- 1Department of Medicine, College of Human Medicine, Michigan State University, East Lansing, MI, United States
- 2Department of Radiology, College of Human Medicine, Michigan State University, East Lansing, MI, United States
The transient receptor potential ankyrin 1 (TRPA1) ion channel has emerged as significant regulators of cardiovascular physiology and pathology. TRPA1 is a non-selective cation channel permeable to calcium ions. A unique feature of the channel is its function as a sensor of various temperature, chemical and mechanical stimuli, while it can also be activated by endogenous inflammatory mediators and reactive oxygen species. Over the last two decades, much progress has been made in illuminating the role of TRPA1 in the regulation of cardiovascular physiology and pathophysiology in addition to its important function in pain sensation. This review provides a comprehensive analysis of recent studies investigating the involvement of TRPA1 channels in various cardiovascular diseases, including myocardial infarction, ischemia-reperfusion injury, myocardial fibrosis, and response to environmental toxins. We discuss the diverse roles of TRPA1 channels in cardiac pathology and highlight their potential as therapeutic targets for cardiovascular disorders. Moreover, we explore the challenges and opportunities linked with targeting TRPA1 channels for treating cardiovascular diseases, alongside future research directions.
1 Introduction
Cardiovascular diseases remain a leading cause of mortality worldwide, necessitating a deeper understanding of their underlying mechanisms and the development of novel therapeutic strategies. Recent studies have implicated transient receptor potential ankyrin 1 (TRPA1) channels, known for their role as sensory receptors (1), in the pathogenesis of cardiovascular diseases (2–4). Found predominantly in nociceptive sensory neurons, TRPA1 channels belong to the transient receptor potential (TRP) ion channel superfamily, known for their role in sensory transduction, notably in pain sensation (5). TRPA1 and TRP melastatin 8 are considered as sensors of cold and cool temperatures, respectively (6–8), and they are involved in the vascular sensation of cold in the cutaneous microvasculature (9, 10). These channels serve as sensitive detectors of various stimuli, orchestrated through specialized receptors decorating the neuronal cell membrane (11, 12). TRPA1 is involved in the pathophysiology of a variety of diseases (13, 14), including renal diseases (15, 16), and clinical trials are currently testing the therapeutic effects of its agonists and antagonists in various neurological and dermatological disorders (17). TRPA1 channels are expressed in various cardiovascular cells, including vascular endothelial cells, vascular smooth muscle cells, cardiac fibroblasts, as well as cardiomyocytes (18, 19).
Structurally, TRPA1 channels exhibit a sophisticated architecture consisting of six transmembrane domains (TM), bordered by intracellular NH2 and COOH termini, with a pivotal pore-forming loop nestled between TM5 and TM6 (20–22). Of particular note is the expansive NH2 terminus, housing 14 (in mice) or 16 (in humans) ankyrin repeat domains, important for protein-protein interactions and imparting structural elasticity (20). Functionally, TRPA1 channels exhibit remarkable sensitivity to a wide range of triggers, facilitated by the presence of specialized receptors on the neuron's cell membrane (1, 23–25). TRPA1 can be covalently and non-covalently activated by various noxious and reactive chemical compounds (25, 26). For example, plants-derived reactive electrophiles such as allyl isothiocyanate (AITC) and cinnamaldehyde activate TRPA1 by covalently binding to its N-terminal cytoplasmic cysteine residues while nonreactive compounds such as menthol and thymol activate TRPA1 without covalent binding (27). By carefully analyzing mutagenesis and deletion constructs, researchers have pinpointed crucial regions that control various functional aspects of TRPA1 channels. These regions regulate calcium sensitivity, responsiveness to electrophiles, phosphorylation, rectification, conductance, and sensitivity to agonists such as AITC and carvacrol. Abnormalities within the realm of TRPA1 lead to specific functional changes, highlighting its contribution to nociception and disorders related to pain. This underscores the critical role TRPA1 plays in sensory transduction. Activation of TRPA1 channels has been shown to influence vascular tone (28, 29), inflammation (16, 30), oxidative stress (29), and cardiac remodeling (31), thereby impacting cardiovascular health. This review focuses on the role of TRPA1 in the development of cardiovascular diseases rather than the underlying regulatory molecular mechanisms as they have been discussed in previous reviews (2, 3).
2 Role of TRPA1 in ischemic heart disease: insights and therapeutic implications
Ischemic heart disease is myocardial injury caused by insufficient coronary blood flow to the heart. Persistent acute myocardial ischemia for a prolonged period can lead to irreversible injury or necrosis of cardiomyocytes, resulting in myocardial infarction (MI). This condition typically results from the obstruction of coronary arteries, often due to rupture of atherosclerotic plaques and subsequent formation of thrombosis. Myocardial ischemia/reperfusion (I/R) injury, resulting from the restoration of blood flow after ischemia, involves complex cellular and molecular processes. Ischemia initiates intracellular and extracellular changes, including calcium overload and ATP depletion, resulting in cellular dysfunction and death. Upon reperfusion, immediate effects like mitochondrial pore permeability and cytokine release exacerbate tissue damage, leading to long-term consequences and progressive cardiomyocyte death (32).
In the context of ischemic heart disease, the role of TRPA1 has garnered considerable interest. In recent studies focusing on the role of TRPA1 channels in MI and myocardial I/R injury, researchers have uncovered valuable insights into the molecular mechanisms underlying this pathological process and its potential therapeutic implications. However, the conclusions from these studies are highly variable.
First of all, a genome-wide association study revealed that TRPA1 variant at rs12541758 is the strongest susceptibility allele for coronary artery disease in humans, which provided the most conspicuous association between TRPA1 and ischemic heart disease (33). TRPA1 channels could be protective against myocardial ischemic injury according to studies. In a study conducted by Lu et al. (34), the presence and role of TRPA1 channels in cardiac myocytes and their involvement in regulating myocardial I/R injury were investigated. Using biochemical analysis techniques and a rat model of cardiac I/R injury, the researchers demonstrated that TRPA1 is indeed present within cardiac myocytes. Activation of TRPA1 reduced myocardial injury in the rat model, while inhibition of TRPA1 blocked the infarct size-sparing effects of morphine, indicating a potential interaction between TRPA1 signaling and analgesic pathways. In isolated cardiac myocytes subjected to hypoxia-reoxygenation, TRPA1 activation reduced cell death during reoxygenation, further supporting the role of TRPA1 in modulating cellular responses to I/R injury (35). However, the beneficial effects of TRPA1 agonists were not observed by Hoebart et al. who conducted a study to unravel the involvement of TRPA1 in MI and I/R injury (36). Through a series of experiments utilizing both pharmacological and genetic approaches, the researchers illuminated potential implications of TRPA1 in myocardial injury and sensory neuron-cardiomyocyte interactions. In pharmacological modulation studies conducted on Sprague-Dawley rats, the administration of TRPA1 agonists and antagonists did not significantly impact infarct size post-I/R injury. Similarly, genetic deletion of TRPA1 in C57BL/6J mice did not yield substantial differences in infarct size compared to wild-type mice. These findings, while suggestive of a potential role for TRPA1, did not establish a direct link between TRPA1 modulation and MI in vivo. In vitro experiments involving sensory neurons derived from dorsal root ganglia and cardiomyocytes provided intriguing insights into the interplay between TRPA1 and cardiomyocyte survival under I/R conditions. While the presence of sensory neurons enhanced cardiomyocyte survival, the specific involvement of TRPA1 in this process remained inconclusive. Additionally, investigations into the role of another sensory neuron receptor, TRP vanilloid 1, did not significantly alter cardiomyocyte protection under IR conditions, further emphasizing the complexity of sensory neuron-cardiomyocyte interactions.
In contrast, some studies demonstrated that TRPA1 channels could be detrimental to MI and myocardial I/R injury. Conklin and colleagues investigated the contribution of TRPA1 channels to myocardial I/R injury using mouse models, including both wild-type and Trpa1 gene knockout (Trpa1−/−) mice (37). Their findings revealed that Trpa1−/− mice exhibited significantly smaller infarct sizes following I/R compared to WT mice. Additionally, immunofluorescence staining revealed the presence of TRPA1 channels in both murine and human hearts, with notable enrichment observed in the intercalated disks of cardiomyocytes. Further experiments using isolated cardiomyocytes showed that TRPA1 deficiency conferred resistance to acrolein-induced toxicity, a known activator of TRPA1 channels. Blocking TRPA1 with a specific antagonist protected cardiomyocytes from acrolein-induced calcium overload and hypercontraction, suggesting a role for TRPA1-mediated calcium dysregulation in myocardial injury during I/R. These findings highlight TRPA1 as a potential therapeutic target for mitigating myocardial I/R injury, potentially through modulation of calcium homeostasis and cellular toxicity pathways.
Studies demonstrated that inhibition of TRPA1 might promote myocardial repair after MI. In a study by Li et al., TRPA1's potential as a therapeutic target for improving outcomes post-MI was explored using murine models (38). Male C57BL/6 mice subjected to MI injury exhibited increased TRPA1 expression, implicating TRPA1 in MI-induced cardiac injury. Treatment with the TRPA1 inhibitor HC-030031 (HC) post-MI resulted in improved cardiac function, reduced myocardial apoptosis, and ameliorated pathological changes, including reduced infarct size and cardiac fibrosis. Mechanistic insights revealed that TRPA1 inhibition promoted angiogenesis and activated the phosphoinositide-3 kinase/protein kinase B signaling pathway, offering potential avenues for therapeutic intervention in MI.
Overall, while the exact role of TRPA1 in ischemic heart disease remains complex and multifaceted, these studies collectively contribute to our understanding of TRPA1's involvement in MI and myocardial I/R injury and offer potential therapeutic targets for mitigating myocardial damage and improving outcomes of MI. The controversial results among studies might be due to the complicated role of TRPA1 in regulating cellular injury and defense during myocardial ischemia. Many factors could be responsible for the contradictory results. The role of TRPA1 may vary from different animal models and may be distinct at different stages of myocardial ischemia and MI. In addition, TRPA1 channels are expressed in various cell types in the heart, including cardiomyocytes, sensory nerve endings, fibroblasts, and macrophages. Future studies using conditional Trpa1 gene knockout mouse models may be helpful to further characterize its role in MI and I/R injury. Possible nonspecific actions of TRPA1 agonists should also be considered and ruled out using specific antagonists or gene knockout mouse models.
3 TRPA1 channels in myocardial fibrosis
Myocardial fibrosis, characterized by the excessive accumulation of collagen and other extracellular matrix proteins in the heart muscle, represents a hallmark feature of congestive heart failure pathogenesis. There are two types of myocardial fibrosis: reactive fibrosis and reparative. Reactive fibrosis refers to interstitial fibrosis mostly due to hypertension or aging, while reparative fibrosis, also known as replacement fibrosis, is responsible for cardiac wound healing and repair after MI. Reactive fibrosis is likely associated with decreased degradation of collagens, whereas reparative fibrosis is mainly due to activation of fibroblasts and increased synthesis of collagens (39, 40). Recent research has shed light on the role of TRPA1 channels in modulating myocardial fibrosis and cardiac dysfunction in response to different pathological stimuli. Studies utilizing animal models and experimental approaches have provided valuable insights into the potential therapeutic implications of targeting TRPA1 in the context of myocardial fibrosis.
Our recent study, utilizing TRPA1 gene knockout mice, has linked TRPA1 to age-related cardiac remodeling and dysfunction have also been linked to TRPA1 (31). We used 12-week-old (young) and 52-week-old (older) Trpa1−/− mice along with WT littermates as their animal models. We evaluated cardiac function and pathology using echocardiography and histological analyses and measured the expression levels of 84 fibrosis-related genes in heart tissue. Our findings reveal that older Trpa1−/− mice exhibited significantly increased left ventricular internal diameter and volume, impaired systolic and diastolic functions, and enhanced cardiac fibrosis compared to older WT mice, despite similar levels of cardiac hypertrophy between the two strains. Analysis of fibrosis-related genes showed significant upregulation of pro-fibrotic genes and downregulation of anti-fibrotic genes in older Trpa1−/− mice compared to older WT mice. These results suggest that TRPA1 deficiency exacerbates age-related myocardial fibrosis, ventricular dilation, and cardiac dysfunction, indicating a potentially anti-fibrotic role of TRPA1 in the context of cardiac aging. The exacerbation of myocardial fibrosis and ventricular dysfunction in older Trpa1−/− mice suggests a protective role of TRPA1 against age-related cardiac pathology (31). In doxorubicin-induced dilated cardiomyopathy rat model, activation of TRPA1 using its agonist cinnamaldehyde alleviated cardiac dysfunction and fibrosis, suggesting a protective role of TRPA1 in myocardial fibrosis and dilated cardiomyopathy (41). Similarly, another study demonstrated that activating TRPA1 attenuates right ventricular fibrosis in a model of monocrotaline-induced pulmonary arterial hypertension through secretion of calcitonin gene-related peptide (42). These findings imply that interventions aimed at enhancing TRPA1 activity or expression may have therapeutic potential for preventing or slowing down myocardial fibrosis and associated cardiovascular complications.
On the other hand, studies highlighted pro-fibrotic effects of TRPA1 in the process of replacement cardiac fibrosis. For instance, Li et al. investigated the role of TRPA1 in driving cardiac myofibroblast transdifferentiation post-MI injury (43). Their findings suggest that TRPA1 activation promotes cardiac myofibroblast transdifferentiation via the calcineurin/nuclear factor of activated T cell signaling pathway. These results imply that targeting TRPA1-mediated myofibroblast activation may represent a potential therapeutic strategy for reducing post-MI fibrotic remodeling and improving cardiac function in MI patients. In a rat model of diabetic cardiomyopathy, Wang et al. demonstrated that TRPA1 deficiency exerts protective effects against cardiac dysfunction and fibrosis (44). Their findings suggest that TRPA1 inhibition may represent a promising therapeutic strategy for mitigating myocardial fibrosis in diabetic patients, potentially reducing the risk of congestive heart failure, and improving overall cardiac function. The identification of the G protein-coupled receptor kinase 5 and nuclear factor of activated T cell signaling pathway as a downstream mediator of TRPA1-related fibrotic changes highlights specific molecular targets for future drug development efforts aimed at modulating TRPA1 activity. In the context of pressure overload-induced cardiac hypertrophy and fibrosis, Wang et al. demonstrated that TRPA1 inhibition attenuates cardiac remodeling and fibrosis post-transverse aortic constriction surgery (45). This suggests that pharmacological targeting of TRPA1 may represent a novel approach for managing pressure overload-induced cardiac diseases, such as hypertrophic cardiomyopathy and congestive heart failure. The identification of calcium-dependent signaling pathways and M2 macrophage transition as mechanisms underlying the protective effects of TRPA1 inhibition provides valuable insights into the complex molecular pathways involved in myocardial fibrosis regulation.
In conclusion, the collective findings from these studies highlight the significance of TRPA1 modulation in myocardial fibrosis and associated cardiac dysfunction. The TRPA1 variant rs574328516 is related to cardiomyopathy in humans according to FinnGen Data Freeze 10 (https://r10.finngen.fi). The role of TRPA1 in cardiac fibrosis varies in different contexts. Overall, TRPA1 channels likely inhibit reactive fibrosis but stimulate reparative fibrosis. Targeting TRPA1 signaling pathways holds promise for developing novel therapeutic interventions aimed at preventing or mitigating myocardial fibrosis-related cardiovascular complications. These diverse findings of previous studies underscore the complexity of TRPA1 signaling in fibrosis regulation and highlight the need for further research to understand its precise role and therapeutic potential in different pathological conditions. Further research is needed to clarify the exact molecular mechanisms underlying TRPA1-mediated fibrotic remodeling and to translate these findings into clinically relevant therapeutic strategies for improving patient outcomes in cardiovascular diseases.
4 TRPA1 agonists: implications for cardiovascular responses
The investigation into the cardiovascular effects of TRPA1 agonists, particularly AITC and cinnamaldehyde, has revealed significant findings with broad implications for cardiovascular physiology and potential therapeutic strategies. Studies have demonstrated that TRPA1 activation by AITC and cinnamaldehyde elicits diverse cardiovascular responses in animal models. In normotensive rats, AITC inhalation evoked atropine-sensitive bradycardia, indicative of parasympathetic reflex activation, whereas spontaneously hypertensive rats exhibited complex brady-tachycardia accompanied by atrial-ventricular block and premature ventricular contractions (46). Furthermore, intravenous administration of AITC induced bradycardia in both rat models, indicating distinct reflex pathways depending on the route of administration (46). Additionally, investigations into cinnamaldehyde's cardiovascular effects revealed its ability to induce relaxation of rat aortic rings and negative inotropic and chronotropic effects on isolated mouse hearts (47). In vivo mouse experiments demonstrated that cinnamaldehyde could induce a systemic depressor response through activating TRPA1 (4) and increase cerebrovascular blood flow by mediating a neurogenic vasodilatation (48).
These findings have several implications for cardiovascular health. Firstly, it suggests that modulation of TRPA1 channels may offer novel strategies for managing hypertension, ischemic heart disease, and peripheral vascular disorders. Another way is that the inhibition of L-type calcium currents by TRPA1 agonists suggests a potential role for TRPA1 channels in modulating cardiac contractility and electrical activity, which could lead to the development of selective TRPA1 modulators or channel blockers for managing cardiac arrhythmias and heart failure. The involvement of TRPA1 agonists in cardiovascular reflexes suggests a potential crosstalk between nociceptive and cardiovascular pathways, providing insights into the pathogenesis of conditions characterized by both pain and cardiovascular dysfunction. Further research in this area may uncover additional therapeutic targets and strategies for improving cardiovascular health and treating related disorders. Furthermore, gaining a deeper understanding of the exact mechanisms through which TRPA1 agonists exert their cardiovascular effects holds the potential to pave the way for the development of more targeted and effective therapeutic interventions. Such advancements have the promise of significantly enhancing outcomes for patients grappling with cardiovascular disease
5 TRPA1 in cardiovascular health: response to environmental toxins
TRPA1 is a versatile protein known for its involvement in various physiological processes, including sensory perception and vasoreactivity. Particularly relevant to cardiovascular health, TRPA1 has emerged as a critical mediator in response to environmental toxins, such as crotonaldehyde and acrolein, commonly found in cigarette smoke and air pollution. Understanding the intricate interplay between TRPA1 activation and cardiovascular outcomes in the context of toxin exposure is essential for deciphering mechanisms underlying toxin-induced cardiovascular toxicity and identifying potential therapeutic targets.
Studies by Jin et al. have shed light on the cardiovascular toxicity of crotonaldehyde, an abundant component of cigarette smoke, and its interaction with TRPA1 (49, 50). Both acute and chronic exposure to crotonaldehyde led to hypotension and augmented relaxations in vascular tissue, with TRPA1 playing a pivotal role in mediating these effects (49, 50). In Trpa1−/− mice, crotonaldehyde exposure had no significant impact on aortic function, highlighting the involvement of TRPA1 in crotonaldehyde-induced cardiovascular effects (49, 50). These findings suggest that TRPA1 activation may represent a key mechanism underlying crotonaldehyde-induced cardiovascular toxicity, emphasizing its potential as a therapeutic target for mitigating the adverse cardiovascular effects of cigarette smoke exposure. Similarly, acrolein, a highly toxic aldehyde present in tobacco smoke and air pollution, has been implicated in cardiovascular toxicity, with TRPA1 playing a crucial role in mediating its effects (51). Studies by Conklin et al. demonstrated that both male and female mice lacking the TRPA1 gene exhibited increased susceptibility to acrolein-induced mortality compared to wild-type mice, suggesting a protective role of TRPA1 (52, 53). Surprisingly, post-acrolein exposure treatment with a TRPA1 antagonist (HC030031) provided significant protection against acrolein-induced mortality (52, 53). Further investigation needs to address the compensatory effects of TRPA1 upon acrolein exposure and to reveal if the protective effects of HC030031 are mediated by TRPA1 inhibition. Additionally, investigations by Kurhanewicz et al. revealed that exposure to acrolein led to cardiac autonomic imbalance and arrhythmia in wild-type mice (54, 55), which was absent in Trpa1−/− mice, underscoring the crucial role of TRPA1 in mediating the cardiovascular effects of acrolein exposure. TRPA1 was also reported to mediate diesel exhaust exposure-induced hypersensitivity of cardiac arrhythmias (56). Ethanol and its metabolite acetaldehyde are known as cardiac toxins, and they can also activate the TRPA1 channel (53, 57). However, it is unclear whether TRPA1 plays a role in the effects of ethanol intake on the cardiovascular system.
Overall, these studies collectively highlight the pivotal role of TRPA1 in mediating cardiovascular responses to environmental toxins, such as crotonaldehyde and acrolein. Understanding the mechanisms underlying TRPA1 activation in response to toxin exposure is crucial for identifying novel therapeutic strategies aimed at mitigating toxin-induced cardiovascular toxicity. Future research efforts should focus on elucidating the intricate signaling pathways and downstream effects of TRPA1 activation in cardiovascular tissues, with the ultimate goal of developing targeted interventions to protect against toxin-induced cardiovascular damage and promote cardiovascular health in toxin-exposed populations.
6 Potential mechanisms underlying the role of TRPA1
The exact mechanism underlying the regulatory role of TRPA1 in cardiovascular health and disease is not well understood and is quite controversial. TRPA1 potentially has direct and/or indirect effects on myocardium. Studies have reported that the roles of TRPA1 are likely mediated by its effects on oxidative stress and inflammation (41). However, the direct effects of TRPA1 remain questionable as a recent study reported that no functional TRPA1 is expressed in cardiomyocytes, which makes a direct effect in cardiomyocytes unlikely (58). It has been confirmed that TRPA1 is abundantly expressed in sensory nerve endings including vagal afferent nerve fibers and that activation of TRPA1 stimulates vagal nerve activity (59–61). Improved vagal nerve activity can exert anti-inflammatory actions through binding to alpha 7 nicotinic acetylcholine receptor on macrophages and other immune cells (59, 62). So far, the indirect effects of TRPA1 through regulating cardiac vagal tone seem to be reasonable but warrant further investigation.
7 Summary
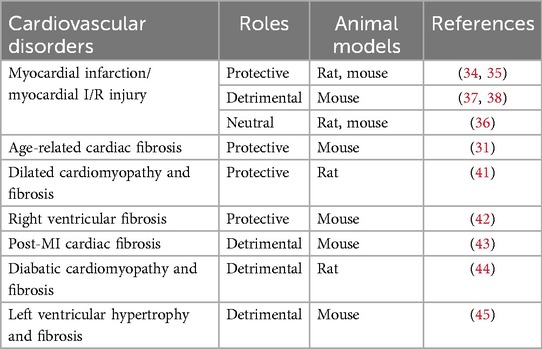
Taken together, TRPA1 channels play diverse roles in cardiovascular physiology and pathology and are involved in the development of cardiac fibrosis, ischemia-reperfusion injury, and arrhythmogenesis. Unlike previous reviews, this article focuses on the role of TRPA1 in the development of cardiovascular diseases. Over the past few years, research has tended to explore the effects of TRPA1 agonists and antagonists in addition to the role of the Trpa1 gene. The roles of TRPA1 in cardiovascular disorders have been summarized in Table 1.
While targeting TRPA1 channels holds promise as a therapeutic approach for cardiovascular diseases, further research is needed to elucidate their precise roles and regulatory mechanisms. Future studies should focus on validating TRPA1 as a viable therapeutic target and exploring novel strategies to modulate TRPA1 activity for the treatment of cardiovascular diseases. As TRPA1 is expressed in various types of cells and tissues, using conditional Trpa1 gene knockout mouse models may provide more precise insights into its role in cardiovascular pathophysiology. Controversial results were observed by using TRPA1 knockout mouse model and agonists/antagonists. Further studies need to confirm the functional status of TRPA1 channels after administrating their agonists and antagonists as their dosage, frequency, and duration may have distinct effects on the receptor. For instance, TRPA1 can be desensitized when exposed to agonists such as AITC, and as such the observed effects of AITC could be due to desensitization of TRPA1 (63). Furthermore, the effects of TRPA1 agonists/antagonists on cardiac sympathetic and parasympathetic nerve activities warrant further clarification. We should be careful that there is a complicated relationship between TRPA1 and L-type calcium channels. For example, the TRPA1 agonist cinnamaldehyde can block L-type calcium channels (47), and the L-type calcium channel blocker nifedipine can activate TRPA1 (64). Therefore, results from experiments testing TRPA1 agonists and antagonists should be carefully interpreted. Several TRPA1 agonists and antagonists have been testing in recent and ongoing clinical trials in various disorders other than cardiovascular diseases (65, 66). When we transform the studies from bench to bedside, the pharmacological effects of TRPA1 agonists and antagonists from animal studies should be carefully interpreted. The therapeutic response to TRPA1 agonists or antagonists may be dependent on the expression level or profile of the TRPA1 protein, therefore, future studies may need to identify specific variants that potentially regulate the expression of TRPA1 in cardiovascular diseases. Comprehensive clinical trials are warranted to evaluate the safety and efficacy of TRPA1-targeted therapies in patients with cardiovascular disorders, paving the way for the development of innovative treatments for these debilitating conditions.
Author contributions
IG: Writing – original draft. CQ: Writing – review & editing. SM: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by a transformational project award from the American Heart Association (#960671).
Conflict of interest
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. (2004) 427(6971):260–5. doi: 10.1038/nature02282
2. Wang Z, Ye D, Ye J, Wang M, Liu J, Jiang H, et al. The TRPA1 channel in the cardiovascular system: promising features and challenges. Front Pharmacol. (2019) 10:1253. doi: 10.3389/fphar.2019.01253
3. Gao S, Kaudimba KK, Guo S, Zhang S, Liu T, Chen P, et al. Transient receptor potential ankyrin type-1 channels as a potential target for the treatment of cardiovascular diseases. Front Physiol. (2020) 11:836. doi: 10.3389/fphys.2020.00836
4. Pozsgai G, Bodkin JV, Graepel R, Bevan S, Andersson DA, Brain Sd. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc Res. (2010) 87(4):760–8. doi: 10.1093/cvr/cvq118
5. Meents JE, Ciotu CI, Fischer MJM. TRPA1: a molecular view. J Neurophysiol. (2019) 121(2):427–43. doi: 10.1152/jn.00524.2018
6. Ma S, Yu H, Zhao Z, Luo Z, Chen J, Ni Y, et al. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol. (2012) 4(2):88–96. doi: 10.1093/jmcb/mjs001
7. Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. (2009) 106(4):1273–8. doi: 10.1073/pnas.0808487106
8. Moparthi L, Sinica V, Moparthi VK, Kreir M, Vignane T, Filipovic MR, et al. The human TRPA1 intrinsic cold and heat sensitivity involves separate channel structures beyond the N-ARD domain. Nat Commun. (2022) 13(1):6113. doi: 10.1038/s41467-022-33876-8
9. Pan Y, Thapa D, Baldissera L Jr, Argunhan F, Aubdool AA, Brain SD. Relevance of TRPA1 and TRPM8 channels as vascular sensors of cold in the cutaneous microvasculature. Pflugers Arch. (2018) 470(5):779–86. doi: 10.1007/s00424-017-2085-9
10. Aubdool AA, Graepel R, Kodji X, Alawi KM, Bodkin JV, Srivastava S, et al. TRPA1 is essential for the vascular response to environmental cold exposure. Nat Commun. (2014) 5:5732. doi: 10.1038/ncomms6732
11. Miyake T, Nakamura S, Zhao M, So K, Inoue K, Numata T, et al. Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat Commun. (2016) 7:12840. doi: 10.1038/ncomms12840
12. Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. (2006) 124(6):1269–82. doi: 10.1016/j.cell.2006.02.023
13. Talavera K, Startek JB, Alvarez-Collazo J, Boonen B, Alpizar YA, Sanchez A, et al. Mammalian transient receptor potential TRPA1 channels: from structure to disease. Physiol Rev. (2020) 100(2):725–803. doi: 10.1152/physrev.00005.2019
14. Xiao R, Zhang B, Dong Y, Gong J, Xu T, Liu J, et al. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell. (2013) 152(4):806–17. doi: 10.1016/j.cell.2013.01.020
15. Ma S, Wang HD. Knockout of Trpa1 exacerbates renal ischemia-reperfusion injury with classical activation of macrophages. Am J Hypertens. (2021) 34(1):110–6. doi: 10.1093/ajh/hpaa162
16. Ma S, Zhang Y, He K, Wang P, Wang HD. Knockout of TRPA1 exacerbates angiotensin II-induced kidney injury. Am J Physiol Renal Physiol. (2019) 317(3):F623–31. doi: 10.1152/ajprenal.00069.2019
17. Koivisto AP, Belvisi MG, Gaudet R, Szallasi A. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat Rev Drug Discov. (2022) 21(1):41–59. doi: 10.1038/s41573-021-00268-4
18. Andrei SR, Sinharoy P, Bratz IN, Damron DS. TRPA1 is functionally co-expressed with TRPV1 in cardiac muscle: co-localization at z-discs, costameres and intercalated discs. Channels (Austin). (2016) 10(5):395–409. doi: 10.1080/19336950.2016.1185579
19. Oguri G, Nakajima T, Kikuchi H, Obi S, Nakamura F, Komuro I. Allyl isothiocyanate (AITC) activates nonselective cation currents in human cardiac fibroblasts: possible involvement of TRPA1. Heliyon. (2021) 7(1):e05816. doi: 10.1016/j.heliyon.2020.e05816
20. Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. (2015) 520(7548):511–7. doi: 10.1038/nature14367
21. Suo Y, Wang Z, Zubcevic L, Hsu AL, He Q, Borgnia MJ, et al. Structural insights into electrophile irritant sensing by the human TRPA1 channel. Neuron. (2020) 105(5):882–94.e5. doi: 10.1016/j.neuron.2019.11.023
22. Cordero-Morales JF, Gracheva EO, Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci U S A. (2011) 108(46):E1184–91. doi: 10.1073/pnas.1114124108
23. Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, Jordt ES. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. (2008) 118(5):1899–910. doi: 10.1172/JCI34192
24. Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. (2004) 41(6):849–57. doi: 10.1016/s0896-6273(04)00150-3
25. Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. (2007) 445(7127):541–5. doi: 10.1038/nature05544
26. Zhao J, Lin King JV, Paulsen CE, Cheng Y, Julius D. Irritant-evoked activation and calcium modulation of the TRPA1 receptor. Nature. (2020) 585(7823):L141–5. doi: 10.1038/s41586-020-2480-9
27. Fine M, Li X. Insights into the irritating mechanisms of TRPA1 revealed by cryo-EM. Neuron. (2021) 109(2):194–6. doi: 10.1016/j.neuron.2020.12.017
28. Rivera-Mancilla E, Al-Hassany L, Marynissen H, Bamps D, Garrelds IM, Cornette J. Functional analysis of TRPA1, TRPM3, and TRPV1 channels in human dermal arteries and their role in vascular modulation. Pharmaceuticals (Basel). (2024) 17(2):156. doi: 10.3390/ph17020156
29. Yang Y, Wang D, Wan J, Ran F, Yang L, Chen S, et al. The role of transient receptor potential ankyrin 1 in age-related endothelial dysfunction. Exp Gerontol. (2021) 154:111517. doi: 10.1016/j.exger.2021.111517
30. Balestrini A, Joseph V, Dourado M, Reese RM, Shields SD, Rouge L, et al. A TRPA1 inhibitor suppresses neurogenic inflammation and airway contraction for asthma treatment. J Exp Med. (2021) 218(4):e20201637. doi: 10.1084/jem.20201637
31. Ma S, Wang DH. Knockout of Trpa1 accelerates age-related cardiac fibrosis and dysfunction. PLoS One. (2022) 17(9):e0274618. doi: 10.1371/journal.pone.0274618
32. Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. (2011) 2(12):e244. doi: 10.1038/cddis.2011.130
33. Wakil SM, Ram R, Muiya NP, Mehta M, Andres E, Mazhar N, et al. A genome-wide association study reveals susceptibility loci for myocardial infarction/coronary artery disease in Saudi Arabs. Atherosclerosis. (2016) 245:62–70. doi: 10.1016/j.atherosclerosis.2015.11.019
34. Lu Y, Piplani H, McAllister SL, Hurt CM, Gross RE. Transient receptor potential ankyrin 1 activation within the cardiac myocyte limits ischemia-reperfusion injury in rodents. Anesthesiology. (2016) 125(6):1171–80. doi: 10.1097/ALN.0000000000001377
35. Andrei SR, Ghosh M, Sinharoy P, Damron DS. Stimulation of TRPA1 attenuates ischemia-induced cardiomyocyte cell death through an eNOS-mediated mechanism. Channels (Austin). (2019) 13(1):192–206. doi: 10.1080/19336950.2019.1623591
36. Hoebart C, Kiss A, Pilz PM, Szabo PL, Podesser BK, Fischer MJM. TRPA1 As target in myocardial infarction. Int J Mol Sci. (2023) 24(3):2516. doi: 10.3390/ijms24032516
37. Conklin DJ, Guo Y, Nystoriak MA, Jagatheesan G, Obal D, Kilfoil PJ, et al. TRPA1 channel contributes to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. (2019) 316(4):H889–99. doi: 10.1152/ajpheart.00106.2018
38. Li R, Liu R, Yan F, Zhuang X, Shi H, Gao X. inhibition of TRPA1 promotes cardiac repair in mice after myocardial infarction. J Cardiovasc Pharmacol. (2020) 75(3):240–9. doi: 10.1097/FJC.0000000000000783
39. Lu L, Guo J, Hua Y, Huang K, Magaye R, Cornell J, et al. Cardiac fibrosis in the ageing heart: contributors and mechanisms. Clin Exp Pharmacol Physiol. (2017) 44(Suppl 1):55–63. doi: 10.1111/1440-1681.12753
40. Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging Dis. (2011) 2(2):158–73.21837283
41. Wang M, Zhao M, Zheng Z, Pan W, Zhang J, Yin Z, et al. TRPA1 deficiency aggravates dilated cardiomyopathy by promoting S100A8 expression to induce M1 macrophage polarization in rats. FASEB J. (2023) 37(6):e22982. doi: 10.1096/fj.202202079RR
42. Li W, Zhang Z, Li X, Cai J, Li D, Du J, et al. CGRP derived from cardiac fibroblasts is an endogenous suppressor of cardiac fibrosis. Cardiovasc Res. (2020) 116(7):1335–48. doi: 10.1093/cvr/cvz234
43. Li S, Sun X, Wu H, Yu P, Wang X, Jiang Z, et al. TRPA1 promotes cardiac myofibroblast transdifferentiation after myocardial infarction injury via the calcineurin-NFAT-DYRK1A signaling pathway. Oxid Med Cell Longev. (2019) 2019:6408352. doi: 10.1155/2019/6408352
44. Wang M, Zhao M, Xu S, Zheng Z, Zhang J, Pan W, et al. TRPA1 deficiency attenuates cardiac fibrosis via regulating GRK5/NFAT signaling in diabetic rats. Biochem Pharmacol. (2023) 214:115671. doi: 10.1016/j.bcp.2023.115671
45. Wang Z, Xu Y, Wang M, Ye J, Liu J, Jiang H, et al. TRPA1 inhibition ameliorates pressure overload-induced cardiac hypertrophy and fibrosis in mice. EBioMedicine. (2018) 36:54–62. doi: 10.1016/j.ebiom.2018.08.022
46. Hooper JS, Stanford KR, Alencar PA, Alves NG, Breslin JW, Dean JB, et al. Nociceptive pulmonary-cardiac reflexes are altered in the spontaneously hypertensive rat. J Physiol. (2019) 597(13):3255–79. doi: 10.1113/JP278085
47. Alvarez-Collazo J, Alonso-Carbajo L, Lopez-Medina AI, Alpizar YA, Tajada S, Nilius B, et al. Cinnamaldehyde inhibits L-type calcium channels in mouse ventricular cardiomyocytes and vascular smooth muscle cells. Pflugers Arch. (2014) 466(11):2089–99. doi: 10.1007/s00424-014-1472-8
48. Aubdool AA, Kodji X, Abdul-Kader N, Heads R, Fernandes ES, Bevan S, et al. TRPA1 activation leads to neurogenic vasodilatation: involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br J Pharmacol. (2016) 173(15):2419–33. doi: 10.1111/bph.13519
49. Jin L, Jagatheesan G, Lynch J, Guo L, Conklin DJ. Crotonaldehyde-induced vascular relaxation and toxicity: role of endothelium and transient receptor potential ankyrin-1 (TRPA1). Toxicol Appl Pharmacol. (2020) 398:115012. doi: 10.1016/j.taap.2020.115012
50. Lynch J, Jin L, Richardson A, Jagatheesan G, Lorkiewicz P, Xie Z, et al. Acute and chronic vascular effects of inhaled crotonaldehyde in mice: role of TRPA1. Toxicol Appl Pharmacol. (2020) 402:115120. doi: 10.1016/j.taap.2020.115120
51. Thompson LC, Walsh L, Martin BL, McGee J, Wood C, Kovalcik K, et al. Ambient particulate matter and acrolein co-exposure increases myocardial dyssynchrony in mice via TRPA1. Toxicol Sci. (2019) 167(2):559–72. doi: 10.1093/toxsci/kfy262
52. Conklin DJ, Haberzettl P, Jagatheesan G, Kong M, Hoyle WG. Role of TRPA1 in acute cardiopulmonary toxicity of inhaled acrolein. Toxicol Appl Pharmacol. (2017) 324:61–72. doi: 10.1016/j.taap.2016.08.028
53. Conklin DJ. Acute cardiopulmonary toxicity of inhaled aldehydes: role of TRPA1. Ann N Y Acad Sci. (2016) 1374(1):59–67. doi: 10.1111/nyas.13055
54. Kurhanewicz N, McIntosh-Kastrinsky R, Tong H, Ledbetter A, Walsh L, Farraj A, et al. TRPA1 mediates changes in heart rate variability and cardiac mechanical function in mice exposed to acrolein. Toxicol Appl Pharmacol. (2017) 324:51–60. doi: 10.1016/j.taap.2016.10.008
55. Kurhanewicz N, Ledbetter A, Farraj A, Hazari M. TRPA1 mediates the cardiac effects of acrolein through parasympathetic dominance but also sympathetic modulation in mice. Toxicol Appl Pharmacol. (2018) 347:104–14. doi: 10.1016/j.taap.2018.03.027
56. Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, et al. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. (2011) 119(7):951–7. doi: 10.1289/ehp.1003200
57. Komatsu T, Uchida K, Fujita F, Zhou Y, Tominaga M. Primary alcohols activate human TRPA1 channel in a carbon chain length-dependent manner. Pflugers Arch. (2012) 463(4):549–59. doi: 10.1007/s00424-011-1069-4
58. Hoebart C, Rojas-Galvan NS, Ciotu CI, Aykac I, Reissig LF, Weninger WJ, et al. No functional TRPA1 in cardiomyocytes. Acta Physiol (Oxf). (2021) 232(4):e13659. doi: 10.1111/apha.13659
59. Silverman HA, Tynan A, Hepler TD, Chang EH, Gunasekaran M, Li JH, et al. Transient receptor potential ankyrin-1-expressing vagus nerve fibers mediate IL-1beta induced hypothermia and reflex anti-inflammatory responses. Mol Med. (2023) 29(1):4. doi: 10.1186/s10020-022-00590-6
60. Kowalski CW, Ragozzino FJ, Lindberg JEM, Peterson B, Lugo JM, McLaughlin RJ, et al. Cannabidiol activation of vagal afferent neurons requires TRPA1. J Neurophysiol. (2020) 124(5):1388–98. doi: 10.1152/jn.00128.2020
61. Birrell MA, Belvisi MG, Grace M, Sadofsky L, Faruqi S, Hele DJ, et al. TRPA1 agonists evoke coughing in Guinea pig and human volunteers. Am J Respir Crit Care Med. (2009) 180(11):1042–7. doi: 10.1164/rccm.200905-0665OC
62. Bazoukis G, Stavrakis S, Armoundas AA. Vagus nerve stimulation and inflammation in cardiovascular disease: a state-of-the-art review. J Am Heart Assoc. (2023) 12(19):e030539. doi: 10.1161/JAHA.123.030539
63. Akopian AN, Ruparel NB, Jeske NA, argreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. (2007) 583(Pt 1):175–93. doi: 10.1113/jphysiol.2007.133231
64. Fajardo O, Meseguer V, Belmonte C, Viana F. TRPA1 channels: novel targets of 1,4-dihydropyridines. Channels (Austin). (2008) 2(6):429–38. doi: 10.4161/chan.2.6.7126
65. Jain SM, Balamurugan R, Tandon M, Mozaffarian N, Gudi G, Salhi Y, et al. Randomized, double-blind, placebo-controlled trial of ISC 17536, an oral inhibitor of transient receptor potential ankyrin 1, in patients with painful diabetic peripheral neuropathy: impact of preserved small nerve fiber function. Pain. (2022) 163(6):e738–47. doi: 10.1097/j.pain.0000000000002470
Keywords: TRPA1, ion channel, cardiovascular disease, myocardial infarction, myocardial fibrosis
Citation: Gellani I, Qian C and Ma S (2024) Unveiling the role of TRPA1 in cardiovascular health and disease: a mini review. Front. Cardiovasc. Med. 11:1416698. doi: 10.3389/fcvm.2024.1416698
Received: 12 April 2024; Accepted: 30 August 2024;
Published: 11 September 2024.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Ari-Pekka Koivisto, Orion Corporation, FinlandCopyright: © 2024 Gellani, Qian and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuangtao Ma, bWFzaHVhbmdAbXN1LmVkdQ==
 Islam Gellani
Islam Gellani Chunqi Qian
Chunqi Qian Shuangtao Ma
Shuangtao Ma