- Department of Cardiovascular Medicine, The Affiliated Qingyuan Hospital (Qingyuan People’s Hospital), Guangzhou Medical University, Qingyuan, Guangdong, China
Aim: Heart failure (HF) is a severe manifestation or late stage of various heart diseases. As an anti-inflammatory nutrient, dietary fiber has been shown to be associated with the progression and prognosis of cardiovascular diseases (CVDs). However, little is known about the relationship between dietary fiber intake and mortality in HF survivors. This study evaluated the association between dietary fiber intake and all-cause and CVD-caused mortality among HF survivors.
Methods: Data for the study were extracted from the National Health and Nutrition Examination Survey 1999–2018. Dietary fiber intake information was obtained by a 24-h dietary recall interview. Death outcomes were ascertained by linkage to National Death Index records through 31 December 2019. Covariates, including sociodemographic, lifestyle, disease history, and laboratory data, were extracted from the database. The weighted univariate and multivariate Cox proportional hazard models were utilized to explore the association between dietary fiber intake and mortality among HF survivors, with hazard ratios and 95% confidence intervals. Further stratified analyses were performed to explore this association based on age, gender, a history of diabetes and dyslipidemia, and duration of HF.
Results: A total of 1,510 patients were included. Up to 31 December 2019, 859 deaths had occurred over a mean follow-up of 70.00 months. After multivariable adjustment, a higher dietary fiber intake was associated with a lower risk of all-cause and CVD-caused mortality in HF survivors, especially in male patients, those aged <60 years old, and those with a history of diabetes and dyslipidemia.
Conclusion: Among HF survivors, higher dietary fiber intake levels may be associated with a good health outcome. More large-scale prospective cohort studies are needed to further explore this benefit relationship.
Introduction
Globally, the prevalence of heart failure (HF) has been increasing over the past few decades. HF is a multi-faceted syndrome characterized by the inability of the heart to pump enough blood and oxygen to support the metabolic demands of other organs and affects approximately 64 million patients worldwide (1). HF is associated with increased morbidity and mortality and places a substantial burden on the healthcare system (2). The total medical costs of HF patients are expected to rise from 20.9 million dollars in 2012 to 53.1 million dollars in 2030 in the USA (3). According to a previous study, the 10-year survival of HF was only 34.9% (4). The proactive prevention of HF is of great significance to improve patient outcomes and reduce the economic burden.
Heart failure is recognized as the final stage in the progression of cardiovascular disease (5). Studies using nutritional strategies based on dietary fiber intake have been proven to be effective for the prevention and treatment of CVDs (6). Several studies reported that a higher dietary fiber intake was related to lower all-cause and CVD mortality among non-CVD populations (7–9). Thus far, evidence on the association between dietary fiber intake and mortality among the CVD population has been limited. A prospective cohort study reported that in patients who survived a myocardial infarction (MI), a greater intake of dietary fiber after an MI was inversely associated with all-cause mortality (10). Another prospective cohort study suggested that dietary fiber derived from grains may be beneficial for slowing the progression of coronary atherosclerosis in postmenopausal women (11). However, the association between dietary fiber intake and all-cause and CVD-caused mortality among patients who have survived HF remains unclear.
In this work, we explore the association between dietary fiber intake and all-cause and CVD-caused mortality among patients who survived HF, using data from the National Health and Nutrition Examination Survey (NHANES) database. This study could lead to a better understanding of the role of dietary fiber in improving the prognosis of HF patients and potentially provides reliable preventive strategies to improve the health outcomes of HF patients.
Methods
Study design and participants
The data for this cohort study were extracted from the NHANES 1999–2018 database. NHANES recruited a representative sample of civilian, community-dwelling members using a complex, multistage design every 2 years, and the primary objective of the study was to assess the health and nutritional status of adults and children in the USA (12). The survey was conducted by the National Center for Health Statistics (NCHS), a part of the Centers for Disease Control and Prevention (CDC). NHANES is a publicly available dataset and was approved by the NCHS Ethics Board, and all participants provided written informed consent.
In this study, data from 1,906 participants aged ≥18 years and diagnosed with HF were extracted from the NHANES 1999–2018. HF was self-reported with “Yes” for the following question about HF in the questionnaire at a Mobile Examination Center (MEC): “Has a doctor ever told you that you had HF?” (13). Of the participants that were initially extracted, 308 were missing dietary fiber intake information, 87 had an extreme energy intake (male: <800 or >6,000 kcal/day; female: <600 or >4,000 kcal/day) (14), and 1 participant was missing survival data. These participants were excluded. Thus, finally, 1,510 eligible HF patients were included for further analyses.
Dietary fiber intake assessment
Total dietary fiber intake was obtained through a 24-h dietary recall interview by a trained interviewer at an NHANES MEC. All the participants were asked to recall the type and amount of food and beverages they consumed in the past 24 h. Dietary fiber intake was calculated according to the US Department of Agriculture (USDA) Food and Nutrient Database for Dietary Studies (15). In this study, total dietary fiber intake was divided into three categories: Q1 (8.8 g), Q2 (13.3 g), and Q3 (19.1 g).
Outcomes and follow-up
The outcomes of this study were all-cause mortality and CVD-caused mortality among HF patients. Mortality data were confirmed through the National Death Index, contact with next of kin, or via the postal system (16, 17). Information regarding the cause of death was collected by a review of medical records by an experienced physician. All-cause mortality among HF patients included mortalities caused by a malignant tumor, CVDs, respiratory disease, Alzheimer's disease, diabetes, nephropathy-related diseases, accidental death, and other causes (18). CVD was defined as the composite of incident non-fatal myocardial infarction, fatal coronary heart disease (CHD), and fatal and non-fatal strokes. A non-fatal myocardial infarction and stroke were confirmed using World Health Organization criteria (19) and the National Survey of Stroke criteria (20), respectively.
Potential covariates
The demographic, lifestyle, disease history, and laboratory data of the participants were extracted from the NHANES database. Smoking status was assessed by the question “Have you smoked at least 100 cigarettes in your life” (answered “yes” or “no”) (21). Participants who answered negatively to the question “Have you had at least 12 alcoholic drinks in 1 year?” were defined as never drinkers. Participants who answered the question “On average, how many times per week did you drink alcohol in the past 12 months?” with “less than once per week” were defined as occasional drinkers and “more than once per week” were defined as regular drinkers (22). Physical activity was expressed as the metabolic equivalent of task (MET) and calculated as follows: physical activity (MET min/week) = recommended MET × exercise time for corresponding activities (min/day) × the number of exercise days per week (days) (23). The history of angina and stroke was adapted from the health questionnaire. Hypertension was defined as systolic blood pressure (SBP) ≥130 mmHg, diastolic blood pressure (DBP) ≥80 mmHg, self-reported, or taking hypotensive drugs (24). Dyslipidemia was defined as total cholesterol (TC) ≥200 mg/dl, triglyceride (TG) ≥150 mg/dl, low-density lipoprotein cholesterol (LDL-C) ≥130 mg/dl, high-density lipoprotein cholesterol (HDL-C) ≤40 mg/dl, or self-reported (25). Diabetes was defined according to the following criteria: self-report of a diabetes diagnosis by a clinician or health professional, glycated hemoglobin (HbA1c) ≥6.5%, fasting glucose ≥126 mg/dl, or 2 h oral glucose tolerance test (OGTT) ≥200 mmol/L (26). Chronic kidney disease (CKD) was defined as estimated glomerular filtration rate <60 ml/min/1.73 m2 and eGFR was determined using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation (27). Patients with a urine albumin-to-creatinine ratio (UACR) ≥30 mg/g were also defined as having CKD (28). Depression was evaluated using the patient health questionnaire (PHQ-9) and a PHQ-9 score ≥10 was defined as being depressed (29). Body mass index (BMI) was classified into four levels: underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–19.9 kg/m2), and obese (≥30 kg/m2) (30).
Statistical analysis
All statistical analyses were conducted by SDMVPSU, SDMVSTRA, and WTDRD1. SDMVSTRA was the confidence interval (CI) that was applied to assess the reliability of an estimate. WTDRD1 refers to the weight for a dietary day of one 2-year sample. SDMVPSU means that the masked variance unit pseudo-substrate is sdmvstra, and the masked variance unit pseudo-primary unit is sdmvpsu.
Continuous data were expressed as mean and standard error (SE) and the weighted t-test was used for comparisons between two groups. Qualitative data were expressed as the number and proportion [n (%)], and χ2 was used for comparison between the two groups. Sensitivity analyses were performed to ascertain whether the results were different before and after imputation (Supplementary Table S1). The weighted Cox proportional-hazards regression models were used to explore the association between dietary fiber intake and all-cause mortality and CVD-caused mortality among HF patients, with hazard ratios (HRs) and 95% CIs. Model 1 was a crude model without adjusting for covariates. Model 2 was adjusted for age, race, education level, marital status, physical activity, stroke, hypertension, diabetes, CKD, energy, hemoglobin, uric acid, WBC, albumin, and drugs for CVDs. Subgroup analyses were further conducted to explore the association based on age, gender, and history of diabetes and dyslipidemia. Two-sided P < 0.05 was considered as statistically significant.
Results
Baseline characteristics
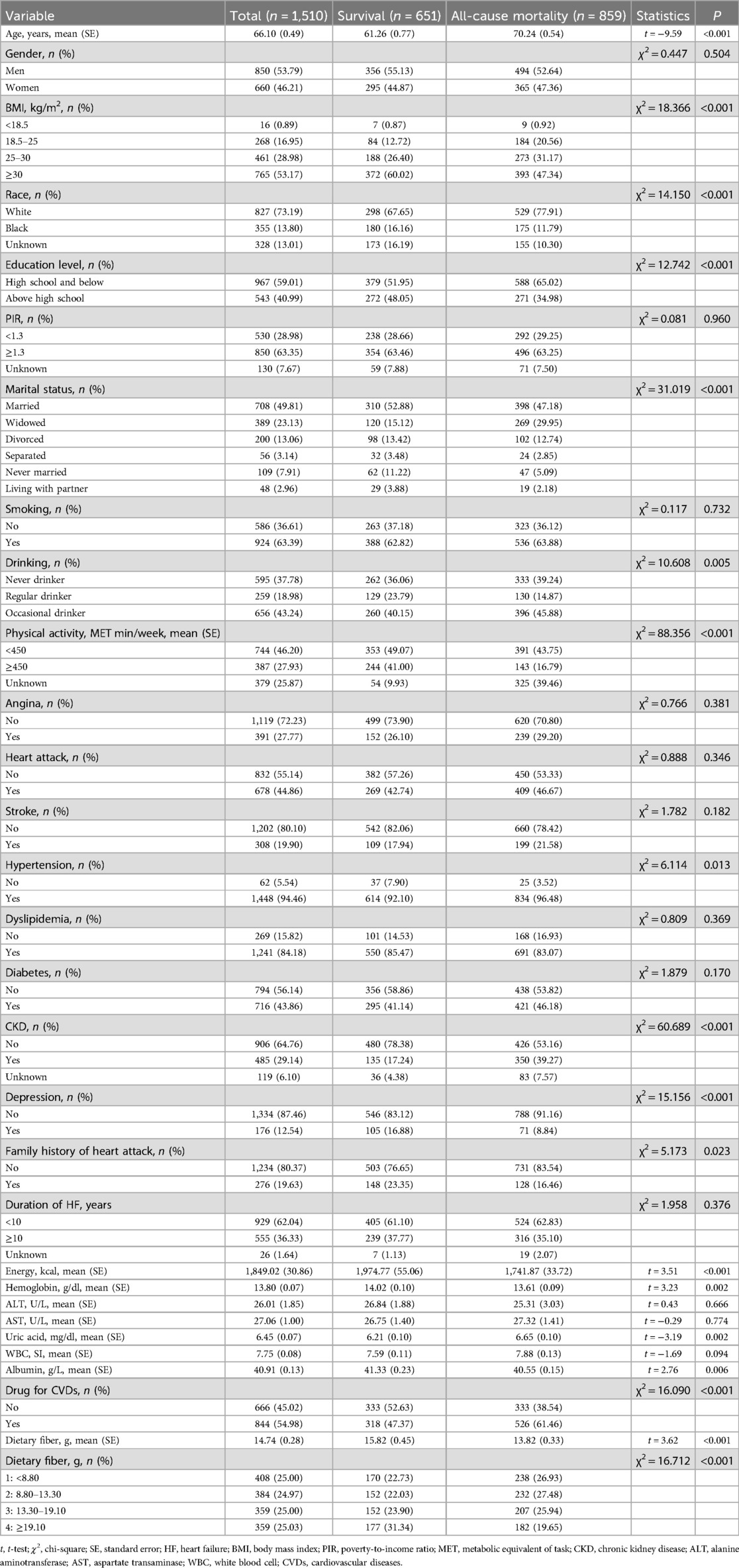
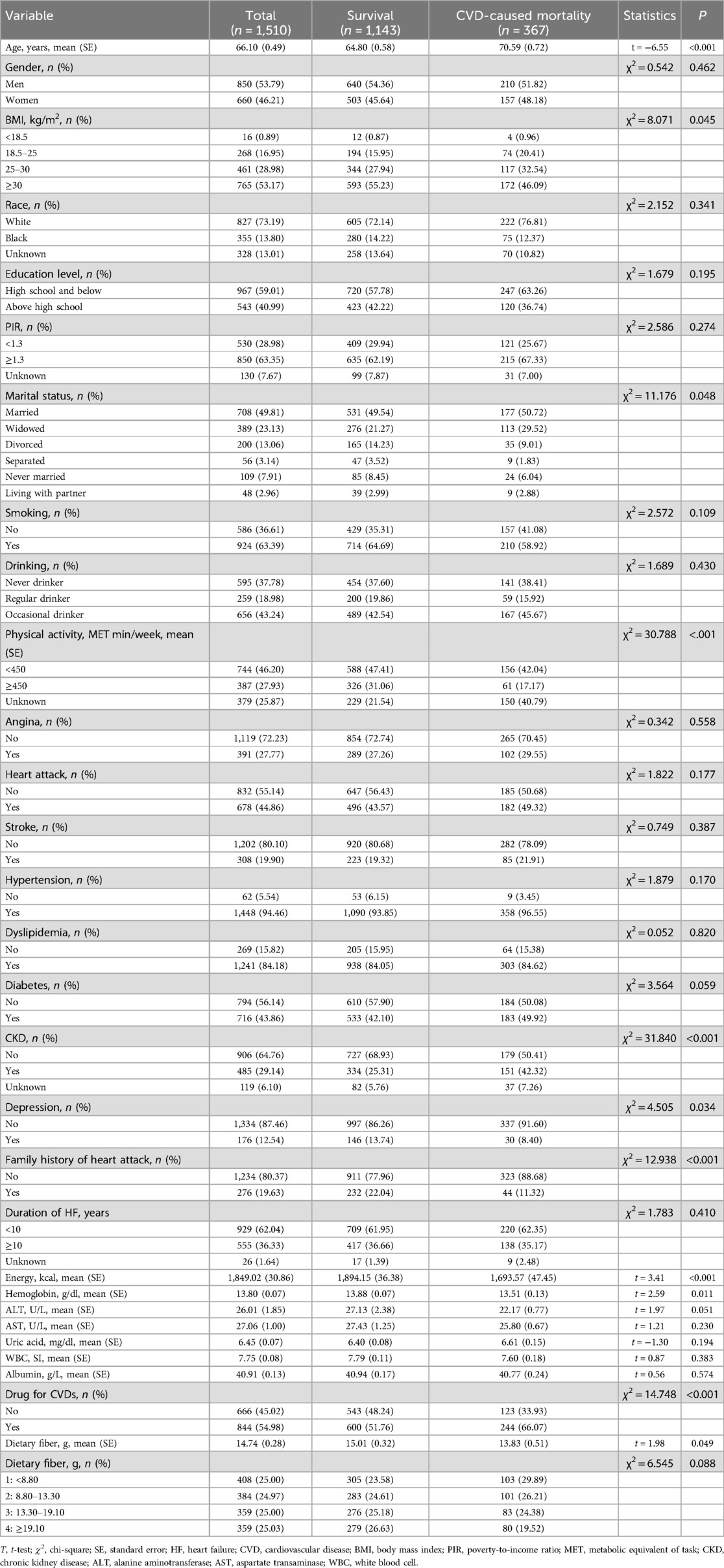
A flow chart of the population screening process is shown in Figure 1. In total, 1,510 HF patients were included with a mean weighted age of 66.10 (0.49) years old and 53.79% were men. The characteristics of the included participants are shown in Tables 1, 2. Notably, the proportion of those who had a high dietary fiber intake in the survival group was significantly higher than in the all-cause and CVD-caused mortality group. Differences were found in age, race, BMI, education, physical activity, energy, hemoglobin, uric acid, albumin, marital status, drinking, history of hypertension, CKD, depression, family heart attack, and CVD drug use between the survival and all-cause mortality groups. For the CVD-caused mortality and survival groups, differences were found in age, BMI, energy, hemoglobin, physical activity, marital status, history of CKD, depression, family heart attack, and CVD drug use (all P < 0.05).
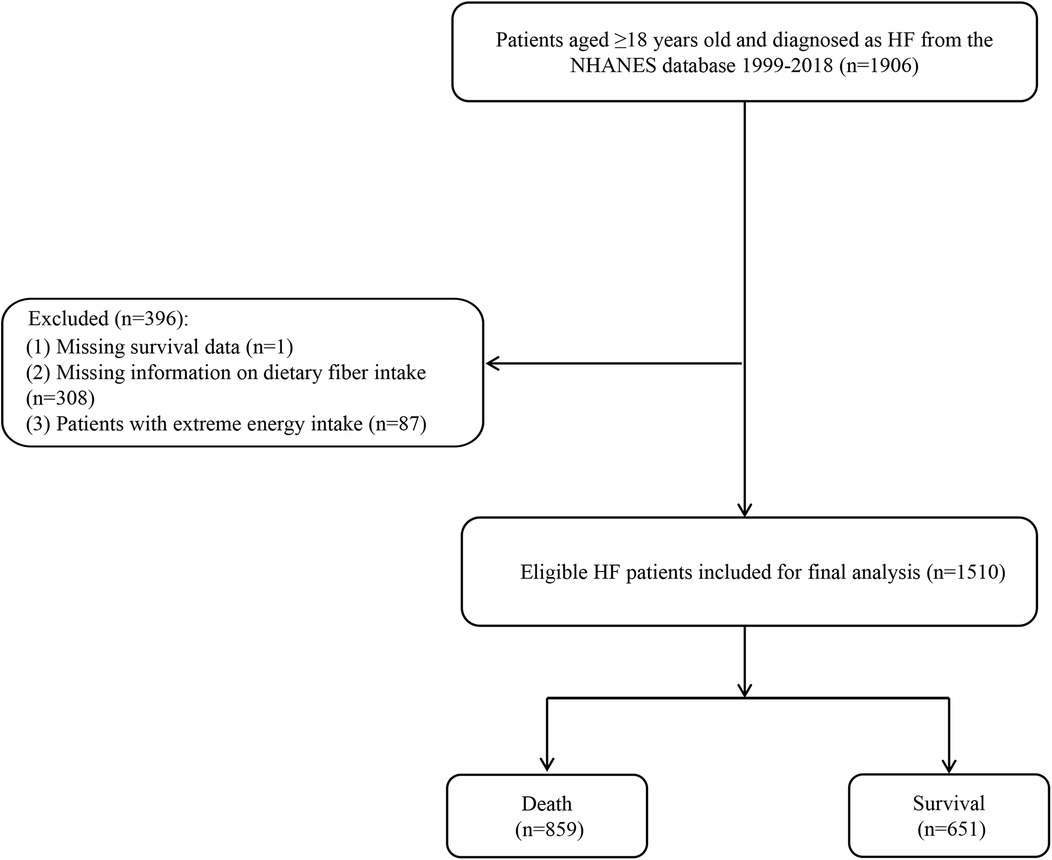
Figure 1. Flow chart of the screening process for the selection of participants in NHANES 1999–2018.
Relationship between dietary fiber intake and mortality among HF patients
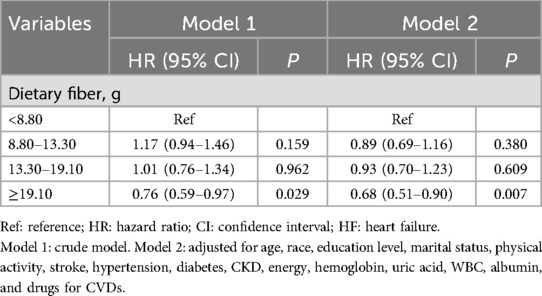
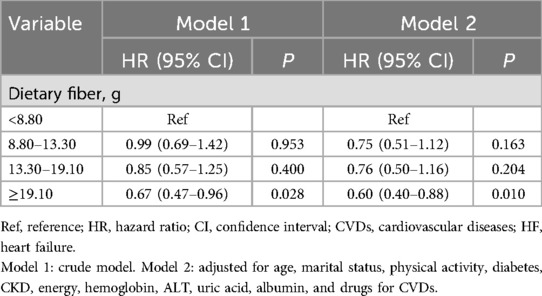
We employed two weighted logistic regression models to investigate the association between dietary fiber intake and all-cause and CVD-caused mortality, as presented in Tables 3, 4. After adjusting for all variables, compared to those in the lowest quartile of dietary fiber intake (<8.80 g), all-cause mortality among those in the highest quartile of dietary fiber intake (≥19.10 g) was reduced by 32% (95% CI: 0.51–0.90); CVD-caused mortality among those in the highest quartile of dietary fiber intake (≥19.10 gm) was reduced by 40% (95% CI: 0.40–0.88) (all P < 0.05).
Subgroup analyses
As depicted in Tables 5, 6, further stratified analyses were conducted to explore the association between dietary fiber intake and all-cause and CVD-caused mortality. Among HF patients who were men, aged <60 years old, and had a duration of HF ≥10 years, the relationship between the highest quartile of dietary fiber intake (≥19.10 g) and a lower risk of all-cause mortality and CVD-caused mortality was more pronounced. Among HF patients with diabetes, all-cause mortality and CVD-caused mortality among those in the highest quartile of dietary fiber intake (≥19.10 g) were reduced by 41% and 46%, respectively.
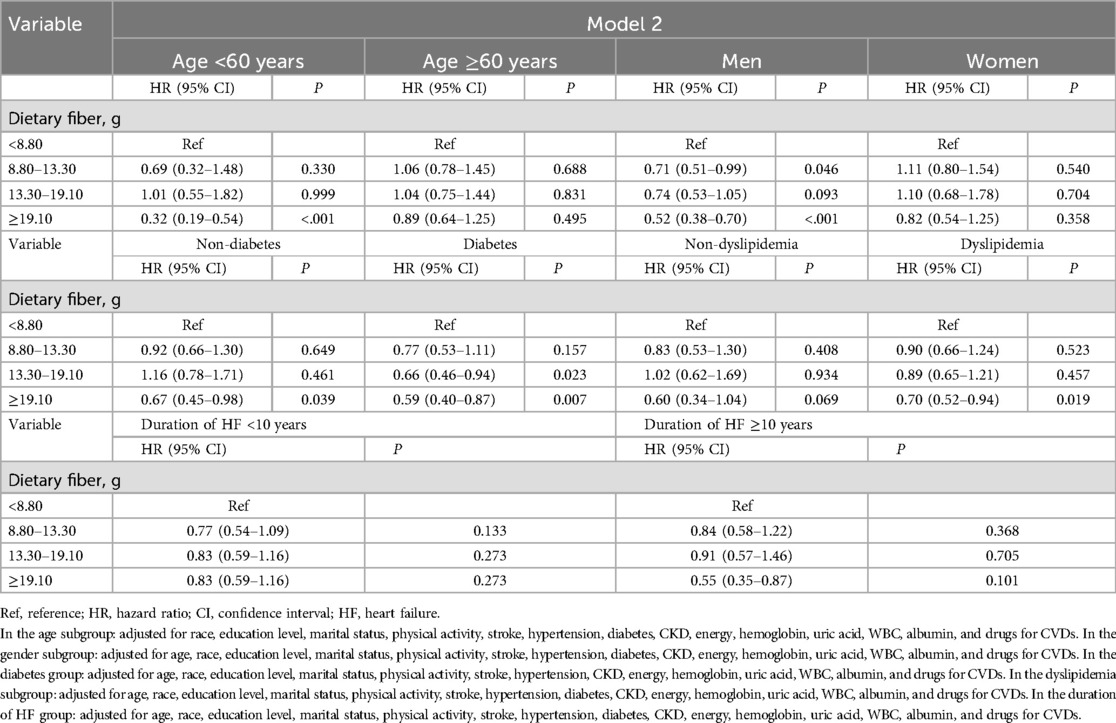
Table 5. Association between dietary fiber intake and all-cause mortality among HF survivors based on age, gender, and a history of diabetes and dyslipidemia.
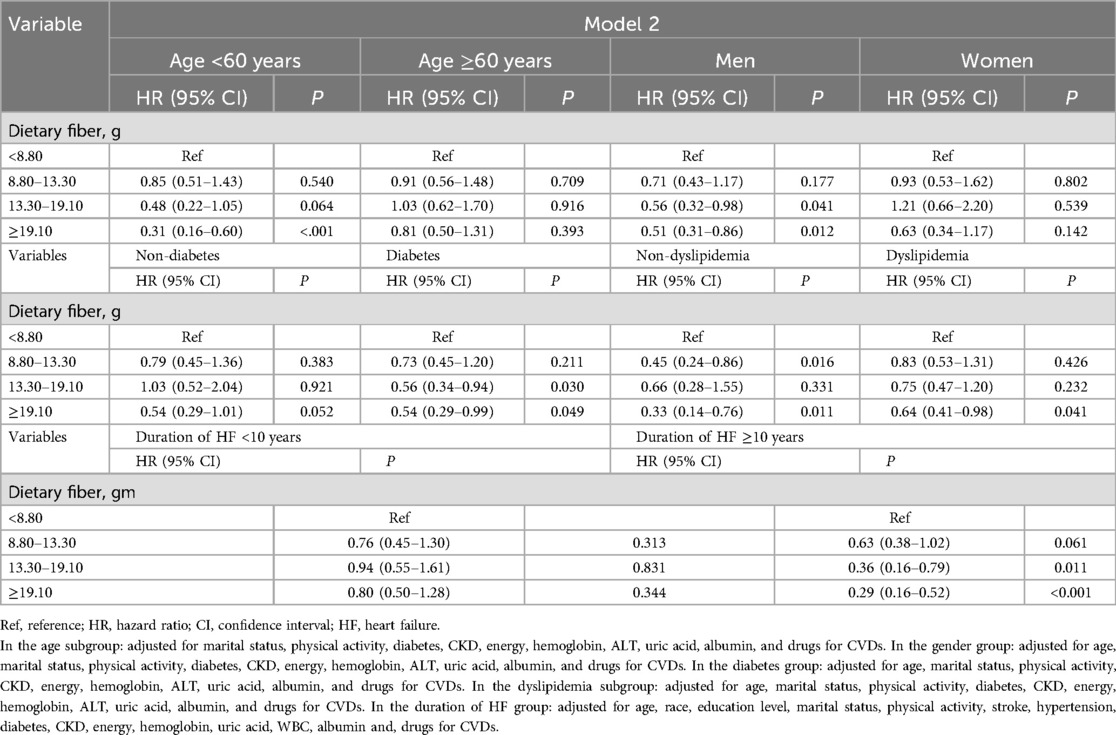
Table 6. Association between dietary fiber and CVD-caused mortality among HF survivors based on age, gender, and a history of diabetes and dyslipidemia.
Discussion
Among HF survivors in this retrospective cohort from the USA, we found that a high dietary fiber intake level was associated with a lower risk of all-cause and CVD-caused mortality. The stratified analyses based on age, gender, a history of diabetes and dyslipidemia, and duration of HF suggested the findings were robust. For the prognostic management of HF survivors, a high dietary fiber intake in their daily diet may yield greater benefits.
Currently, a small number of studies have focused on dietary intervention for the risk of death in patients with HF, but no consistent conclusions have been reached. Chang et al. (31) explored the association between adherence to the Mediterranean diet (aMED) with all-cause mortality in patients with HF and observed that a Mediterranean diet was not associated with mortality; however, among the aMED components, a lower red/processed meat intake was associated with higher mortality risk. Sun and Du (32) assessed the association of dietary magnesium intake and vitamin D levels with the risk of mortality in HF patients and suggested that vitamin D and magnesium levels may have a joint effect on improving the prognosis of HF patients. Dietary fiber contains a unique blend of bioactive components including resistant starches, vitamins, minerals, phytochemicals, and antioxidants (33). As a result, studies regarding their potential health benefits have received considerable attention in the last several decades. Epidemiological and clinical studies reported that the consumption of dietary fiber was inversely related to CVDs. The Zutphen study conducted by Streppel et al. suggested that a higher dietary fiber intake was associated with a lower risk of both CHD and all-cause mortality (34). Every additional 10 g of dietary fiber intake per day reduced coronary heart disease mortality by 7% and all-cause mortality by 9%. Another study from the Nurses’ Health Study documented 761 CHD cases, and, after adjusting for age and smoking, increased whole-grain intake was associated with a decreased risk of CHD (35). The consumption of whole-grain products is often associated with beneficial effects for consumer health. Dietary fiber is a vital component present in whole grains and is believed to be responsible for these health benefits (36). Two previous prospective cohort studies also examined whether the intake of whole-grain foods was related to CVD-caused morbidity and mortality (37, 38). Both studies reported consistent results that high levels of dietary fiber-rich food intake were inversely associated with cardiovascular-related morbidity and mortality.
Although the mechanism of the progression of HF has not been fully elucidated, disturbances in the metabolic and inflammatory pathways seem to play a vital role (39). The gut microbiota, including the bacteria that reside in the gastrointestinal tract, may influence these pathways (40). The interaction between the gut microbiome and the host determines key physiological processes in human metabolism, including inflammatory response, metabolic function, and disease susceptibility (41). The maintenance and improvement of the balance of intestinal flora has a positive regulatory effect on physiological processes in the body. The intestinal microbiome is largely regulated by diet, and a dietary fiber intervention can change the composition and abundance of intestinal microorganisms in the human body to a certain extent (42). Heart and intestinal failure are closely related. The current leaky gut hypothesis for HF holds that intestinal edema and barrier dysfunction caused by HF cause the translocation of intestinal flora components into the host’s circulatory system, resulting in endotoxemia and ultimately aggravating systemic inflammation (43).
To further evaluate the robustness of the results, we examined the association between dietary fiber and all-cause and CVD-caused mortality in a specific population of HF patients. After adjusting for all the covariates, we also found an inverse association between dietary fiber and all-cause and CVD-caused mortality among HF patients, especially among men and those younger than 60 years old. Among the HF survivors with a history of diabetes and dyslipidemia, a high dietary fiber intake was associated with a lower risk for all-cause and CVD-caused mortality. A previous study reported that there was a gender difference in HF (44). Except for those aged 80 years and older, the incidence of HF was lower in women than in men in all age groups. One possible reason for the higher incidence rate in men than in women is that men may have more independent risk factors for CVDs than women, such as smoking, alcohol abuse, binge eating, and hypertension (44). In addition, postmenopausal women have higher levels of estrogen, which has an anti-atherosclerotic effect and plays a protective role in blood vessels (45). Maintaining a high dietary fiber intake may be beneficial for men.
Diabetes is a systemic disease that can lead to systemic vascular lesions. Myocardial metabolism disorders caused by diabetes can reduce the pumping function of myocardial cells, which is an important cause of HF. Dietary fiber can increase the sensitivity of peripheral tissues to insulin and improve insulin resistance, thereby reducing blood glucose levels. In addition, dietary fiber can reduce the activities of intestinal digestive enzymes and promote the repair of islet function (46). When the human body maintains a state of dyslipidemia for a long time, cholesterol in the blood may be deposited on the arterial wall to form atherosclerotic plaques and eventually lead to a variety of CVDs (47). Dietary fiber plays an important role in maintaining healthy blood lipids by binding to the bile and preventing cholesterol from being absorbed (48). For HF patients with a history of diabetes and dyslipidemia, a higher dietary fiber intake is a beneficial way to maintain their cardiovascular health.
Using the data from the NHANES database, we explored the association between dietary fiber intake and mortality among HF survivors. It is essential for clinicians, policymakers, and patients with HF to be aware of the benefits of dietary fiber for cardiovascular health management. In addition, it would be beneficial to add fiber-enriched foods to the diet of HF patients such as fresh fruits and vegetables, cereals, nuts, and legumes. However, our study has several limitations. First, as we used a retrospective cohort, we could not include all variables that might affect HF development. Second, the study had a limited sample size; however, the sufficient follow-up duration ensures a sufficient sample size for mortality outcome events. Third, due to the unavailability of NHANES data, we were unable to distinguish between types of dietary fiber. Previous studies have shown that dietary fiber from different sources has different compositions, structures, and rational properties and that its regulatory effects on body metabolism and intestinal flora are also different. Future studies need to consider the different types of dietary fiber when further exploring the association between dietary fiber and the prognosis of patients with HF (49). Finally, the NHANES database did not distinguish the degree of HF and whether the patients received surgical treatment was also unknown. Thus, the relationship between dietary fiber and different degrees of HF needs to be further explored.
Conclusion
A high dietary fiber intake level was related to a lower risk of all-cause and CVD-caused mortality among HF survivors. Sufficient dietary fiber intake may be beneficial for maintaining heart health, especially in men, patients with a duration of HF ≥10 years, and those with a history of diabetes and dyslipidemia. Larger studies in the future are needed to evaluate the association between dietary fiber intake and mortality among HF patients.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://wwwn.cdc.gov/nchs/nhanes/.
Ethics statement
The requirement of ethical approval was waived by Affiliated Qingyuan Hospital, Guangzhou Medical University, Qingyuan People's Hospital as this study used publicly anonymous data. The data is used according to the user agreement of the original database. The participants provided their written informed consent to participate in this study.
Author contributions
SW: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing, Funding acquisition. YR: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. YZ: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by The Science and Technology Planning Project of Guangdong Province of China (A2022162).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1406511/full#supplementary-material
References
1. Castiglione V, Aimo A, Vergaro G, Saccaro L, Passino C, Emdin M. Biomarkers for the diagnosis and management of heart failure. Heart Fail Rev. (2022) 27(2):625–43. doi: 10.1007/s10741-021-10105-w
2. Emmons-Bell S, Johnson C, Prevalence RG. Incidence and survival of heart failure: a systematic review. Heart. (2022) 108(17):1351–60. doi: 10.1136/heartjnl-2021-320131
3. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. (2016) 13(6):368–78. doi: 10.1038/nrcardio.2016.25
4. Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail. (2019) 21(11):1306–25. doi: 10.1002/ejhf.1594
5. Sanches Machado d'Almeida K, Ronchi Spillere S, Zuchinali P, Corrêa Souza G. Mediterranean diet and other dietary patterns in primary prevention of heart failure and changes in cardiac function markers: a systematic review. Nutrients. (2018) 10(1):58. doi: 10.3390/nu10010058
6. Simão AF, Précoma DB, Andrade JP, Correa Filho H, Saraiva JF, Oliveira GM. I cardiovascular prevention guideline of the Brazilian society of cardiology—executive summary. Arq Bras Cardiol. (2014) 102(5):420–31. doi: 10.5935/abc.20140067
7. Eshak ES, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, et al. Dietary fiber intake is associated with reduced risk of mortality from cardiovascular disease among Japanese men and women. J Nutr. (2010) 140(8):1445–53. doi: 10.3945/jn.110.122358
8. Miyazawa I, Miura K, Miyagawa N, Kondo K, Kadota A, Okuda N, et al. Relationship between carbohydrate and dietary fibre intake and the risk of cardiovascular disease mortality in Japanese: 24-year follow-up of Nippon Data80. Eur J Clin Nutr. (2020) 74(1):67–76. doi: 10.1038/s41430-019-0424-y
9. Huang T, Xu M, Lee A, Cho S, Qi L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med. (2015) 13:59. doi: 10.1186/s12916-015-0294-7
10. Li S, Flint A, Pai JK, Forman JP, Hu FB, Willett WC, et al. Dietary fiber intake and mortality among survivors of myocardial infarction: prospective cohort study. Br Med J. (2014) 348:g2659. doi: 10.1136/bmj.g2659
11. Erkkilä AT, Herrington DM, Mozaffarian D, Lichtenstein AH. Cereal fiber and whole-grain intake are associated with reduced progression of coronary-artery atherosclerosis in postmenopausal women with coronary artery disease. Am Heart J. (2005) 150(1):94–101. doi: 10.1016/j.ahj.2004.08.013
12. Hong C, Zhu H, Zhou X, Zhai X, Li S, Ma W, et al. Association of blood urea nitrogen with cardiovascular diseases and all-cause mortality in USA adults: results from NHANES 1999–2006. Nutrients. (2023) 15(2):461. doi: 10.3390/nu15020461
13. Zhu Y, Chen Z, Chen S, Fu G, Wang Y. Combined effects of physical activity and sedentary behavior on all-cause mortality in heart failure patients: a cohort study of national health and nutrition examination survey analysis. Front Cardiovasc Med. (2022) 9:1027995. doi: 10.3389/fcvm.2022.1027995
14. Yuan S, Ming-Wei L, Qi-Qiang H, Larsson SC. Egg, cholesterol and protein intake and incident type 2 diabetes Mellitus: results of repeated measurements from a prospective cohort study. Clin Nutr. (2021) 40(6):4180–6. doi: 10.1016/j.clnu.2021.01.041
15. Zhang H, Wei X, Pan J, Chen X, Sun X. Anemia and frailty in the aging population: implications of dietary fiber intake (findings of the US nhanes from 2007 to 2018). BMC Geriatr. (2023) 23(1):634. doi: 10.1186/s12877-023-04352-9
16. Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the national death index and equifax nationwide death search. Am J Epidemiol. (1994) 140(11):1016–9. doi: 10.1093/oxfordjournals.aje.a117191
17. Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, et al. Test of the national death Index. Am J Epidemiol. (1984) 119(5):837–9. doi: 10.1093/oxfordjournals.aje.a113804
18. Li B, Chen L, Hu X, Tan T, Yang J, Bao W, et al. Association of Serum uric acid with all-cause and cardiovascular mortality in diabetes. Diabetes Care. (2023) 46(2):425–33. doi: 10.2337/dc22-1339
19. Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mähönen M, Ngu Blackett K, et al. World health organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. (2011) 40(1):139–46. doi: 10.1093/ije/dyq165
20. Walker AE, Robins M, Weinfeld FD. The national survey of stroke. Clinical findings. Stroke. (1981) 12(2 Pt 2 Suppl 1):I13–44.7222164
21. Shen Q, Xu Q, Li G, Ren L, Zhang Z, Zhang Y, et al. Joint effect of 25-hydroxyvitamin D and secondhand smoke exposure on hypertension in non-smoking women of childbearing age: nhanes 2007–2014. Environ Health. (2021) 20(1):117. doi: 10.1186/s12940-021-00803-1
22. Anderson ML, Chang BH, Kini N. Alcohol and drug use among deaf and hard-of-hearing individuals: a secondary analysis of NHANES 2013–2014. Subst Abus. (2018) 39(3):390–7. doi: 10.1080/08897077.2018.1442383
23. Mendes MA, da Silva I, Ramires V, Reichert F, Martins R, Ferreira R, et al. Metabolic equivalent of task (mets) thresholds as an indicator of physical activity intensity. PLoS One. (2018) 13(7):e0200701. doi: 10.1371/journal.pone.0200701
24. Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. (2020) 30(3):160–4. doi: 10.1016/j.tcm.2019.05.003
25. Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW, et al. American Association of Clinical Endocrinologists’ guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocr Pract. (2012) 18(Suppl 1):1–78. doi: 10.4158/EP.18.S1.1
26. McClure ST, Schlechter H, Oh S, White K, Wu B, Pilla SJ, et al. Dietary intake of adults with and without diabetes: results from NHANES 2013–2016. BMJ Open Diabetes Res Care. (2020) 8(1):e001681. doi: 10.1136/bmjdrc-2020-001681
27. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
28. Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. (2016) 165(7):473–81. doi: 10.7326/M16-0273
29. Wang K, Zhao Y, Nie J, Xu H, Yu C, Wang S. Higher Hei-2015 score is associated with reduced risk of depression: result from NHANES 2005–2016. Nutrients (2021) 13(2):348. doi: 10.3390/nu13020348
30. He K, Pang T, Huang H. The relationship between depressive symptoms and BMI: 2005–2018 NHANES data. J Affect Disord. (2022) 313:151–7. doi: 10.1016/j.jad.2022.06.046
31. Chang CY, Lee CL, Liu WJ, Wang JS. Association of adherence to the Mediterranean diet with all-cause mortality in subjects with heart failure. Nutrients. (2022) 14(4):842. doi: 10.3390/nu14040842
32. Sun L, Du J. Magnesium status, serum vitamin D concentration and mortality among congestive heart failure patients: a cohort study from NHANES 2007–2018. Magnesium Res. (2024) 37(2):61–75. doi: 10.1684/mrh.2024.0528
33. Lattimer JM, Haub MD. Effects of dietary fiber and its components on metabolic health. Nutrients. (2010) 2(12):1266–89. doi: 10.3390/nu2121266
34. Streppel MT, Ocké MC, Boshuizen HC, Kok FJ, Kromhout D. Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 Y: the Zutphen study. Am J Clin Nutr. (2008) 88(4):1119–25. doi: 10.1093/ajcn/88.4.1119
35. Liu S, Stampfer MJ, Hu FB, Giovannucci E, Rimm E, Manson JE, et al. Whole-grain consumption and risk of coronary heart disease: results from the nurses’ health study. Am J Clin Nutr. (1999) 70(3):412–9. doi: 10.1093/ajcn/70.3.412
36. Nirmala Prasadi PV, Joye IJ. Dietary fibre from whole grains and their benefits on metabolic health. Nutrients. (2020) 12(10):3045. doi: 10.3390/nu12103045
37. Fraser GE, Sabaté J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The adventist health study. Arch Intern Med. (1992) 152(7):1416–24. doi: 10.1001/archinte.1992.00400190054010
38. Jacobs DR J, Meyer KA, Kushi LH, Folsom AR. Whole-grain intake may reduce the risk of ischemic heart disease death in postmenopausal women: the Iowa women’s health study. Am J Clin Nutr. (1998) 68(2):248–57. doi: 10.1093/ajcn/68.2.248
39. Aukrust P, Yndestad A, Ueland T, Damås JK, Gullestad L. Anti-inflammatory trials in chronic heart failure. Heart Fail Monit. (2006) 5(1):2–9.16547529
40. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. (2016) 535(7610):56–64. doi: 10.1038/nature18846
41. Brown EM, Clardy J, Xavier RJ. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe. (2023) 31(2):173–86. doi: 10.1016/j.chom.2023.01.009
42. Gagnon E, Mitchell PL, Manikpurage HD, Abner E, Taba N, Esko T, et al. Impact of the gut microbiota and associated metabolites on cardiometabolic traits, chronic diseases and human longevity: a Mendelian randomization study. J Transl Med. (2023) 21(1):60. doi: 10.1186/s12967-022-03799-5
43. Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. (2019) 16(3):137–54. doi: 10.1038/s41569-018-0108-7
44. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker-Kleiner D, Kaye DM, et al. Sex differences in heart failure. Eur Heart J. (2019) 40(47):3859–68c. doi: 10.1093/eurheartj/ehz835
45. Sobenin IA, Myasoedova VA, Orekhov AN. Phytoestrogen-rich dietary supplements in anti-atherosclerotic therapy in postmenopausal women. Curr Pharm Des. (2016) 22(2):152–63. doi: 10.2174/1381612822666151112150520
46. Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. (2000) 342(19):1392–8. doi: 10.1056/NEJM200005113421903
47. Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, et al. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European atherosclerosis society (EAS). Eur Heart J. (2011) 32(14):1769–818. doi: 10.1093/eurheartj/ehr158
48. Albrink MJ, Ullrich IH. Interaction of dietary sucrose and fiber on serum lipids in healthy young men fed high carbohydrate diets. Am J Clin Nutr. (1986) 43(3):419–28. doi: 10.1093/ajcn/43.3.419
Keywords: dietary fiber, heart failure, all-cause mortality, CVD-caused mortality, NHANES
Citation: Wang S, Ruan Y and Zhang Y (2025) Association between dietary fiber intake and all-cause and CVD-caused mortality among heart failure survivors: a cohort study from the NHANES database. Front. Cardiovasc. Med. 11:1406511. doi: 10.3389/fcvm.2024.1406511
Received: 25 March 2024; Accepted: 13 December 2024;
Published: 10 April 2025.
Edited by:
Massimo Iacoviello, University of Foggia, ItalyCopyright: © 2025 Wang, Ruan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shulin Wang, d2FuZ3NodWxpbjA3MTNAb3V0bG9vay5jb20=
 Shulin Wang
Shulin Wang Yun Ruan
Yun Ruan