitness. (2022) 62(11):1568–75. doi: 10.23736/s0022-4707.22.13424-9
28. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. (2017) 317(2):165–82. doi: 10.1001/jama.2016.19043
29. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. (2020) 75(2):285–92. doi: 10.1161/hypertensionaha.119.14240
30. Cao L, Li X, Yan P, Wang X, Li M, Li R, et al. The effectiveness of aerobic exercise for hypertensive population: a systematic review and meta-analysis. J Clin Hypertens. (2019) 21(7):868–76. doi: 10.1111/jch.13583
31. Gomes Neto M, Menezes M, Carvalho V. Dance therapy in patients with chronic heart failure: a systematic review and a meta-analysis. Clin Rehabil. (2014) 28(12):1172–9. doi: 10.1177/0269215514534089
32. Semlitsch T, Jeitler K, Hemkens LG, Horvath K, Nagele E, Schuermann C, et al. Increasing physical activity for the treatment of hypertension: a systematic review and meta-analysis. Sports Med. (2013) 43(10):1009–23. doi: 10.1007/s40279-013-0065-6
33. Bangalore S, Messerli FH, Franklin SS, Mancia G, Champion A, Pepine CJ. Pulse pressure and risk of cardiovascular outcomes in patients with hypertension and coronary artery disease: an INternational VErapamil SR-trandolapril STudy (INVEST) analysis. Eur Heart J. (2009) 30(11):1395–401. doi: 10.1093/eurheartj/ehp109
34. Reboldi G, Gentile G, Angeli F, Ambrosio G, Mancia G, Verdecchia P. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. (2011) 29(7):1253–69. doi: 10.1097/HJH.0b013e3283469976
35. Fei X, Mingxin L, Chao L, Hongjuan L. Effects of exercise on blood lipid for patients with hypertension: a network metaanalysis. Chin J Evidence-Based Med. (2021) 21(12):1424–31. doi: 10.7507/1672-2531.202109036
36. Vítovec J, Spinar J. First-dose hypotension after angiotensin-converting enzyme (ACE) inhibitors in chronic heart failure: a comparison of enalapril and perindopril. Slovak Investigator Group. Eur J Heart Fail. (2000) 2(3):299–304. doi: 10.1016/s1388-9842(00)00095-7
37. Waclawovsky G, Pedralli ML, Eibel B, Schaun MI, Lehnen AM. Effects of different types of exercise training on endothelial function in prehypertensive and hypertensive individuals: a systematic review. Arq Bras Cardiol. (2021) 116(5):938–947. English, Portuguese. doi: 10.36660/abc.20190807
38. Si XB, Liu W. Relationship between blood lipid and arterial stiffness in hypertension. Clin Invest Med. (2019) 42(3):E47–e55. doi: 10.25011/cim.v42i3.33092
39. Yuanyuan C, Zengwu W, Jian Jun Li M, Ping Y, Yuqing Z, Yong L, et al. China Expert consensus on comprehensive management of blood pressure and lipid in patients with hypertension. Chin J Hypertens. (2019) 27(07):605–14. doi: 10.16439/j.cnki.1673-7245.2019.07.002
40. Shan C, Peizhen Z. Research progress on the effect of exercise training on blood lipid metabolism. Chin Prev Med. (2019) 20(09):881–6. doi: 10.16506/j.1009-6639.2019.09.025
41. Honghong L, Lijuan X, Qinghang W, Birong Z. Correlation between monocyte to high-density lipoprotein cholesterol ratio, flow-mediated dilation and arteriosclerosis in young and middle-aged patients with essential hypertension. Chin J Hypertens. (2023) 31(07):642–8. doi: 10.16439/j.issn.1673-7245.2023.07.008
42. Zhou Y, Wang L, Jia L, Lu B, Gu G, Bai L, et al. The monocyte to high-density lipoprotein cholesterol ratio in the prediction for atherosclerosis: a retrospective study in adult Chinese participants. Lipids. (2021) 56(1):69–80. doi: 10.1002/lipd.12276
43. Tessier S, Riesco E, Lacaille M, Pérusse F, Weisnagel J, Doré J, et al. Impact of walking on adipose tissue lipoprotein lipase activity and expression in pre- and postmenopausal women. Obes Facts. (2010) 3(3):191–9. doi: 10.1159/000314611
44. Franczyk B, Gluba-Brzózka A, Ciałkowska-Rysz A, Ławiński J, Rysz J. The impact of aerobic exercise on HDL quantity and quality: a narrative review. Int J Mol Sci. (2023) 24:5. doi: 10.3390/ijms24054653
45. Muscella A, Stefàno E, Marsigliante S. The effects of exercise training on lipid metabolism and coronary heart disease. Am J Physiol Heart Circ Physiol. (2020) 319(1):H76–h88. doi: 10.1152/ajpheart.00708.2019
46. Kumanyika S, Dietz WH. Solving population-wide obesity—progress and future prospects. N Engl J Med. (2020) 383(23):2197–200. doi: 10.1056/NEJMp2029646
47. Lüscher TF. Novel insights into body fat distribution and cardiometabolic risk. Eur Heart J. (2019) 40(34):2833–6. doi: 10.1093/eurheartj/ehz634
48. Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, adipose tissue and vascular dysfunction. Circ Res. (2021) 128(7):951–68. doi: 10.1161/circresaha.121.318093
49. Liangliang W, Yu H, Wei G, Xingmin W, Ning F, Guixue Z, et al. Interaction between obesity/central obesity and hypertension. Prev Med. (2022) 34(02):129–34. doi: 10.19485/j.cnki.issn2096-5087.2022.02.005
50. Zampino M, AlGhatrif M, Kuo PL, Simonsick EM, Ferrucci L. Longitudinal changes in resting metabolic rates with aging are accelerated by diseases. Nutrients. (2020) 12:10. doi: 10.3390/nu12103061
51. Aydemir T, Sahin M, Aydemir O. Determination of hypertension disease using chirp z-transform and statistical features of optimal band-pass filtered short-time photoplethysmography signals. Biomed Phys Eng Express. (2020) 6:6. doi: 10.1088/2057-1976/abc634
52. Brito LC, Peçanha T, Fecchio RY, Pio-Abreu A, Silva G, Mion-Junior D, et al. Comparison of morning versus evening aerobic-exercise training on heart rate recovery in treated hypertensive men: a randomized controlled trial. Blood Press Monit. (2021) 26(5):388–92. doi: 10.1097/mbp.0000000000000545
53. Wen J, Yujia C. Correlation between heart rate and blood pressure in patients with hypertension during exercise. Prevent Treatment Cardio-Cerebral-Vascular Dis. (2016) 16(04):306–7, 9. doi: 10.3969/j.issn.1009-816x.2016.04.20
54. Grassi G, Dell'Oro R, Bombelli M, Cuspidi C, Quarti-Trevano F. High blood pressure with elevated resting heart rate: a high risk “Sympathetic” clinical phenotype. Hypertens Res. (2023) 46(10):2318–25. doi: 10.1038/s41440-023-01394-9
55. Yanping W, Dongyang Z, Xiujun H, Yuchun Z, Yonghua X. Effects of diversified health education combined with personalized aerobic exercise on health behavior and psychological stress of hypertension patients. Chin J Health Care Med. (2021) 23(02):183–5. doi: 10.3969/j.issn.1674-3245.2021.02.023
Keywords: dance therapy, hypertension, blood pressure, blood lipid, meta-analysis
Citation: Wang J, Yin Y, Yu Z, Lin Q and Liu Y (2024) Does dance therapy benefit the improvement of blood pressure and blood lipid in patients with hypertension? A systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1421124. doi: 10.3389/fcvm.2024.1421124
Received: 21 April 2024; Accepted: 3 October 2024;
Published: 24 October 2024.
Edited by:
Giuseppe Caminiti, Università telematica San Raffaele, ItalyReviewed by:
Giuseppe Marazzi, IRCCS San Raffaele, ItalyMele Look, University of Hawaii at Mānoa, United States
Copyright: © 2024 Wang, Yin, Yu, Lin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yikun Yin, MTM2NTM0NDE5NUBxcS5jb20=
†These authors have contributed equally to this work
 Jialin Wang
Jialin Wang Yikun Yin
Yikun Yin Zhengze Yu
Zhengze Yu Qihan Lin4,†
Qihan Lin4,†