- 1Department of Cardiology, Rabin Medical Center, Petah-Tikva and the Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- 2Clinical Cardiovascular Research Center, University of Rochester Medical Center, Rochester, NY, United States
Background and aims: Long QT syndrome (LQTS) and coronary artery disease (CAD) are both associated with increased risk of ventricular tachyarrhythmia. However, there are limited data on the incremental risk conferred by CAD in adult patients with congenital LQTS. We aimed to investigate the risk associated with CAD and life threatening events (LTEs) in patients with LQTS after age 40 years.
Methods: The risk of LTEs (comprising aborted cardiac arrest, sudden cardiac death, or appropriate defibrillator shock) from age 40 through 75 years was examined in 1,020 subjects from the Rochester LQTS registry, categorized to CAD (n = 137) or no-CAD (n = 883) subgroups.
Results: Survival analysis showed that patients with CAD had a significantly higher cumulative event rate of LTEs from 40 to 75 years (35%) compared with those without CAD (7%; p < 0.001 for the overall difference during follow-up). Consistently, multivariate analysis showed that the presence of CAD was associated with a 2.5-fold (HR = 2.47; p = 0.02) increased risk of LTEs after age 40 years. Subgroup analyses showed that CAD vs. no CAD was associated with a pronounced >4-fold (p = 0.008) increased risk of LTEs among LQTS patients with a lower-range QTc (<500 ms). The increased risk of LTEs associated with CAD was not significantly different among the 3 main LQTS genotypes. Patient treatment was suboptimal, with only 63% on β-blockers and 44% on non-selective β-blockers.
Conclusions: Our findings suggest that CAD is associated with a higher risk of LTEs in LQTS patients, with the risk being more pronounced in those with QTc <500 ms.
Introduction
Congenital long QT syndrome (LQTS) is an inherited channelopathy characterized by the prolongation of action potential duration, which can lead to early after depolarizations and potentially life-threatening ventricular arrhythmias. Individuals carrying pathogenic or likely pathogenic variants of LQTS may experience palpitations, syncope, or sudden cardiac death. Previous studies have shown that the risk of life threatening cardiac events (LTEs) among patients with LQTS is influenced by age, gender, corrected QT interval (QTc), genotype, mutation location, environmental factors and therapy (1). The primary focus of these studies was on the initial four decades of life, leaving a scarcity of data regarding predictors of LTEs in individuals with LQTS beyond the age of 40 years (2). Possible factors that may affect the risk of life threatening arrhythmic events in LQTS patients especially after the fourth decade of life include comorbid conditions that accumulate during the years such as diabetes mellitus, hypertension, smoking, hyperlipidemia and coronary artery disease (CAD).
Beyond the age of 40 years, CAD becomes the primary cause of sudden cardiac death (SCD). Prior research has indicated that an abnormally prolonged QTc may also play a role as a causative factor in the pathophysiology of SCD in patients with CAD (3). Thus, in this study we sought to examine whether CAD contributes to the risk of life threatening arrhythmic events after the fourth decade of life beyond the risk associated with congenital LQTS during this time period.
Methods
Study population
The study population was drawn from the Rochester LQTS Registry and involved enrolled LQTS subjects who were followed up after the age of 40 years. Enrolled LQTS subjects had either genetically confirmed pathogenic or likely pathogenic LQTS variants or a QTc ≥450 ms in males or ≥470 ms in females. Patients with multiple LQTS-causing mutations (n = 32) and patients with LQTS genotype other than LQTS 1–3 genotype (n = 24) were excluded. The final study cohort comprised 1,020 patients.
Data collection and follow-up
Clinical data were collected as previously described (2). Briefly, A comprehensive medical history was gathered upon enrollment, and continuous clinical data were collected using prospectively designed forms during each visit or medical contact at yearly intervals. The duration of the QT corrected for heart rate (QTc) by use of Bazett's formula was assessed from a 12-lead ECG obtained at enrollment and assessed again from ECGs recorded during follow up. The QTc that was chosen for the current study was determined by the maximum QTc duration recorded before the age of 40 years or by the first ECG recorded after the age of 40 in patients lacking prior ECG data.
Data on non-LQTS comorbidities were collected through baseline and follow-up questionnaires that are sent to enrolled subjects ≥40 years old at the yearly follow-up assessment. Patients were identified as having CAD if they had a history of acute coronary syndrome, coronary artery angioplasty, coronary artery bypass graft or had chest pain and were prescribed nitroglycerine. The presence of CAD was assessed as a time dependent variable (including the date when CAD diagnosis was made according to filled forms).
Follow-up data regarding β-blocker therapy included the initiation date and, if applicable, the discontinuation date. Information on other LQTS-related therapies including pacemaker or ICD implantation, and left cervical sympathetic denervation was also recorded prospectively.
Pathogenic or likely pathogenic LQTS variants were identified using standard genetic tests performed in academic molecular-genetic laboratories affiliated with the Rochester Long QT Syndrome Registry. Patients with variants of uncertain significance (VUS) were excluded from the study unless their QTc was ≥450 ms for males or ≥470 ms for females. Genetic testing included comprehensive LQTS gene panels or targeted mutation testing, utilizing methods such as Sanger sequencing, Next-Generation Sequencing (NGS), and occasionally Multiplex Ligation-dependent Probe Amplification (MLPA).
Endpoints
The primary end point of the study was the occurrence of a first LTE, comprising aborted cardiac arrest (ACA) requiring external defibrillation as part of the resuscitation, LQTS-related SCD (abrupt in onset without evident cause if witnessed or death that was not explained by any other cause if it occurred in an unwitnessed setting) or an appropriate ICD shock between the ages of 40 and 75 years. Events that occurred during an acute myocardial infarction (MI) were excluded.
All participants provided informed consent, consenting to inclusion in the registry and participation in subsequent clinical studies.
Statistical analysis
To explore the unadjusted relationship between LTE and time-dependent CAD, Simon and Makuch plot is displayed, which is an expansion of the Kaplan-Meier plot with respect to time-dependent covariates. Mantel-Byar test was used for comparison of survival data with a time-dependent covariate. To assess the independent association of clinical and genetic factors with the initial incidence of a LTE, multivariable Cox proportional hazards modeling was conducted with decade was born (before January 1970 and after January 1970) strata and robust standard errors were utilized to account for clustering of patients within family. Best stepwise variable selection method was applied selecting from the following list of potential covariates based on literature review and clinical judgement: Age, sex, QTc ≥500 ms, CAD, hypertension, diabetes mellitus, hyperlipidemia, mitral valve prolapse documented by echo, heart disease other than LQTS or CAD, asthma, autoimmune disorder, smoking, obstructive sleep apnea, menopause, cancer, high alcohol intake, syncope prior to age 40 years, time-dependent β-blocker use, and genotype mutations. The reduced model included all covariates at the <0.05 significant level, but forcing sex, history of syncope before age 40 years, time-dependent β-blocker use, and genotype mutations. Furthermore, specified subgroup analyses and test of interactions were investigated for LTE outcome.
All statistical analyses were two-sided, and significance was determined at a p-value of <0.05. The analyses were conducted using SAS software (version 9.4, SAS Institute, Cary, North Carolina).
Results
Clinical characteristics
The clinical characteristics of 1,020 study patients and the frequency of comorbidities before age 40 years and during follow up by CAD are presented in Table 1.
The 137 patients with CAD exhibited significant differences in baseline characteristics when compared with 883 patients with no-CAD. Patients with CAD had longer QTc duration, and displayed a higher frequency of comorbidities, including diabetes mellitus, hypertension, hyperlipidemia, smoking, history of stroke and asthma when compared to patients with no-CAD. Conversely, no-CAD patients displayed a greater proportion of females. Among patients with CAD, 43% had a history of MI, 63% underwent coronary angioplasty, and 25% underwent coronary artery bypass grafting.
Genotype
Out of the total, 800 (78%) individuals underwent genotyping. Among them, 710 (89%) were found to carry an LQTS 1–3 mutation and were classified as LQT1, LQT2, or LQT3. The remaining 90 (11%) individuals who were genotyped but did not carry an LQTS mutation were classified as “no mutation found (tested)”. There were 220 (22%) LQTS patients that were not tested genetically and were thus categorized as unknown mutation status (not tested). Patients without CAD displayed a greater proportion of LQT1 and LQT2 related mutations compared with patients with CAD.
Clinical course of LQTS patients with CAD vs. no CAD after age 40 years
During a mean (±SD) follow up of 21.4 (±11.7) years, 97 patients experienced any cardiac event, and 60 patients experienced an LTE after age 40. Among the 60 patients who experienced an LTE, 29 had an ACA, 4 experienced SCD related to LQTS, and 33 received appropriate shocks from an ICD. Patients with CAD more frequently received β-blockers (mainly selective β-blockers), pacemakers and ICDs when compared to no-CAD patients. The proportion of patients who experienced syncope, ACA, any cardiac event, LTEs or death was significantly higher in the CAD vs. no CAD group (Table 1).
Figure 1 presents the Simon and Makuch estimates for LTEs by time-dependent CAD. The cumulative probability of LTE at age 75 was significantly higher in CAD patients (35%) than among no-CAD patients (7%, Mantle-Byar p < 0.001).
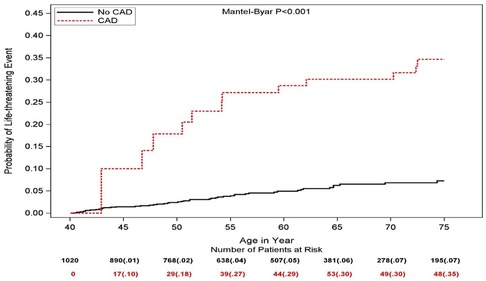
Figure 1. Simon and Makuch estimates for life threatening cardiac events by time-dependent coronary artery disease (values in parentheses are probability estimates).
Multivariate analysis (Table 2) consistently showed that the presence of CAD was a powerful predictor of LTEs in LQTS patients after age 40 years and was independently associated with a 2.5-fold increased risk of LTE (p = 0.02). A more prolonged QTc (≥500 ms) was also identified to be a powerful independent predictor of LTE (HR = 3.54; p < 0.001), whereas other known LQTS related known risk factors, including sex, prior syncope, and genotype were not associated with a statistically significant increased risk after adjustment for the presence of CAD (Table 2).
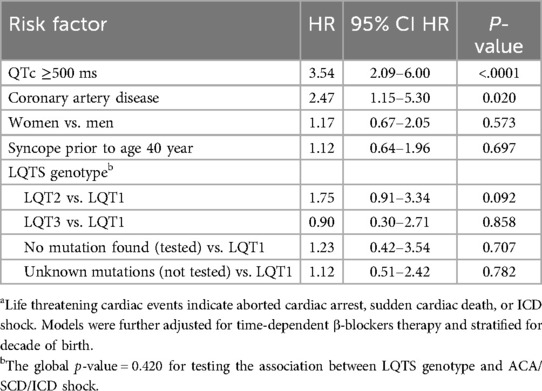
Table 2. Multivariate analysis: risk factors for life threatening cardiac events among all LQTS patientsa.
Other CAD risk factors such as hypertension, diabetes mellitus, and hyperlipidemia were not associated with a statistically significant increased risk of LTEs and therefore were not included in the final LTE model (Table 2).
Subgroup analysis
Subgroup analysis (Figure 2) did not show a statistically significant difference in the correlation between CAD and LTE across risk subsets of LQTS patients, encompassing QTc duration, history of syncope prior to age 40 years, β-blocker utilization, gender, and LQTS genotype (all p-values for risk subset-by-CAD interaction >0.10). Nevertheless, the increased risk of LTEs associated with CAD was pronounced (>4-fold risk increase) in lower risk LQTS patients (with QTc <500 ms) and among those with the LQT2 genotype (Figure 2). Thus, among patients with QTc <500 ms, the cumulative risk of LTEs from age 40 to 75 was 37% in the CAD vs. only 3% in the no CAD subgroup (p < 0.001 for the overall difference during follow-up (Figure 3A). In contrast, among patients with QTC ≥500 ms, there was no statistically significant difference in the cumulative risk of LTE between the CAD (24%) and no-CAD (17%) subgroups (p = 0.482, Figure 3B).
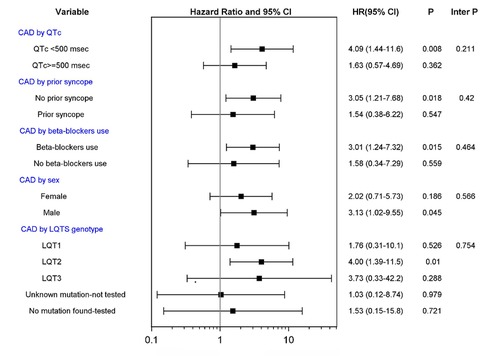
Figure 2. CAD vs. no CAD and risk of life threatening cardiac events in subgroups of patients. CAD, coronary artery disease; LQTS, long QT syndrome. Tests of interactions were performed using a single interaction term, except for the model investigating CAD by LQTS genotype using 4 interaction terms.
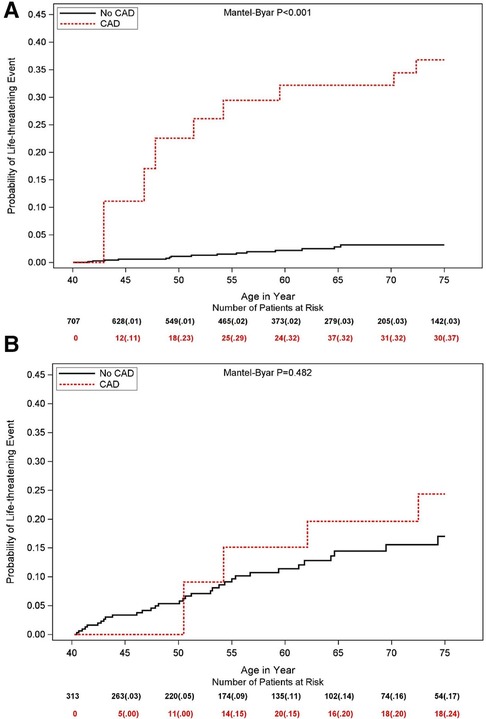
Figure 3. Simon and Makuch estimates for life threatening cardiac events by time-dependent coronary artery disease in patients with (A) QTc <500 ms and (B) QTc ≥500 ms. Values in parentheses are probability estimates.
In an exploratory analysis, we reviewed data on unaffected family members of LQTS patients who were followed after age 40 from the LQTS Registry. These individuals did not have LQTS (no confirmed LQTS-causing genetic mutation and no QTc ≥450 ms in males or ≥470 ms in females). Among the 599 subjects, 55 had CAD and 544 did not. Over a mean follow-up period of 22 ± 12 years, there were only 4 LTE episodes based on the earlier definition. Two patients with CAD and two without CAD experienced appropriate ICD shocks. This very low LTE rate among LQTS-negative family members highlights that the original cohort of LQTS patients included true LQTS-related life-threatening events, rather than ACS-related or scar-related LTEs.
Discussion
Our study provides several important implications for risk stratification and management of patients with long QT syndrome after age 40 years. We have shown that from age 40 to 75 years the presence and/or development of CAD is associated with a pronounced incremental risk for LTE compared to the risk associated with LQTS in this age-group without CAD. These findings were independent of known LQTS risk factors and were consistent in all risk subsets analyzed. Nevertheless, the incremental risk associated with CAD appears to be very pronounced (≥4-fold risk increase) in lower risk LQTS patients (with QTc <500 ms). These findings suggest that more careful follow-up and monitoring for arrhythmic events is required in adult LQTS patients who develop CAD.
Incremental effect of CAD in LTE in adult patients with LQTS
Most studies evaluating the clinical course and prognosis of subjects with LQTS have focused on the first 4 decades of life. Although it has been suggested that carriers of this congenital disorder have a low risk of ventricular arrhythmias or SCD after the age of 40 years, research conducted within the international LQTS cohort demonstrated that individuals carrying an LQTS mutation faced a fourfold higher risk of LTEs between the ages of 40 and 60 compared with genotype-negative subjects in the same age group (2).
There is lack of data on the effect of cardiovascular comorbidities on the risk of LTEs among LQTS patients after the age of 40 years. A previous study analyzing data from the International Long QT Syndrome Registry has suggested that CAD may augment the risk of cardiac events in LQTS patients at the age range of 41–80 years (4). In this study we included 641 LQTS patients (of whom 87 patients had CAD); CAD vs. no CAD was associated with a 2-fold increased risk of cardiac events dominated by syncope. In this study, however, the risk of LTEs was not assessed due to a smaller sample and a lower event rate. The present study including 1,020 LQTS patients found that CAD was associated with a a statistically significant 2.5-fold increase in the risk of LTEs, thus supporting previous findings and extending the results for the robust endpoint of LTEs.
Diabetes mellitus may also affect ventricular repolarization (5, 6); Ouelett G. et al, showed that the development of diabetes mellitus in adult patients with LQTS was not associated with an increased risk of any cardiac events (dominated mainly by syncope events) (7). In our study, focusing on the endpoint of LTEs, we found similar results, that diabetes mellitus was not associated with increased risk of LTEs among LQTS patients. Furthermore, additional established CVD risk factors, including smoking and hypertension were not shown to confer increased risk for LTE in our LQTS population after further adjustment for the presence of CAD.
In the current study, 6% of the patients experienced an LTE, with a higher incidence observed in those with CAD (12%) compared to those without CAD (5%). Thus, it appears that the rates of LTEs are somewhat higher than those reported in studies with follow-up before age 40 (8, 9).
Additionally, the use of β-blockers and left cardiac sympathetic denervation (LCSD) after age 40 and during follow-up was less frequent than in studies involving patients under 40 (8, 10, 11). However, direct comparison between these studies and the current one is challenging due to differences in patient age, characteristics, QT interval lengths, follow-up duration, and endpoint definitions.
It should be noted that the population in this study was not optimally treated with β-blockers: only 63% of patients received β-blockers, and just 44% were treated with non-selective β-blockers. Patients with CAD more frequently received β-blockers (mainly selective beta blockers) compared to no-CAD patients.
Possible mechanisms linking CAD risk in LQTS
There are several pathophysiological substrates that contribute to the development of LTEs in patients with CAD including arrhythmias initiated by transient ischemia, arrhythmias occurring during the acute and convalescent phases of MI, after scar formation and in the setting of ischemic cardiomyopathy (3). Previous studies have suggested that abnormally prolonged ventricular repolarization may be a causative factor in the pathophysiology of SCD in patients with CAD. Chugh et al. have found that an abnormal QTC prolongation (>470 ms) was associated with a 5-fold increased risk of SCD in a population based case-control study of patients with CAD (12). In addition, clinical and experimental evidence show that ischemia/reperfusion injury is associated with prolongation of repolarization in the affected area that may contribute to the generation of triggered activity leading to polymorphic ventricular tachycardia (13). It is possible that there is a mechanistic link between QT prolongation associated with ischemia/reperfusion injury and the abnormal regulation of potassium, sodium, and calcium ions flux across myocellular membranes as appears in the congenital long QT syndrome, thus facilitating the generation of triggered activity leading to ventricular arrhythmias.
Effect of QTc duration and genotype on CAD-related risk
In the current study we found that the presence or development of CAD was associated with a statistically significant pronounced increased risk of subsequent LTEs among patients with QTc <500 ms (HR = 4.09, p = 0.008). The mechanism underlying the observed pronounced increase in the risk associated with CAD in lower risk LQTS patients (i.e., with a shorter QTc duration) is unclear. It is possible that patients with a very prolonged QTc (≥500 ms) have already a very high risk of LTE, regardless of the presence of CAD, while the effect of ischemia/injury on repolarization dynamics is more pronounced in LQTS patients who have a baseline lower risk for arrhythmic events. Adult LQTS patients with QTC <500ms are considered to have low risk for cardiac events and therefore may be followed, monitored and treated (with β-blockers) less intensively than patients with QTc ≥500 ms, but when they develop CAD their risk for cardiac events increases dramatically.
Limitations
The Rochester Long QT Syndrome Registry is continually collecting follow up data on non-LQTS comorbidities including CAD for enrolled subjects older than 40 years since January 2004. For the diagnosis of CAD we relied on data from prespecified medical questionnaire. We did not collect data on the severity of CAD per coronary angiogram, data on multiple PCI procedures, residual inducible ischemia or type of MI and thus were not able to perform subgroup analyses according to CAD severity. There were 220 (22%) LQTS patients that were not tested genetically and were thus categorized as unknown mutation status (not tested). We did not gather data on left ventricular ejection fraction. While we did not have complete data on all episodes of ventricular arrhythmias, among the 60 patients who experienced LTEs, 29 episodes of VF and 20 episodes of Torsades de Pointes were recorded, suggesting that most events were related to LQTS rather than monomorphic ventricular tachycardia (VT). Nevertheless, we cannot rule out that the larger risk of LTE among CAD patients with QTc <500 ms might be related to a higher incidence of scar-related monomorphic VT. There is a high rate of LTEs among LQTS patients with CAD in the present study compared to other studies that could be related to age-related factors and follow-up duration. However, it is still possible that non-LQTS-related LTEs were included, despite our careful review of this endpoint. We also did not collect data on whether an electrophysiological study was conducted following syncopal events.
Clinical implications and future directions
The present study is among the first to assess the relationship between CAD and life-threatening cardiac arrhythmic events among LQTS patients after the age of 40 years.
In this study we demonstrate that CAD contributes to the risk of life threatening cardiac events beyond the risk conferred by congenital LQTS. CAD was associated with a 2.5 fold increased risk of LTEs after age 40. We suggest that LQTS patients who develop CAD are at increased risk for LTEs and should be followed, monitored and treated more intensively (focusing on dose optimization of nonselective β-blockers). Further research is needed to understand determinants and mechanisms underlying the increased risk of cardiac arrhythmias in these patients.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: no public access. Requests to access these datasets should be directed toQmFyc2hlc2hldGFAZ21haWwuY29t.
Ethics statement
The studies involving humans were approved by University of Rochester Medical Center, Rochester, NY. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AB: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. IG: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing. MB: Conceptualization, Methodology, Writing – review & editing. KB: Investigation, Writing – review & editing. AE: Methodology, Writing – review & editing. GG: Methodology, Writing – review & editing. AC: Conceptualization, Formal Analysis, Methodology, Writing – review & editing. BP: Conceptualization, Formal Analysis, Methodology, Validation, Writing – review & editing. SM: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Writing – review & editing. MA: Conceptualization, Methodology, Writing – review & editing. WZ: Conceptualization, Methodology, Writing – review & editing. GG: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The content of this manuscript has been presented in part at the ESC Congress 2023, European Heart Journal, Volume 44, Issue Supplement_2, November 2023, ehad655.636.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wilde AAM, Semsarian C, Marquez MF, Shamloo AS, Ackerman MJ, Ashley EA, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) expert consensus statement on the state of genetic testing for cardiac diseases. Europace. (2022) 24(8):1307–67. doi: 10.1093/europace/euac030
2. Goldenberg I, Moss AJ, Bradley J, Polonsky S, Peterson DR, McNitt S, et al. Long-QT syndrome after age 40. Circulation. (2008) 117(17):2192–201. doi: 10.1161/CIRCULATIONAHA.107.729368
3. Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. (2012) 125(8):1043–52. doi: 10.1161/CIRCULATIONAHA.111.023846
4. Sze E, Moss AJ, Goldenberg I, McNitt S, Jons C, Zareba W, et al. Long QT syndrome in patients over 40 years of age: increased risk for LQTS-related cardiac events in patients with coronary disease. Ann Noninvasive Electrocardiol. (2008) 13(4):327–31. doi: 10.1111/j.1542-474X.2008.00250.x
5. Xiao J, Luo X, Lin H, Zhang Y, Lu Y, Wang N, et al. MicroRNA miR-133 represses HERG K+ channel expression contributing to QT prolongation in diabetic hearts. J Biol Chem. (2011) 286(32):28656. doi: 10.1074/jbc.A111.700015
6. Gordin D, Forsblom C, Ronnback M, Groop PH. Acute hyperglycaemia disturbs cardiac repolarization in type 1 diabetes. Diabet Med. (2008) 25(1):101–5. doi: 10.1111/j.1464-5491.2007.02322.x
7. Ouellet G, Moss AJ, Jons C, McNitt S, Mullally J, Fugate T, et al. Influence of diabetes mellitus on outcome in patients over 40 years of age with the long QT syndrome. Am J Cardiol. (2010) 105(1):87–9. doi: 10.1016/j.amjcard.2009.08.657
8. Mazzanti A, Maragna R, Vacanti G, Monteforte N, Bloise R, Marino M, et al. Interplay between genetic substrate, QTc duration, and arrhythmia risk in patients with long QT syndrome. J Am Coll Cardiol. (2018) 71(15):1663–71. doi: 10.1016/j.jacc.2018.01.078
9. Wang M, Peterson DR, Pagan E, Bagnardi V, Mazzanti A, McNitt S, et al. Assessment of absolute risk of life-threatening cardiac events in long QT syndrome patients. Front Cardiovasc Med. (2022) 9:1–11. doi: 10.3389/fcvm.2022.988951
10. Martinez K, Bains S, Giudicessi JR, Bos JM, Neves R, Ackerman MJ. Spectrum and prevalence of side effects and complications with guideline-directed therapies for congenital long QT syndrome. Heart Rhythm. (2022) 19(10):1666–72. doi: 10.1016/j.hrthm.2022.06.008
11. Dusi V, Dagradi F, Spazzolini C, Crotti L, Cerea P, Giovenzana FLF, et al. Long QT syndrome: importance of reassessing arrhythmic risk after treatment initiation. Eur Heart J. (2024) 45(29):2647–56. doi: 10.1093/eurheartj/ehae289
12. Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, et al. Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease. Circulation. (2009) 119(5):663–70. doi: 10.1161/CIRCULATIONAHA.108.797035
Keywords: long QT syndrome, coronary artery disease, sudden cardiac death, ventricular arrhythmia, risk factors
Citation: Barsheshet A, Goldenberg I, Bjelic M, Buturlin K, Erez A, Goldenberg G, Chen AY, Polonsky B, McNitt S, Aktas M, Zareba W and Golovchiner G (2024) Coronary artery disease and the risk of life-threatening cardiac events after age 40 in long QT syndrome. Front. Cardiovasc. Med. 11:1418428. doi: 10.3389/fcvm.2024.1418428
Received: 16 April 2024; Accepted: 7 October 2024;
Published: 22 October 2024.
Edited by:
Rui Providencia, University College London, United KingdomReviewed by:
Veronica Dusi, University of Turin, ItalyKamran Shamsa, University of California, Los Angeles, United States
Copyright: © 2024 Barsheshet, Goldenberg, Bjelic, Buturlin, Erez, Goldenberg, Chen, Polonsky, McNitt, Aktas, Zareba and Golovchiner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alon Barsheshet, YmFyc2hlc2hldGFAZ21haWwuY29t
 Alon Barsheshet
Alon Barsheshet Ilan Goldenberg2
Ilan Goldenberg2 Bronislava Polonsky
Bronislava Polonsky Wojciech Zareba
Wojciech Zareba Gregory Golovchiner
Gregory Golovchiner