- 1Department of Cardiology, Shandong Corps Hospital of Chinese People’s Armed Police Forces, Jinan, Shandong, China
- 2Cardiology Department and Experimental Animal Center, Liaocheng People’s Hospital of Shandong University and Liaocheng Hospital Affiliated to Shandong First Medical University, Liaocheng, Shandong, China
- 3School of Clinical Medicine, Shandong Second Medical University, Weifang, Shandong, China
Both de Winter syndrome and Wellens syndrome mainly indicate severe stenosis in the proximal segment of the anterior descending coronary artery. However, as research deepens, the accuracy and specificity of diagnosing proximal left anterior descending coronary artery (LAD) culprit lesions separately by de Winter syndrome or Wellens syndrome are challenged. The patient in this case developed both syndromes in a short period of time, and imaging showed significant stenosis of the proximal LAD, indicating a culprit lesion. The successive appearance of these two special electrocardiogram changes may increase the accuracy and specificity of diagnosing LAD as a culprit lesion, and the short-term occurrence of these two special electrocardiogram changes also suggests that the culprit lesion may be incomplete occlusion. In addition, de Winter syndrome is prone to missed diagnosis, while Wellens syndrome is prone to misdiagnosis or underestimation of its risk.
Introduction
De Winter syndrome is a special type of acute myocardial infarction that indicates severe lesions in the proximal segment of the left anterior descending coronary artery (LAD). Early identification of it is of great significance for preventing sudden death from coronary artery disease (CAD). However, de Winter syndrome is an easily overlooked and missed diagnosis. Wellens syndrome is prone to progress to acute ST segment elevation extensive anterior myocardial infarction. These two special types of electrocardiogram (ECG) changes mainly indicate severe stenosis in proximal LAD and are considered to be critical conditions, such as ST segment elevation myocardial infarction (STEMI) (1–4). In clinical practice, de Winter or Wellens syndrome is usually detected separately, and there are few reports of both types of ECG patterns occurring consecutively in the same patient. However, our hospital recently treated a patient with non-ST segment elevation myocardial infarction (NSTEMI) and recorded in detail this rare example in the patient's ECGs before and after the onset of the disease. Moreover, by reviewing literature and analyzing its mechanism, we further deepened our understanding of the clinical significance of these two ECG manifestations.
Case presentation
The patient, a 77-year-old man, was admitted due to “sudden unstable chest pain.” Resting for 15–20 min could alleviate the patient's chest tightness in the 2 months before admission. Half an hour before admission, the patient experienced chest tightness again during physical activity and was admitted to the emergency department with chest tightness lasting for 40 min. This patient had a history of hypertension for more than 10 years and oral administration of 80 mg valsartan and 5 mg amlodipine tablets daily has controlled his blood pressure (BP) to normal. The patient has a smoking history of 50 years, accompanied by 10 cigarettes per day, and his mother has a history of cerebral hemorrhage. Upon admission, physical examination showed a BP of 154/75 mmHg and heart rate of 68 beats/min; no significant abnormalities were observed during cardiopulmonary auscultation. There was no edema in both lower limbs. An ECG examination immediately upon admission showed sinus rhythm and ST-T changes, and the patient was preliminarily diagnosed with acute coronary syndrome (ACS). The patient refused a coronary intervention examination and received dual antiplatelet, statin, and nitrate therapy. The blood TNI titer was monitored and increased to 0.727 ng/ml (0–0.023 ng/ml) 12 h after admission. Fasting blood glucose was 4.95 mmol/L. The titer of low-density lipoprotein cholesterol was 2.54 mmol/L. The blood indicators of liver and kidney function were found to be normal. Cardiac Doppler ultrasound indicated normal cardiac structure and function. Three days after admission, the patient agreed to undergo coronary intervention treatment. Coronary angiography (CAG) showed 50% stenosis in the distal left main trunk (LM), 95% stenosis alongside a blurred lesion edge in the proximal LAD (Figure 1A), and approximately 75% stenosis in the middle segment of the right coronary artery (RCA) (Figure 1B). LAD was identified as the criminal vessel and one stent was inserted (Figure 2A). Later, one stent was inserted into the middle RCA (Figure 2B). After discharge, the patient underwent standardized secondary prevention and treatment for CAD and no further chest tightness occurred.

Figure 1. Coronary angiography. (A) Stenosis of 95% in the proximal LAD, with blurred lesion margins. (B) Stenosis of 75% in the middle RCA, with a regular lesion margin. LAD, left anterior descending artery; RCA, right coronary artery.
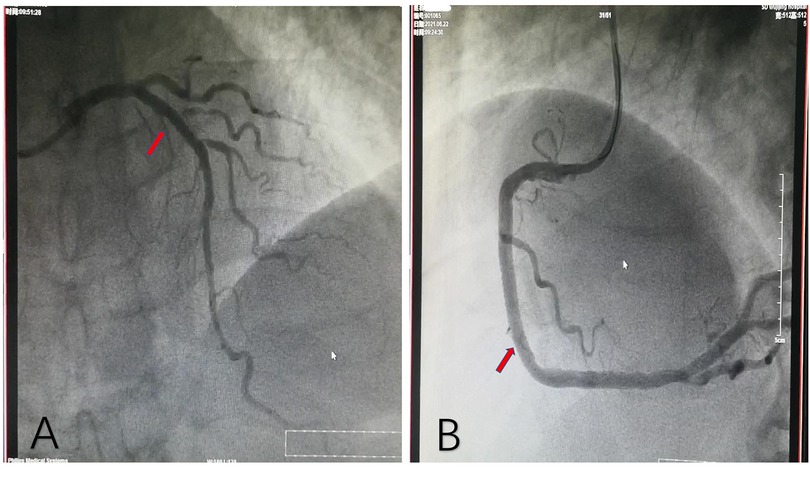
Figure 2. Coronary angiography after stent implantation. (A) One stent implanted in the proximal LAD. (B) Another stent implanted in the middle RCA. LAD, left anterior descending artery; RCA, right coronary artery.
The changes in the ECG pattern of this patient before and after hospitalization were identified. Compared with the ECG (Figure 3A) 3 months before admission, the ECG of the patient at admission (continuous chest tightness for 30 min) showed an upward sloping depression of 2 mm in STV2–4 (J-point depression) accompanied by symmetrical tall and peaked T-waves and an elevation of nearly 1 mm in STAVR (de Winter syndrome) (Figure 3B). The patient's chest tightness lasted for 40 min, and the ECG pattern 30 min after admission (chest tightness relieved for 20 min) showed that the changes in the ST segment disappeared, replaced by bidirectional TV2 and inverted TV3−V4 (Figure 3C). The T-wave changes persisted for more than 4 days (Wellens syndrome) (Figures 3C–E). The patient’s ECG pattern showed an upright TV2–4 (Figure 3F) 33 days after the onset of the disease.
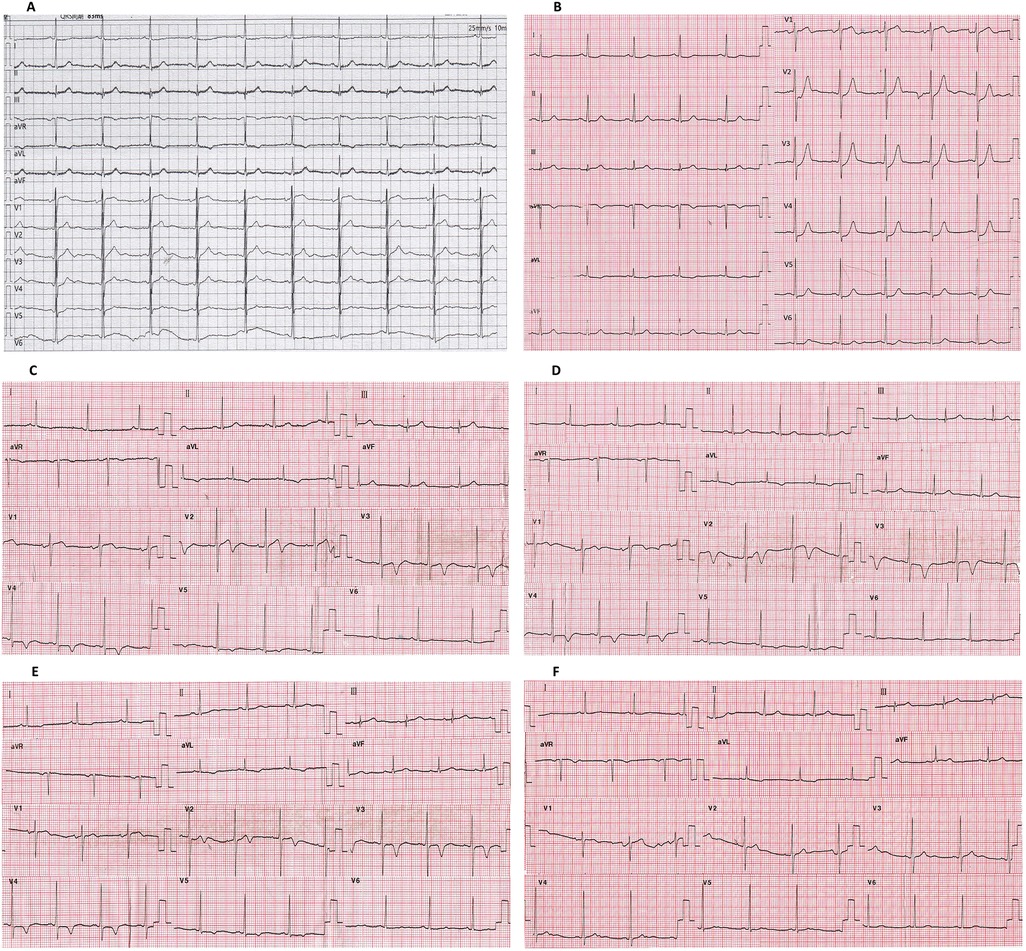
Figure 3. Changes in the ECG of this patient. (A) Patient's asymptomatic ECG before hospitalization. (B) ECG at admission (persistent chest tightness for 30 min). (C) ECG after 1 h of hospitalization (chest tightness relieved for 20 min). (D) ECG after 12 h of hospitalization. (E) ECG after 4 days of hospitalization (1 day after percutaneous intervention). (F) ECG 1 month after percutaneous intervention. De Winter syndrome is shown in (B). Wellens syndrome is shown in (C–E). ECG, electrocardiogram; PCI, percutaneous intervention.
Discussion
Electrocardiogram changes in de Winter syndrome and Wellens syndrome
De Winter syndrome and Wellens syndrome are two distinct ECG patterns named after their respective discoverers. The main feature of de Winter syndrome is an upward sloping STV1−V6 depression (1–3 mm J-point downward shift), accompanied by symmetrical tall and peaked T-waves. Most cases also exhibit a STAVR elevation of 1–2 mm (1, 5). In contrast, the main features of Wellens syndrome are bidirectional or symmetrical and deeply inverted TV2−V3 after angina relief, which may extend to leads V4–V6 (5, 6). In this case, the patient's ECG initially showed changes in STV2−V4, AVR consistent with de Winter syndrome, 30 min after symptom onset. However, subsequent ECGs recorded 20 min to 4 days after symptom relief displayed TV2−V4 changes consistent with Wellens syndrome. The patient presented with these two special ECG manifestations in a short period of time.
Sensitivity and specificity of de Winter syndrome and Wellens syndrome in diagnosing underlying lesions in proximal LAD
Although de Winter syndrome and Wellens syndrome are classified as non-ST segment elevation acute coronary syndromes, both indicate severe stenosis in the proximal LAD and are considered equivalent to STEMI (7, 8). CAG should be performed as early as possible and further revascularization therapy such as percutaneous coronary intervention (PCI) should be performed (6–9).
De Winter syndrome is found in 2% of patients with acute proximal LAD occlusion (1) and 3.4% of patients with acute anterior myocardial infarction (10); however, there are also reports that de Winter syndrome is occasionally found in patients with proximal-RCA occlusion (11), LAD branch embolism (12), LM or left circumflex artery lesions (13), STEMI thrombolysis with LAD occlusion (14), and myocarditis (15). Early studies have shown that de Winter syndrome can predict 95%–100% acute proximal LAD occlusion (16, 17). However, a subsequent meta-analysis showed that de Winter's positive predictive rate for LAD occlusion was only 50%–85.7% (17). Previous studies have shown that Wellens syndrome occurs in 14%–18% of ACS patients (6, 18) and 8.8% of NSTEMI patients (4). Two-thirds of the culprit lesions in Wellens syndrome are located in the LAD and one-third of the culprit lesions in Wellens syndrome are located in the proximal LAD. The sensitivity and specificity for predicting LAD underlying lesions were 24.6% and 96.2%, respectively (4). However, other studies have shown that 31% of patients with Wellens syndrome have normal coronary arteries or experience non-obstructive CAD (3). Therefore, as people's understanding gradually increases, the specificity for diagnosing proximal LAD occlusive lesions, whether it is de Winter syndrome or Wellens syndrome, is being challenged.
There are few reports of consecutive occurrence of de Winter syndrome and Wellens syndrome in the same patient; however, the culprit lesions in the cases reported so far have been located in the proximal LAD and were not completely occluded (19–23). Therefore, we speculate that the sequential presence of these two ECG patterns may not only enhance the accuracy and specificity of diagnosing proximal LAD lesions, but also indicate that the culprit lesion is not completely occluded. Whether this represents a completely occluded lesion that reopened within a short period remains unclear and should be clarified.
Easily misdiagnosed de Winter syndrome and preferably misdiagnosed or underestimated Wellens syndrome
De Winter syndrome is an electrocardiogram pattern similar to the hyperacute phase of myocardial infarction. Although it holds significant clinical value, the time window for observing this special ECG change is very short, with an average reported time of 1.5 h, as reported in the literature (1). Monitoring the ECG changes of the patient in this case revealed that de Winter syndrome was recorded 30 min after the onset of chest tightness. Wellens syndrome was subsequently recorded 30 min later (after 20 min of chest tightness relief), with an interval of only 30 min between the two different ECG patterns. Wellens syndrome typically occurs after the resolution of angina rather than during symptom onset, and this ECG pattern can persist for several weeks (8). Furthermore, Wellens syndrome is characterized solely by T-wave changes. Without comparison to pre-onset or normal ECGs, these changes can easily be misinterpreted as other conditions or non-specific T-wave changes. In addition, Wellens syndrome is only characterized by T-wave changes, which, in the absence of pre-onset or baseline ECG, can be misinterpreted considered as other conditions or non-specific T-wave changes. In this case, although the patient showed Wellens syndrome on multiple follow-up ECGs after admission, there was no dynamic evolution and the patient remained in an asymptomatic state. Therefore, in clinical practice, de Winter syndrome is often misdiagnosed and Wellens syndrome is easily misdiagnosed or underestimated. This case highlights the importance of vigilance among clinicians when encountering Wellens syndrome on a patient’s ECG. Even if the patient is asymptomatic and there are no dynamic changes in the follow-up ECGs, it is still necessary to investigate recent symptoms of chest tightness or chest pain and any previous ECG changes to identify high-risk patients in a timely manner. The uniqueness of this case lies in the sequential occurrence of de Winter syndrome and Wellens syndrome within a short period of time. These dynamic ECG changes provide sufficient basis for judging the patient's condition.
Underlying mechanisms of de Winter syndrome and Wellens syndrome
The pathophysiological mechanisms of de Winter syndrome need to be clarified. It is generally believed that the ST changes are hyperacute ECG changes caused by acute total occlusion of the proximal LAD; however, ST elevation does not occur due to short-term reperfusion of coronary artery occlusion or the presence of collateral circulation supply (7, 8). Another study (11) suggests several potential mechanisms for de Winter syndrome, including anatomical variations in Purkinje fibers and delayed endocardial conduction. Ischemic ATP depletion may also play a role, as it prevents the activation of ATP-sensitive potassium channels in the muscle membrane. In addition, the area of transmural ischemia is so large that it fails to generate damaging currents toward the precordial leads and instead produces upward currents to the AVR lead. The mechanism of Wellens syndrome is also not yet fully understood. Potential mechanisms include severe stenosis of the proximal LAD, coronary artery spasm (24), or myocardial reperfusion injury (25), which may cause myocardial stunning or edema after myocardial ischemia (7, 26), resulting in abnormal T-wave repolarization.
The patient in this case presented with sequential ECG changes consistent with de Winter syndrome and Wellens syndrome. The underlying mechanism may involve the formation of blood clots at unstable plaques in the proximal LAD, leading to acute exacerbation or complete occlusion of the lumen and resulting in acute, severe myocardial ischemia. This resulted in de Winter syndrome being displayed on the ECG. The rapid reduction in thrombus size results in decreased vascular stenosis or the rapid reopening of completely occluded vascular lumens. After partial recovery from myocardial ischemia, myocardial stunning occurs, causing abnormal repolarization of myocardial cells and ECG changes, which evolves into Wellens syndrome. After PCI, myocardial perfusion was restored effectively, leading to a gradual improvement in myocardial cell activity normalization of cardiac cell repolarization. As a result, the ECG returned to its pre-hospitalization state. This case demonstrated a progression of ECG changes from “roughly normal → de Winter syndrome → Wellens syndrome → roughly normal,” further deepening our understanding of the clinical significance and pathogenesis of de Winter syndrome and Wellens syndrome.
Conclusion
The sequential appearance of de Winter syndrome and Wellens syndrome within a short time frame may enhance the specificity and accuracy of diagnosing underlying lesions in the proximal LAD. In addition, it suggests that the LAD may be in a state of non-complete occlusion. De Winter syndrome susceptible to missed diagnosis, while Wellens syndrome is prone to misdiagnosis or underestimation of the patient's condition.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Liaocheng People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the patient is unable to sign in writing, but their verbal consent has been obtained. The manuscript presents research on animals that does not require ethical approval for their study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FW: Writing – original draft, Funding acquisition. XZ: Writing – review & editing. HP: Writing – review & editing. YW: Formal Analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received funding support from Fei Wang and was supported by the Liaocheng Key R&D Program Policy Guidance Project (No. 2024YD24) and Shandong Province Traditional Chinese Medicine Science and Technology Development Plan Project (No. 20190906).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. de Winter RJ, Verouden NJ, Wellens HJ, Wilde AA. A new ECG sign of proximal LAD occlusion. N Engl J Med. (2008) 359(19):2071–3. doi: 10.1056/NEJMc0804737
2. Verouden NJ, Koch KT, Peters RJ, Henriques JP, Baan J, van der Schaaf RJ, et al. Persistent precordial “ hyperacute” T-waves signify proximal left anterior descending artery occlusion. Heart. (2009) 95(20):1701–6. doi: 10.1136/hrt.2009.174557
3. Arshad S, Ferrick NJ, Monrad ES, Fisher JD, Krumerman A, Ferrick KJ. Prevalence and association of the Wellens’ sign with coronary artery disease in an ethnically diverse urban population. J Electrocardiol. (2020) 62:211–5. doi: 10.1016/j.jelectrocard.2020.09.002
4. Kobayashi A, Misumida N, Aoi S, Kanei Y. Prevalence and clinical implication of Wellens’ sign in patients with non-ST-segment elevation myocardial infarction. Cardiol Res. (2019) 10(3):135–41. doi: 10.14740/cr856
5. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Glob Heart. (2018) 13(4):305–38. doi: 10.1016/j.gheart.2018.08.004
6. de Zwaan C, Bar FW, Wellens HJ. Characteristic electrocardiographic pattern indicating a critical stenosis high in left anterior descending coronary artery in patients admitted because of impending myocardial infarction. Am Heart J. (1982) 103(4 Pt 2):730–6. doi: 10.1016/0002-8703(82)90480-X
7. Tzimas G, Antiochos P, Monney P, Eeckhout E, Meier D, Fournier S, et al. Atypical electrocardiographic presentations in need of primary percutaneous coronary intervention. Am J Cardiol. (2019) 124(8):1305–14. doi: 10.1016/j.amjcard.2019.07.027
8. Lawner BJ, Nable JV, Mattu A. Novel patterns of ischemia and STEMI equivalents. Cardiol Clin. (2012) 30(4):591–9. doi: 10.1016/j.ccl.2012.07.002
9. Tziakas D, Chalikias G, Al-Lamee R, Kaski JC. Total coronary occlusion in non ST elevation myocardial infarction: Time to change our practice?. Int J Cardiol. (2021) 329:1–8. doi: 10.1016/j.ijcard.2020.12.082
10. Xu J, Wang A, Liu L, Chen Z. The de Winter electrocardiogram pattern is a transient electrocardiographic phenomenon that presents at the early stage of ST-segment elevation myocardial infarction. Clin Cardiol. (2018) 41(9):1177–84. doi: 10.1002/clc.23002
11. Tsutsumi K, Tsukahara K. Is the diagnosis ST-segment elevation or non-ST-segment elevation myocardial infarction? Circulation. (2018) 138(23):2715–7. doi: 10.1161/CIRCULATIONAHA.118.037818
12. Alahmad Y, Sardar S, Swehli H. De Winter T-wave electrocardiogram pattern due to thromboembolic event: a rare phenomenon. Heart Views. (2020) 21(1):40–4. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_90_19
13. Xu N, You Y, Wang XG. Coronary occlusion analysis of De Winter syndrome. Chin J Cardiac Pacing Electrophysiol. (2019) 33(5):419–21. doi: 10.13333/j.cnki.cjcpe.2019.05.006
14. Zhao YT, Wang L, Yi Z. Evolvement to the de Winter electrocardiographic pattern. Am J Emerg Med. (2016) 34(2):330–2. doi: 10.1016/j.ajem.2015.11.057
15. García-Izquierdo E, Parra-Esteban C, Mirelis JG, Fernández-Lozano I. The De Winter ECG pattern in the absence of acute coronary artery occlusion. Can J Cardiol. (2018) 34(2):209.e1–e3. doi: 10.1016/j.cjca.2017.11.014
16. Goebel M, Bledsoe J, Orford JL, Mattu A, Brady WJ. A new ST-segment elevation myocardial infarction equivalent pattern? Prominent T wave and J-point depression in the precordial leads associated with ST-segment elevation in lead aVr. Am J Emerg Med. (2014) 32:287.e5–e8. doi: 10.1016/j.ajem.2013.09.037
17. Morris NP, Body R. The De Winter ECG pattern: morphology and accuracy for diagnosing acute coronary occlusion: systematic review. Eur J Emerg Med. (2017) 24:236–42. doi: 10.1097/MEJ.0000000000000463
18. de Zwaan C, Bär FW, Janssen JH, Cheriex EC, Dassen WR, Brugada P, et al. Angiographic and clinical characteristics of patients with unstable angina showing an ECG pattern indicating critical narrowing of the proximal LAD coronary artery. Am Heart J. (1989) 117(3):657–65. doi: 10.1016/0002-8703(89)90742-4
19. Li XY, Li X, Man QS, Li Y, Long Y. An electrocardiogram case of De Winter’s T-waves evolving into Wellens’ waves. Zhonghua Xin Xue Guan Bing Za Zhi. (2019) 47(11):918–20. Chinese. doi: 10.3760/cma.j.issn.0253-3758.2019.11.013
20. Zhu Y, Luo S, Huang B. Evolution of de Winter into Wellens on electrocardiogram-what happened? JAMA Intern Med. (2021) 181(12):1647–9. doi: 10.1001/jamainternmed.2021.5734
21. Samadov F, Akaslan D, Cincin A, Tigen K, Sarı I. Acute proximal left anterior descending artery occlusion with de Winter sign. Am J Emerg Med. (2014) 32(1):110.e1–3. doi: 10.1016/j.ajem.2013.08.024
22. Nini L, Chao L, Yanxin Z, Wenjuan W. Case of de Winter ST-T change evolving into Wellens ST-T change. Chin J Cardiac Pacing Electrophysiol. (2022) 36:489–90. doi: 10.13333/j.cnki.cjcpe.2022.05.026
23. Yijiao J, Yunlang D, Xin Z. De Winter electrocardiogram combined with Wellens electrocardiogram: a case report. Chin J Cardiovasc Med. (2021) 26:582–4. doi: 10.3969/j.issn.1007-5410.2021.06.018
24. Abulaiti A, Aini R, Xu H, Song Z. A special case of Wellens’ syndrome. J Cardiovasc Dis Res. (2013) 4(1):51–4. doi: 10.1016/j.jcdr.2013.02.016
25. Alexander J, Rizzolo D. Wellens syndrome. JAAPA. (2023) 36(2):25–9. doi: 10.1097/01.JAA.0000911188.18646.31
Keywords: de Winter syndrome, Wellens syndrome, proximal LAD lesion, culprit lesion, specificity, incomplete occlusion, short-term
Citation: Wang F, Zhang X, Pang H and Wang Y (2025) Evolution of de Winter syndrome to Wellens syndrome: a case report and literature review. Front. Cardiovasc. Med. 11:1415306. doi: 10.3389/fcvm.2024.1415306
Received: 10 April 2024; Accepted: 16 December 2024;
Published: 9 January 2025.
Edited by:
Vincenzo Santinelli, IRCCS San Donato Polyclinic, ItalyReviewed by:
Rade Babic, University of Belgrade, SerbiaRicardo Adrian Nugraha, Airlangga University, Indonesia
Copyright: © 2025 Wang, Zhang, Pang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuehai Wang, d3loXzEyMzQ1NjdAMTI2LmNvbQ==
 Fei Wang
Fei Wang Xuesong Zhang2
Xuesong Zhang2 Yuehai Wang
Yuehai Wang