
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 19 April 2024
Sec. Cardiac Rhythmology
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1406192
Background: Atrial fibrillation (AF) is a common cardiac arrhythmia that is associated with obesity and obstructive sleep apnea syndrome (OSA). Obesity and OSA may increase the risk of AF by affecting cardiovascular health.
Methods: The study used the Mendelian randomization (MR) approach, combined with two-sample and multivariable analyses, to assess the relationships between obesity, OSA, and AF. The study utilized GWAS data and applied various statistical methods for the analysis.
Results: The study found that obesity increased the risk of OSA, which in turn significantly increased the risk of AF. Through mediating MR analysis, it was found that OSA played a certain role in the causal relationship between obesity and AF, with about 6.4% of the risk of AF being mediated by OSA.
Conclusion: This study highlights the relationships among obesity, OSA, and AF, providing useful guidance for future clinical researches.
Atrial fibrillation (AF) is a common cardiac arrhythmia characterized by rapid and irregular heartbeats, frequently observed in elderly individuals or patients with concomitant cardiovascular diseases such as coronary artery disease and hypertension (1). The global prevalence of AF is approximately 0.5%, with an expected rate of up to 9% in individuals aged 65 years or older (2). The increasing incidence of AF poses a significant threat to public health and socioeconomic burden worldwide. Thromboembolism caused by AF is one of the most severe complications in patients with this condition.
The pathogenesis of AF is multifaceted, involving abnormalities in the conduction system, the effects of the autonomic nervous system, and abnormal ionic current metabolism in cardiomyocytes (3–6). Current research focuses on changes in ion channels, extracellular matrix, and intracellular signaling pathways. Risk factors for AF include Systemic arterial hypertension, coronary artery disease, heart failure, including heart failure with preserved ejection fraction, chronic kidney disease, obesity, obstructive sleep apnea (OSA) (7–9).
OSA is a prevalent sleep breathing disorder characterized by recurrent apnea and airway obstruction during sleep, resulting in decreased sleep quality and daytime sleepiness. The primary cause of OSA is the relaxation and collapse of the upper airway tissues, leading to airway narrowing or occlusion and subsequent apnea or shallow breathing (10). OSA is often associated with cardiovascular diseases such as obesity, hypertension, and diabetes (11). Recent studies have identified OSA as an independent risk factor for AF, with a close relationship between the two conditions (12). Patients with severe OSA have a two to five times higher risk of developing AF compared to normal individuals. Furthermore, sleep apnea has been linked to AF recurrence, particularly within the first year after recurrence (13). Untreated sleep apnea may exacerbate heart disease symptoms and increase the risk of heart disease, emphasizing the importance of sleep apnea interventions in patients with AF.
Obesity plays a crucial role in the pathogenesis of AF by adversely affecting cardiovascular hemodynamics and cardiac structure and function, thereby increasing the incidence of AF (14). It may also be associated with electroanatomical remodeling in obese patients. A meta-analysis on obesity and AF, which included 603,510 samples, demonstrated that obesity increases the likelihood of AF (15). Another study with medium and long-term follow-up results revealed that for every unit increase in body mass index (BMI), the risk of AF increased by 3% (95% CI, 1%–5%) (16). Obesity is also a significant risk factor for OSA, as it leads to the accumulation of fat in the neck, tongue, and larynx areas, causing airway obstruction.
In conclusion, AF is a prevalent cardiac arrhythmia with a multifaceted pathogenesis and various risk factors, including obstructive sleep apnea and obesity. The close relationship between these conditions highlights the importance of early intervention and management to reduce the incidence and recurrence of AF and its associated complications.
Mendelian randomization (MR) is an increasingly popular research method in the biomedical field. This method uses the random assignment pattern of human genes, or genetic variation, as an instrumental variable to reduce confounding factors, eliminate non-random errors, and improve the causal inference between exposure and outcome. As a result, MR can more accurately assess the effect of factors of concern on outcomes (17). MR has several advantages over traditional observational studies (18, 19). Therefore, this study used MR to investigate the relationship between OSA, obesity, and AF, with the hope of gaining new insights into interventions for the prevention of AF.
To assess mediating effects, this study employed a two-step and multivariate MR approach. In the first step, we conducted MR studies using two-sample MR to examine the correlations between exposure and outcome, exposure and mediating variables, and mediating variables and outcome. If evidence of mediating effects was present in the two-step MR, we then employed multivariate Mendelian randomization (MVMR). Similar to traditional MR studies, the instrumentation of exposure and potential mediating variables is subject to three main assumptions. First, the instrument should be associated with the corresponding phenotype. Second, single nucleotide polymorphisms (SNPs) should not be confounded. Third, the association of SNPs with mediating variables and outcomes should occur only through their effects on exposure and mediating variables. Mediated MR results will be considered valid only if all three assumptions are met simultaneously.
The genome-wide association study (GWAS) data for AF used in this study were obtained from a biobank-based study of European origin with more than one million participants. The study identified 111 independent risk variants at 142 loci associated with AF, using a total of 60,620 AF cases and 970,216 controls (20). The data were screened for genome-wide level significance (p < 5 × 10−8) and removal of linkage disequilibrium (r2 < 0.001).
The GWAS data for OSA and obesity were obtained from the FinnGen database, a comprehensive health data platform in Finland. The goal of FinnGen is to provide a large, high-quality data resource for medical research by collecting, integrating, and analyzing clinical, genomic, and health records of the Finnish population, the cut-off AF, OSA, obesity are classified according to the Tenth Revision of the International Classification of Diseases (ICD-10/ICD-9) published by the World Health Organization for the classification of diseases and health-related issues.
In this study, we employed five different models, including inverse variance weighted (IVW), MR-Egger regression, weighted median, simple mode, and weighted mode, to explore causal correlations (21) and assess the causal relationships between exposure, mediators, and outcomes. The IVW method is the predominant MR analysis method used to combine the Wald ratios of individual SNPs (22). The MR-Egger method can be used to identify and correct for potential pleiotropy, and the weighted median method can provide consistent causal estimates when the highest 50% of the weights analyzed are from invalid instrumental variables. However, since the analysis was conducted assuming that all variants are valid instruments and instrumental variables may have directional pleiotropy (23), the estimates may be biased (24).
To assess whether the analysis is heterogeneous, we used Cochran's Q-values to assess the presence of heterogeneity. We also performed other sensitivity analyses, such as outlier test MR-PRESSO analysis, to identify and correct potential outliers and to avoid potential horizontal multicollinearity. Additionally, we used different conditioning plots, such as scatter plots, leave-one-out plots, and forest plots, to show the relationship between SNPs-exposure associations and SNPs-outcome associations, to reduce the effect of errors and improve the reliability and accuracy of the experiment and the contribution of each instrumental variable to the overall causal estimate.
In this study, 8 SNPs were included in the MR study on obesity and AF. The results indicated a positive correlation between obesity and AF. In the IVW method, the association was significant [p = 1.38 × 10−4; OR, 95% Confidence Interval (CI) = 1.11 (1.05, 1.17)], in the MR Egger method, the association was not statistically significant [p = 1.72 × 10−1; OR, 95% CI = 1.15 (0.96, 1.36)], and in the Weighted median method, the results were significant [p = 1.41 × 10−4; OR, 95% CI = 1.11 (1.05, 1.17)].
For the study on obesity and obstructive sleep apnea (OSA), 8 SNPs were included. The results showed a positive correlation between obesity and AF. In the IVW method, the association was significant [p = 5.99 × 10−11; OR, 95% CI = 1.38 (1.25, 1.52)], in the MR Egger method, the association was significant [p = 3.40 × 10−5; OR, 95% CI = 1.57 (1.23, 1.76)], and in the Weighted median method, the results were significant [p = 7.80 × 10−5; OR, 95% CI = 1.47 (1.35, 1.60)].
For the study on OSA and AF, 5 SNPs were included. The results indicated a positive correlation between OSA and AF. In the IVW method, the association was significant [p = 9.40 × 10−5; OR, 95% CI = 1.18 (1.07, 1.3)], in the MR Egger method, the association was significant [p = 6.50 × 10−3; OR, 95% CI = 1.23 (1.01, 1.36)], and in the Weighted median method, the results were significant [p = 5.60 × 10−3; OR, 95% CI = 1.05 (1.09, 1.15)]. The results are shown in Forest Plot, Figure 1. and the trend of MR results is shown in the scatter plot, Figure 2.
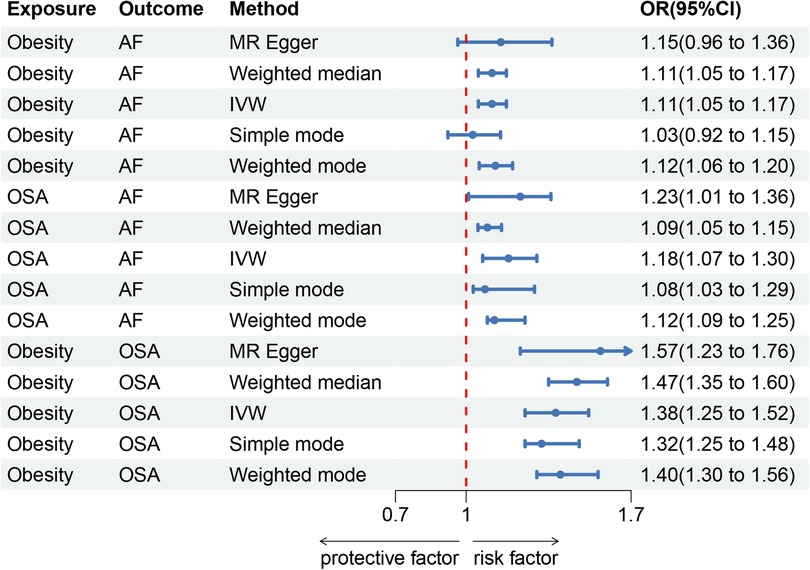
Figure 1. Forest map. (A) Obesity and AF forest map, (B) OSA and AF forest map, (C) Obesity and OSA forest map.
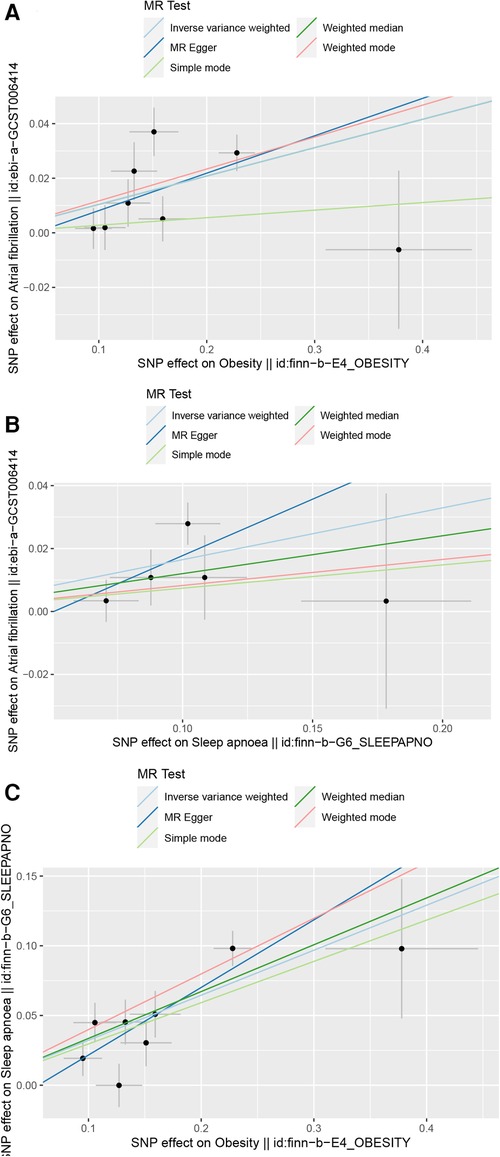
Figure 2. Scatter plot. (A) Scatter plot of obesity and AF, (B) Scatter plot of OSA and AF, (C) Scatter plot of obesity and OSA.
In the MR-Egger pleiotropy test, the intercept p-value was 0.71 for obesity and AF, indicating no pleiotropy bias in assessing the effect of obesity on AF using the IVW method. The intercept p-value was 0.271 for obesity and OSA, and in the sensitivity analysis of OSA and AF, the intercept p-value was 0.52 for the IVW method. Our results in the pleiotropy test were >0.05, and none of the data were confounded by pleiotropy. In the MR-PRESSO outlier test, p = 0.11 for obesity and AF, p = 0.13 for obesity and OSA, and p = 0.29 for OSA and AF, indicating no outliers were found in all three groups. There was no heterogeneity in Cochran's Q-test for obesity vs. AF (p = 0.051), obesity vs. OSA (p = 0.057), and OSA vs. AF (p = 0.26). Our results remained stable in the leave-one-out plot (Figure 3).
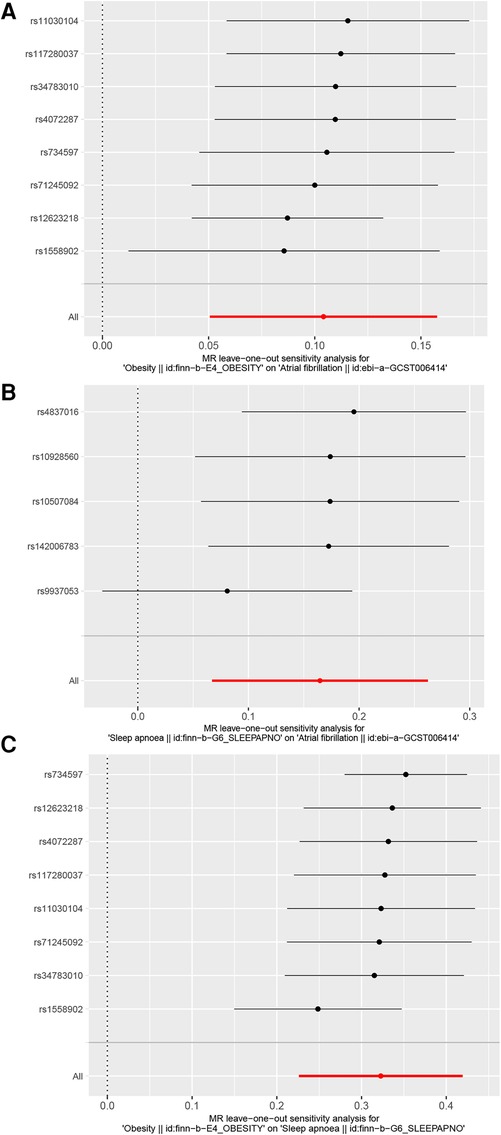
Figure 3. Leave-one-out plot. (A) Leave-one-out plot of obesity and AF, (B) Leave-one-out plot of OSA and AF, (C) Leave-one-out plot of obesity and OSA.
In the mediation effect analysis, we obtained an OSA mediation share of 6.4% by multivariate mediation MR. The results of the mediation analysis showed that OSA as a mediating variable has a role in the causal relationship of obesity on AF, and approximately 6.4% of the risk of obesity causing AF was due to OSA-mediated effects.
We further conducted several sensitivity analyses to ensure the robustness of our findings. Outlier test MR-PRESSO analysis was performed to identify and correct potential outliers, and multiple validity tests were conducted to assess the reliability and accuracy of the experiment. We also used different conditioning plots, such as scatter plots, leave-one-out plots, and forest plots, to show the relationship between SNPs-exposure associations and SNPs-outcome associations, to reduce the effect of errors and improve the reliability and accuracy of the experiment.
In conclusion, our study provides evidence of a causal relationship between obesity and AF, with OSA serving as a mediating variable. Our findings suggest that reducing obesity and preventing or treating OSA may help prevent the development of AF. Further studies are needed to explore the mechanisms underlying this relationship and to develop effective interventions for the prevention and treatment of AF.
OSA can lead to increased cardiac load, which may contribute to the development of AF. The frequent apnea and hypopnea events in OSA patients can lead to repeated arousals from sleep, resulting in increased sympathetic activity, blood pressure, and heart rate (25). This can cause an increase in the left atrial pressure and volume, leading to atrial remodeling and increased susceptibility to AF (26). Furthermore, OSA can cause poor oxygenation and hemodynamic instability, which may contribute to the development of AF. The intermittent hypoxemia and hypercapnia in OSA patients can cause endothelial dysfunction, oxidative stress, and inflammation, which can lead to vascular damage and a prothrombotic state. This can increase the risk of thromboembolic events, which can lead to the development of AF (27).
Overall, the mechanism by which OSA causes AF is complex and multifactorial, involving alterations in cardiac electrophysiology and structure, autonomic nervous system disorders, increased cardiac load, poor oxygenation, and hemodynamic instability (28–30). Further research is needed to better understand the underlying mechanisms and develop effective interventions for the prevention and treatment of AF in OSA patients.
OSA may also contribute to the development of AF by increasing cardiac load, poor oxygenation, and hemodynamic instability. Patients with OSA are usually associated with cardiovascular diseases such as obesity, hypertension, and diabetes, which are themselves risk factors for AF (31). OSA in the treatment of AF may also reduce the efficacy of antiarrhythmic drugs, electrical cardioversion, and catheter ablation in the treatment of AF, thus increasing the recurrence rate of AF (32), and thus the outcome is poorer in patients with OSA combined with AF. This may be related to factors such as disturbances in the autonomic nervous system and alterations in cardiac electrophysiology and structure due to OSA (33).
In the relationship between obesity and AF, obesity has been shown to be an independent risk factor for AF in several studies (34), and the mechanism may involve several aspects such as alterations in cardiac structure and function, inflammatory response, and endocrine abnormalities (35). First, obesity can lead to structural and functional alterations in the heart, including atrial enlargement, myocardial fibrosis, and left ventricular hypertrophy. These alterations increase the risk of developing and recurring AF (36). A study of more than 25,000 patients with cardiovascular disease showed that the risk of AF increased by 20% for every 5-unit increase in BMI (37). Another study suggests that the effect of obesity on AF may be related to abdominal fat. This study found that abdominal fat area was positively associated with the incidence of AF. In addition, obesity can lead to inflammatory responses and endocrine abnormalities (38), factors that are also associated with the development and recurrence of AF. Obesity causes adipocytes to secrete a variety of inflammatory factors, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and C-reactive protein (CRP) (39). These factors can lead to myocardial fibrosis and electrophysiological abnormalities, which can increase the risk of AF (40). Obesity can also lead to endocrine abnormalities, such as insulin resistance and abnormal insulin secretion, and these factors are also associated with the development and recurrence of AF (41, 42). Finally, obesity also affects the outcome and prognosis of treatment for AF. One study found that obesity decreased the response of patients with AF to antiarrhythmic drug therapy, thereby increasing the risk of recurrence (43, 44).
Moreover, the MR approach used in this study assumes that the genetic variants used as instrumental variables are only related to the exposure variable and not to any confounding factors or other pathways that may affect the outcome variable. However, there may still be pleiotropic effects of these genetic variants, which can lead to biased estimates of causal effects. Therefore, further validation and replication of the findings are needed using other methods and data sources.
In conclusion, our study underscores the potential impact of obesity and OSA on the occurrence of AF, which holds significant implications for clinical practice. For example, clinical interventions such as weight reduction and the prevention or treatment of OSA may aid in mitigating the risk of AF occurrence and recurrence in patients. These considerations can be integrated into patient management plans, particularly for individuals already diagnosed with AF or deemed at risk for the condition. Early screening, especially for obese and OSA patients, including those with cardiovascular disease risk factors, can be beneficial as it may help prevent the onset of AF through timely interventions. Moreover, personalized treatment is paramount: comprehending the interplay between obesity, OSA, and AF can empower clinicians to tailor treatment plans that optimize cardiovascular health management for patients. Lastly, further research is imperative: this study sets the stage for deeper exploration of the relationships between obesity, OSA, and AF. Subsequent investigations can delve into the biological mechanisms underpinning these associations and devise more efficacious intervention strategies.
This study provides new insights into the potential role of OSA as a mediating variable in the relationship between obesity and AF, and highlights the importance of addressing obesity as a modifiable risk factor for both OSA and AF. Interventions aimed at reducing obesity may have a beneficial effect on reducing the incidence of OSA and AF. However, further studies are needed to explore the optimal interventions for the prevention and treatment of obesity, OSA, and AF, and to identify potential targets for therapeutic interventions.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
TL: Methodology, Writing – original draft. LR: Data curation, Writing – original draft. YG: Methodology, Writing – original draft. WC: Project administration, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Antman EM, Leopold JA, Sauer WH, Zei PC. Atrial fibrillation—what is it and how is it treated? N Engl J Med. (2022) 387(16):e38. doi: 10.1056/NEJMp2212939
2. Wang L, Ze F, Li J, Mi L, Han B, Niu H, et al. Trends of global burden of atrial fibrillation/flutter from global burden of disease study 2017. Heart. (2021) 107(11):881–7. doi: 10.1136/heartjnl-2020-317656
3. Glasscock E, Voigt N, McCauley MD, Sun Q, Li N, Chiang DY, et al. Expression and function of Kv1.1 potassium channels in human atria from patients with atrial fibrillation. Basic Res Cardiol. (2015) 110(5):505. doi: 10.1007/s00395-015-0505-6
4. Dobrev D, Ravens U. Remodeling of cardiomyocyte ion channels in human atrial fibrillation. Basic Res Cardiol. (2003) 98(3):137–48. doi: 10.1007/s00395-003-0409-8
5. Linz D, Elliott AD, Hohl M, Malik V, Schotten U, Dobrev D, et al. Role of autonomic nervous system in atrial fibrillation. Int J Cardiol. (2019) 287:181–8. doi: 10.1016/j.ijcard.2018.11.091
6. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. (2014) 114(9):1500–15. doi: 10.1161/CIRCRESAHA.114.303772
7. Elliott AD, Middeldorp ME, Van Gelder IC, Albert CM, Sanders P. Epidemiology and modifiable risk factors for atrial fibrillation. Nat Rev Cardiol. (2023) 20(6):404–17. doi: 10.1038/s41569-022-00820-8
8. Crea F. Novel risk factors for atrial fibrillation, conduction disturbances, sudden coronary death, and device infection. Eur Heart J. (2022) 43(47):4853–7. doi: 10.1093/eurheartj/ehac734
9. Mariani MV, Pierucci N, Piro A, Trivigno S, Chimenti C, Galardo G, et al. Incidence and determinants of spontaneous cardioversion of early onset symptomatic atrial fibrillation. Medicina. (2022) 58(11):1513. doi: 10.3390/medicina58111513
10. Jun JC. Dying with OSA, or from it: a cautionary note about novel hypoxia metrics. Am J Respir Crit Care Med. (2022) 206(12):1563–4. doi: 10.1164/rccm.202206-1052LE
11. Huang B, Liu H, Scherlag BJ, Sun L, Xing S, Xu J, et al. Atrial fibrillation in obstructive sleep apnea: neural mechanisms and emerging therapies. Trends Cardiovasc Med. (2021) 31(2):127–32. doi: 10.1016/j.tcm.2020.01.006
12. Prystowsky EN, Padanilam BJ, Fogel RI. Treatment of atrial fibrillation. JAMA. (2015) 314(3):278–88. doi: 10.1001/jama.2015.7505
13. Latif Z, Modest AM, Ahn A, Thomas R, Tieu H, Tung P. Effect of widespread sleep apnea screening on progression of atrial fibrillation. Am J Cardiol. (2022) 182:25–31. doi: 10.1016/j.amjcard.2022.07.034
14. Lavie CJ, Pandey A, Lau DH, Alpert MA, Sanders P. Obesity and atrial fibrillation prevalence, pathogenesis, and prognosis: effects of weight loss and exercise. J Am Coll Cardiol. (2017) 70(16):2022–35. doi: 10.1016/j.jacc.2017.09.002
15. Wong CX, Sullivan T, Sun MT, Mahajan R, Pathak RK, Middeldorp M, et al. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: a meta-analysis of 626,603 individuals in 51 studies. JACC Clin Electrophysiol. (2015) 1(3):139–52. doi: 10.1016/j.jacep.2015.04.004
16. Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, et al. The long- and short-term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (women’s health study). J Am Coll Cardiol. (2010) 55(21):2319–27. doi: 10.1016/j.jacc.2010.02.029
17. Zhang H. Pros and cons of Mendelian randomization. Fertil Steril. (2023) 119(6):913–6. doi: 10.1016/j.fertnstert.2023.03.029
18. Ference BA, Ray KK, Catapano AL, Ference TB, Burgess S, Neff DR, et al. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med. (2019) 380(11):1033–42. doi: 10.1056/NEJMoa1806747
19. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR statement. JAMA. (2021) 326(16):1614–21. doi: 10.1001/jama.2021.18236
20. Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. (2018) 50(9):1234–9. doi: 10.1038/s41588-018-0171-3
21. Zoccali C. The challenge of Mendelian randomization approach. Curr Med Res Opin. (2017) 33(sup3):5–8. doi: 10.1080/03007995.2017.1378514
22. Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. (2017) 26(5):2333–55. doi: 10.1177/0962280215597579
23. Zhao SS, Holmes MV, Zheng J, Sanderson E, Carter AR. The impact of education inequality on rheumatoid arthritis risk is mediated by smoking and body mass index: Mendelian randomization study. Rheumatology. (2022) 61(5):2167–75. doi: 10.1093/rheumatology/keab654
24. Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. (2018) 27(R2):R195–208. doi: 10.1093/hmg/ddy163
25. Marulanda-Londoño E, Chaturvedi S. The interplay between obstructive sleep apnea and atrial fibrillation. Front Neurol. (2017) 8:668. doi: 10.3389/fneur.2017.00668
26. Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American heart association. Circulation. (2021) 144(3):e56–67. doi: 10.1161/CIR.0000000000000988
27. Yang Y, Ning Y, Wen W, Jia Y, Chen X, Huang M, et al. CPAP is associated with decreased risk of AF recurrence in patients with OSA, especially those younger and slimmer: a meta-analysis. J Interv Card Electrophysiol. (2020) 58(3):369–79. doi: 10.1007/s10840-020-00738-6
28. Patel D, Mohanty P, Di Biase L, Shaheen M, Lewis WR, Quan K, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol. (2010) 3(5):445–51. doi: 10.1161/CIRCEP.109.858381
29. Parise BK, Ferreira NL, Drager LF. The cardiovascular impact of obstructive sleep apnea in women: current knowledge and future perspectives. Sleep Med Clin. (2023) 18(4):473–80. doi: 10.1016/j.jsmc.2023.06.008
30. Hertel JN, Linz B, Isaksen J, Jerltorp K, Leonhardt C, Gottlieb L, et al. Inhibition of the acetylcholine-regulated potassium current prevents transient apnea-related atrial arrhythmogenic changes in a porcine model. Heart Rhythm. (2024) 26:S1547-5271(24)00087-0. doi: 10.1016/j.hrthm.2024.01.033
31. May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhythmogenesis: mechanistic insights. Chest. (2017) 151(1):225–41. doi: 10.1016/j.chest.2016.09.014
32. Linz D, Linz B, Hohl M, Böhm M. Atrial arrhythmogenesis in obstructive sleep apnea: therapeutic implications. Sleep Med Rev. (2016) 26:87–94. doi: 10.1016/j.smrv.2015.03.003
33. Goudis CA, Ketikoglou DG. Obstructive sleep and atrial fibrillation: pathophysiological mechanisms and therapeutic implications. Int J Cardiol. (2017) 230:293–300. doi: 10.1016/j.ijcard.2016.12.120
34. Ergün G, Başaran Ö, Doğan V, Doğan MM, Biteker M. Obesity and atrial fibrillation. Int J Cardiol. (2016) 223:159–60. doi: 10.1016/j.ijcard.2016.08.064
35. Middeldorp ME, Kamsani SH, Sanders P. Obesity and atrial fibrillation: prevalence, pathogenesis, and prognosis. Prog Cardiovasc Dis. (2023) 78:34–42. doi: 10.1016/j.pcad.2023.04.010
36. Narkiewicz K. Diagnosis and management of hypertension in obesity. Obes Rev. (2006) 7(2):155–62. doi: 10.1111/j.1467-789X.2006.00226.x
37. Ardissino M, Reddy RK, Slob EAW, Patel KHK, Ryan DK, Gill D, et al. Sleep disordered breathing, obesity and atrial fibrillation: a Mendelian randomisation study. Genes. (2022) 13(1):104. doi: 10.3390/genes13010104
38. Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. (2017) 16(1):120. doi: 10.1186/s12933-017-0604-9
39. Scridon A, Dobreanu D, Chevalier P, Şerban RC. Inflammation, a link between obesity and atrial fibrillation. Inflamm Res. (2015) 64(6):383–93. doi: 10.1007/s00011-015-0827-8
40. Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. (2015) 12(4):230–43. doi: 10.1038/nrcardio.2015.2
41. Csige I, Ujvárosy D, Szabó Z, Lőrincz I, Paragh G, Harangi M, et al. The impact of obesity on the cardiovascular system. J Diabetes Res. (2018) 2018:3407306. doi: 10.1155/2018/3407306
42. Kachur S, Lavie CJ, de Schutter A, Milani RV, Ventura HO. Obesity and cardiovascular diseases. Minerva Med. (2017) 108(3):212–28. doi: 10.23736/S0026-4806.17.05022-4
43. Wang SY, Giugliano RP. Non-vitamin K antagonist oral anticoagulant for atrial fibrillation in obese patients. Am J Cardiol. (2020) 127:176–83. doi: 10.1016/j.amjcard.2020.04.016
44. Pan KL, Singer DE, Ovbiagele B, Wu YL, Ahmed MA, Lee M. Effects of non-vitamin K antagonist oral anticoagulants versus warfarin in patients with atrial fibrillation and valvular heart disease: a systematic review and meta-analysis. J Am Heart Assoc. (2017) 6(7):e005835. doi: 10.1161/JAHA.117.005835
Keywords: obesity, obstructive sleep apnea, AF, mediated Mendelian randomization, causal relationship
Citation: Li T, Rong L, Gao Y and Cheng W (2024) The causal relationship between obesity, obstructive sleep apnea and atrial fibrillation: a study based on mediated Mendelian randomization. Front. Cardiovasc. Med. 11:1406192. doi: 10.3389/fcvm.2024.1406192
Received: 24 March 2024; Accepted: 8 April 2024;
Published: 19 April 2024.
Edited by:
Danilo Menichelli, Sapienza University of Rome, ItalyReviewed by:
Vincenzo Mirco La Fazia, Texas Cardiac Arrhythmia Institute, United States© 2024 Li, Rong, Gao and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Cheng Y2gtd2VpMDQwNEBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.