- 1School of Pharmacy, College of Health Sciences, Debre Berhan University, Debre Berhan, Ethiopia
- 2Department of Midwifery, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Introduction: Based on office blood pressure (BP) values, hypertension is categorized into three stages: stage 1 (140–159/90–99 mmHg), stage 2 (160–179/100–109 mmHg), and stage 3 (≥180/≥110 mmHg). Malignant hypertension (MHT) is characterized by extreme BP elevation (systolic blood pressure above 200 mmHg and diastolic blood pressure above 130 mmHg) and acute microvascular damage affecting various organs, particularly the retinas, brain, and kidneys.
Objectives: The pathogenesis, predisposing variables, therapy, and preventive strategies for MHT were examined in this review.
Conclusions and recommendations: Malignant hypertension requires prompt and efficient treatment because it is the most severe kind of hypertension that affects target organs. At the same time, there are a number of alternatives available for treating MHT. The International Society of Hypertension 2020 and European Society of Cardiology/European Society of Hypertension 2018 recommendations suggest using labetalol and nicardipine as the first-line choice, with urapidil and nitroprusside serving as alternative medications. Elevated risk of MHT has been linked to many socio-demographic and genetic factors.
1 Introduction
According to the 2023 European Society of Hypertension (ESH) guideline, hypertension (HTN) is defined as a recurrent office systolic blood pressure (SBP) value of 140 mmHg and diastolic blood pressure (DBP) value of 90 mmHg. Based on office blood pressure (BP) values, HTN is categorized into three stages: stage 1 (140–159/90–99 mmHg), stage 2 (160–179/100–109 mmHg), and stage 3 (≥180/≥110 mmHg) (1).
Malignant hypertension (MHT) is characterized by extreme BP elevation (SBP above 200 mmHg and DBP above 130 mmHg) and acute microvascular damage affecting various organs, particularly the retinas, brain, and kidneys (2–4).
The 2018 European Society of Cardiology (ESC)/ESH guideline differentiates MHT from “hypertension urgencies and emergencies” through the severity of damage to the organs, which is referred to as hypertension-multi-organ damage (retinopathy, microangiopathy, disseminated intravascular coagulation, encephalopathy, abrupt heart failure, or acute deterioration in renal function). It is a medical emergency with a poor prognosis if left untreated (5).
MHT requires emergency medical attention to limit organ damage and other complications related to severely high blood pressure (6). Vascular injury is mainly caused by the loss of autoregulation of blood flow, which typically occurs in individuals with uncontrolled and chronic hypertension (3, 7, 8).
There is limited data on MHT so the diagnostic and therapeutic guidelines are primarily based on consensus. The different definitions of MHT in the guidelines are listed in Table 1.
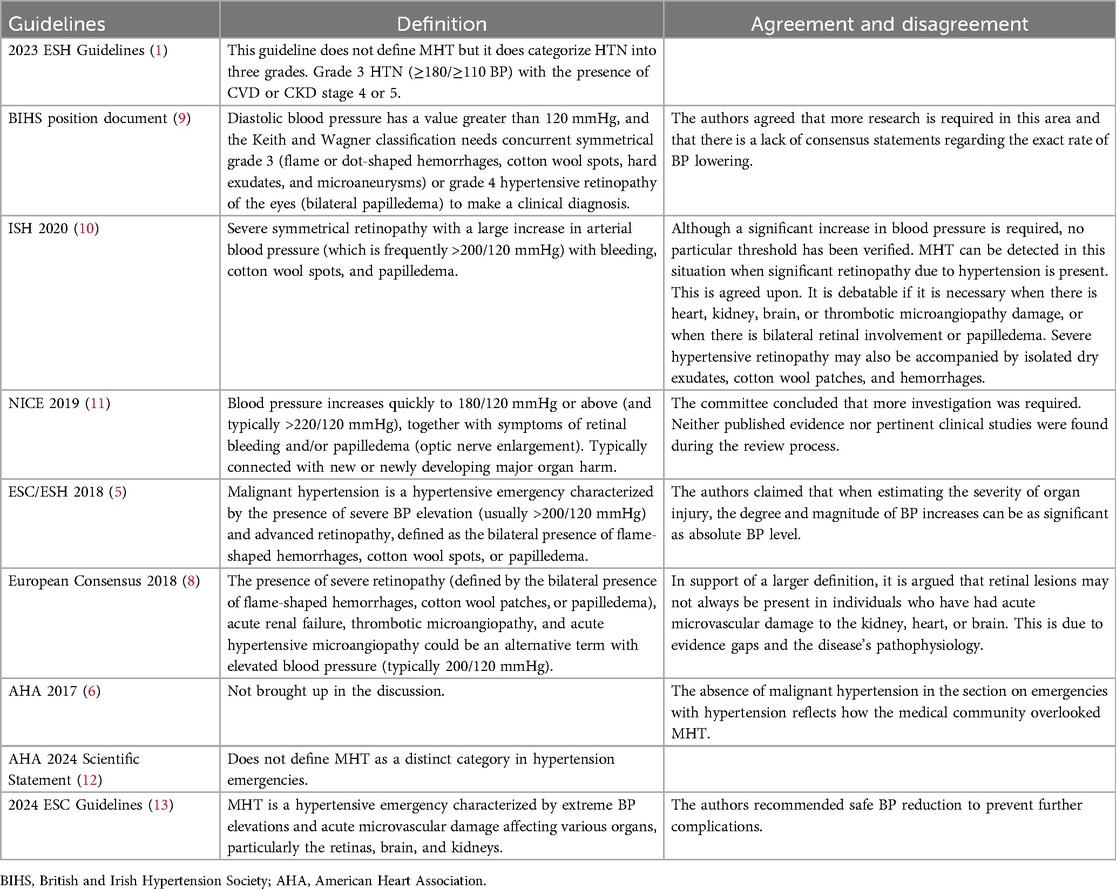
Table 1. Agreements and disagreements regarding the definition of malignant hypertension in current guideline/consensus documents (3).
2 Epidemiology of malignant hypertension
Although the prevalence and incidence data for MHT are scarce, a study conducted in Amsterdam (Netherlands) and Birmingham (United Kingdom) revealed an incident rate of 2/100,000 new cases per year, with up to fourfold higher rates among patients of Black African/Afro-Caribbean ethnicities (14). In the Afro-American population, 7.3/100,000 new cases per year were reported. A worse disease prognosis and greater disease predisposition were also observed (15).
3 Predisposing factors of malignant hypertension
Malignant hypertension mainly occurs in individuals with a history of uncontrolled high blood pressure and those who missed or discontinued their antihypertensive medications (16, 17). In addition, patients with stroke, structural heart disease, thyroid disorders, brain bleeding, renal artery disease, traumatic brain injury, Conn's syndrome, Cushing's syndrome, pheochromocytoma, and substance and medication withdrawal may experience MHT (17). In addition, pregnancy is another known precipitating factor of MHT (3, 18).
The study conducted in Birmingham included 460 patients diagnosed with MHT from 1958 to May 2016 and indicated that the study participants’ ethnic differences, advanced age, prior use of antihypertensive medication, duration of hypertension, and presence of proteinuria were strong predictors of MHT outcomes (19). There are more pronounced abnormalities in macrovascular and microvascular function in patients with MHT (which seem to be both endothelium-dependent and endothelium-independent) compared with patients with hypertension and healthy controls (20).
3.1 Socio-demographic factors
According to a study conducted on the Birmingham registry of patients with MHT, white Caucasian patients were more likely than African-Caribbean and South Asian patients to experience papilledema-related ocular problems (19). A study in Nigeria indicated patients with MHT had stressful lifestyles, were members of lower socio-economic groups, were older, had higher body mass indexes (BMI), had high BP, and had shorter in-time diagnoses of MHT (21).
Patients who had higher serum creatinine levels, who were current smokers, and who had no medical insurance had a significant association with MHT complications (22). A study conducted by Hertz et al. using the National Health and Nutrition Examination Survey (NHANES) 1999–2002 data indicated higher prevalence rates of hypertension in Black people compared to white people and a growing disparity in BP control among those treated using pharmacological agents (23).
3.2 Genetic factors
According to a study conducted using the Bordeaux, Birmingham, and Amsterdam MHT registries, ethnic minorities had a higher risk of MHT (15). Black patients had a higher incidence and more complications than white patients (22). In the USA, non-Hispanic Black patients had poor BP control compared to non-Hispanic white patients, and a higher prevalence of HTN was observed among non-Hispanic Black patients (24, 25).
Kalinowski et al. reported that Black patients’ endothelial cells had an increased release of both oxygen-free radicals and peroxynitrite and a reduced release of biologically active nitric oxide (NO). The study implied that there are differences in vascular function among patients of different races (26).
Another factor contributing to racial/ethnic pathophysiological differences in the mechanisms of HTN was BMI. The BMI of non-Hispanic Black patients was higher compared to non-Hispanic white patients and Chinese and Asian patients, resulting in a high prevalence of HTN (27).
Seven single-nucleotide polymorphisms (G589S, R597H, T594M, R624C, G442V, E632G, and V434M) were identified in the β subunit of the epithelial sodium channel (ENaC) (SCNN1β gene). The threonine-to-methionine point one mutation has only shown an association with HTN at position 594 (T594M). Persons of African origin have approximately 6% of the T594M allele, which is associated with elevated HTN risk in the African-American population, but this has not been found in any white patients (28).
4 Pathophysiology of malignant hypertension
There are various factors associated with the pathogenesis of MHT, although the underlying mechanism leading to MHT is not fully understood. Fibrinoid arteriolar necrosis in vascular tissue beds is the hallmark pathophysiological marker of MHT (9). Appropriate tissue blood perfusion is maintained by the auto-regulatory arterial and arteriolar processes which prevent the increase in pressure from being transmitted to the smaller, more distal vessels. During severe HTN, this autoregulation eventually fails and the vascular wall of arterioles and capillaries becomes damaged due to the rise in BP. The vascular endothelium then allows plasma constituents (including fibrinoid material) to enter the vascular wall, thereby narrowing or obliterating the vascular lumen (29).
Macrovascular and microvascular endothelium dysfunction is one of the abnormalities found in MHT patients (20). Thrombotic microangiopathies have resulted from the activation of the renin–angiotensin–aldosterone system (RAAS) and impairment of the intravascular prothrombotic state (agglutination/coagulation) along with excessive endothelial injury (20, 30) (Figure 1).
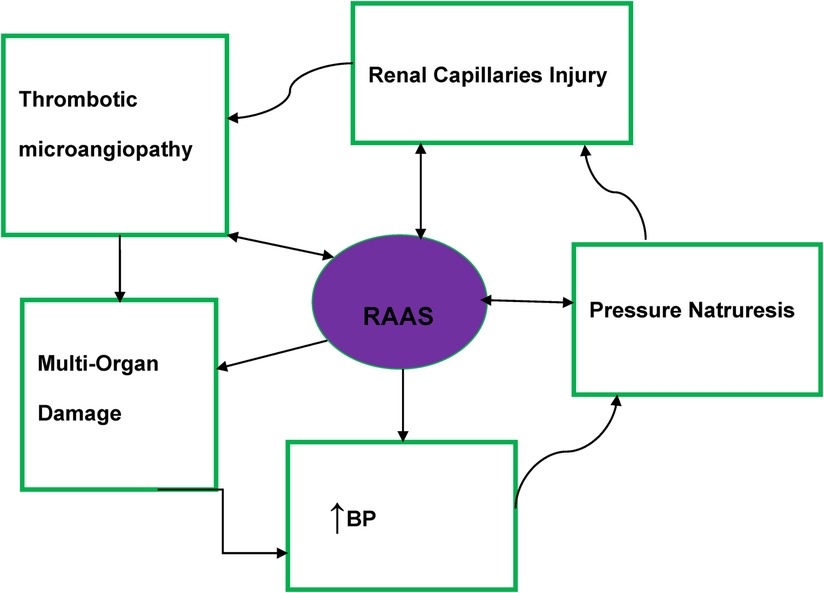
Figure 1. Malignant hypertension pathophysiology diagram. RAAS, renin–angiotensin–aldosterone system; BP, blood pressure.
Significant activation of the RAAS is evident in patients with MHT and may also contribute to the development of fibrinoid necrosis (31). In patients with MHT, there is evidence that irrespective of the impact of BP elevation on plasma renin activity, angiotensin 2-dependent aldosterone secretion was more profound than in patients with severe HTN (32, 33).
The basic pathophysiology of MHT is summarized in Figure 1.
4.1 Symptoms of malignant hypertension
In many cases, there are no symptoms associated with high blood pressure. However, this is not the case with MHT (16). During the MHT phase, a visual disturbance is one of the MHT symptoms. Retinopathy secondary to MHT is divided into grades A and B. Grade A retinopathy includes arteriolar narrowing and focal constriction of the retina and it corresponds to non-MHT changes. Grade B retinopathy corresponding to MHT includes linear flame-shaped hemorrhages, exudates, and/or cotton wool spot changes with or without papilledema (34).
Furthermore, sudden onset of headache, nausea, vomiting, visual disturbances, and visual field loss [in some cases, the symptoms depend on the damaged organ (e.g., brain) and include restlessness, confusion, seizures, and coma] are the signs and symptoms of MHT encephalopathy (35, 36).
MHT also results from acute renal failure (37). Malignant hypertensive patients have been shown to have severe renal dysfunction and a good cardiovascular risk profile compared to patients with controlled HTN (38). A long follow-up period and close BP control are required to preserve renal function (32, 39).
Clinically, thrombotic microangiopathy (TMA) is defined as microangiopathic hemolytic anemia, thrombocytopenia, and increased serum lactate dehydrogenase (LDH), all of which are frequently seen in patients with MHT (30).
Pulmonary edema has also been associated with MHT (38).
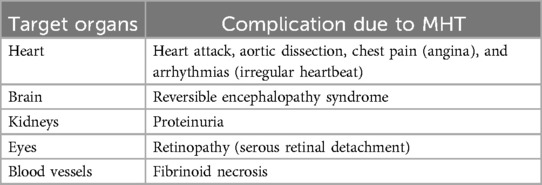
5 Complications of malignant hypertension
Macrovascular and microvascular function abnormalities are one of the major complications of MHT (20, 40). Malignant hypertension can also lead to stroke and encephalopathy (20, 41), heart damage (angina, heart attack, and arrhythmia), kidney failure, vision damage (permanent blindness), and pulmonary edema (16, 17, 20, 40, 42, 43) (Table 2).
A single-center retrospective study conducted by Mishima et al. indicated that patients with MHT had a wide range of distribution patterns in the brainstem and 60% of the patients had posterior reversible encephalopathy syndrome (PRES) (44) (Table 2).
6 Prognostic factors of malignant hypertension
The prognosis is typically favorable for those who obtain an early diagnosis and suitable antihypertensive medication. Due to the disease's tendency to advance quickly and produce irreversible end organ damage, the interval between diagnosis and therapy is crucial (3). The death rate for untreated MHT is 80% within 2 years. MHT can be fatal even with treatment; one study found that across 25 US hospitals, the hospital death rate was close to 7% and the readmission rate was 37% within 90 days (15).
7 Assessment of malignant hypertension
7.1 Diagnosis of malignant hypertension
In many patients, the initial diagnosis of MHT is frequently missed which results in the diagnosis only being established when target organ damage occurs (14).
Some evidence suggests that a diagnosis of MHT can be made when a patient presents with acutely and quickly raised BP in cardiovascular disease (CVD) and chronic kidney disease (CKD) stages 4 and 5, respectively (1).To differentiate between a hypertensive urgency, emergency, or MHT, healthcare providers must perform a comprehensive assessment. A patient’s symptoms will determine the kinds of investigation tests they undergo, which could include an electrocardiogram (EKG), a chest x-ray, an eye exam to check for signs of bleeding or damage to the eyes, a neurological exam to assess brain function, a physical examination, a toxicology serum drug level study, a urinalysis, liver function tests, and renal function tests (17).
Malignant hypertension is diagnosed clinically when there is evidence of organ damage from a fundoscopic eye examination, a chest x-ray, urine analysis, blood tests, and high blood pressure (taken using both arms) at or above 180/120 mmHg (1, 2, 45).
In addition, one of several possible presentations is also hypertension-mediated multi-organ damage (2, 5). Typically, concurrent bilateral grade 3 (flame or dot-shaped hemorrhages, cotton wool spots, hard exudates, and microaneurysms) or grade 4 hypertensive retinopathy (bilateral papilledema), as defined by Keith and Wagner's classification, is required for a clinical diagnosis of MHT (9).
8 Malignant hypertension treatment and prevention methods
There are limited therapeutic options specifically indicated for MHT (3, 46), and its treatment depends on expert decision-making (11). According to the National Institute for Health and Care Excellence (NICE) guideline, there is no consensus on the BP targets, BP reduction frequency, or type of drugs to administer to patients when treating MHT. However, the goal of therapy for MHT is to rapidly lower the mean arterial pressure by approximately 10%–15% in the first hour, and by no more than 25% compared with baseline by the end of the first day of treatment (47) (Table 3).
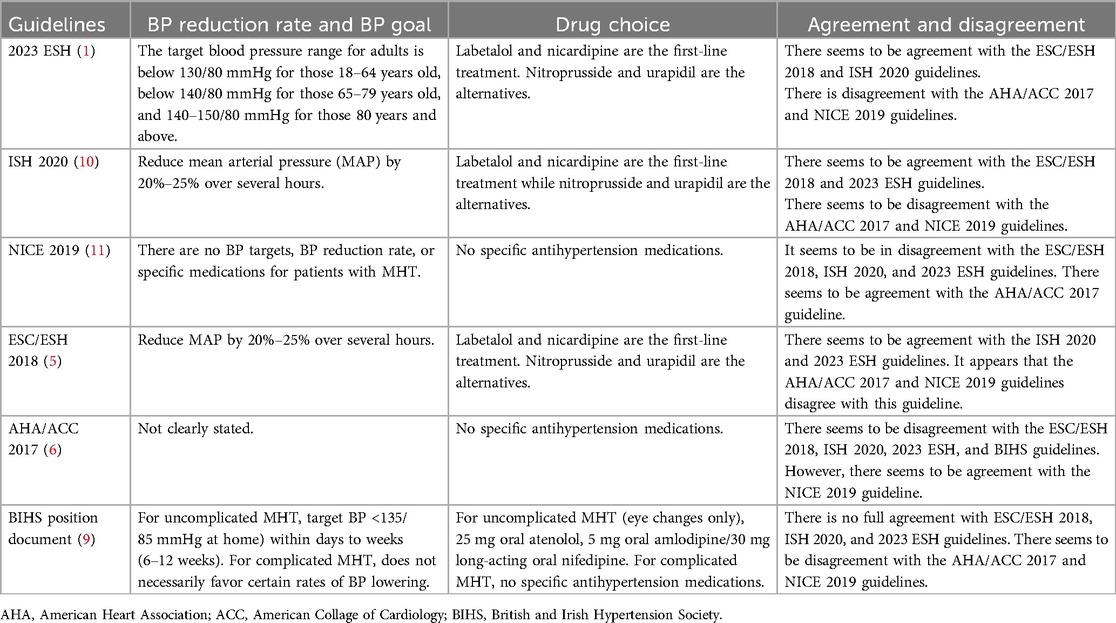
Table 3. Agreement and disagreement in latest hypertension guidelines regarding BP reduction rate and BP target in malignant hypertension.
According to the British and Irish Hypertension Society regarding the speed of BP reduction and BP targets in uncomplicated MHT (eye changes alone), BP is to be lowered within days, aiming for a gradual reduction to reach the target BP within weeks. For example, within 24 h, BP should be lowered to <200/120 mmHg, within a week to <160/100 mmHg, and then to <140/90 mmHg within 6–12 weeks (9) (Table 3).
The latest hypertension guidelines provide a BP goal and the rate of BP reduction during treatment. Large and sudden reductions in BP must be avoided in patients with MHT as this can exacerbate ischemic lesions. The gradual increase in BP control allowed gradual healing of necrotizing vascular lesions (8) (Table 3).
Since MHT constitutes a hypertensive emergency, the ESC position document on the management of hypertensive emergencies advises using intravenous BP-lowering medicines in patients with acute presentations. Because there are no randomized controlled trials on various treatment approaches, the position paper does recognize that any recommendations are based on clinical experience and consensus (8, 42) (Table 3).
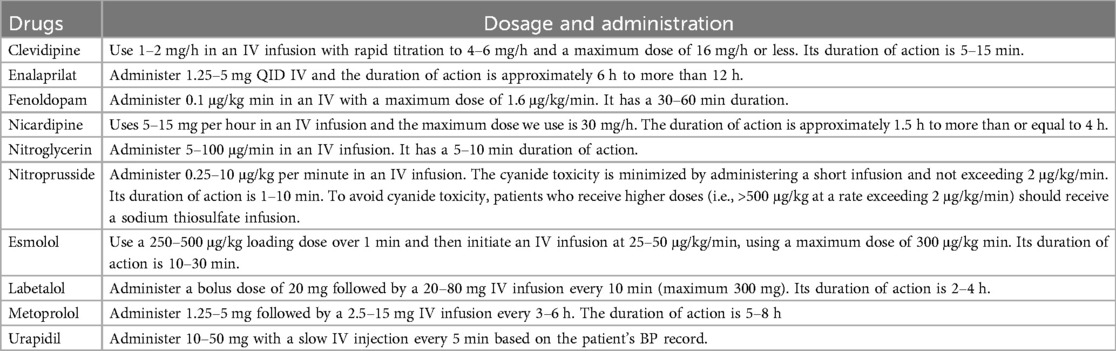
Parenteral antihypertensive drugs are used for the treatment of MHT (46, 48–51) (Table 4).
Once the target BP range is achieved in patients with MHT, oral antihypertensive treatment is progressively introduced at the clinician's discretion (46, 50) (Table 4).
Based on the reviewed works in the literature above and using the latest hypertension guidelines, the current treatment protocol for MHT is depicted in Figure 2 (5, 8, 46, 52, 53). A retrospective cohort study conducted by Endo et al. reported that in individuals experiencing hypertensive emergencies, early administration of renin-angiotensin system (RAS) inhibitors aids in the recovery of renal function following an abrupt decrease in estimated glomerular filtration rate (eGFR). In addition, compared to calcium channel blockers (CCB), renin-angiotensin system inhibitor (RASi) has a stronger positive impact on 2-year renal survival (54).
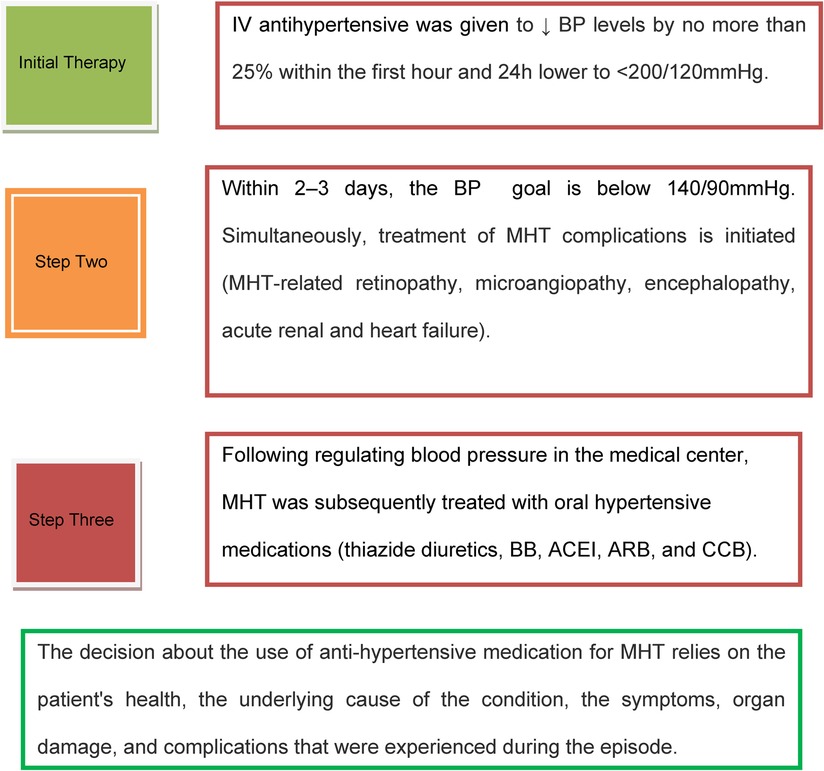
Figure 2. Malignant hypertension treatment protocol. IV, intravenous; MHT, malignant hypertension; BP, blood pressure; BB, beta-blocker; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker.
Non-pharmacological prevention modalities can also prevent MHT. Dr. Walter Kempner began using “The Rice Diet,” named thus because the patients consumed a bowl of white rice with every meal, to treat patients with kidney disease and MHT in 1934 while he was a physician at Duke Hospital. At the time, there was no other treatment for these conditions. Dr. Kempner realized that the best way to avoid and treat these disorders would be to follow a diet low in added salt and fat (55).
Therefore, it is advisable that patients with MHT frequently check their blood pressure, take their medicine as directed, eat a nutritious diet low in saturated fat and salt, stop smoking if they already do, and maintain a healthy weight (16).
Clinically, a significant risk factor for MHT is the self-discontinuation of antihypertensive medication by patients who have previously experienced MHT. Thus, long-term continuous follow-up is very important for prevention of MHT.
9 Conclusion and future perspectives
Malignant hypertension requires prompt and efficient treatment because it is the most severe kind of hypertension that affects target organs. There are a number of alternatives available for treating MHT, with the International Society of Hypertension (ISH) 2020 and ESC/ESH 2018 recommendations suggesting the use of labetalol and nicardipine as the first-line choice, with urapidil and nitroprusside serving as alternative medications.
An elevated risk of MHT has been linked to many socio-demographic factors and genetic factors. The international scientific community and governmental and non-governmental organizations, particularly those involved in hypertension research, should examine the new international MHT studies and conduct additional MHT research.
Author contributions
AT: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 ESH guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European Society of Hypertension. J Hypertens. (2023) 41(12):1874–2071. doi: 10.1097/HJH.0000000000003480
2. Cremer A, Amraoui F, Lip GY, Morales E, Rubin S, Segura J. From malignant hypertension to hypertension-MOD: a modern definition for an old but still dangerous emergency. J Hum Hypertens. (2016) 30(8):463–6. doi: 10.1038/jhh.2015.112
3. Boulestreau R, van den Born BJH, Lip GY, Gupta A. Malignant hypertension: current perspectives and challenges. J Am Heart Assoc. (2022) 11(7):e023397. doi: 10.1161/JAHA.121.023397
4. European Society of Cardiology. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur Heart J. (2024). https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/Elevated-Blood-Pressure-and-Hypertension (cited November 16, 2024)
5. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39(33):3021–104. doi: 10.1093/eurheartj/ehy339
6. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. (2018) 71(6):1269–324. doi: 10.1161/HYP.0000000000000066
7. Rubin S, Cremer A, Boulestreau R, Rigothier C, Kuntz S, Gosse P. Malignant hypertension: diagnosis, treatment and prognosis with experience from the Bordeaux cohort. J Hypertens. (2019) 37(2):316–24. doi: 10.1097/HJH.0000000000001913
8. van den Born BH, Lip GYH, Brguljan-Hitij J, Cremer A, Segura J, Morales E, et al. ESC council on hypertension position document on the management of hypertensive emergencies. Eur Heart J Cardiovasc Pharmacother. (2019) 5(1):37–46. doi: 10.1093/ehjcvp/pvy032
9. Kulkarni S, Glover M, Kapil V, Abrams SML, Partridge S, McCormack T, et al. Management of hypertensive crisis: British and Irish hypertension society position document. J Hum Hypertens. (2022) 37(10):863–79. doi: 10.1038/s41371-022-00776-9
10. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. (2020) 75(6):1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
11. The National Institute for Health and Care Excellence. Hypertension in Adults: Diagnosis and Management. NICE guidelines (2019). Available online at: https://www.nice.org.uk/guidance/ng136/resources/hypertension-in-adults-diagnosis-and-management-pdf-66141722710213 (cited May 23, 2023)
12. Bress AP, Anderson TS, Flack JM, Ghazi L, Hall ME, Laffer CL, et al. The management of elevated blood pressure in the acute care setting: a scientific statement from the American Heart Association. Hypertension. (2024) 81(8):e94–e106. doi: 10.1161/HYP.0000000000000238
13. McEvoy JW, McCarthy CP, Bruno RM, Brouwers S, Canavan MD, Ceconi C, et al. 2024 ESC guidelines for the management of elevated blood pressure and hypertension. Eur Heart J. (2024) 45(38):3912–4018. doi: 10.1093/eurheartj/ehae178
14. Shantsila A, Lip GY. Malignant hypertension revisited—does this still exist? Am J Hypertens. (2017) 30(6):543–9. doi: 10.1093/ajh/hpx008
15. Domek M, Gumprecht J, Lip GYH, Shantsila A. Malignant hypertension: does this still exist? J Hum Hypertens. (2020) 34(1):1–4. doi: 10.1038/s41371-019-0267-y
16. Prestige ER. Malignant Hypertension—Causes, Symptoms, Complications. Texas: Prestige ER (2021). Available online at: https://prestigeer.org/2021/03/19/malignant-hypertension-causes-symptoms-complications/ (cited March 31, 2023)
17. Cleveland Clinic. Malignant Hypertension. Cleveland Clinic (2023). Available online at: https://my.clevelandclinic.org/health/diseases/22285-malignant-hypertension (cited March 31, 2023)
18. Agrawal A, Wenger NK. Hypertension during pregnancy. Curr Hypertens Rep. (2020) 22(9):64. doi: 10.1007/s11906-020-01070-0
19. Shantsila A, Shantsila E, Beevers DG, Lip GYH. Predictors of 5-year outcomes in malignant phase hypertension. J Hypertens. (2017) 35(11):2310–4. doi: 10.1097/HJH.0000000000001446
20. Shantsila A, Dwivedi G, Shantsila E, Butt M, Beevers DG, Lip GY. Persistent macrovascular and microvascular dysfunction in patients with malignant hypertension. Hypertension. (2011) 57(3):490–6. doi: 10.1161/HYPERTENSIONAHA.110.166314
21. Kadiri S, Olutade BO, Osobamiro O. Factors influencing the development of malignant hypertension in Nigeria. J Hum Hypertens. (2000) 14(3):171–4. doi: 10.1038/sj.jhh.1000963
22. van den Born BJ, Koopmans RP, Groeneveld JO, van Montfrans GA. Ethnic disparities in the incidence, presentation, and complications of malignant hypertension. J Hypertens. (2006) 24(11):2299–304. doi: 10.1097/01.hjh.0000249710.21146.38
23. Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. (2005) 165(18):2098–104. doi: 10.1001/archinte.165.18.2098
24. Balfour PC, Rodriguez CJ, Ferdinand KC. The role of hypertension in race-ethnic disparities in cardiovascular disease. Curr Cardiovasc Risk Rep. (2015) 9(4):18. doi: 10.1007/s12170-015-0446-5
25. Saeed A, Dixon D, Yang E. Racial disparities in hypertension prevalence and management: a crisis control? Am Coll Cardiol. (2020) 2021. doi: 10.1001/archinte.165.18.2098
26. Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function. Circulation. (2004) 109(21):2511–7. doi: 10.1161/01.CIR.0000129087.81352.7A
27. Stevens J, Truesdale KP, Katz EG, Cai J. Impact of body mass index on incident hypertension and diabetes in Chinese Asians, American whites, and American blacks: the people’s republic of China study and the atherosclerosis risk in communities study. Am J Epidemiol. (2008) 167(11):1365–74. doi: 10.1093/aje/kwn060
28. Nesbitt S, Victor RG. Pathogenesis of hypertension in African Americans. Congest Heart Fail. (2004) 10(1):24–9. doi: 10.1111/j.1527-5299.2004.02021.x
29. Pickering GW. The pathogenesis of malignant hypertension. Circulation. (1952) 6(4):599–612. doi: 10.1161/01.CIR.6.4.599
30. Akimoto T, Muto S, Ito C, Takahashi H, Takeda S, Ando Y, et al. Clinical features of malignant hypertension with thrombotic microangiopathy. Clin Exp Hypertens. (2011) 33(2):77–83. doi: 10.3109/10641963.2010.503303
31. González R, Morales E, Segura J, Ruilope LM, Praga M. Long-term renal survival in malignant hypertension. Nephrol Dial Transplant. (2010) 25(10):3266–72. doi: 10.1093/ndt/gfq143
32. Amraoui F, Bos S, Vogt L, van den Born BJ. Long-term renal outcome in patients with malignant hypertension: a retrospective cohort study. BMC Nephrol. (2012) 13:71. doi: 10.1186/1471-2369-13-71
33. van den Born BJ, Koopmans RP, van Montfrans GA. The renin-angiotensin system in malignant hypertension revisited: plasma renin activity, microangiopathic hemolysis, and renal failure in malignant hypertension. Am J Hypertens. (2007) 20(8):900–6. doi: 10.1016/j.amjhyper.2007.02.018
34. Tajunisah I, Patel DK. Malignant hypertension with papilledema. J Emerg Med. (2013) 44(1):164–5. doi: 10.1016/j.jemermed.2011.05.042
35. Krieglsteiner S, Nussbaumer K, Haring H, Aichner F. Hypertensive emergencies. Lancet. (2000) 356(9239):1443. doi: 10.1016/S0140-6736(05)74085-X
36. Acelajado MC, Calhoun DA. Resistant hypertension, secondary hypertension, and hypertensive crises: diagnostic evaluation and treatment. Cardiol Clin. (2010) 28(4):639–54. doi: 10.1016/j.ccl.2010.07.002
37. Gassanov N, Pollok M, Er F. Acute renal failure associated with malignant hypertension. Dtsch Med Wochenschr. (2009) 134(44):2224–7. doi: 10.1055/s-0029-1241930
38. Amraoui F, Van Der Hoeven NV, Van Valkengoed IG, Vogt L, Van Den Born BJ. Mortality and cardiovascular risk in patients with a history of malignant hypertension: a case-control study. J Clin Hypertens. (2014) 16(2):122–6. doi: 10.1111/jch.12243
39. van der Merwe W, van der Merwe V. Malignant hypertension: a preventable emergency. N Z Med J. (2013) 126(1380):39–45.24126748
40. Boulestreau R, Śpiewak M, Januszewicz A, Kreutz R, Guzik TJ, Januszewicz M, et al. Malignant hypertension: a systemic cardiovascular disease. J Am Coll Cardiol. (2024) 83(17):1688–701. doi: 10.1016/j.jacc.2024.02.037
41. Polgreen LA, Suneja M, Tang F, Carter BL, Polgreen PM. Increasing trend in admissions for malignant hypertension and hypertensive encephalopathy in the United States. Hypertension. (2015) 65(5):1002–7. doi: 10.1161/HYPERTENSIONAHA.115.05241
42. Shantsila A, Lip GYH. Malignant hypertension: not quite an obsolete diagnosis yet. J Hypertens. (2019) 37(2):282–3. doi: 10.1097/HJH.0000000000001974
43. Saito T, Hasebe N. Malignant hypertension and multiorgan damage: mechanisms to be elucidated and countermeasures. Hypertens Res. (2021) 44(1):122–3. doi: 10.1038/s41440-020-00555-4
44. Mishima E, Funayama Y, Suzuki T, Mishima F, Nitta F, Toyohara T, et al. Concurrent analogous organ damage in the brain, eyes, and kidneys in malignant hypertension: reversible encephalopathy, serous retinal detachment, and proteinuria. Hypertens Res. (2021) 44(1):88–97. doi: 10.1038/s41440-020-0521-2
45. Gosse P, Coulon P, Papaioannou G, Litalien J, Lemetayer P. Impact of malignant arterial hypertension on the heart. J Hypertens. (2011) 29(4):798–802. doi: 10.1097/HJH.0b013e3283430b12
46. Lewek J, Bielecka-Dąbrowa A, Maciejewski M, Banach M. Pharmacological management of malignant hypertension. Expert Opin Pharmacother. (2020) 21(10):1189–92. doi: 10.1080/14656566.2020.1732923
47. Kaplan NM, Victor RG, Flynn JT. Kaplan’s Clinical Hypertension. 11th ed. Philadelphia: Wolters Kluwer (2015).
48. Varon J. Treatment of Acute Severe Hypertension. Drugs. (2008) 68(3):283–97. doi: 10.2165/00003495-200868030-00003
49. Marik PE, Varon J. Hypertensive Crises. Chest. (2007) 131(6):1949–62. doi: 10.1378/chest.06-2490
50. Gosse P, Boulestreau R, Brockers C, Puel C, Rubin S, Cremer A. The pharmacological management of malignant hypertension. J Hypertens. (2020) 38(11):2325–30. doi: 10.1097/HJH.0000000000002547
51. Prodan G, Leucuța D-C, Marc G, Simu H, Nastasă C, Oniga O. The Impact of urapidil use in hypertension prehospital emergency intervention. Farmacia J. (2017) 65(6):896–99.
52. Aronow WS. Treatment of hypertensive emergencies. Ann Transl Med. (2017) 5(Suppl 1):S5. doi: 10.21037/atm.2017.03.34
53. Chakraborty DS, Lahiry S, Choudhury S. Hypertension clinical practice guidelines (ISH, 2020): what is new? Med Princ Pract. (2022) 30(6):579–84. doi: 10.1159/000518812
54. Endo K, Hayashi K, Hara Y, Miyake A, Takano K, Horikawa T, et al. Impact of early initiation of renin-angiotensin blockade on renal function and clinical outcomes in patients with hypertensive emergency: a retrospective cohort study. BMC Nephrol. (2023) 24(1):68. doi: 10.1186/s12882-023-03117-1
Keywords: malignant hypertension, risk factors, complication, treatment, prevention strategies
Citation: Tsige AW and Ayele SG (2024) Malignant hypertension: current challenges, prevention strategies, and future perspectives. Front. Cardiovasc. Med. 11:1409212. doi: 10.3389/fcvm.2024.1409212
Received: 29 March 2024; Accepted: 4 December 2024;
Published: 24 December 2024.
Edited by:
Komal Marwaha, Texas Tech University Health Sciences Center, United StatesCopyright: © 2024 Tsige and Ayele. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abate Wondesen Tsige, QWJhdGV3b25kZXNlbkBkYnUuZWR1LmV0
 Abate Wondesen Tsige
Abate Wondesen Tsige Siraye Genzeb Ayele
Siraye Genzeb Ayele