
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 05 July 2024
Sec. Structural Interventional Cardiology
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1416613
 Serdar Farhan1
Serdar Farhan1 Michael Freilich2
Michael Freilich2 Gennaro Giustino1
Gennaro Giustino1 Birgit Vogel1
Birgit Vogel1 Usman Baber1
Usman Baber1 Samantha Sartori1
Samantha Sartori1 Haroon Kamran1
Haroon Kamran1 Roxana Mehran1
Roxana Mehran1 George Dangas1
George Dangas1 Prakash Krishnan1
Prakash Krishnan1 Annapoorna Kini1
Annapoorna Kini1 Samin K. Sharma1*
Samin K. Sharma1*
Introduction: High-risk percutaneous coronary interventions (HRPCI) are a potential treatment option for patients with reduced left ventricular ejection fraction (LVEF) and coronary artery disease. The extent to which such intervention is coupled with improvement in LVEF and associated with favorable outcomes is unknown.
Methods: We aimed to characterize the incidence and correlates of LVEF improvement after Impella-guided HRPCI, and compare clinical outcomes in patients with versus without LVEF improvement. Data on consecutive patients undergoing Impella-guided HRPCI from a single center registry were analyzed. LVEF-improvement was defined as an absolute increase of LVEF of ≥10% measured at ≥30‐days after intervention. The primary outcome was a composite of all‐cause death, myocardial infarction or target vessel revascularization within 1-year.
Results: Out of 161 consecutive patients undergoing Impella-guided HRPCI from June 2008 to December 2017, 43% (n = 70) demonstrated LVEF-improvement (baseline LVEF of 25.09 ± 6.19 to 33.30 ± 11.98 post intervention). Patients without LVEF-improvement had higher frequency of previous MI (61.5% vs. 37.1%, p = 0.0021), Q-waves on ECG (17.6% vs. 5.7%, p = 0.024) and higher SYNTAX scores (30.8 ± 17.6 vs. 25.2 ± 12.2; p = 0.043). After correction of these confounders by multivariable analysis, no significant differences were found regarding the composite endpoint in patients with versus without LVEF-improvement (34.9% vs. 38.3%; p = 0.48).
Discussion: In this single-center retrospective analysis, we report the following findings. First, LVEF improvement of at least 10% was documented in over 40% of patients undergoing Impella supported high-risk PCI. Second, a history of MI, Q-waves on admission ECG, and higher baseline SYNTAX scores were independent correlates of no LVEF improvement. Third, one year rates of adverse CV events were substantial and did not vary by the presence or absence of LVEF improvement Prospective studies with longer follow-up are needed to elucidate the impact of LVEF improvement on clinical outcomes.
Up to 20% of patients with complex coronary artery disease are deemed poor surgical candidates, leading this subset of the population to be underserved with regards to coronary revascularization (1). The reasons underlying this fact are multifaceted and can be traced to advanced age, multiple medical co-morbidities, left ventricular dysfunction, decompensated heart failure, among others (2). Such patients suffer a markedly higher rate of adverse outcomes, even if a percutaneous coronary intervention (PCI) is sought (1, 3). However, with recent advances in mechanical circulatory support-assisted PCI, the ability of improved clinical outcomes in this population remains a possibility. The added hemodynamic stability provided by these devices provides additional support not previously available.
There is scarce data available evaluating whether these interventions are associated with an improvement in left ventricular (LV) function and subsequent clinical outcomes. Findings from a randomized trial demonstrated that reverse LV remodeling occurred in 51% of patients undergoing high-risk PCI with Impella support, which was associated with a reduction in 30-day adverse events (4). However, the extent to which these benefits are generalizable to an unselected cohort and durable over longer-term follow-up remains unknown. Therefore, we aimed to investigate the correlates of LVEF change and the association between LVEF improvement and 1-year clinical events in patients undergoing Impella-supported high-risk PCI at our institution.
The study cohort was selected from a prospective registry maintained at Mount Sinai Heart. All patients who underwent Impella-supported PCI were selected for eligibility for inclusion in the present analysis.
Between June 2008 and December 2017, a total of 328 patients underwent Impella 2.5® (Abiomed Inc. Danvers, Massachusetts) supported PCI at Mount Sinai Hospital. High-risk PCI was defined according to our institutional algorithm Complex PCI (Long calcified lesion, Bifurcation lesion, Unprotected LM lesion, SVG lesion) with concomitant LVEF >35%; Complex PCI or High SYNTAX score >32/STS risk for mortality >5% or extensive revascularization with concomitant LVEF 20%–35%; or simple or complex PCI or inoperable patient with concomitant LVEF <20% (Supplementary Figure S1). The inclusion criteria for the present analysis were (i) underlying CAD undergoing Impella-supported PCI (ii) LVEF measurement before and at least 30 days after the procedure. Patients who expired within index hospitalization and those with missing LVEF evaluation during follow-up were excluded. For the purpose of the present analysis, patients were grouped according to LVEF improvement of at least 10% (delta LVEF >10%) vs. less than 10% (delta LVEF <10%). LVEF was calculated either via the Simpson method using transthoracic echocardiogram or MUGA nuclear medicine scans by plotting red blood cell technetium using a gated ECG approach.
An institutional review board approved the study.
The endpoint of interest was a composite of all-cause death, myocardial infarction (MI), or target vessel revascularization (TVR) within 1-year of follow-up. MI was defined according to the 3rd universal definition of MI (5) and TVR was defined according to the academic research consortium (ARC) (6). Follow-up information was captured via telephone calls by trained research coordinators at one year after index PCI. Source documents were obtained for those patients reporting any adverse events. All information was then forwarded to a clinical events committee for formal adjudication.
Continuous variables are presented as mean ± SD. Categorical variables are presented as percentages. Chi-square test was used to compare differences between categorical variables. The independent-samples t-test was used to compare continuous variables with normal distribution, and the Mann-Whitney test was used to compare continuous variables without normal distribution. Crude 1-year event rates were calculated using the Kaplan-Meier method and a log-rank test to assess differences. A multivariate linear regression analysis with purposeful selection of variables was used to identify independent correlates of LVEF change (delta LVEF, calculated as the difference in LVEF measurement between follow-up and baseline).
Out of 328 patient who underwent Impella-supported PCI a total of 161 eligible patients with baseline LVEF 25.1 ± 6.2 with a median follow-up of 112 days were included in the study. Baseline and procedural characteristics of patient included vs. not included in the analysis are presented in a Supplementary Table S1. Multivessel disease was present in 88.6% of patients. Baseline and procedural characteristics are presented in Tables 1, 2. LVEF improvement of greater than 10% was observed in 70 patients (43%). This group showed LVEF of 39.1 ± 11.2% vs. 24.5 ± 6.5% in the group without delta LVEF <10% (p ≤ 0.0001) (Figure 1 and Supplementary Figure S2). There were no significant differences between groups with regards to age, sex, cardiovascular risk factors, renal impairment, anemia, history of peripheral as well as cerebrovascular disease or clinical presentation. Upon further review, patients from the delta-LVEF <10% group showed a significantly higher prevalence of previous MI (61.5% vs. 37.1%, p = 0.0021) and Q waves on admission ECG (17.6% vs. 5.7%, p = 0.024). PCI was successful in 87.3% and multivessel PCI was performed in 52.5% of the study population. Procedural characteristics, including stent length, bifurcation lesion, severe calcification, and stent diameter were similar between groups. Furthermore, the SYNTAX score was significantly higher in patients from delta-LVEF <10% compared to delta-LVEF ≥10% group (30.8 ± 17.6 vs. 25.2 ± 12.2; p = 0.043) (Table 1). There was a non-significant trend for lower residual SYNTAX score in the delta-LVEF ≥10% group compared to delta-LVEF <10% (Table 1). Clinical outcomes are presented in Supplementary Table S2. There were no significant differences in the composite endpoint of death, MI, or TVR at 1-year (Figure 2). In a multivariable linear regression model, history of prior MI, Q-waves on admission ECG and higher baseline SYNTAX score were independent correlates of LVEF change (Table 2). There were no significant differences in the composite endpoint of all-cause death, MI, and TVR over one year between patients from delta-LVEF ≥10% and delta-LVEF <10% (34.9% vs. 38.3%, p = 0.481).
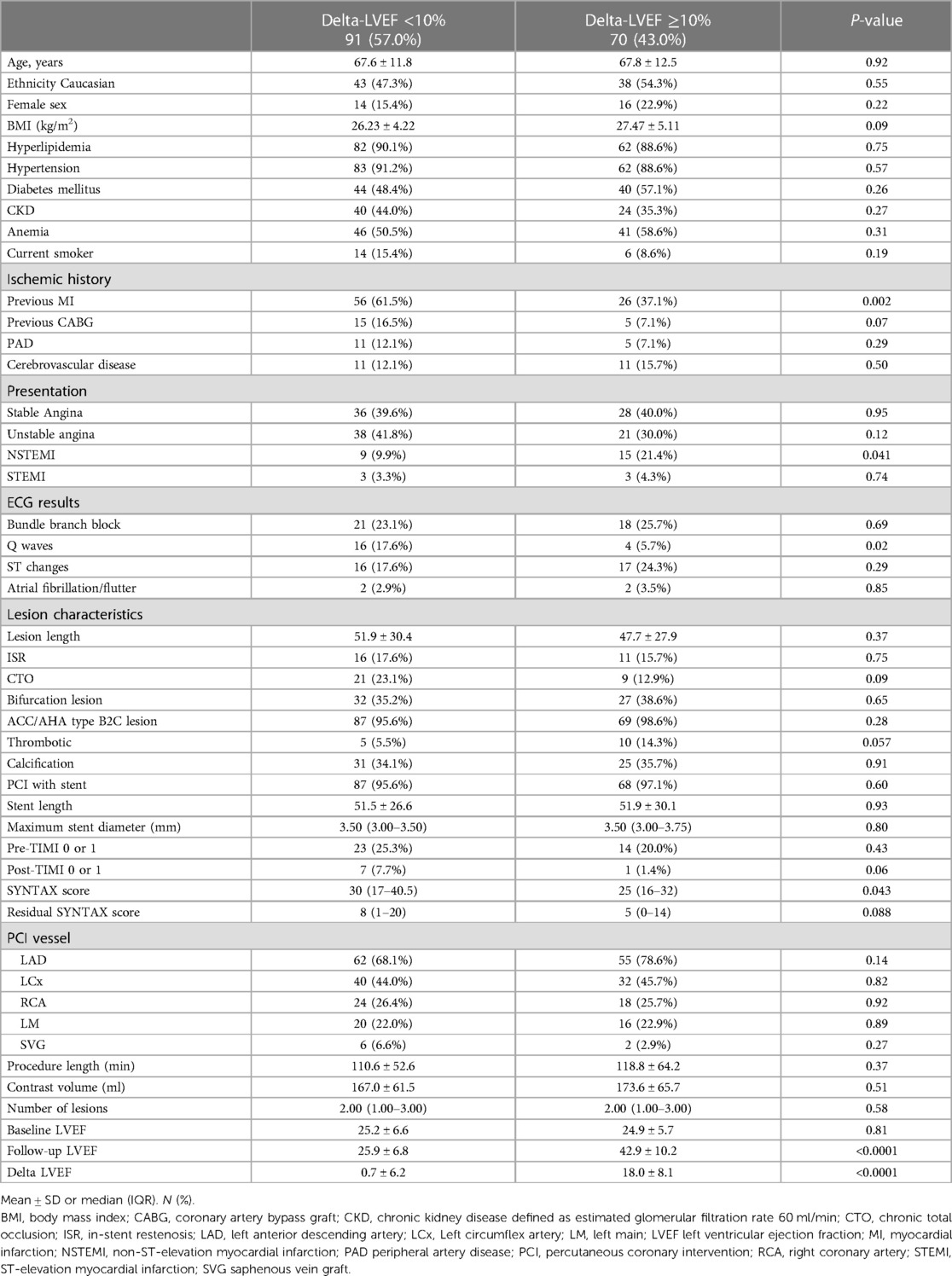
Table 1 Clinical and procedural characteristics of patients according to left ventricular function improvement.
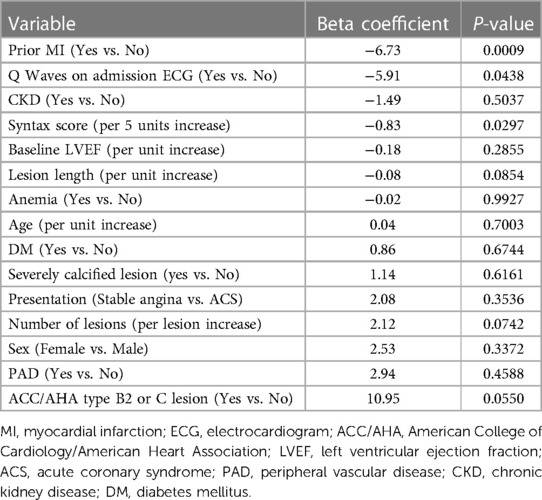
Table 2 Linear regression analysis of correlates of left ventricular function improvement in patients undergoing Impella-guided high-risk percutaneous coronary intervention.
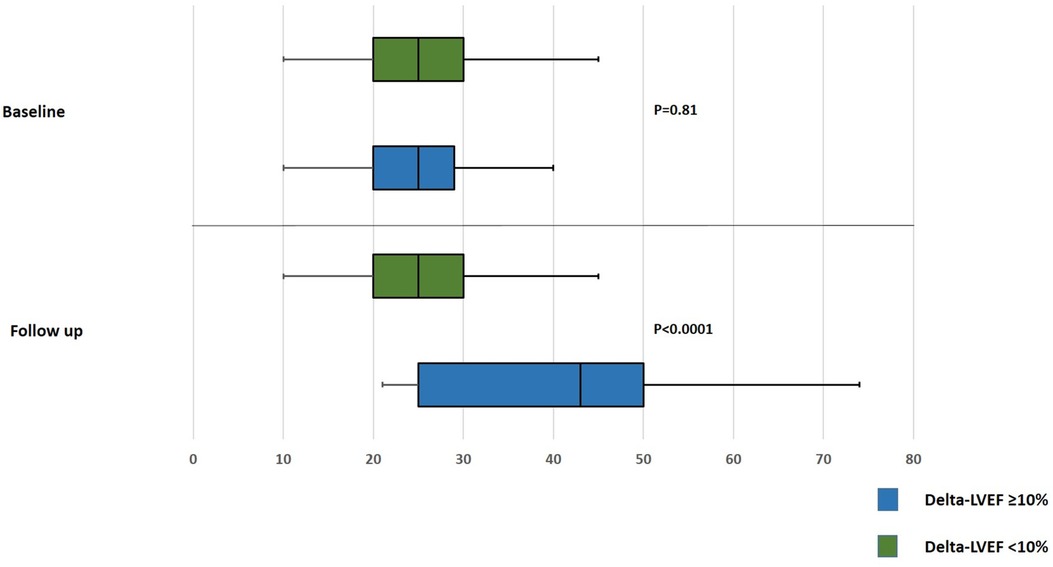
Figure 1 Box-whiskers plot for comparing baseline and follow up left ventricular ejection fraction after Impella-guided percutaneous coronary intervention. LVEF, left ventricular ejection fraction.
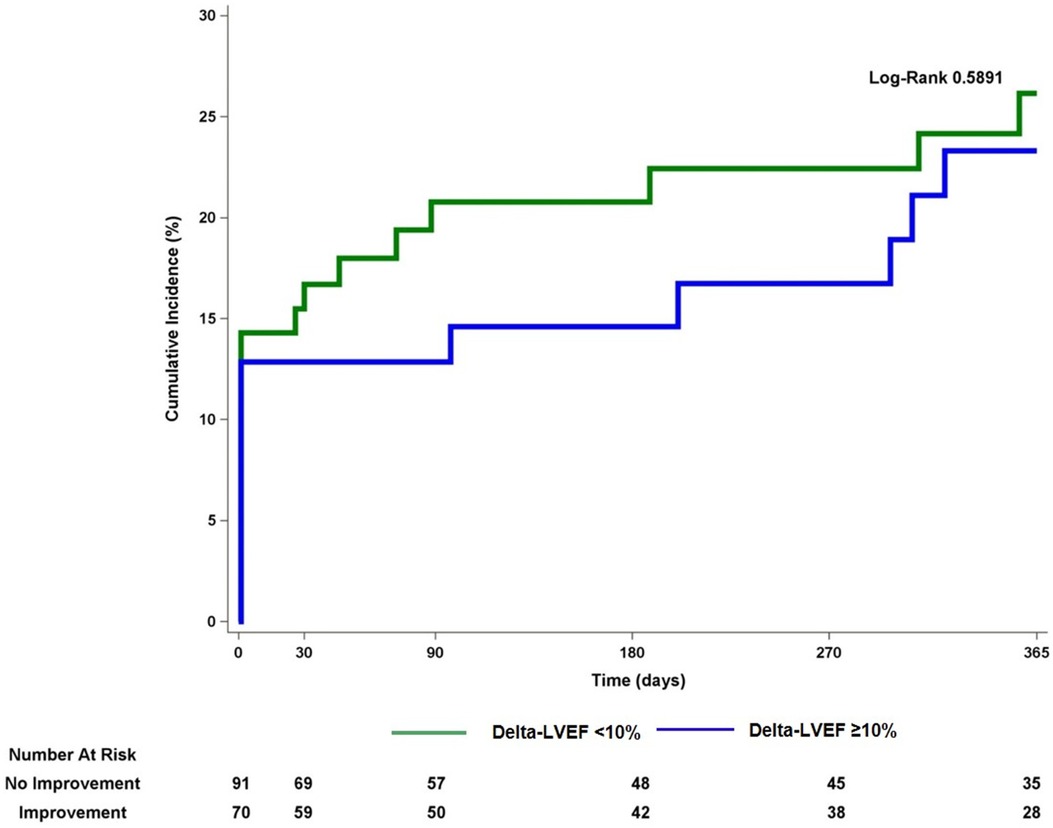
Figure 2 Kaplan-Maier curve for the composite endpoint of all-cause death, myocardial infarction or target vessel revascularization comparing patients with vs. without improvement in left ventricular ejection fraction. LVEF, left ventricular ejection fraction.
In this single-center retrospective analysis, we report the following findings: First, LVEF improvement of at least 10% was documented in over 40% of patients undergoing Impella supported high-risk PCI. Second, a history of MI, Q-waves on admission ECG, and higher baseline SYNTAX score were independent correlates of no LVEF improvement. Third, one-year rates of adverse CV events were substantial and did not vary by the presence or absence of LVEF improvement.
Patients with complex CAD and concomitant left ventricular dysfunction are usually characterized by significant comorbidity, thus rendering surgical revascularization prohibitive or very high risk, with high evidence of mortality noted (1). A meta-analysis of randomized clinical trials and registry studies comparing CABG vs. PCI vs. medical therapy in patients with CAD and LVEF ≤40% showed more favorable outcomes with surgical revascularization (3, 7). However, the majority included studies that did not utilize mechanical circulatory devices for the PCI group, resulting in higher rates of incomplete revascularization (3, 7). The introduction of mechanical circulatory support devices made such patients more amendable for PCI with complete revascularization (8–10). Recently, Burzotta et al. found an improvement of LVEF in about 70% of patients undergoing high-risk PCI by Impella support (11). Additionally, the authors found that completeness of revascularization measured by the British Cardiovascular Intervention Society (BCIS) Jeopardy Score (JS) was associated with improvements of LVEF and clinical outcomes at a mean follow-up of 14 months (11). Moreover, in a pooled analysis of the PROTECT II trial and the cVAD registry, Russo et al. showed that low baseline LVEF, absence of congestive heart failure, and the number of treated vessels were independent correlates of LVEF improvement (12). All-cause death in our study was 3.81% at 12 months as compared to 10.5% in the study of Burzotta et al. This difference might be attributed to the higher rate of acute coronary syndrome patients (73%) and higher rate of left main (LM) interventions (44%) in the study of Burzotta et al. (11) as compared to the present study.
Mechanical circulatory devices provide additional hemodynamic stability not previously available, allowing for the opportunity of complete revascularization. This was confirmed in Burzotta et al., where Impella-guided PCI resulted in a higher rate of complete revascularization (11). This proves to be important as a sub-study of the ACUITY trial showed complete revascularization measured by residual SYNTAX score was associated with improved 1-year outcomes, while a residual SYNTAX score of >8 was associated with poor prognosis (13). In the present study, residual SYNTAX score was slightly lower in LVEF improvement patients without reaching statistical significance. Furthermore, both a history of myocardial infarction and Q-waves on admission ECG were significant negative correlates of LVEF improvement in the present study. Both parameters indicate developed scar tissue, making an expectation of LVEF improvement less likely.
Previously the OAT trial showed no difference in the composite endpoint of all-cause death, re-infarction, or heart failure readmission when PCI was compared to medical management only in patients deemed high risk who were less than one month after an MI with a total occlusion of the infarcted artery (14). In an ancillary study, the authors found that myocardial viability was associated with the improvement of LVEF regardless of assigned treatment (15). The REVIVED trial showed no decrease in all-cause mortality or hospitalization for HF when comparing PCI plus optimal medical therapy vs. optimal medical therapy alone, in patient with LVEF ≤35% and extensive coronary artery disease (16). Similarly, others were also not able to document an association of LVEF restoration after revascularization with improved clinical outcome (17–19). However, sample size, mode of revascularization by surgery vs. PCI, the variability of the measured endpoints, and follow up duration might be the reason for the differing findings obtained as compared to the present investigation (4, 17–19). Improvement of clinical outcomes with revascularization over medical therapy became evident only after long-term follow-up, as highlighted in the extension of the STICH trial (20). Furthermore, the definition of LVEF improvement varied between the studies (4, 17–19). Our study showed similar results with regards to no improvement in clinical outcomes even in patients with significant LVEF improvement.
We are aware of several limitations of the present analysis. First, the data provided herein were derived from a single-center observational study, which limits the generalizability of our results. Furthermore, due to the retrospective design, several unmeasured confounders might have affected the results obtained in this analysis. Despite the encouraging results of the recently published DanGer Shock trial the present analysis excluded patients in shock. However, ongoing Randomized trials e.g., PROTCT IV are awaited to provide definitive answers on the impact of mechanical support device assisted high-risk PCI on changes in LVEF and subsequently on clinical outcomes. Second, we did not systematically evaluate preoperative scores e.g., the society of thoracic surgery mortality score and EURO-Score, providing additional opportunities for confounders. Furthermore, we did not evaluate imaging or hemodynamic parameters e.g., end-diastolic and end-systolic volumes, right ventricular function, and systolic pressure, which have been associated with better predictive value for outcomes after high-risk PCI (21, 22). Third, analyses with respect to clinical outcomes are underpowered, introducing the possibility of a type II error. Fourth, due to the small sample size of our study, our results are hypothesis-generating rather than conclusive. Larger studies such as the PROTCT IV trial would potentially address this issue. Fifth, our follow-up post intervention was only one year, and significant value would be derived in future studies with longer term follow-up. Furthermore, such interventions might have an impact on quality of life measures. However, this prospective registry did not include such metrics during follow up which is a limitation of the present study. Finally, despite obtaining stress test for all patients (excluding those with NSTEMI and STEMI) before the procedure, systematic viability testing was not performed. Adding viability testing might have impacted our findings.
History of MI, Q-waves on admission ECG and higher SYNTAX score were negative correlates of LVEF improvement in patients undergoing Impella-guided high-risk PCI. An increase of LVEF did not translate into an improvement of clinical outcomes in this patient population. Further research is warranted to elucidate predictors of LVEF improvement and their impact of on clinical outcomes in patients with ischemic heart disease undergoing high-risk intervention.
The datasets presented in this article are not readily available because only people on IRB had access to this data and is under strict protection by Mount Sinai Hospital. Requests to access these datasets should be directed toc2VyZGFyLmZhcmhhbkBtb3VudHNpbmFpLm9yZw==.
The studies involving humans were approved by Mount Sinai Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
SF: Writing – review & editing, Writing – original draft. MF: Writing – review & editing, Writing – original draft. GG: Writing – review & editing, Writing – original draft. BV: Writing – review & editing, Writing – original draft. UB: Writing – review & editing, Writing – original draft. SS: Writing – review & editing, Writing – original draft. HK: Writing – review & editing, Writing – original draft. RM: Writing – review & editing, Writing – original draft. GD: Writing – review & editing, Writing – original draft. PK: Writing – review & editing, Writing – original draft. AK: Writing – review & editing, Writing – original draft. SS: Writing – review & editing, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
GD has received consulting fees and honoraria from Johnson & Johnson, Sanofi, Covidien, The Medicines Company, Merck, CSL Behring, AstraZeneca, Medtronic, Abbott, Bayer, Boston Scientific, Osprey Medical, and GE Healthcare; and research grant support from Sanofi, Bristol-Myers Squibb, and Eli Lilly & Company/Daiichi-Sankyo. RM has received institutional research grant support from Eli Lilly, AstraZeneca, The Medicines Company, BMS/Sanofi-Aventis, consulting fees from AstraZeneca, Bayer, CSL Behring, Janssen Pharmaceuticals Inc., Merck & Co., Osprey Medical Inc., Watermark Research Partners. She also serves as a Scientific Advisory Board member for Abbott Laboratories, Boston Scientific Corporation, Covidien, Janssen Pharmaceuticals, The Medicines Company and Sanofi-Aventis. SS has received speakers honorarium from Abbott vascular Inc., Boston, Scientific Corporation, Cardiovascular Systems, Inc. (CSI).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1416613/full#supplementary-material
1. Waldo SW, Secemsky E, O'Brien C, Kennedy K, Pomerantsev E, Sundt T, et al. Surgical ineligibility and mortality among patients with unprotected left main or multivessel coronary artery disease undergoing percutaneous coronary intervention. Circulation. (2014) 130(25):2295–301. doi: 10.1161/CIRCULATIONAHA.114.011541
2. Patterson T, McConkey H, Ahmed-Jushuf F, Moschonas K, Nguyen H, Karamasis G, et al. Long-term outcomes following heart team revascularization recommendations in complex coronary artery disease. J Am Heart Assoc. (2019) 8(8):e011279. doi: 10.1161/JAHA.118.011279
3. Wolff G, Dimitroulis D, Andreotti F, Kolodziejczak M, Jung C, Scicchitano P, et al. Survival benefits of invasive versus conservative strategies in heart failure in patients with reduced ejection fraction and coronary artery disease: a meta-analysis. Circ Heart Fail. (2017) 10(1):e003255. doi: 10.1161/CIRCHEARTFAILURE.116.003255
4. Daubert MA, Massaro J, Liao L, Pershad A, Mulukutla S, Ohman EM, et al. High-risk percutaneous coronary intervention is associated with reverse left ventricular remodeling and improved outcomes in patients with coronary artery disease and reduced ejection fraction. Am Heart J. (2015) 170(3):550–8. doi: 10.1016/j.ahj.2015.06.013
5. Thygesen K, Alpert JS, Jaffe A, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Eur Heart J. (2012) 33(20):2551–67. doi: 10.1093/eurheartj/ehs184
6. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, Anne van Es G, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. (2007) 115(17):2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313
7. Bangalore S, Guo Y, Samadashvili Z, Blecker S, Hannan EL. Revascularization in patients with multivessel coronary artery disease and severe left ventricular systolic dysfunction: everolimus-eluting stents versus coronary artery bypass graft surgery. Circulation. (2016) 133(22):2132–40. doi: 10.1161/CIRCULATIONAHA.115.021168
8. O'Neill WW, Kleiman NS, Moses J, Henriques JPS, Dixon S, Massaro J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. (2012) 126(14):1717–27. doi: 10.1161/CIRCULATIONAHA.112.098194
9. Dixon SR, Henriques JPS, Mauri L, Sjauw K, Civitello A, Kar B, et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (the PROTECT I trial): initial U.S. experience. JACC Cardiovasc Interv. (2009) 2(2):91–6. doi: 10.1016/j.jcin.2008.11.005
10. Baumann S, Werner N, Ibrahim K, Westenfeld R, Al-Rashid A, Sinning JM, et al. Indication and short-term clinical outcomes of high-risk percutaneous coronary intervention with microaxial Impella(R) pump: results from the German Impella(R) registry. Clin Res Cardiol. (2018) 107(8):653–7. doi: 10.1007/s00392-018-1230-6
11. Burzotta F, Russo G, Ribichini F, Piccoli A, D'Amario D, Paraggio L, et al. Long-term outcomes of extent of revascularization in complex high risk and indicated patients undergoing Impella-protected percutaneous coronary intervention: report from the Roma-Verona registry. J Interv Cardiol. (2019). 2019:5243913. doi: 10.1155/2019/5243913
12. Russo JJ, Prasad M, Doshi D, Karmpaliotis D, Parikh MA, Ali ZA, et al. Improvement in left ventricular function following higher-risk percutaneous coronary intervention in patients with ischemic cardiomyopathy. Catheter Cardiovasc Interv. (2019) 96(4):764–70. doi: 10.1002/ccd.28557
13. Genereux P, Palmerini T, Caixeta A, Rosner G, Green P, Dressler O, et al. Quantification and impact of untreated coronary artery disease after percutaneous coronary intervention: the residual SYNTAX (synergy between PCI with Taxus and cardiac surgery) score. J Am Coll Cardiol. (2012) 59(24):2165–74. doi: 10.1016/j.jacc.2012.03.010
14. Hochman JS, Lamas GA, Buller CE, Dzavik V, Reynolds HR, Abramsky SJ, et al. Coronary intervention for persistent occlusion after myocardial infarction. N Engl J Med. (2006) 355(23):2395–407. doi: 10.1056/NEJMoa066139
15. Udelson JE, Pearte CA, Kimmelstiel CD, Kruk M, Kufera JA, Forman SA, et al. The occluded artery trial (OAT) viability ancillary study (OAT-NUC): influence of infarct zone viability on left ventricular remodeling after percutaneous coronary intervention versus optimal medical therapy alone. Am Heart J. (2011) 161(3):611–21. doi: 10.1016/j.ahj.2010.11.020
16. Perera D, Clayton T, O'Kane PD, Greenwood JP, Weerackody R, Ryan M, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. (2022) 387(15):1351–60. doi: 10.1056/NEJMoa2206606
17. Rizzello V, Poldermans D, Biagini E, Schinkel AFL, Boersma E, Boccanelli A, et al. Prognosis of patients with ischaemic cardiomyopathy after coronary revascularisation: relation to viability and improvement in left ventricular ejection fraction. Heart. (2009) 95(15):1273–7. doi: 10.1136/hrt.2008.163972
18. Joshi K, Alam I, Ruden E, Gradus-Pizlo I, Mahenthiran J, Kamalesh M, et al. Effect of improvement in left ventricular ejection fraction on long-term survival in revascularized patients with ischaemic left ventricular systolic dysfunction. Eur J Echocardiogr. (2011) 12(6):454–60. doi: 10.1093/ejechocard/jer045
19. Samady H, Elefteriades JA, Abbott B, Mattera JA, McPherson CA, Wackers FJT, et al. Failure to improve left ventricular function after coronary revascularization for ischemic cardiomyopathy is not associated with worse outcome. Circulation. (1999) 100(12):1298–304. doi: 10.1161/01.cir.100.12.1298
20. Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. (2016) 374(16):1511–20. doi: 10.1056/NEJMoa1602001
21. Schwietz T, Spyridopoulos J, Pfeiffer S, Laskowski R, Palm S, De Rosa S, et al. Risk stratification following complex PCI: clinical versus anatomical risk stratification including “post PCI residual SYNTAX-score” as quantification of incomplete revascularization. J Interv Cardiol. (2013) 26(1):29–37. doi: 10.1111/j.1540-8183.2013.12014.x
22. Bagai A, Armstrong PW, Stebbins A, Mahaffey KW, Hochman JS, Weaver WD, et al. Prognostic implications of left ventricular end-diastolic pressure during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: findings from the assessment of pexelizumab in acute myocardial infarction study. Am Heart J. (2013) 166(5):913–9. doi: 10.1016/j.ahj.2013.08.006
Keywords: high-risk percutaneous coronary interventions, Impella, left ventricular ejection fraction, mechanical circulatory devices, SYNTAX score
Citation: Farhan S, Freilich M, Giustino G, Vogel B, Baber U, Sartori S, Kamran H, Mehran R, Dangas G, Krishnan P, Kini A and Sharma SK (2024) Change in left ventricular function and outcomes following high-risk percutaneous coronary intervention with Impella-guided hemodynamic support. Front. Cardiovasc. Med. 11:1416613. doi: 10.3389/fcvm.2024.1416613
Received: 12 April 2024; Accepted: 14 June 2024;
Published: 5 July 2024.
Edited by:
Dimitrios Terentes-Printzios, University of Oxford, United KingdomReviewed by:
Paul Guedeney, Hôpitaux Universitaires Pitié Salpêtrière, France© 2024 Farhan, Freilich, Giustino, Vogel, Baber, Sartori, Kamran, Mehran, Dangas, Krishnan, Kini and Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samin K. Sharma, c2FtaW4uc2hhcm1hQG1vdW50c2luYWkub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.