
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 26 June 2024
Sec. Pediatric Cardiology
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1408543
Aim: Periprocedural and postinterventional care of patients undergoing closure of patent foramen ovale (PFO) varies significantly across care providers. Same-day discharge (SDD) after transcatheter interventions is an evolving concept. This study aimed to assess the same-day discharge rate and incidence of complications in patients undergoing PFO closure with intracardiac echocardiography (ICE) using the Gore®Cardioform Septal Occluder (GSO) device. The secondary aim was to analyse the efficacy of femoral vein closure with Perclose ProGlide.
Methods: Patients who underwent PFO closure with the GSO device at a university hospital in Stockholm, Sweden, were retrospectively included between March 1, 2017, and June 30, 2020, all with cryptogenic stroke as the indication for the procedure. All patients underwent PFO closure with conscious sedation and local anaesthesia. The indication for all patients was a cryptogenic stroke. Periprocedural imaging was performed using ICE and fluoroscopy in all patients. Patient characteristics and periprocedural data were collected from patient charts. Patients were kept on bed rest for 4–6 h post-intervention. Transthoracic echocardiography and clinical examination, including groin status, were performed before discharge. No clinical routine follow-up was performed the day following the intervention. Clinical follow-up was done by phone call two weeks after the procedure, and echocardiographic follow-up was done after 12 months. Data were analysed using linear and logistic regression models.
Results: In total, 262 patients were included, of which 246 (94%) had SDD. 166 patients (63%) received the ProGlide™ system for femoral vein access closure. Post-procedural arrhythmias occurred in 17 (6%) patients, and vascular complications in 9 patients (3%). The overall closure rate at follow-up was 98.5%. 25 out of 264 patients (9.5%) had to be readmitted within the first eight weeks after PFO closure, 16 due to atrial fibrillation warranting electric cardioversion, one due to an arteriovenous fistula that was operated, four due to chest pain/pain at the access site, and four patients developed fever. There was no difference in SDD among patients who received ProGlide™ vs. patients who did not receive ProGlide™.
Conclusion: SDD appears safe after transcatheter PFO closure with the GSO device with high procedural success rates. Low rates of complications and readmissions make the intervention suitable for this patient-friendly and cost-effective concept.
The first clinical report of a paradoxical embolisation across a persistent foramen ovale (PFO) leading to systemic embolisation through the fossa Sylvii was written by Cohnheim in 1877 (1), as well as its importance in other clinical settings such as Platypnea–orthodeoxia syndrome and decompression sickness (2–4).
PFO closure can be performed surgically using direct sutures or patch closures (5), often requiring extended hospital stays, or as a catheter-based intervention with dedicated PFO closure devices, which has become the primary treatment option during the last two decades (5). Antiplatelet therapy has previously been the recommended therapy for patients with cryptogenic stroke (6, 7). Several randomised controlled trials and meta-analyses have reported a lower risk for recurrent ischemic stroke after PFO closure compared to medical therapy (8–12), leading to an increased number of PFO closures in the last five years.
Developments within several areas of interventional cardiology have aided in making procedures less invasive, allowing for early discharge from the hospital. Vascular closure devices (VCD) provide reliable access site control and allow for rapid mobilisation after procedures. These improvements have allowed for an increasing possibility of same-day discharge (SDD) after catheter-based interventions (13–18). An extensive review and summary discussing the feasibility of SDD after numerous transcatheter procedures was published by Asbeutah et al. (18).
This study aimed to assess the same-day discharge feasibility and, safety, and incidence of complications in patients undergoing PFO closure with intracardiac echocardiography (ICE) using the Gore®Cardioform Septal Occluder (GSO) device. The secondary aim was to analyse the efficacy of femoral vein access closure with Perclose ProGlide™.
An ethical permit from the Swedish Ethical Review Authority has been approved with the registration number: (2022-02255-01).
This retrospective, single-centre, register-based cohort study included patients from the Swedish registry of congenital heart disease (SWEDCON) (19). The SWEDCON register is a National Quality Registry frequently used for research projects due to its high quality and good follow-up data. It consists of subregisters for adult patients with congenital heart defects, children with congenital heart defects, paediatric cardiothoracic surgery, foetal register and a register for cardiomyopathies. Patients who underwent transcatheter PFO closure between March 1st 2017, and June 30th 2020, at the Karolinska University Hospital, Stockholm, were included for analysis. Cryptogenic stroke was the indication for all PFO closures in this study.
In total, 262 patients (98 females, 164 males) were included, of which 261 received a GSO device. Mean age at intervention was 46.3 years (SD ± 10.4), mean BMI 25.5 (SD ± 4.2). Cardiovascular risk factors such as arterial hypertension were noted in 32 patients (12.2%), Diabetes mellitus in 3 patients (1.1%), hypercholesterolemia in 29 patients (11%) and smoking in 11 patients (4%), see Table 1.
All patients underwent TTE and TOE with administration of agitated saline as a diagnostic evaluation after cryptogenic stroke to verify the PFO diagnosis. Before the intervention, all patients were evaluated at a multidisciplinary team conference by an interventional cardiologist, neurologist, clinical physiologist and neuroradiologist, where the decision to proceed with percutaneous PFO closure was made.
All interventions were performed in a monoplane catheterisation laboratory using conscious sedation and local anaesthesia. Femoral venous access was obtained in all patients using a micropuncture technique with ultrasound guidance to minimise puncture-related complications. Two ipsilateral punctures were performed to allow access for the PFO-closure device and ICE catheter for intraprocedural imaging. An 11 Fr introducer was inserted into the femoral vein for device delivery. An ICE catheter (Abbot View Flex, Abbott Vascular, CA, USA) was introduced through a 9 Fr introducer and placed in the right atrium. Right heart catheterisation and the PFO closure were performed according to IFU.
After the procedure, patients were observed in the cardiology ward or daycare facility for 4–6 h, including a bedside TTE, to confirm the position of the device in situ and the absence of pericardial effusion. Patients were kept on strict bed rest for the first hour, followed by mobilisation. The antiplatelet regimen used for most patients was lifelong acetylsalicylic acid (160 mg once daily) combined with clopidogrel (75 mg once daily) for six months. Depending on the patient's comorbidity and preexisting therapy, anticoagulation (e.g., apixaban or coumadin) was continued with life-long acetylsalicylic acid.
Access site closure was performed at the sole discretion of the treating interventionist, using preclosure with either the Perclose ProGlide™ system (Abbott Vascular, Santa Clara, California, USA) for each femoral venous puncture site or a figure-of-eight suture see Figure 1.
Baseline and periprocedural characteristics were retrospectively collected from patient charts. Bleeding or puncture-related complications were analysed following the Bleeding Academic Research Consortium Definition for Bleeding (BARC) criteria (20).
Categorical variables were analysed using logistic regression models and described as proportions (percentages). Continuous variables were analysed using linear regression models and expressed as mean values with standard deviations (SDs). In all analyses, P-values < 0.05 were considered statistically significant. All data obtained were registered in Microsoft Excel® (Microsoft, Redmond, Washington, USA). Analyses were performed using STATA software (version 16.1 Stata Corp., College Station, Texas, USA).
SDD was achieved in 246/262 patients (94%). In patients with VCD, SDD was achieved in 159/166 patients (96%) and in 87/96 patients (91%) with figure-of-eight suture (p = 0.1).
The PFO device used most often was the 25 mm device in 164 patients (62%), followed by the 30 mm device in 93 patients (35%) and the 20 mm device in 4 patients (3%).
Vascular complications were seen in 9 patients (3%), 4 of these in patients with VCD and 5 in Non-VCD patients (p = 0.24).
Procedure-related atrial arrhythmias occurred in 17 (6%) patients, atrial fibrillation in 16 patients, and atrial flutter in one patient.
Periprocedural characteristics are summarised in Table 2. Complications depending on the femoral access closure approach are shown in Table 3. A comparison of SDD rates between patients receiving a VCD and patients with no VCD is shown in Table 4.
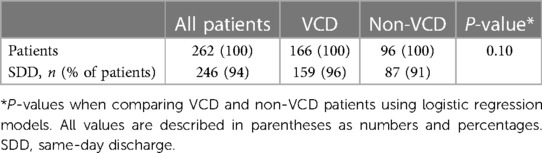
Table 4 Comparison of same-day discharge rates between patients receiving a VCD and patients with no VCD.
SDD was achieved in 246 (93.9%) patients. Among the patients with delayed discharge (i.e., no SDD), the following complications were registered: vascular complications (n = 3), late procedure starting time (n = 4), pain in the groin (n = 2), epigastric pain (n = 1), drainage of pericardial effusion (n = 2), post-procedural atrial fibrillation (n = 1), post-procedural desaturation (n = 1), post-procedural pulmonary complications (n = 1), and personal reasons (n = 1). The reasons for post-procedural readmissions and emergency room (ER) visits were atrial fibrillation (arrhythmia n = 16, 6.1%), fever (n = 4, 1.5%) or chest pain/pain at the access site (n = 4, 1.5%). One patient was operated on due to an AV fistula five weeks post-PFO closure.
The Perclose ProGlide™ system was used for access site closure in 166 patients, five (3%) of whom suffered vascular complications. Three had minor venous bleeding after application of the Perclose ProGlide™ and were treated with additional figure-of-eight sutures in the catheterisation laboratory. Technical failure at deployment of the ProGlide™ system occurred in one patient, and the access site was closed with a figure-of-eight suture instead. One patient was readmitted to the hospital due to vascular complications and underwent surgery due to an arteriovenous (AV) fistula five weeks post-PFO closure. No other patient had to be readmitted due to vascular complications. There were two type 3b (cardiac tamponade) bleeding complications and eight type 1 bleeding complications, according to BARC (20). There were no differences in complications between patients who received the ProGlide™ device and those who did not (Table 3).
The findings of this study support that SDD after PFO closure can be safely achieved in a majority of patients. Early, or SDD, is evolving for numerous transcatheter interventions such as aortic valve implantations, coronary intervention mitral valve procedures, and PFO closure (13–17). PFO closure is performed as right heart catheterisation from the venous side, with considerably smaller delivery catheters than transcatheter aortic valve implantations or mitral valve procedures, making the intervention suitable for early discharge.
Three previous studies have evaluated SDD after PFO closure using different PFO devices: Gore Helex Occluder and the Amplatzer Occluder (21); Amplatzer PFO occluder (22), Gore Cardioform Septal Occluder and Amplatzer PFO Occluder (23). The success rate of PFO closures in this study was higher than in previous studies. The higher use of GSO devices in this study could have contributed to the higher success rates, as the GSO's different design with Polytetrafluoroethylene optimises for complete closure.
All patients in this study underwent PFO closure with conscious sedation and general anaesthesia, which can facilitate early discharge. Postoperative nausea and vomiting (PONV) is a common adverse effect of general anaesthesia affecting up to 80% of patients (24) and may prolong hospital stay. The primary risk factor for PONV is the use of volatile anaesthetics. Performing the intervention with conscious sedation and local anaesthesia facilitates SDD by reducing the rate of PONV. An argued advantage of general anaesthesia with intubation is the possibility of performing TOE, but it can prolong procedural duration. Other options are TOE without general anaesthesia and/or with only a brief TOE exam at the end of the procedure (18).
At our institution, ICE and fluoroscopy are the standard protocols for imaging of the atrial septum, enabling procedures without TOE and general anaesthesia.
The perioperative imaging guided by ICE rather than TOE reduces the necessity for general anaesthesia and lessens the risk of associated complications (25, 26). Hence, the use of ICE during all the procedures in this study possibly contributed to the low rate of complications and high rate of SDD. This is supported by Blusztein et al., presenting that ICE and avoiding general anaesthesia were highly and significantly associated with SDD (23). They reported SDD in 382/554 patients (68.9%), with ICE used in 503/554 patients (90.7%), ICE and TOE were used in another three patients, and the remaining patients were treated using fluoroscopy or TOE, concluding that SDD was more common in patients with ICE.
In a retrospective study by Barker et al., SDD was achieved in 456/467 patients (97.6%) (22). Similar to our study, patients underwent PFO closure using local anaesthesia and conscious sedation. Periprocedural imaging in the study by Barker et al. was done using ICE and fluoroscopy in 86/467 patients (18.4%); the remaining 381 patients underwent closure with fluoroscopy alone.
High success rates of SDD rates were seen in the study by Barker et al. (22), in our study and in the subgroup of patients with local anaesthesia in the study by Bluzstein et al (23), suggesting that local anaesthesia is associated with SDD.
An extensive overview discussing the advantages and disadvantages of ICE for PFO closure has been published by Egidy Assenza (27). Another approach was described by Achim et al., using fluoroscopy as imaging during the procedure with a short TOE control before device release to assess device positioning and stability. This approach lead to significantly shorter procedural length in the fluoroscopy-guided group with no difference in procedural complications, including death, major bleeding, device dislodgement, stroke or clinically relevant peripheral embolisation between the two groups (p = .99) (28).
Applying a VCD such as the ProGlide™ Perclose system can further increase the possibility and safety of early discharge. Sekhar et al. reported the device to be safe and effective for femoral artery closure after heart catheterisation before early discharge (29). In our study, there was no difference overall in SDD rate between the patients who received the ProGlide™ device and those who did not receive the ProGlide™ device.
Late vascular problems were rarely seen in our study. One patient (0.38%) was diagnosed with an AV fistula one week after the procedure and underwent vascular surgery five weeks after the initial procedure.
Arrhythmia was the most common complication seen after the PFO closures in this study. The most encountered arrhythmias after PFO closure are known to be atrial tachycardias such as supraventricular tachycardia (SVT), atrial fibrillation (AFib) and atrial flutter (Af) (REF). Readmission rates are mainly driven by atrial fibrillation (AFib) requiring treatment, as demonstrated in this study and supported by Abrahamyan et al. (30). There is a low risk of clinically significant atrioventricular disturbances such as complete atrioventricular block (AVB) requiring pacemaker implantation (30). The risk of SVT requiring medical therapy or electrical cardioversion was reported to be around 1.5% in a large study by Szkutnik (31); late AVB requiring pacemaker was seen in 2/739 patients (0.27%) after 4.3 years respectively 1.5 years after Amplatzer device implantation. This reflects the results in our study, where acute onset AF delayed hospital discharge in one patient and caused ER visits in another sixteen patients.
This study is mainly limited by the retrospective and observational design, which is susceptible to confounding. There was no randomisation to either SDD or VCD, which complicates the interpretation of the result.
This retrospective study demonstrates the safety and feasibility of SDD in patients undergoing PFO closure for cryptogenic stroke with the GSO device. SDD after PFO closure can lead to healthcare savings and better utilisation of healthcare resources.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Etikprövningsmyndigheten Box 2110 750 02 Uppsala. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
KS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. GS: Methodology, Supervision, Writing – review & editing. AD: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Writing – review & editing. MS: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing. DV: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
KS has received research and travel grants for this study from Karolinska Institutet, the Swedish Heart-Lung Foundation, Stiftelsen Samariten Anna-Lisa and Arne Gustafssons Stiftelsen. MS is a proctor and advisory board member for WL Gore. WL Gore and Abbott have not seen or reviewed this manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACT, activated clotting time; AV, arteriovenous fistula; AFib, atrial fibrillation; Af, atrial flutter; ASD, atrial septal defect; BMI kg/m2, body mass index; cAVB, complete atrioventricular block; CT, computed tomography; DRG, diagnoses related groups; ED, early discharge; Fr, French; GSO, Gore Septal Occluder; ICE, intracardiac echocardiography; MRI, magnetic resonance imaging; PCI, percutaneous coronary intervention; PFO, persistent foramen ovale; PONV, post-operative nausea and vomiting; RCT, randomised controlled trial; SD, standard deviation; SDD, same-day discharge; SVT, supraventricular tachycardia; TAVI, transcatheter aortic valve implantation; TTE, transthoracic echocardiography; TOE, Transoesophageal echocardiography; VCD, vascular closure device; VSD, ventricular septal defect.
1. Cohnheim J. Thrombose und embolie in den vorlesungen uber allgemine pathologie; ein handbuch fur artzte und studierende. Berlin Hirschwald. (1877) 1:134.
2. Seward JB, Hayes DL, Smith HC, Williams DE, Rosenow EC 3rd, Reeder GS, et al. Platypnea-orthodeoxia: clinical profile, diagnostic workup, management, and report of seven cases. Mayo Clin Proc. (1984) 59(4):221–31. doi: 10.1016/S0025-6196(12)61253-1
3. Moon RE, Camporesi EM, Kisslo JA. Patent foramen ovale and decompression sickness in divers. Lancet. (1989) 1(8637):513–4. doi: 10.1016/S0140-6736(89)90064-0
4. Agrawal A, Palkar A, Talwar A. The multiple dimensions of platypnea-orthodeoxia syndrome: a review. Respir Med. (2017) 129:31–8. doi: 10.1016/j.rmed.2017.05.016
5. Giblett JP, Williams LK, Kyranis S, Shapiro LM, Calvert PA. Patent foramen ovale closure: state of the art. Interv Cardiol. (2020) 15:e15. doi: 10.15420/icr.2019.27
6. Sun YP, Homma S. Patent foramen ovale and stroke. Circ J. (2016) 80(8):1665–73. doi: 10.1253/circj.CJ-16-0534
7. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45(7):2160–236. doi: 10.1161/STR.0000000000000024
8. Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, et al. Patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. (2017) 377(11):1033–42. doi: 10.1056/NEJMoa1707404
9. Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. (2013) 368(12):1092–100. doi: 10.1056/NEJMoa1301440
10. Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, et al. Long-term outcomes of patent foramen ovale closure or medical therapy after stroke. N Engl J Med. (2017) 377(11):1022–32. doi: 10.1056/NEJMoa1610057
11. Lee PH, Song JK, Kim JS, Heo R, Lee S, Kim DH, et al. Cryptogenic stroke and high-risk patent foramen ovale: the DEFENSE-PFO trial. J Am Coll Cardiol. (2018) 71(20):2335–42. doi: 10.1016/j.jacc.2018.02.046
12. Ahmad Y, Howard JP, Arnold A, Shin MS, Cook C, Petraco R, et al. Patent foramen ovale closure vs. Medical therapy for cryptogenic stroke: a meta-analysis of randomized controlled trials. Eur Heart J. (2018) 39(18):1638–49. doi: 10.1093/eurheartj/ehy121
13. Barbanti M, van Mourik MS, Spence MS, Iacovelli F, Martinelli GL, Muir DF, et al. Optimising patient discharge management after transfemoral transcatheter aortic valve implantation: the multicentre European FAST-TAVI trial. EuroIntervention. (2019) 15(2):147–54. doi: 10.4244/EIJ-D-18-01197
14. Baekke PS, Jørgensen TH, Søndergaard L. Impact of early hospital discharge on clinical outcomes after transcatheter aortic valve implantation. Catheter Cardiovasc Interv. (2021) 98(2):E282–e90. doi: 10.1002/ccd.29403
15. Marmagkiolis K, Kilic ID, Ates I, Kose G, Iliescu C, Cilingiroglu M. Feasibility of same-day discharge approach after transcatheter mitral valve repair procedures. J Invasive Cardiol. (2021) 33(2):E123–E6.33443488
16. Serletis-Bizios A, Durand E, Cellier G, Tron C, Bauer F, Glinel B, et al. A prospective analysis of early discharge after transfemoral transcatheter aortic valve implantation. Am J Cardiol. (2016) 118(6):866–72. doi: 10.1016/j.amjcard.2016.06.035
17. Landes U, Kornowski R. Same day discharge: how much less is more for TAVR patients? Catheter Cardiovasc Interv. (2021) 97(5):948–9. doi: 10.1002/ccd.29658
18. Asbeutah AA, Junaid M, Hassan F, Avila Vega J, Efeovbokhan N, Khouzam RN, et al. Same day discharge after structural heart disease interventions in the era of the coronavirus-19 pandemic and beyond. World J Cardiol. (2022) 14(5):271–81. doi: 10.4330/wjc.v14.i5.271
19. Swedish registry of congenital heart disease. Available online at: https://www.ucr.uu.se/swedcon/ (Accessed June 18, 2024).
20. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. (2011) 123(23):2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449
21. Ponnuthurai FA, van Gaal WJ, Burchell A, Mitchell AR, Wilson N, Ormerod OJ. Safety and feasibility of day case patent foramen ovale (PFO) closure facilitated by intracardiac echocardiography. Int J Cardiol. (2009) 131(3):438–40. doi: 10.1016/j.ijcard.2007.07.141
22. Barker M, Muthuppalaniappan AM, Abrahamyan L, Osten MD, Benson LN, Bach Y, et al. Periprocedural outcomes of fluoroscopy-guided patent foramen ovale closure with selective use of intracardiac echocardiography. Can J Cardiol. (2020) 36(10):1608–15. doi: 10.1016/j.cjca.2019.12.032
23. Blusztein D, Sarwary S, Parikh DS, Garcia S, Price MJ, Nayak K, et al. Safety of same-day hospital discharge post patent foramen ovale closure: findings from a multicenter study. Am J Cardiol. (2023) 208:118–23. doi: 10.1016/j.amjcard.2023.09.045
24. Weibel S, Rücker G, Eberhart LH, Pace NL, Hartl HM, Jordan OL, et al. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis. Cochrane Database Syst Rev. (2020) 10(10):Cd012859. doi: 10.1002/14651858.CD012859.pub2
25. Bartel T, Müller S, Biviano A, Hahn RT. Why is intracardiac echocardiography helpful? Benefits, costs, and how to learn. Eur Heart J. (2014) 35(2):69–76. doi: 10.1093/eurheartj/eht411
26. Medford BA, Taggart NW, Cabalka AK, Cetta F, Reeder GS, Hagler DJ, et al. Intracardiac echocardiography during atrial septal defect and patent foramen ovale device closure in pediatric and adolescent patients. J Am Soc Echocardiogr. (2014) 27(9):984–90. doi: 10.1016/j.echo.2014.05.017
27. Egidy Assenza G, Spinardi L, Mariucci E, Balducci A, Ragni L, Ciuca C, et al. Transcatheter closure of PFO and ASD: multimodality imaging for patient selection and perioperative guidance. J Cardiovasc Dev Dis. (2021) 8(7):78. doi: 10.3390/jcdd8070078
28. Achim A, Hochegger P, Kanoun Schnur SS, Moser L, Stark C, Pranevičius R, et al. Transesophageal echocardiography-guided versus fluoroscopy-guided patent foramen ovale closure: a single center registry. Echocardiography. (2023) 40(7):657–63. doi: 10.1111/echo.15630
29. Sekhar A, Sutton BS, Raheja P, Mohsen A, Anggelis E, Anggelis CN, et al. Femoral arterial closure using ProGlide® is more efficacious and cost-effective when ambulating early following cardiac catheterization. Int J Cardiol Heart Vasc. (2016) 13:6–13. doi: 10.1016/j.ijcha.2016.09.002
30. Abrahamyan L, Barker M, Dharma C, Lee DS, Austin PC, Asghar A, et al. Real world long-term outcomes among adults undergoing transcatheter patent foramen closure with amplatzer PFO occluder. Int J Cardiol. (2023) 371:109–15. doi: 10.1016/j.ijcard.2022.09.033
Keywords: PFO closure, same-day discharge, intracardiac echocardiography, stroke prevention, Gore Septal Occluder (GSO), vascular closure device (VCD)
Citation: Steiner K, Sjöberg G, Damlin A, Settergren M and Verouhis D (2024) Same-day discharge after percutaneous closure of persistent foramen ovale using intracardiac echocardiography and the Gore Septal Occluder. Front. Cardiovasc. Med. 11:1408543. doi: 10.3389/fcvm.2024.1408543
Received: 28 March 2024; Accepted: 11 June 2024;
Published: 26 June 2024.
Edited by:
Petru Liuba, Lund University, SwedenReviewed by:
Stanimir Georgiev, Technical University Munich, Germany© 2024 Steiner, Sjöberg, Damlin, Settergren and Verouhis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristoffer Steiner, a3Jpc3RvZmZlci5zdGVpbmVyQGtpLnNl
†ORCID:
Kristoffer Steiner
orcid.org/0000-0002-8799-652X
Gunnar Sjöberg
orcid.org/0000-0002-7077-1404
Anna Damlin
orcid.org/0000-0002-8124-5864
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.