- 1Guang’anmen Hospital, China Academy of Chinese Medicine Sciences, Beijing, China
- 2Department of Anatomy, School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Significance: Since the advent of Optical Coherence Tomography (OCT) two decades ago, there has been substantial advancement in our understanding of intravascular biology. Identifying culprit lesion pathology through OCT could precipitate a paradigm shift in the treatment of patients with Acute Coronary Syndrome. Given the technical prowess of OCT in the realm of cardiology, bibliometric analysis can reveal trends and research focal points in the application of OCT for cardiovascular diseases. Concurrently, meta-analyses provide a more comprehensive evidentiary base, supporting the clinical efficacy of OCT-guided Percutaneous Coronary Intervention (PCI).
Design: This study employs a dual approach of Bibliometric and Meta-analysis.
Methods: Relevant literature from 2003 to 2023 was extracted from the Web of Science Core Collection (WoSCC) and analyzed using VOSviewer, CiteSpace, and R for publication patterns, countries, institutions, authors, and research hotspots. The study compares OCT-guided and coronary angiography-guided PCI in treating adult coronary artery disease through randomized controlled trials (RCTs) and observational studies. The study has been reported in the line with PRISMA and AMSTAR Guidelines.
Results: Adhering to inclusion and exclusion criteria, 310 publications were incorporated, demonstrating a continual rise in annual output. Chinese researchers contributed the most studies, while American research wielded greater influence. Analysis of trends indicated that research on OCT and angiography-guided PCI has become a focal topic in recent cohort studies and RCTs. In 11 RCTs (n = 5,277), OCT-guided PCI was not significantly associated with a reduction in the risk of Major Adverse Cardiac Events (MACE) (Odds ratio 0.84, 95% CI 0.65–1.10), cardiac death (0.61, 0.36–1.02), all-cause death (0.7, 0.49–1.02), myocardial infarction (MI) (0.88, 0.69–1.13), target lesion revascularization (TLR) (0.94, 0.7–1.27), target vessel revascularization (TVR) (1.04, 0.76–1.43), or stent thrombosis (0.72, 0.38–1.38). However, in 7 observational studies (n = 4,514), OCT-guided PCI was associated with a reduced risk of MACE (0.66, 0.48–0.91) and TLR (0.39, 0.22–0.68).
Conclusion: Our comprehensive review of OCT in cardiovascular disease literature from 2004 to 2023, encompassing country and institutional origins, authors, and publishing journals, suggests that OCT-guided PCI does not demonstrate significant clinical benefits in RCTs. Nevertheless, pooled results from observational studies indicate a reduction in MACE and TLR.
1 Introduction
Recent evidence suggests that MACE in chronic ischemic heart disease correlates more with the overall atherosclerotic burden than with specific flow-limiting luminal lesions (1–6). Traditional models simplistically link CAD complications to severe obstructions from narrow atherosclerotic plaques (7–10). However, this perspective is increasingly recognized as overly reductionist. Longitudinal studies on the natural progression of individual coronary plaques have revealed that even those lesions perceived as high-risk and potentially ischemia-inducing maintain stability over several years, seldom progressing to instability or resulting in MACE (11–16). The limitations of angiography in direct PCI, including inaccurate assessments of lesion morphology and the underlying mechanisms of STEMI, as well as suboptimal recognition of post-stent outcomes, underscore the necessity for a more holistic understanding of atherosclerosis within the entire arterial system (17).
OCT provides the highest resolution (1–15 μm) among current intravascular imaging technologies, enabling detailed exploration of microscopic vascular structures (18). In cardiovascular clinical applications, the significance of OCT encompasses: (1) Comprehensive plaque assessment: OCT provides detailed information about plaque size, type, and composition, aiding in understanding the total burden of atherosclerosis, not merely localized stenosis (19); (2) Vulnerable plaque identification: OCT can provide detailed views of potentially hazardous plaques by analyzing tissue characteristics, such as the size of the lipid core and the thickness of the fibrous cap (20); (3) Enhanced risk stratification: The detailed plaque and vascular information provided by OCT can help more accurately assess the risk of cardiovascular events, thus improving the accuracy of risk stratification (21); (4) Complementing traditional imaging techniques: By offering direct observation of vessel walls and plaques, OCT supplements the limitations of traditional imaging methods, providing a more comprehensive cardiovascular health assessment (22). Thus, OCT is not only a potent diagnostic tool but also adds a new dimension to the risk assessment and management of cardiovascular diseases. Its application highlights a deeper and more nuanced understanding of cardiac diseases, contributing to the refinement of existing risk stratification methods for greater precision.
We analyzed trends and applications of OCT in cardiovascular treatment over the past two decades using bibliometric techniques (23, 24). Our meta-analysis indicates OCT as a prominent focus in recent PCI trials. Previous studies comparing OCT-guided with angiography-guided PCI treatment in Meta-analyses have encountered several issues. Firstly, they did not include all significant related studies. Secondly, these meta-analyses did not separate observational studies from RCTs, a methodological rigor essential for enhancing the credibility of results. Therefore, we conducted a stringent Meta-analysis, differentiating RCTs from observational studies, aiming to provide more accurate and reliable evidence to guide clinical practice and future research directions.
2 Methods
2.1 Data sources and search strategy
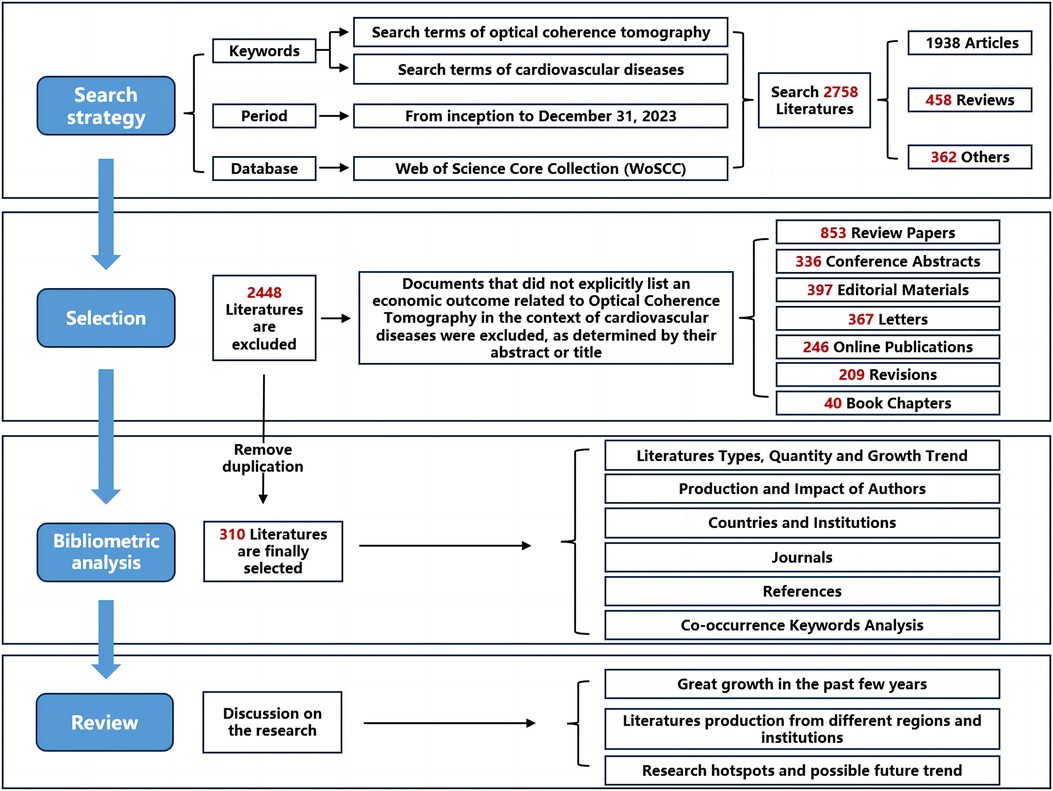
The Web of Science, esteemed for its extensive interdisciplinary coverage, comprehensive citation indexing, and rich analytical metrics, serves as an exemplary database for bibliometric analysis. This resource enables researchers to identify hotspots and trends within their respective fields. Our study utilized data retrieved from the WoSCC database concerning OCT and cardiovascular diseases for bibliometric analysis. To mitigate data variability due to updates, search activities, data extraction, and downloading were conducted on the same day. The types of literature studied were confined to articles and reviews. The search strategy, specific outcomes, and search terms are detailed in Figure 1 (refer to Supplementary eMethods S1). Overall, 2,758 literature sources were analyzed, with 310 articles ultimately included and downloaded in text format (complete records and referenced citations).
2.2 Data analysis and visualization
In this study, the bibliometrix package in R (version 4.3.2) was utilized to analyze major countries, active authors and institutions, contributing journals, and keyword trends (25). Additionally, CiteSpace (version 6.1), a Java-based freeware developed by Chen (26), was employed for clustering and burst analysis of keywords. Collectively, these two software programs facilitated visual analyses, offering deep insights into the advancements in OCT research within the cardiovascular field and uncovering research frontiers using extensive data.
2.3 Meta-analysis
This work has been reported in line with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines (27, 28). A systematic review and meta-analysis were conducted on data from 11 RCTs and 7 observational studies. These 18 cohorts were identified through searches of electronic databases including PubMed, Cochrane Library, Embase, and Web of Science, employing a combination of text and MeSH headings in the search strategy (refer to Supplementary eMethods S2 and eTableS1). For this study, primary outcomes of interest were MACE, Cardiac death, and All-cause death, with secondary endpoints including Myocardial Infarction (MI), TVR, TLR, and stent thrombosis. All details regarding the search strategy, data extraction, and study selection are presented in the Supplementary (eMethods S3–S5).
2.4 Statistical analysis
The outcomes of interest were dichotomous variables, and rates of events with the total sample size were extracted for analysis. The Mantel-Haenszel method's random-effects model was employed to calculate Odds Ratio (OR) and their 95% Confidence Intervals (CI). For inter-study variance, Restricted Maximum Likelihood (REML) was used. An OR estimate and its corresponding 95% CI not including the vertical line at 1 (p-value < 0.05) was considered statistically significant. The extent of heterogeneity was approximated using the I2 test, with 0%–40% indicating negligible, 30%–60% moderate, 50%–90% substantial, and 75%–100% considerable heterogeneity. Given the limited number of studies included, a funnel plot for pre-specified publication bias analysis was deemed inappropriate.
2.5 Cardiovascular clinical research and patients involvement
Following the completion of our initial manuscript, we consulted a patient with cardiovascular disease and a frontline cardiovascular clinical scholar, both of whom suggested acceptance or implementation of PCI for coronary artery disease. The feedback received indicated that the certainty of the evidence presented in our study was highly useful for evaluating the efficacy of OCT-guided vs. angiography-guided PCI in the treatment of acute coronary syndrome (ACS).
3 Results
3.1 Bibliometric results
3.1.1 Annual growth trends in publications
From 2003 to 2023, a total of 2,758 papers were retrieved from the WoSCC database. After eliminating duplicates and other types of literature, 310 articles were ultimately included for analysis, comprising 246 articles and 64 reviews. Figure 2A displays the annual statistics of publications in this field, revealing a trend in three distinct phases: (1) From 2004 to 2012, the annual publication count did not exceed 10 papers; (2) From 2013 to 2019, the number of annual publications remained relatively stable; (3) A notable increase in publication volume was observed from 2020 to 2023, with a significant spike exceeding 40 papers in 2022. By fitting the data to construct a publication trend, results indicate a high correlation between the annual number of publications and the years (y = 0.0541x ^2 + 0.8396x − 1.0807, R^2 = 0.8841) (Figure 2B). The publication trend suggests that by 2024, over 400 articles on this topic are projected to be published, signifying an increasing scholarly focus on this field over time.
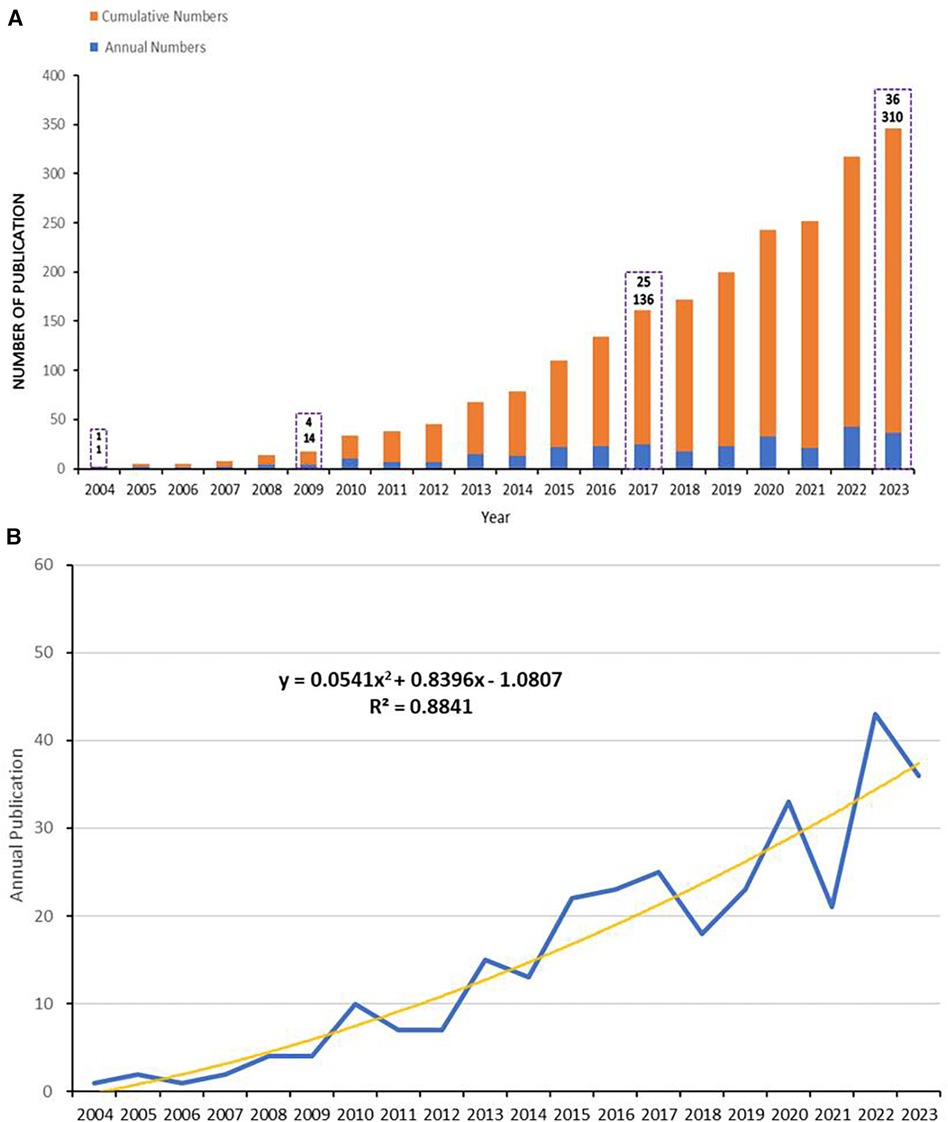
Figure 2 Annual publications, and fitting curves. (A) Cumulative (in orange-red) and annual (in blue) number of publications from 2004 to 2023. (B) The trend curve fitted according to the number of publications, depicted using polynomial fitting in Microsoft Excel 2019.
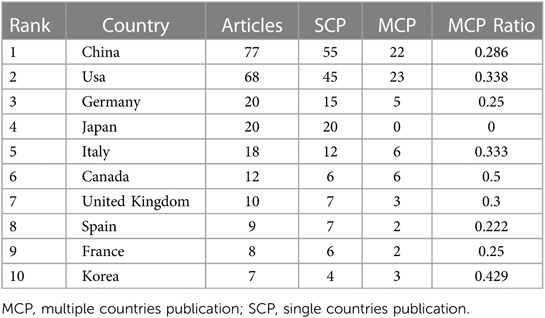
In this field, the top 10 countries accounted for over 80% of the total publication output compared to all other countries combined. Statistically, the five countries and regions with the most published articles were China (77 articles), the United States (68 articles), Germany, Japan, and Italy (Table 1). In terms of the growth rate in the number of publications (Figure 3A), the United States consistently maintained a high output, slightly outperforming China, while Germany, Italy, and Japan showed relatively stable production levels. Moreover, among the top 20 countries for corresponding authors, those with the highest proportions of multiple countries publication (MCP) relative to their total publication output were Canada, the United States, Italy, the United Kingdom, and China. Although the U.S. had the most MCPs (23 articles), it did not rank first in MCP ratio. While China had the highest total number of articles, it had fewer publications in collaboration with other countries (22 articles), thus a lower MCP Ratio (Table 1). Among the limited international collaborations from China, those with the United States were the most frequent (Figure 3B).
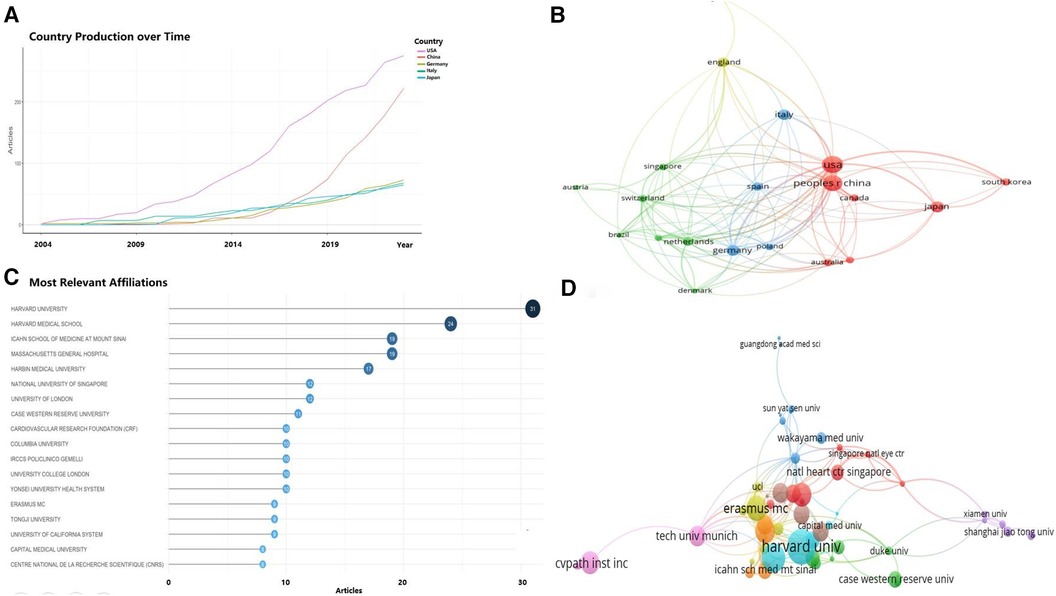
Figure 3 Analysis of countries/regions engaged in OCT research. (A) Top 5 countries with the largest number of publications over time; (B) country cooperation network; (C) top 18 institutions by number of publications; (D) institutional collaboration-network.
These articles were authored by 659 institutions, among which 21 institutions published at least 5 articles each. The top 10 institutions alone authored 176 articles, accounting for 56.8% of the total (Figure 3C). The institutions with the highest number of publications included Harvard University, Harvard Medical School, Harbin Medical University, Icahn School of Medicine, Massachusetts General Hospital, National University of Singapore, University College London, Case Western Reserve University, and Columbia University, all with over 10 articles each. Chinese institutions such as Harbin Medical University, Tongji University, and Capital Medical University each produced more than 8 articles. As depicted in Figure 3D, institutional collaboration was more extensive than inter-country cooperation, with Harvard University and Harbin Medical University engaging in significant collaborations with numerous universities and research centers in China, as well as institutions in the UK, the US, and other countries.
3.1.2 Author analysis
Figure 4A, created using VOSviewer software, visualizes the author collaboration network in OCT research within the cardiovascular field. The minimum criterion for an author's inclusion was set at 10 publications, encompassing nearly 2000 contributing authors. Among the top 10 most productive authors, Professor Mehran Roxana possessed the highest m-INDEX; Professor Yu Bo boasted the greatest G-index; and Professor Virmani Renu held the highest h-index and total citations (TC) (Figures 4A-B).
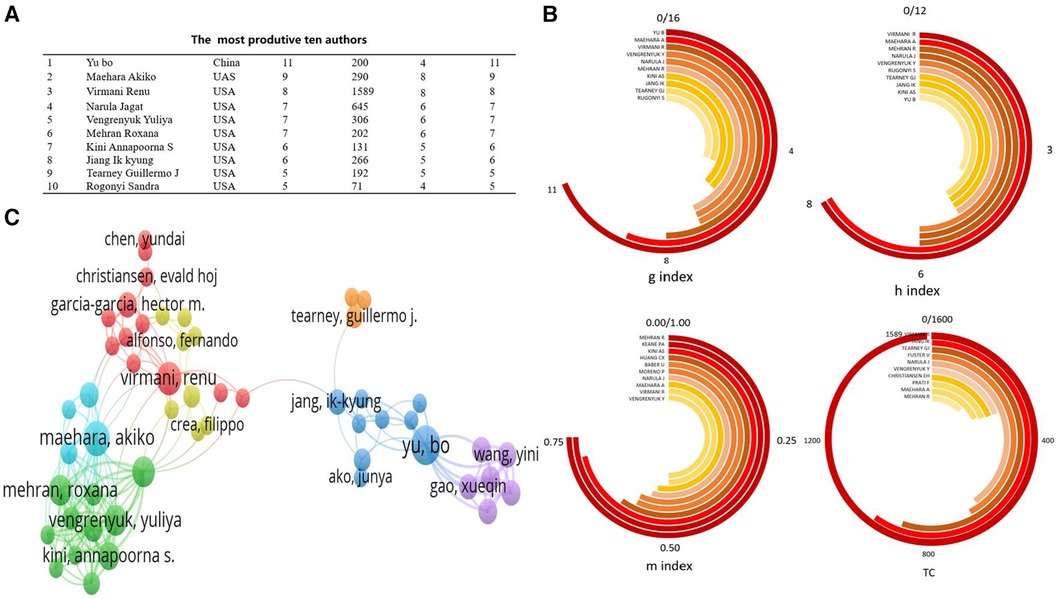
Figure 4 Analysis of authors engaged in OCT research. (A) the most produtive ten authors; (B) Under Various Indices, the Top 10 Most Productive Authors; (C) author's cooperation network.
Figure 4C presents the network map of co-cited authors, where higher weightage of a co-cited author corresponds to larger labels and circles in the visualization. In the field of OCT research, prominent figures like Professor Yu Bo from China, and Professor Maehara Akiko and Professor Virmani Renu from the United States, hold significant influence and citation weight. Among the authors with the highest publication volumes, Professor Yu Bo is the only one from China, while the others are predominantly from the United States. Many of these authors have collaborated on publications in journals such as the “New England Journal of Medicine” and “JACC Cardiovascular Imaging” (29, 30). This indicates close collaboration among authors within this field.
3.1.3 Analysis of journals
According to our analysis, 179 journals published papers related to OCT and cardiovascular diseases. The top 5 most productive journals — “International Journal of Cardiology”, “Frontiers in Cardiovascular Medicine”, “Catheterization and Cardiovascular Interventions”, “Revista Espanola de Cardiologia”, and “Scientific Reports” — showed notable publication numbers and growth trends, as depicted in Figure 5A. Figure 5B illustrates the journal's thematic distribution through a dual-map overlay, with citing journals positioned on the left and cited journals on the right of the map. The labels represent journals covering specific themes, and colored lines trace the reference pathways. Two distinct citation pathways are evident. Two green citation paths indicate that studies from medical/clinical/surgical journals are often cited by those in molecular physiology/medical/clinical journals.
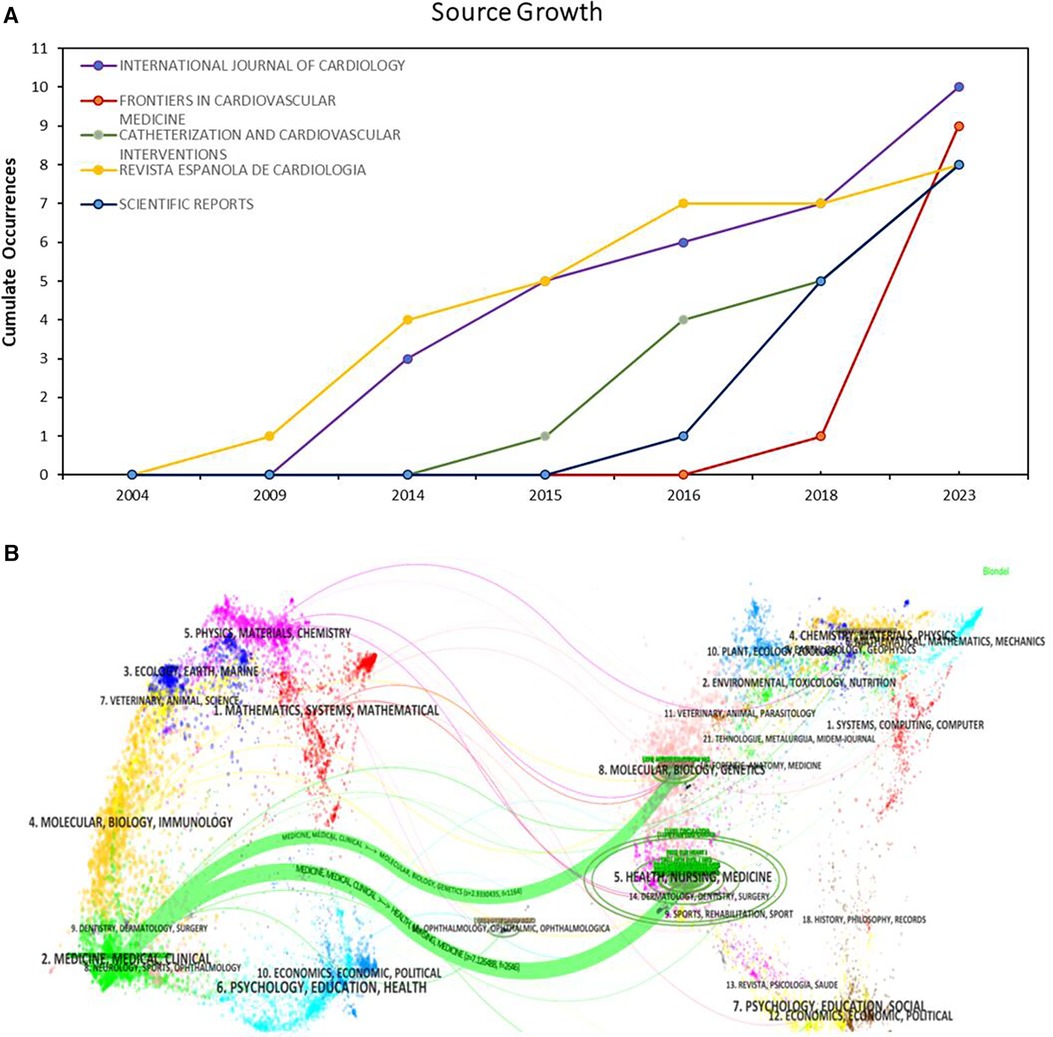
Figure 5 Journal distribution. (A) Growth Trends of the Top 5 Productive Journals; (B) dual map overlay for journals.
3.1.4 Citation analysis
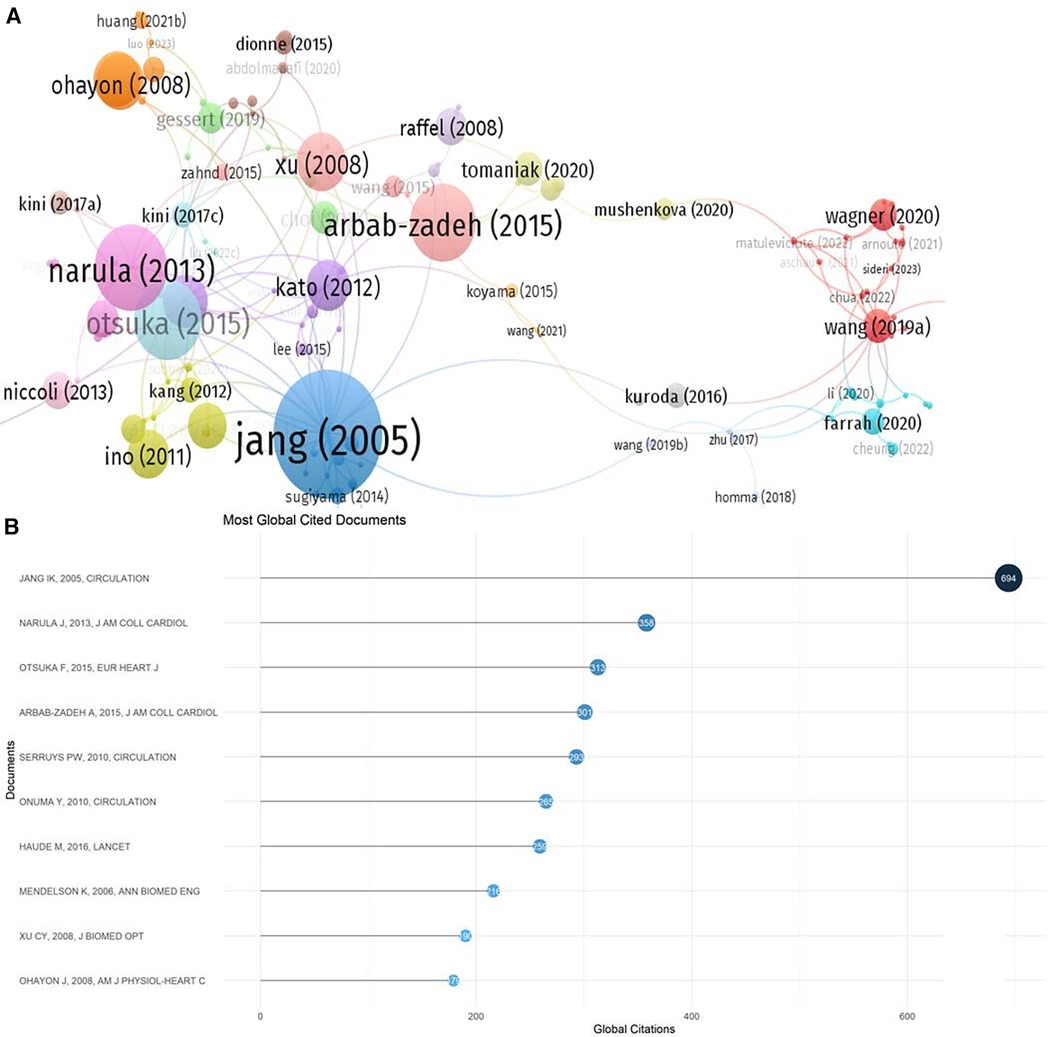
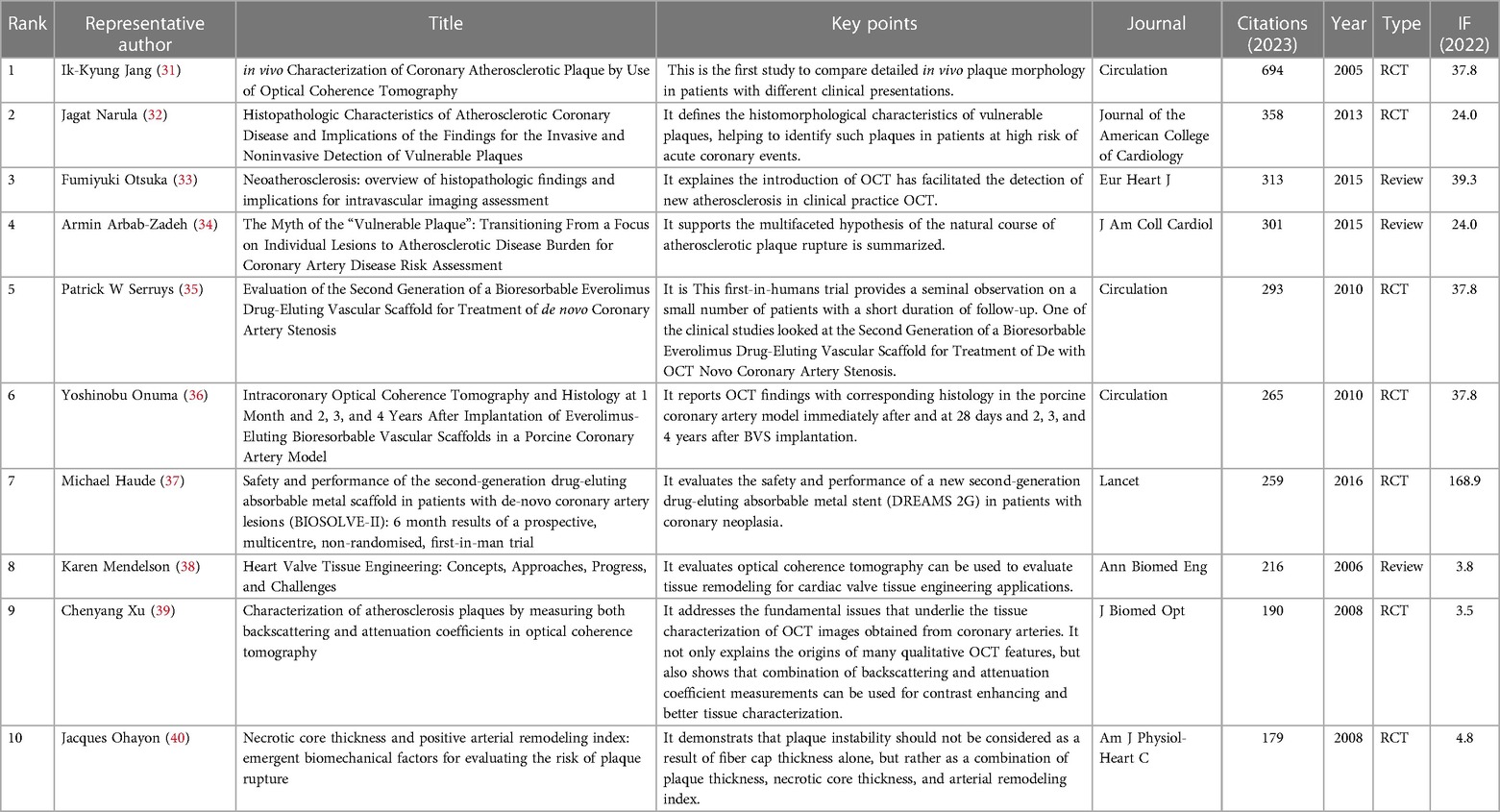
The results of the citation analysis are presented in Figure 6 and Table 2. Among the top 10 most cited articles, 6 are clinical trial studies, with one published in “The Lancet" (37), three in “Circulation" (31, 35, 36), and other high-impact journals. Three reviews discussed the application of OCT technology in detecting atherosclerosis in clinical practice. The 2005 randomized controlled trial by Professor Ik-Kyung Jang, “in vivo Characterization of Coronary Atherosclerotic Plaque by Use of Optical Coherence Tomography,” ranks first with 694 citations.
3.1.5 Co-occurrence analysis
In the study of the structure of scientific knowledge, keyword co-occurrence analysis is an effective bibliometric method to grasp current hotspots. We analyzed the co-occurrence of keywords in the field and the top 50 keywords (Figure 7A), centering around OCT. Figure 7B employs a log-likelihood ratio analysis to generate eight clusters, including: coronary artery disease, deep learning, coronary stenosis, heart transplantation, plaque rupture, OCT, congenital heart disease, and cardiovascular diseases. Burst analysis of keywords was also conducted, revealing overall trends in OCT research in the cardiovascular field, encompassing topics like bare metal stents, acute myocardial infarction, intravascular ultrasound, aortic valve implantation, artery disease, elevation myocardial infarction, coronary disease, and coronary artery disease (Figure 7C).
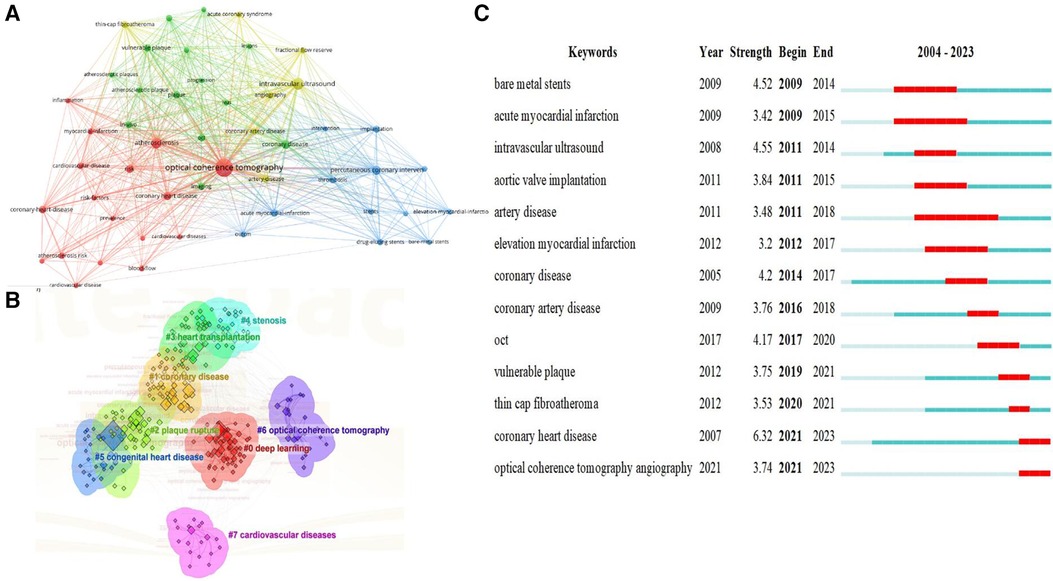
Figure 7 Visualized analysis of keywords and literature related to OCT and cardiovascular diseases. (A) Co-occurrence network of terms in 310 publications; nodes represent keywords (top 50), and lines denote co-occurrence relationships; (B) keyword clustering analysis; (C) the burst strength and duration of the top 13 keywords with the strongest citation bursts.
3.1.6 Changes in trends of research in the recent years
The thematic word analysis method was employed to explore the core issues in OCT research within the cardiovascular field. Figure 8A indicates that well-developed themes focus on atherosclerotic diseases, blood pressure, stent implantation, and plaque characteristics. The impact of surgery, post-operative care, and survival on disease treatment and prognosis are also noteworthy. Emerging research in areas such as molecular biology and cell biology is also beginning to emerge. Researchers are focusing on the roles and potential molecular mechanisms of “inflammation, oxidative stress, mitochondria, cytokines, and metabolism” in disease development.
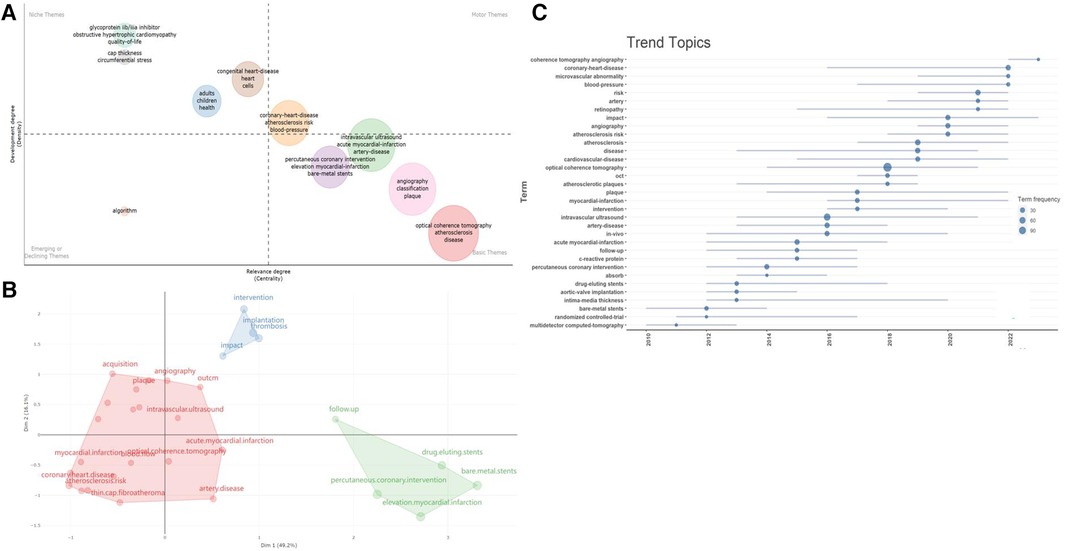
Figure 8 Analysis of research directions. (A) Thematic analysis related to cardiovascular diseases and OCT. The horizontal and vertical axes represent centrality and density, respectively. The first quadrant represents mature themes, the second quadrant is less significant to the current field, the third quadrant possibly represents emerging or fading themes, and the fourth quadrant is fundamental but less significant themes; (B) conceptual structure map of Keyword Plus; (C) timeline of research dynamics in the field of OCT and cardiovascular diseases.
Moreover, using multidimensional scaling, we categorized the most frequently occurring keywords and generated a conceptual structure map, resulting in three clusters (Figure 8B). Current research continues to focus on clinical manifestations, diagnosis, interventions, and prognosis of diseases like “coronary stenosis, acute myocardial infarction, atherosclerosis” (red cluster), as well as exploring pathogenic mechanisms and intervention methods related to diseases, such as “interventional methods, post-stent thrombosis formation, and potential impacts of PCI” (blue-green cluster).
Additionally, we visualized the temporal trends of keywords (Figure 8C). In the past five years, new trends in the field include coherence tomographic vascular scanning technology, coronary heart disease, microvascular lesions, vascular pressure, retinal arteriolar abnormalities, atherosclerotic risk, as well as the etiology, pathomechanisms, and clinical outcomes of cardiovascular diseases, all of which are worthy areas for continued exploration. The thematic word analysis method was employed to explore the core issues in OCT research within the cardiovascular field. Figure 8A indicates that well-developed themes focus on atherosclerotic diseases, blood pressure, stent implantation, and plaque characteristics. The impact of surgery, post-operative care, and survival on disease treatment and prognosis are also noteworthy. Emerging research in areas such as molecular biology and cell biology is also beginning to emerge. Researchers are focusing on the roles and potential molecular mechanisms of “inflammation, oxidative stress, mitochondria, cytokines, and metabolism” in disease development.
Moreover, using multidimensional scaling, we categorized the most frequently occurring keywords and generated a conceptual structure map, resulting in three clusters (Figure 8B). Current research continues to focus on clinical manifestations, diagnosis, interventions, and prognosis of diseases like “coronary stenosis, acute myocardial infarction, atherosclerosis” (red cluster), as well as exploring pathogenic mechanisms and intervention methods related to diseases, such as “interventional methods, post-stent thrombosis formation, and potential impacts of PCI” (blue-green cluster).
Additionally, we visualized the temporal trends of keywords (Figure 8C). In the past five years, new trends in the field include coherence tomographic vascular scanning technology, coronary heart disease, microvascular lesions, vascular pressure, retinal arteriolar abnormalities, atherosclerotic risk, as well as the etiology, pathomechanisms, and clinical outcomes of cardiovascular diseases, all of which are worthy areas for continued exploration.
3.2 Meta results
3.2.1 Description of included trials
Of 4,350 citations, we reviewed 1,385 after removal of duplicates. We excluded an additional 1,367 studies on the basis of the title and abstract level screening and a priori selection criteria (Figure 9). Finally, we included 11 RCTs (n = 5,277) (29, 41–50), and 7 observational studies (n = 4,514) (51–58).
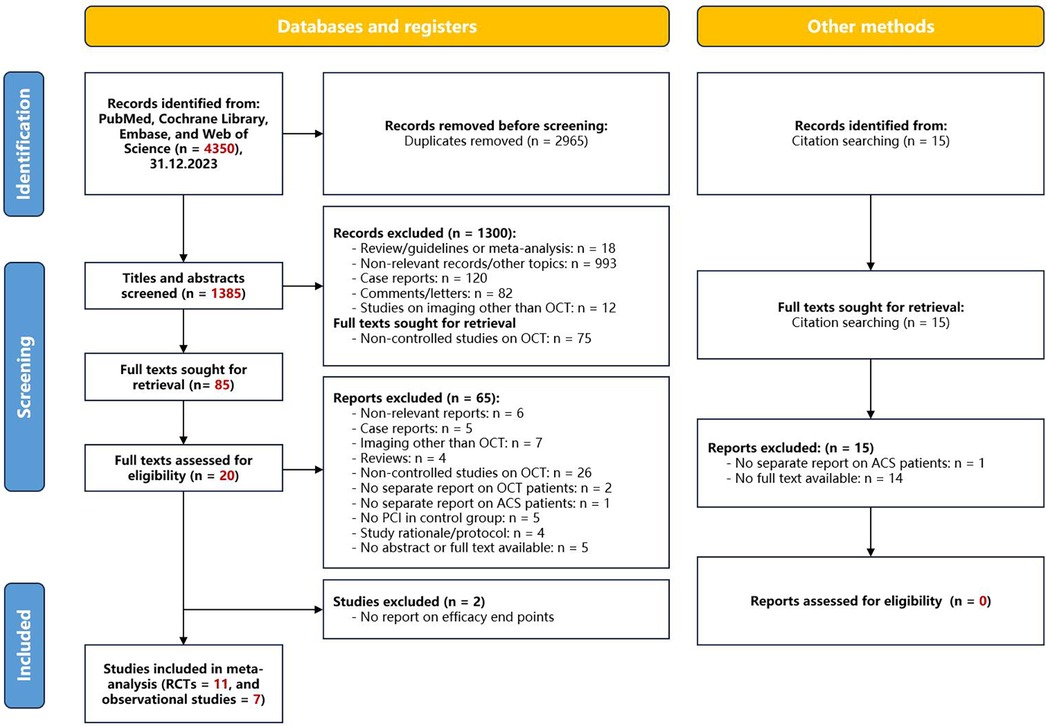
Figure 9 Flowchart of study selection. OCT, optical coherence tomography; PCI, percutaneous coronary intervention; ACS, acute coronary syndrome.
During the quality assessment process, a thorough evaluation of the methodological rigor of each study played a crucial role in enhancing the credibility of the results. Our bias risk assessment revealed that 36% (4 out of 11) of the trials raised some concerns regarding the randomization process, and 43% (3 out of 7) of the observational studies exhibited lower evidence quality regarding outcomes (refer to Supplementary eFigure S1 and eTable S2).
3.2.2 Patient-level baseline characteristics and procedural data
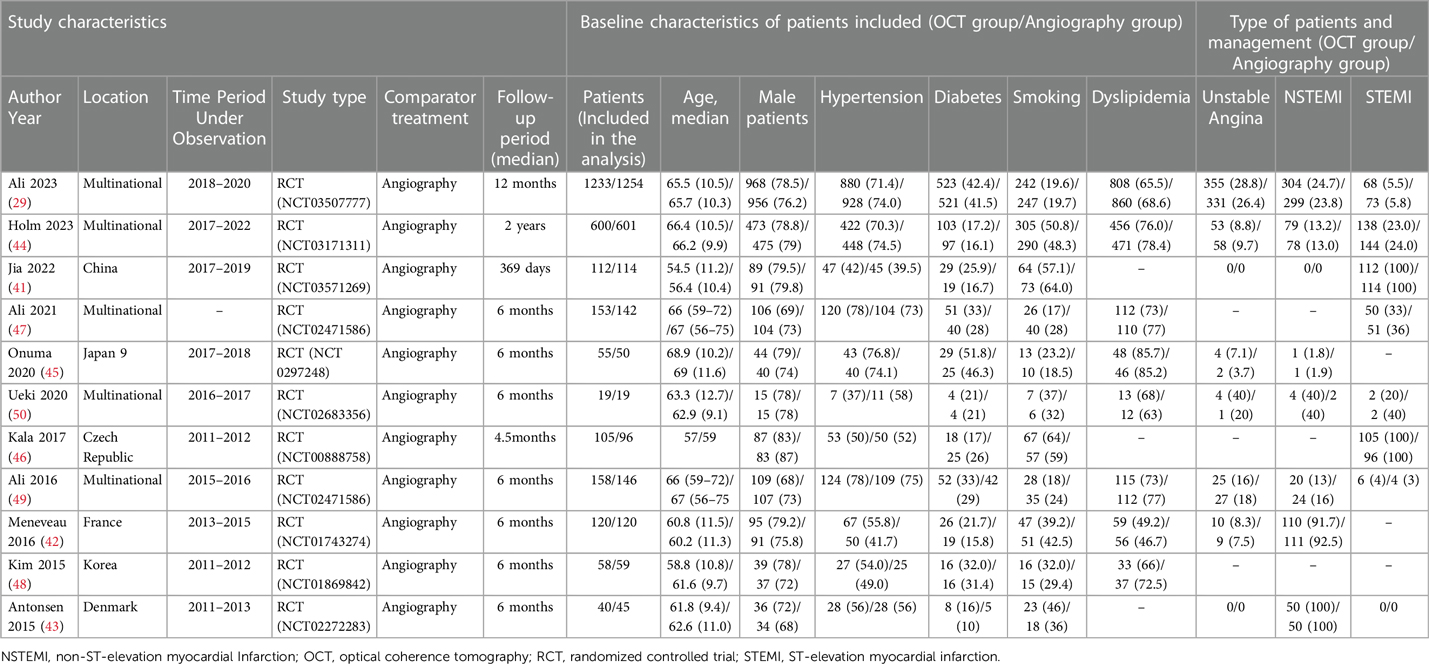
The 11 articles included in this study collectively encompassed 5,277 patients with coronary artery lesions. Table 3 summarizes the baseline characteristics. The median age ranged from 54.5 to 69 years, with 77.2% being male. Cardiovascular risk factor analysis indicated that 31.5% of the patients had diabetes, 63.1% had dyslipidemia, and 69.0% had hypertension, with 31.7% being current or former smokers. STEMI was the predominant type of ACS, followed by NSTEMI and unstable angina. All patients underwent invasive treatment. A total of 2,653 patients received OCT-guided therapy, and 2,640 patients underwent angiography-guided PCI. Stent implantation was the primary strategy for vascular revascularization. The follow-up period ranged from 3 to 25 months. The characteristics of the observational studies are available in Supplementary eTable S3.
3.2.3 MACE
Six trials (n = 2,109) reported MACE (41, 43, 44, 46–48). Compared with coronary angiography, OCT-guided PCI was not associated with a significant reduction in MACE (OR 0.84, 95% CI 0.65 to 1.10; p = 0.515, I2 = 0%, non-relevant heterogeneity, high certainty, see Figure 10A). However, the observational studies, comprising 5 studies with 3,674 patients, painted a different picture (51, 52, 54–56). In contrast to coronary angiography, OCT-guided PCI showed a reduction in the risk of MACE (OR 0.66, 95% CI 0.48–0.91; p = 0.243, I2 = 26.7%, non-relevant heterogeneity, moderate certainty, see Figure 11A).
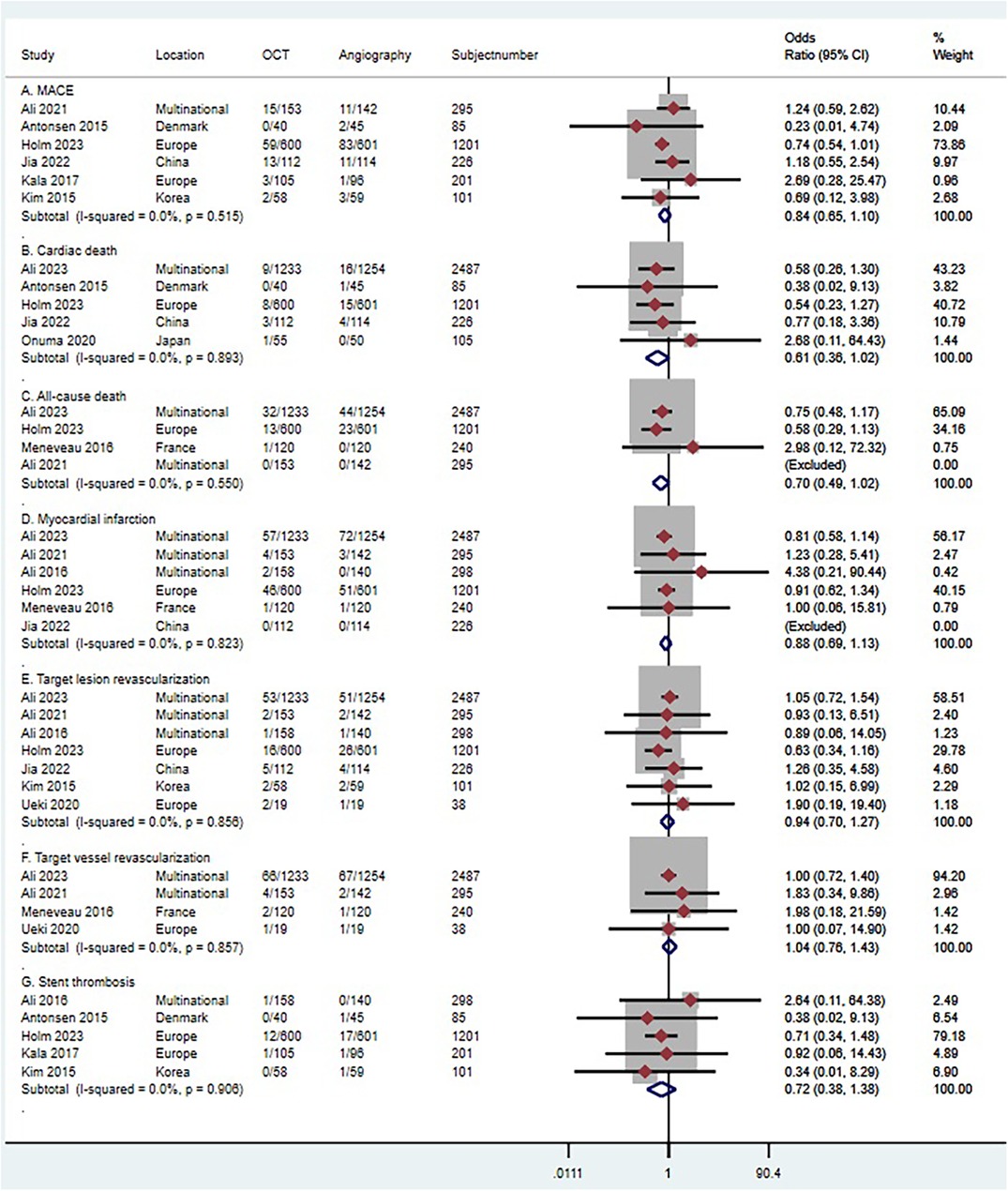
Figure 10 Forest plot comparing OCT guided with coronary angiography guided PCI in RCT studies. Data obtained from RCTs using fixed effect meta-analysis and expressed as OR. (A) MACE; (B) cardiac death; (C) all-cause death; (D) myocardial infarction; (E) target lesion revascularization (F) target vessel revascularization; (G) stent thrombosis. CI, confidence interval; OCT, optical coherence tomography; PCI, Percutaneous coronary intervention.
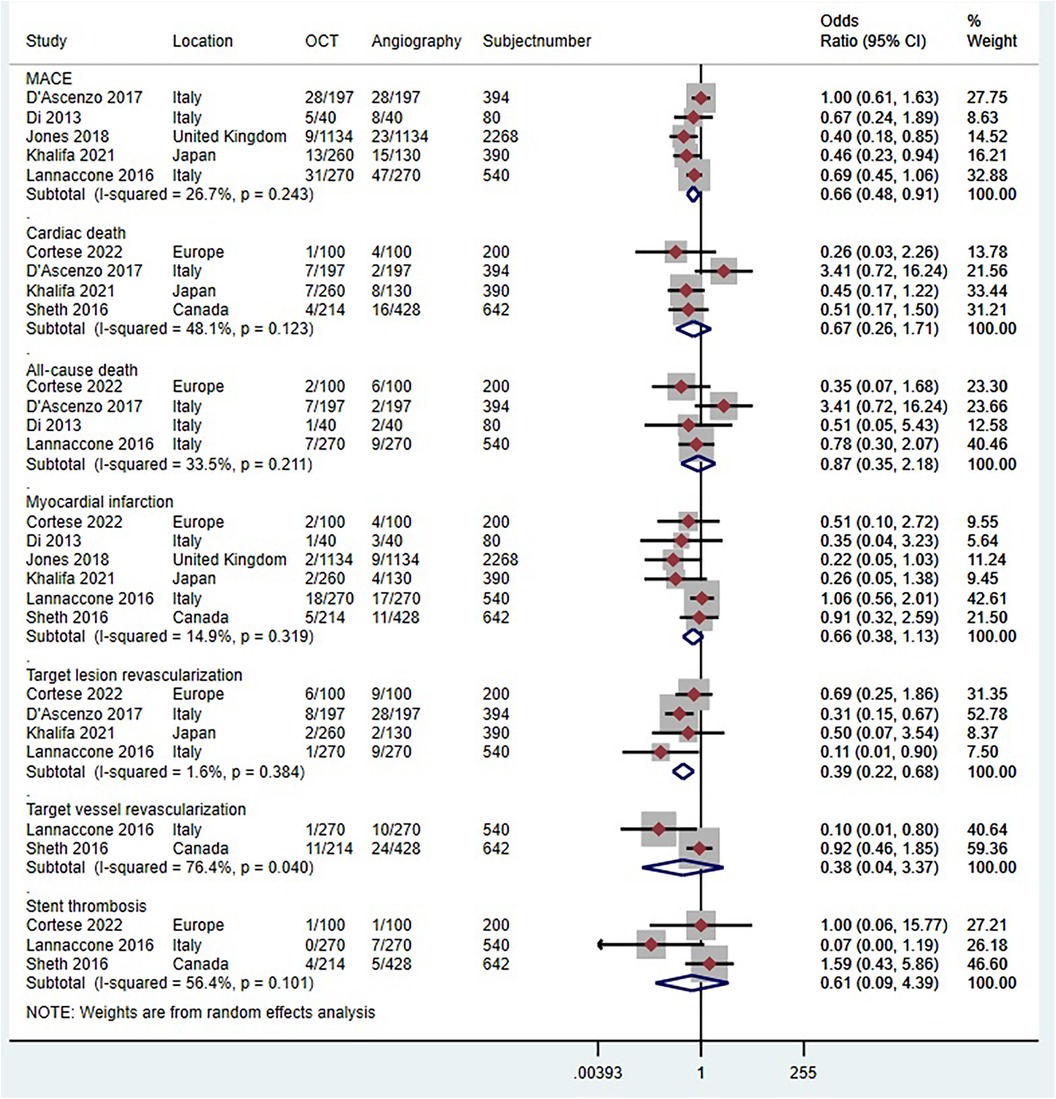
Figure 11 Forest plot comparing OCT guided with coronary angiography guided PCI for all-cause death. Data obtained from RCTs using fixed effect meta-analysis and expressed as OR. CI, confidence interval; OCT, optical coherence tomography; PCI, percutaneous coronary intervention.
3.2.4 Cardiac death
Five trials (n = 4,104) reported cardiac death (29, 41, 43–45). Compared with coronary angiography, OCT-guided PCI was not associated with a significant reduction in cardiac death (OR 0.61, 95% CI 0.36–1.02; p = 0.893, I2 = 0%, non-relevant heterogeneity, high certainty, see Figure 10B). Observational studies (51–53, 57), which included 4 studies with 1,626 patients, also demonstrated that OCT-guided PCI was not significantly associated with a reduction in the risk of cardiac death when compared to coronary angiography (OR 0.67, 95% CI 0.27–1.71; p = 0. 123, I2 = 48.1%, indicating moderate heterogeneity and moderate certainty, as shown in Figure 11B).
3.2.5 All-cause death
Four trials (n = 4,223) reported all-cause death (29, 42, 44, 47). Compared with coronary angiography, OCT-guided PCI was not associated with a significant reduction in all-cause death (OR 0.7, 95% CI 0.49–1.02; p = 0.550, I2 = 0%, non-relevant heterogeneity, high certainty, see Figure 10C). Observational studies (52, 54, 55, 57), which included 4 studies with 1,214 patients, also demonstrated that OCT-guided PCI was not significantly associated with a reduction in the risk of all-cause death when compared to coronary angiography (OR 0.87, 95% CI 0.35–2.18; p = 0. 211, I2 = 33.5%, indicating moderate heterogeneity and moderate certainty, as shown in Figure 11C).
3.2.6 MI
Six trials (n = 4,747) reported MI (29, 41, 42, 44, 47, 49). Compared with coronary angiography, OCT-guided PCI was not associated with a significant reduction in MI (OR 0.88, 95% CI 0.69 to 1.13; p = 0.823, I2 = 0%, non-relevant heterogeneity, high certainty, see Figure 10D). Observational studies (51, 53–57), which included 6 studies with 4,120 patients, also demonstrated that OCT-guided PCI was not significantly associated with a reduction in the risk of MI when compared to coronary angiography (OR 0.66, 95% CI 0.38–1.13; p = 0. 319, I2 = 14.9%, indicating low heterogeneity and moderate certainty, as shown in Figure 11D).
3.2.7 TLR
Seven trials (n = 4,646) reported TLR (29, 41, 44, 47–50). Compared with coronary angiography, OCT-guided PCI was not associated with a significant reduction in TLR (OR 0.94, 95% CI 0.7–1.27; p = 0.856, I2 = 0%, non-relevant heterogeneity, high certainty, see Figure 10E). However, the results from the observational studies, which included 4 studies with 1,524 patients, showed differing outcomes (51, 52, 54, 57). In contrast to coronary angiography, OCT-guided PCI showed a reduction in the risk of TLR (OR 0.39, 95% CI 0.22–0.68; p = 0.384, I2 = 1.6%, non-relevant heterogeneity, high certainty, see Figure 11E).
3.2.8 TVR
Four trials (n = 3,060) reported TVR (29, 42, 47, 50). Compared with coronary angiography, OCT-guided PCI was not associated with a significant reduction in TVR (OR 1.04, 95% CI 0.76–1.43; p = 0.857, I2 = 0%, non-relevant heterogeneity, high certainty, see Figure 10F). Observational studies (53, 54), which included 2 studies with 1,182 patients, also demonstrated that OCT-guided PCI was not significantly associated with a reduction in the risk of TVR when compared to coronary angiography (OR 0.38, 95% CI 0.04–3.37; p = 0. 04, I2 = 76.4%, indicating high heterogeneity and low certainty, as shown in Figure 11F).
3.2.9 Stent thrombosis
Five trials (n = 1,886) reported stent thrombosis (43, 44, 46, 48, 49). Compared with coronary angiography, OCT-guided PCI was not associated with a significant reduction in stent thrombosis (OR 0.72, 95% CI 0.38–1.38; p = 0.906, I2 = 0%, non-relevant heterogeneity, high certainty, see Figure 10G). Observational studies (53, 54, 57), which included 3 studies with 1,382 patients, also demonstrated that OCT-guided PCI was not significantly associated with a reduction in the risk of stent thrombosis when compared to coronary angiography (OR 0.61, 95% CI 0.09–4.39; p = 0. 101, I2 = 56.4%, indicating moderate heterogeneity and moderate certainty, as shown in Figure 11G).
4 Discussion
OCT, with its high-resolution imaging (10–20 μm), accurately identifies vascular features like thrombi, lipids, and calcium deposits (59–63). In this study, we analyzed 2,758 articles related to OCT and cardiovascular diseases from the WoSCC. In-depth analyses were conducted on these articles by country, institution, journal, author, and keywords using Bibliometrix R software and CiteSpace. This comprehensive exploration revealed the knowledge structure, research hotspots, and emerging trends in the field, laying the groundwork for future strategies in disease prevention and treatment. Our study found that OCT, as a guiding tool for PCI, has become a focal point in recent cohorts and randomized trials, which was further confirmed in our subsequent meta-analysis. After including 11 RCTs and 7 observational studies, we concluded that OCT-guided PCI did not demonstrate significant association with better clinical outcomes. Although the point estimate and the upper bound of the confidence interval hinted at a possible reduction in MACE, cardiac death, all-cause death, MI, TLR, or stent thrombosis with OCT guided PCI, this did not reach statistical significance. However, the meta-analysis of observational studies showed a significant reduction in MACE and TLR.
4.1 Advantages and limitations of bibliometric analysis
The United States led in the publication output related to OCT and cardiovascular diseases, also exhibiting the highest proportion of international collaboration. Moreover, China's publication numbers are rapidly growing, likely influenced by recent expert consensus from Chinese cardiology societies emphasizing the importance of OCT in PCI (64). Among the top 10 institutions with the highest publication output, 7 were from the USA, while the remaining were from other countries (China, Singapore, and the UK). Professor Yu Bo from China was the most prolific among the top 10 corresponding authors, followed by authors from the USA. Professor Mehran Roxana held the highest m-INDEX, Professor Yu Bo the largest G-index, and Professor Virmani Renu the highest h-index and total citations. Additionally, among the top 10 most cited papers, one was published in “The Lancet" (37), and three in “Circulation” and other high-impact journals (31, 35, 36). Professor Ik-Kyung Jang's 2005 paper “in vivo Characterization of Coronary Atherosclerotic Plaque by Use of Optical Coherence Tomography” ranked highest in citations (31).
Thematic word trend analysis over the past 20 years in the cardiovascular field has centered on eight key terms: coronary artery disease, deep learning, coronary stenosis, heart transplantation, plaque rupture, OCT, congenital heart disease, and cardiovascular diseases. Burst analysis of keywords indicated that studies on OCT and angiography-guided PCI have become hot topics in recent cohorts and randomized trials. Well-developed themes focus on atherosclerotic diseases, blood pressure, stent implantation, and plaque characteristics. The field's attention to the treatment and prognosis of diseases such as “coronary stenosis, acute myocardial infarction, atherosclerosis” post-operation and for survival is also noteworthy.
However, this study has limitations. The primary data for the bibliometric analysis was sourced from the WoSCC. Although the WoSCC includes over 11,000 authoritative and high-impact international academic journals with extensive coverage and powerful analysis features, its singular source may lead to potential article omissions from other databases. Additionally, researchers manually removed papers deemed irrelevant to the study objectives, which might introduce selection bias. Despite these limitations, our study comprehensively analyzes the current state and progress of OCT in cardiovascular research, aiding in identifying future research directions.
4.2 Advantages and limitations of meta-analysis
OCT has shown significant technical advantages in the application of cardiovascular diseases (30, 65). Compared to traditional coronary angiography, OCT provides higher-resolution spatial three-dimensional images, critical in accurately assessing plaque composition and morphology. Importantly, OCT optimizes angioplasty of bifurcation lesions, avoiding the common issues of perspective shortening and image overlap in traditional angiography (66). These technical strengths theoretically endow OCT with significant clinical application potential. However, in actual clinical practice, these theoretical advantages of OCT have not entirely translated into clinical benefits. Our meta-analysis of RCTs revealed that OCT-guided PCI did not exhibit significant clinical benefits in MACE, Cardiac death, All-cause death, MI, TLR, TVR, and Stent thrombosis, compared to angiography-guided PCI. Although studies suggest that OCT-guided PCI can achieve a larger minimum lumen diameter (MLD) (53), its use also leads to longer procedural times and higher contrast agent dosages (67), increasing perioperative risks such as early mortality, emergency coronary artery bypass grafting, cancer, and contrast-induced nephropathy (68, 69). These risks might overshadow the clinical benefits of OCT. However, the meta-analysis of observational studies indicated a significant reduction in MACE and TLR with OCT-guided PCI, aligning with previous research (67, 70).
4.2.1 Limitations
When interpreting the results of our meta-analysis, its inherent limitations must be considered. Firstly, the included trials varied in participant populations, outcome definitions, and follow-up periods, potentially affecting comparability and generalizability. Secondly, pre-planned overall and subgroup analyses were conducted at the study level, not at the individual patient level, precluding precise assessment of the specific impact of stent size pre and post PCI guided by OCT on cardiovascular outcomes. Lastly, variations in intravascular imaging guidance standards among different trials could also influence the results.
4.3 Comparisons with other studies
Although many meta-analyses have studied intravascular imaging-guided PCI, a systematic review of 24 meta-analyses showed that only 9 focused specifically on RCTs (71). Given the potential introduction of confounding factors in observational studies (71), we conducted separate meta-analyses of evidence from RCTs and observational studies for OCT-guided PCI. This approach differs from previous meta-analyses, showing OCT's significant advantages are more pronounced in observational studies (67, 70), consistent with previous high-quality RCTs (29, 49, 53).
Our results, compared with the study led by Niels R. Holm, showed differences in MACE outcomes (44). The fundamental reason is that calculating OR values directly using incidence rates might differ from results reported in that study, as Cox regression analysis incorporates specific time points of events, often overlooked in simple calculations (44, 72). Additionally, the Cox model typically considers multiple covariates potentially influencing outcomes, such as patient age, gender, and medical history (73). This might be one reason our study did not show a significant clinical advantage of OCT. Furthermore, with a median follow-up time of only 1–2 years in the studies included, detecting statistically significant differences between the two interventions would require longer follow-up and higher event rates.
5 Conclusion
In summary, this study primarily employed bibliometric analysis to examine literature published over the past twenty years on OCT and cardiovascular diseases. It identified specific countries, institutions, authors, and journals that have made significant contributions to this field during this period. It was found that OCT as a guiding tool for PCI has become a hot topic in recent cohorts and randomized trials, prompting subsequent meta-analyses. However, OCT-guided PCI did not demonstrate significant clinical benefits, with only the meta-analysis of observational studies suggesting a reduction in MACE and TLR.
Author contributions
WL: Conceptualization, Writing – original draft, Writing – review & editing. CC: Data curation, Formal Analysis, Writing – original draft. JW: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. JL: Formal Analysis, Methodology, Writing – review & editing. CL: Conceptualization, Methodology, Writing – review & editing. XZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by National Natural Science Foundation of China (Key Program) (No. 82230124), Central High-level Hospital of Traditional Chinese Medicine Clinical research and achievement transformation ability improvement project - TCM clinical evidence-based research project (No. HLCMHPP2023083).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1414205/full#supplementary-material
References
1. Stone PH, Libby P, Boden WE. Fundamental pathobiology of coronary atherosclerosis and clinical implications for chronic ischemic heart disease management-the plaque hypothesis: a narrative review. JAMA Cardiol. (2023) 8(2):192–201. doi: 10.1001/jamacardio.2022.3926 (published Online First: 2022/12/15).36515941
2. Libby P. Molecular bases of the acute coronary syndromes. Circulation. (1995) 91(11):2844–50. doi: 10.1161/01.cir.91.11.2844 (published Online First: 1995/06/01).7758192
3. Arbab-Zadeh A, Fuster V. The risk Continuum of atherosclerosis and its implications for defining CHD by coronary angiography. J Am Coll Cardiol. (2016) 68(22):2467–78. doi: 10.1016/j.jacc.2016.08.069 (published Online First: 2016/12/03).27908353
4. Mortensen MB, Dzaye O, Steffensen FH, Bøtker HE, Jensen JM, Rønnow Sand NP, et al. Impact of plaque burden versus stenosis on ischemic events in patients with coronary atherosclerosis. J Am Coll Cardiol. (2020) 76(24):2803–13. doi: 10.1016/j.jacc.2020.10.021 (published Online First: 2020/12/12).33303068
5. Villines TC, Rodriguez Lozano P. Transitioning from stenosis to plaque burden in the cardiac CT era: the changing risk paradigm. J Am Coll Cardiol. (2020) 76(24):2814–16. doi: 10.1016/j.jacc.2020.10.030 (published Online First: 2020/12/12).33303069
6. Ferraro R, Latina JM, Alfaddagh A, Michos ED, Blaha MJ, Jones SR, et al. Evaluation and management of patients with stable angina: beyond the ischemia paradigm: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 76(19):2252–66. doi: 10.1016/j.jacc.2020.08.078 (published Online First: 2020/11/07).33153586
7. Zhang Q, Wang L, Wang S, Cheng H, Xu L, Pei G, et al. Signaling pathways and targeted therapy for myocardial infarction. Signal Transduct Targeted Ther. (2022) 7(1):78. doi: 10.1038/s41392-022-00925-z (published Online First: 2022/03/12).
8. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010 (published Online First: 2020/12/15).33309175
9. Mackman N, Bergmeier W, Stouffer GA, Weitz JI. Therapeutic strategies for thrombosis: new targets and approaches. Nat Rev Drug Discovery. (2020) 19(5):333–52. doi: 10.1038/s41573-020-0061-0 (published Online First: 2020/03/07).32132678
10. Sabatine MS, Braunwald E. Thrombolysis in myocardial infarction (TIMI) study group: JACC focus seminar 2/8. J Am Coll Cardiol. (2021) 77(22):2822–45. doi: 10.1016/j.jacc.2021.01.060 (published Online First: 2021/06/05).34082913
11. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. (2011) 364(3):226–35. doi: 10.1056/NEJMoa1002358 (published Online First: 2011/01/21).21247313
12. Oemrawsingh RM, Cheng JM, García-García HM, van Geuns RJ, de Boer SP, Simsek C, et al. Near-infrared spectroscopy predicts cardiovascular outcome in patients with coronary artery disease. J Am Coll Cardiol. (2014) 64(23):2510–8. doi: 10.1016/j.jacc.2014.07.998 (published Online First: 2014/12/17).25500237
13. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. (2012) 367(11):991–1001. doi: 10.1056/NEJMoa1205361 (published Online First: 2012/08/29).22924638
14. Stone PH, Saito S, Takahashi S, Makita Y, Nakamura S, Kawasaki T, et al. Prediction of progression of coronary artery disease and clinical outcomes using vascular profiling of endothelial shear stress and arterial plaque characteristics: the PREDICTION study. Circulation. (2012) 126(2):172–81. doi: 10.1161/circulationaha.112.096438 (published Online First: 2012/06/23).22723305
15. Erlinge D, Maehara A, Ben-Yehuda O, Bøtker HE, Maeng M, Kjøller-Hansen L, et al. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): a prospective natural history study. Lancet (London, England). (2021) 397(10278):985–95. doi: 10.1016/s0140-6736(21)00249-x (published Online First: 2021/03/15).33714389
16. Kedhi E, Berta B, Roleder T, Hermanides RS, Fabris E, IJsselmuiden AJJ, et al. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: the COMBINE OCT-FFR trial. Eur Heart J. (2021) 42(45):4671–79. doi: 10.1093/eurheartj/ehab433 (published Online First: 2021/08/05).34345911
17. Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, et al. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. (2018) 14(6):656–77. doi: 10.4244/eijy18m06_01 (published Online First: 2018/06/26).
18. Fujimoto JG, Pitris C, Boppart SA, Brezinski ME. Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia. (2000) 2(1-2):9–25. doi: 10.1038/sj.neo.7900071 (published Online First: 2000/08/10).10933065
19. Terashima M, Kaneda H, Suzuki T. The role of optical coherence tomography in coronary intervention. Korean J Intern Med. (2012) 27(1):1–12. doi: 10.3904/kjim.2012.27.1.1 (published Online First: 2012/03/10).22403493
20. Cilingiroglu M, Oh JH, Sugunan B, Kemp NJ, Kim J, Lee S, et al. Detection of vulnerable plaque in a murine model of atherosclerosis with optical coherence tomography. Catheter Cardiovasc Interv. (2006) 67(6):915–23. doi: 10.1002/ccd.20717 (published Online First: 2006/04/08).16602128
21. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. (2014) 7(10):1025–38. doi: 10.1016/j.jcmg.2013.11.014 (published Online First: 2014/07/24).25051948
22. Wang J, Yuan S, Qi J, Zhang Q, Ji Z. Advantages and prospects of optical coherence tomography in interventional therapy of coronary heart disease (review). Exp Ther Med. (2022) 23(4):255. doi: 10.3892/etm.2022.11180 (published Online First: 2022/03/10).35261627
23. Donthu N, Kumar S, Pattnaik D, Weng ML. A bibliometric retrospection of marketing from the lens of psychology: insights from psychology & marketing. Psychol Mark. (2021) 38(5):834–65. doi: 10.1002/mar.21472
24. Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. (2021) 133:285–96. doi: 10.1016/j.jbusres.2021.04.070
25. Aria M, Cuccurullo C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. (2017) 11(4):959–75. doi: 10.1016/j.joi.2017.08.007
26. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. (2004) 6:101 Suppl 1(Suppl 1):5303–10. doi: 10.1073/pnas.0307513100
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
28. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
29. Ali ZA, Landmesser U, Maehara A, Matsumura M, Shlofmitz RA, Guagliumi G, et al. Optical coherence tomography-guided versus angiography-guided PCI. N Engl J Med. (2023) 389(16):1466–76. doi: 10.1056/NEJMoa2305861 (published Online First: 2023/08/27).37634188
30. Gruslova AB, Singh S, Hoyt T, Vela D, Vengrenyuk Y, Buja LM, et al. Accuracy of OCT core labs in identifying vulnerable plaque. JACC Cardiovasc Imaging. (2023) 17(4):448–50. doi: 10.1016/j.jcmg.2023.10.005 (published Online First: 2023/11/09).37943235
31. Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. (2005) 111(12):1551–5. doi: 10.1161/01.Cir.0000159354.43778.69 (published Online First: 2005/03/23).15781733
32. Narula J, Nakano M, Virmani R, Kolodgie FD, Petersen R, Newcomb R, et al. Histopathologic characteristics of atherosclerotic coronary disease and implications of the findings for the invasive and noninvasive detection of vulnerable plaques. J Am Coll Cardiol. (2013) 61(10):1041–51. doi: 10.1016/j.jacc.2012.10.054 (published Online First: 2013/03/12).23473409
33. Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, et al. Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J. (2015) 36(32):2147–59. doi: 10.1093/eurheartj/ehv205 (published Online First: 2015/05/23).25994755
34. Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque": transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol. (2015) 65(8):846–55. doi: 10.1016/j.jacc.2014.11.041 (published Online First: 2015/01/21).25601032
35. Serruys PW, Onuma Y, Ormiston JA, de Bruyne B, Regar E, Dudek D, et al. Evaluation of the second generation of a bioresorbable everolimus drug-eluting vascular scaffold for treatment of de novo coronary artery stenosis: six-month clinical and imaging outcomes. Circulation. (2010) 122(22):2301–12. doi: 10.1161/circulationaha.110.970772 (published Online First: 2010/11/26).21098436
36. Onuma Y, Serruys PW, Perkins LE, Okamura T, Gonzalo N, García-García HM, et al. Intracoronary optical coherence tomography and histology at 1 month and 2, 3, and 4 years after implantation of everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: an attempt to decipher the human optical coherence tomography images in the ABSORB trial. Circulation. (2010) 122(22):2288–300. doi: 10.1161/circulationaha.109.921528 (published Online First: 2010/10/27).20975003
37. Haude M, Ince H, Abizaid A, Toelg R, Lemos PA, von Birgelen C, et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet. (2016) 387(10013):31–9. doi: 10.1016/s0140-6736(15)00447-x (published Online First: 2015/10/17).26470647
38. Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng. (2006) 34(12):1799–819. doi: 10.1007/s10439-006-9163-z (published Online First: 2006/10/21).17053986
39. Xu C, Schmitt JM, Carlier SG, Virmani R. Characterization of atherosclerosis plaques by measuring both backscattering and attenuation coefficients in optical coherence tomography. J Biomed Opt. (2008) 13(3):034003. doi: 10.1117/1.2927464 (published Online First: 2008/07/08).18601548
40. Ohayon J, Finet G, Gharib AM, Herzka DA, Tracqui P, Heroux J, et al. Necrotic core thickness and positive arterial remodeling index: emergent biomechanical factors for evaluating the risk of plaque rupture. Am J Physiol Heart Circ Physiol. (2008) 295(2):H717–27. doi: 10.1152/ajpheart.00005.2008 (published Online First: 2008/07/01).18586893
41. Jia H, Dai J, He L, Xu Y, Shi Y, Zhao L, et al. EROSION III: a multicenter RCT of OCT-guided reperfusion in STEMI with early infarct artery patency. JACC Cardiovasc Interv. (2022) 15(8):846–56. doi: 10.1016/j.jcin.2022.01.298 (published Online First: 2022/04/04).35367176
42. Meneveau N, Souteyrand G, Motreff P, Caussin C, Amabile N, Ohlmann P, et al. Optical coherence tomography to optimize results of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome: results of the multicenter, randomized DOCTORS study (does optical coherence tomography optimize results of stenting). Circulation. (2016) 134(13):906–17. doi: 10.1161/circulationaha.116.024393 (published Online First: 2016/08/31).27573032
43. Antonsen L, Thayssen P, Maehara A, Hansen HS, Junker A, Veien KT, et al. Optical coherence tomography guided percutaneous coronary intervention with nobori stent implantation in patients with non-ST-segment-elevation myocardial infarction (OCTACS) trial: difference in strut coverage and dynamic malapposition patterns at 6 months. Circ Cardiovasc Interv. (2015) 8(8):e002446. doi: 10.1161/circinterventions.114.002446 (published Online First: 2015/08/09).26253735
44. Holm NR, Andreasen LN, Neghabat O, Laanmets P, Kumsars I, Bennett J, et al. OCT Or angiography guidance for PCI in Complex bifurcation lesions. N Engl J Med. (2023) 389(16):1477–87. doi: 10.1056/NEJMoa2307770 (published Online First: 2023/08/27).37634149
45. Onuma Y, Kogame N, Sotomi Y, Miyazaki Y, Asano T, Takahashi K, et al. A randomized trial evaluating online 3-dimensional optical frequency domain imaging-guided percutaneous coronary intervention in bifurcation lesions. Circ Cardiovasc Interv. (2020) 13(12):e009183. doi: 10.1161/circinterventions.120.009183 (published Online First: 2020/12/05).33272034
46. Kala P, Cervinka P, Jakl M, Kanovsky J, Kupec A, Spacek R, et al. OCT Guidance during stent implantation in primary PCI: a randomized multicenter study with nine months of optical coherence tomography follow-up. Int J Cardiol. (2018) 250:98–103. doi: 10.1016/j.ijcard.2017.10.059 (published Online First: 2017/10/29).29079414
47. Ali ZA, Karimi Galougahi K, Maehara A, Shlofmitz RA, Fabbiocchi F, Guagliumi G, et al. Outcomes of optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation: one-year results from the ILUMIEN III: OPTIMIZE PCI trial. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. (2021) 16(13):1085–91. doi: 10.4244/eij-d-20-00498 [published Online First: 2020/06/17].32540793
48. Kim JS, Shin DH, Kim BK, Ko YG, Choi D, Jang Y, et al. Randomized comparison of stent strut coverage following angiography- or optical coherence tomography-guided percutaneous coronary intervention. Rev Esp Cardiol (English ed). (2015) 68(3):190–7. doi: 10.1016/j.rec.2014.07.025 (published Online First: 2014/12/10).
49. Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet (London, England). (2016) 388(10060):2618–28. doi: 10.1016/s0140-6736(16)31922-5 (published Online First: 2016/11/04).27806900
50. Ueki Y, Yamaji K, Barbato E, Nef H, Brugaletta S, Alfonso F, et al. Randomized comparison of optical coherence tomography versus angiography to guide bioresorbable vascular scaffold implantation: the OPTICO BVS study. Cardiovasc Revasc Med. (2020) 21(10):1244–50. doi: 10.1016/j.carrev.2020.03.023 (published Online First: 2020/03/25).32205067
51. Khalifa AKM, Kubo T, Shimamura K, Ino Y, Kishk YT, Hasan-Ali H, et al. Impact of optical coherence tomography imaging on decision-making during percutaneous coronary intervention in patients presented with acute coronary syndromes. Circ J. (2021) 85(10):1781–88. doi: 10.1253/circj.CJ-20-0942 (published Online First: 2021/01/22).33473095
52. D'Ascenzo F, Iannaccone M, De Filippo O, Leone AM, Niccoli G, Zilio F, et al. Optical coherence tomography compared with fractional flow reserve guided approach in acute coronary syndromes: a propensity matched analysis. Int J Cardiol. (2017) 244:54–8. doi: 10.1016/j.ijcard.2017.05.108 (published Online First: 2017/06/21).
53. Sheth TN, Kajander OA, Lavi S, Bhindi R, Cantor WJ, Cheema AN, et al. Optical coherence tomography-guided percutaneous coronary intervention in ST-segment-elevation myocardial infarction: a prospective propensity-matched cohort of the thrombectomy versus percutaneous coronary intervention alone trial. Circ Cardiovasc Interv. (2016) 9(4):e003414. doi: 10.1161/circinterventions.115.003414 (published Online First: 2016/04/09).27056766
54. Iannaccone M, D'Ascenzo F, Frangieh AH, Niccoli G, Ugo F, Boccuzzi G, et al. Impact of an optical coherence tomography guided approach in acute coronary syndromes: a propensity matched analysis from the international FORMIDABLE-CARDIOGROUP IV and USZ registry. Catheter Cardiovasc Interv. (2017) 90(2):E46–e52. doi: 10.1002/ccd.26880 (published Online First: 2016/12/29).28029210
55. Di Giorgio A, Capodanno D, Ramazzotti V, Imola F, Mallus MT, Stazi F, et al. Optical coherence tomography guided in-stent thrombus removal in patients with acute coronary syndromes. Int J Cardiovasc Imaging. (2013) 29(5):989–96. doi: 10.1007/s10554-013-0191-0 (published Online First: 2013/02/16).23412883
56. Jones DA, Rathod KS, Koganti S, Hamshere S, Astroulakis Z, Lim P, et al. Angiography alone versus angiography plus optical coherence tomography to guide percutaneous coronary intervention: outcomes from the Pan-London PCI cohort. JACC Cardiovasc Interv. (2018) 11(14):1313–21. doi: 10.1016/j.jcin.2018.01.274 (published Online First: 2018/07/22).30025725
57. Cortese B, de la, Torre Hernandez JM, Lanocha M, Ielasi A, Giannini F, Campo G, et al. Optical coherence tomography, intravascular ultrasound or angiography guidance for distal left main coronary stenting. The ROCK cohort II study. Catheter Cardiovasc Interv. (2022) 99(3):664–73. doi: 10.1002/ccd.29959 (published Online First: 2021/09/29).34582631
58. Floré V, Brown AJ, Giblett JP, Liou K, Cranley J, Hoole SP, et al. Clinical outcomes of bioresorbable vascular scaffolds implanted with routine versus selective optical coherence tomography guidance: results from a single-centre experience. EuroIntervention. (2019) 14(17):1776–83. doi: 10.4244/eij-d-18-00262 (published Online First: 2018/10/31).
59. Ali ZA, Karimi Galougahi K, Maehara A, Shlofmitz RA, Ben-Yehuda O, Mintz GS, et al. Intracoronary optical coherence tomography 2018: current Status and future directions. JACC Cardiovasc Interv. (2017) 10(24):2473–87. doi: 10.1016/j.jcin.2017.09.042 (published Online First: 2017/12/23).29268880
60. Wijns W, Shite J, Jones MR, Lee SW, Price MJ, Fabbiocchi F, et al. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J. (2015) 36(47):3346–55. doi: 10.1093/eurheartj/ehv367 (published Online First: 2015/08/06).26242713
61. Prati F, Romagnoli E, Burzotta F, Limbruno U, Gatto L, La Manna A, et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II study. JACC Cardiovasc Imaging. (2015) 8(11):1297–305. doi: 10.1016/j.jcmg.2015.08.013 (published Online First: 2015/11/14).26563859
62. Prati F, Romagnoli E, La Manna A, Burzotta F, Gatto L, Marco V, et al. Long-term consequences of optical coherence tomography findings during percutaneous coronary intervention: the centro per la Lotta contro l'infarto—optimization of percutaneous coronary intervention (CLI-OPCI) LATE study. EuroIntervention. (2018) 14(4):e443–e51. doi: 10.4244/eij-d-17-01111 (published Online First: 2018/04/11).29633940
63. Ino Y, Kubo T, Matsuo Y, et al. Optical coherence tomography predictors for edge restenosis after everolimus-eluting stent implantation. Circ Cardiovasc Interv. (2016) 9(10):e004231. doi: 10.1161/circinterventions.116.004231 (published Online First: 2016/10/01).27688261
64. Chinese Society of Cardiology, Chinese Medical Association; Editorial Board of Chinese Journal of Cardiology. Chinese Expert consensus on the application of optical coherence tomography in the interventional diagnosis and treatment of coronary artery disease. Zhonghua Xin Xue Guan Bing Za Zhi (2023)51(2):109–24. doi: 10.3760/cma.j.cn112148-20220602-00436 (published Online First: 2023/02/16)36789590
65. Araki M, Park SJ, Dauerman HL, Uemura S, Kim JS, Di Mario C, et al. Optical coherence tomography in coronary atherosclerosis assessment and intervention. Nat Rev Cardiol. (2022) 19(10):684–703. doi: 10.1038/s41569-022-00687-9 (published Online First: 2022/04/23).35449407
66. Otake H. Optical coherence tomography-guided percutaneous coronary intervention: evidence and clinical trials. Interv Cardiol Clin. (2023) 12(2):225–36. doi: 10.1016/j.iccl.2022.12.004 (published Online First: 2023/03/16).36922063
67. Attar A, Hosseinpour A, Azami P, Kohansal E, Javaheri R. Clinical outcomes of optical coherence tomography versus conventional angiography guided percutaneous coronary intervention: a meta-analysis. Curr Probl Cardiol. (2024) 49(2):102224. doi: 10.1016/j.cpcardiol.2023.102224
68. Khan SU, Agarwal S, Arshad HB, Akbar UA, Mamas MA, Arora S, et al. Intravascular imaging guided versus coronary angiography guided percutaneous coronary intervention: systematic review and meta-analysis. BMJ (Clinical Research ed). (2023) 383:e077848. doi: 10.1136/bmj-2023-077848 (published Online First: 2023/11/17).37973170
69. Johnson LW, Moore RJ, Balter S. Review of radiation safety in the cardiac catheterization laboratory. Cathet Cardiovasc Diagn. (1992) 25(3):186–94. doi: 10.1002/ccd.1810250304 (published Online First: 1992/03/01).1571973
70. Macherey-Meyer S, Meertens MM, Heyne S, Braumann S, Tichelbäcker T, Wienemann H, et al. Optical coherence tomography-guided versus angiography-guided percutaneous coronary intervention in acute coronary syndrome: a meta-analysis. Clin Res Cardiol. (2023). doi: 10.1007/s00392-023-02272-7 (published Online First: 2023/08/01).
71. Mintz GS, Bourantas CV, Chamié D. Intravascular imaging for percutaneous coronary intervention guidance and optimization: the evidence for improved patient outcomes. J Soc Cardiovasc Angiogr Interv. (2022) 1(6):100413. doi: 10.1016/j.jscai.2022.100413
72. Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in cox regression models. Ann Transl Med. (2018) 6(7):121. doi: 10.21037/atm.2018.02.12
Keywords: optical coherence tomography, cardiovascular diseases, bibliometric analysis, meta-analysis, percutaneous coronary intervention
Citation: Lian W, Chen C, Wang J, Li J, Liu C and Zhu X (2024) Application of optical coherence tomography in cardiovascular diseases: bibliometric and meta-analysis. Front. Cardiovasc. Med. 11:1414205. doi: 10.3389/fcvm.2024.1414205
Received: 8 April 2024; Accepted: 24 June 2024;
Published: 9 July 2024.
Edited by:
Pasqualino Sirignano, Sapienza University of Rome, Italy© 2024 Lian, Chen, Wang, Li, Liu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Wang, d2FuZ2ppZTAxMDNAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Wenjing Lian
Wenjing Lian Cong Chen
Cong Chen Jie Wang
Jie Wang Jun Li
Jun Li Chao Liu1
Chao Liu1 Xueying Zhu
Xueying Zhu