- 1Department of Cardiovascular Medicine, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Cardiovascular Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Background: Inflammation is considered to play an important role in chronic obstructive pulmonary disease (COPD) and acute myocardial infarction (AMI), but the relationship between inflammation and poor prognosis in these patients has not yet been studied.
Methods: We enrolled AMI patients combined with COPD and divided them into three groups according to the tertiles of neutrophil-to-lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and monocyte to lymphocyte ratio (MLR) respectively. Logistic regression analyses were used to identify risk factors for in-hospital all-cause death in these patients. Covariates were adjusted stepwise to determine the association between inflammatory markers and poor prognosis. Also, the receiver operating characteristic (ROC) curve was used to evaluate the greatest predictive indicator for all-cause death.
Results: A total of 281 AMI patients combined with COPD were enrolled, of which 31 experienced in-hospital mortality. The risk of all-cause death was significantly higher among those with higher NLR. The highest tertile of NLR was significantly associated with an increased risk of all-cause death (all P < 0.05). This association remained significant after adjusting for confounding factors [Odds Ratio (OR): 10.571, 95% confidence interval (CI): 2.307–48.442, P = 0.002]. Moreover, compared to MLR and PLR, NLR had the highest predictive value for all-cause death [area under the curve (AUC): 0.764, 95% CI: 0.681–0.847].
Conclusion: In AMI patients combined with COPD, elevated levels of inflammation were associated with increased all-cause mortality. Compared to other inflammatory indicators, NLR may provide a more superior predictive value.
1 Introduction
Chronic obstructive pulmonary disease (COPD) progressively inflames the respiratory system, affecting the airways, alveoli, and microvascular system. The incidence and mortality rates of COPD are increasing annually, causing approximately 3 million deaths worldwide. Despite improvement in treatment, the disease continues to impose substantial social and economic burdens (1, 2). Acute myocardial infarction (AMI) presents a significant challenge to global public health, with increasing incidence rates in our country (3). The pathological basis of AMI is the rupture of atherosclerotic plaques in the coronary arteries, resulting in the formation of complete, incomplete, or partial obstructive thrombi, which consequently cause a clinical syndrome of myocardial ischemia and necrosis (4).
Comorbidities are a crucial component of COPD, with cardiovascular diseases (CVD) being notably prevalent. Special attention is needed for AMI (5). Although the primary characteristic of COPD is localized pulmonary inflammation, previous studies have shown that it also leads to systemic spillover of elevated acute-phase proteins levels. This systemic inflammation can cause atherogenesis, promoting the development and expansion of arterial plaques. Recent researches highlight the indispensable role that chronic inflammation plays in the development of various diseases, including diabetes, CVD, and pulmonary diseases. The neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and monocyte to lymphocyte ratio (MLR) are recognized as crucial biomarkers of inflammation, reflecting the state of immunological equilibrium and prognosis (6, 7). Elevated NLR level is associated with a higher risk of all-cause and disease-specific mortality. Ball MK et al. found that high NLR in COPD patients is associated with an increased risk of AMI, stroke, and death (8). PLR is acknowledged as a marker of inflammation and atherosclerosis, with prognostic value for cardiovascular events (9). MLR has been recognized as a significant independent risk factor for all-cause and cardiac death (6).
Inflammation is a key factor in the pathogenesis of both AMI and COPD. Systemic inflammation and oxidative stress can promote the formation of atherosclerotic plaques in patients with COPD. Previous research has found that patients with both COPD and AMI have a higher long-term mortality rate (10), even after adjusting for recognized prognostic factors. However, no studies have yet explored whether this adverse prognosis is directly related to the inflammatory response.
Therefore, it is important to explore inflammation biomarkers for prognosis in AMI patients combined with COPD. However, there is limited research exploring the clinical characteristics of these patients, and the influence of inflammatory response on the prognosis of AMI patients with COPD lacks sufficient and reliable evidence. Therefore, this study aims to initiate a single-center study, retrospectively enrolled AMI patients combined with COPD from the First Affiliated Hospital of Xi'an Jiaotong University and analyzed the impact of NLR, PLR and MLR on short-term prognosis in these patients. This research tends to evaluate whether inflammatory markers can serve as predictors of adverse outcomes in these patients.
2 Methods
2.1 Population and groups
This is a cross-sectional study that included patients diagnosed with type I AMI combined with COPD at the First Affiliated Hospital of Xi'an Jiaotong University from January 2013 to January 2024. The study followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University (No: XJTU1AF2023LSK-515).
The criteria for inclusion were as follow: (1) type I AMI patients combined with COPD; (2) 18–80 years. The criteria for exclusion were as follows: (1) incomplete clinical data; (2) recent history of infection within the last month; (3) combined with malignant tumors, rheumatic diseases, hematological diseases, and severe liver and renal insufficiency (Figure 1).
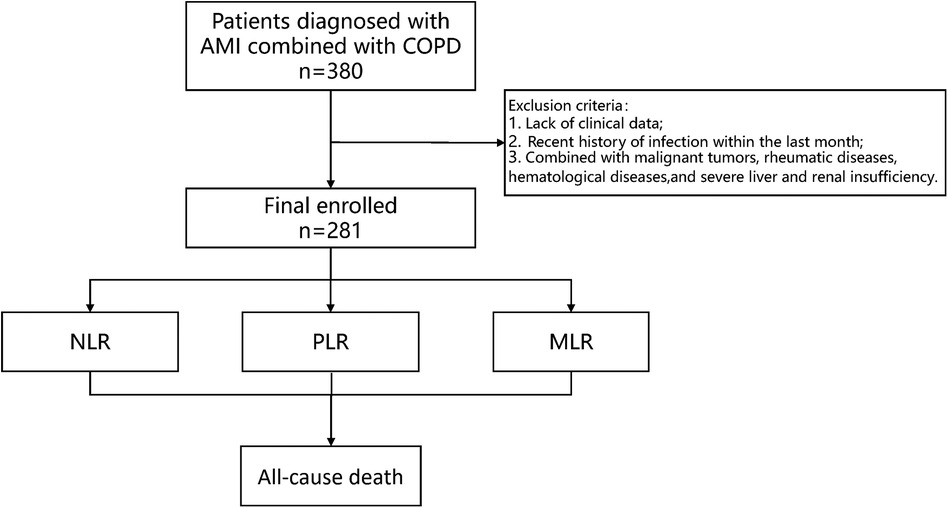
Figure 1 Study protocol. The flowchart of enrollment of study patients. AMI, acute myocardial infarction; COPD, chronic obstructive pulmonary disease.
2.2 Data collection
On admission, all patients were assessed for demographic characteristics (age, gender), comorbidities (hypertension, diabetes, stroke, arrhythmia, smoking history), cardiac function and laboratory data. The laboratory data included complete blood count, liver and kidney functions, glycated hemoglobin, blood lipids. The primary endpoint of this study was the incidence of in-hospital all-cause death, which was obtained from the discharge records.
2.3 Measuring inflammation indicators based on blood cell counts
Blood cell counts were collected from patients at their admission to the hospital, including the total counts of neutrophil, lymphocyte, monocyte and platelet. Subsequently, composite inflammation ratios (including NLR, PLR and MLR) were calculated. The calculation of NLR is neutrophil count/lymphocyte count. The calculation of PLR is platelet count/lymphocyte count. The calculation of MLR is monocyte count/lymphocyte count.
2.4 Statistical analysis
Continuous variables were expressed as mean ± standard deviation for normally distribution data and quartiles for non-normally distribution data. Categorical variables were expressed as percentages. Descriptive statistics analyses were performed based on the levels of NLR, PLR and MLR, with subgroup differences were explored using Chi-square tests, Analysis of Variance, or Kruskal-Wallis H-test. Logistic regression analysis was performed to assess the association between inflammation ratios and all-cause death. And regression models were built, we performed three regression models: Model 1: unadjusted model; Model 2: adjusted for age, gender, and smoking, hypertension, diabetes, stroke, arrythmia; Model 3: adjusted for model 2, and creatinine (Cr), low-density lipoprotein (LDL), lactate dehydrogenase (LDH), creatine kinase (CK), CK-MB, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and cardiac function. We used an alpha level of 0.05 for the inclusion of variables and 0.1 for the exclusion of variables, employing a backward stepwise method to build the regression model. The statistical analyses were performed by SPSS 27.0 (IBM Corp., NY). All analyses were two-sided and P < 0.05 was considered statistically significant.
3 Results
3.1 Clinical characteristics of study participants
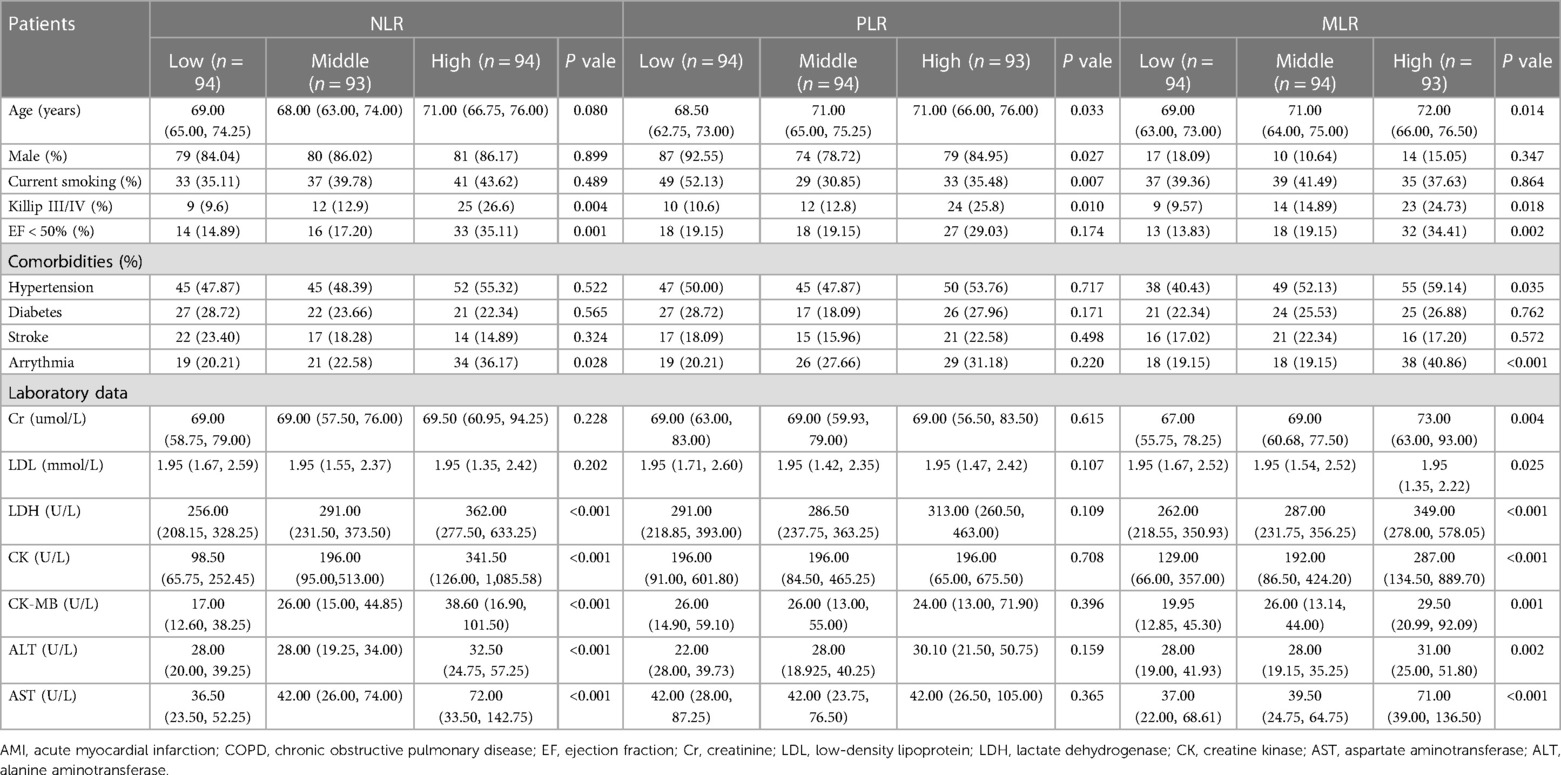
Baseline data for all patients were shown in Table 1, including 281 AMI patients combined with COPD. Patients within the high NLR group had poorer cardiac function, and these patients were also likely to have higher levels of liver enzymes, and cardiac enzymes. Also, arrhythmia was more common in the high NLR group (all P < 0.05).
The elderly and females were more likely to be in the high PLR group. Also, those with poor heart function, and arrhythmias were more likely to have higher MLR. These patients also tended to have higher levels of liver enzymes, and myocardial enzymes (all P < 0.05).
3.2 The association between composite inflammation ratios and all-cause death
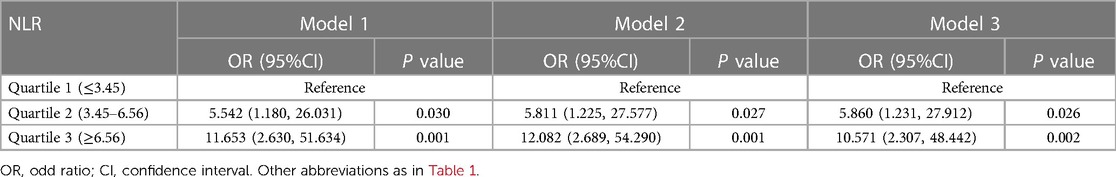
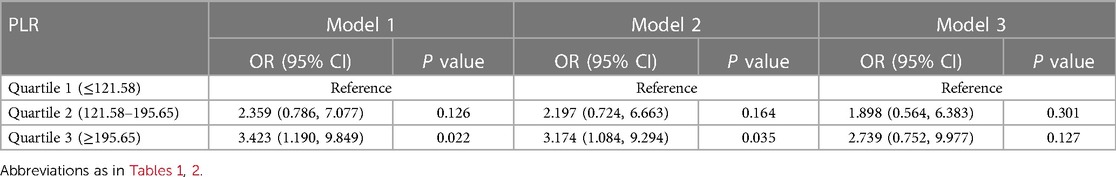
A total of 31 (11.0%) patients died during their hospital stay, of which 9 died within 24 h of admission. Logistic regression analysis was used to assess the association between all-cause death and inflammation markers (Figure 2). The higher NLR was significantly associated with an increased risk of all-cause death [Odds Ratio (OR) = 11.653, 95% CI: 2.630–51.634, P = 0.001]. After adjusting for demographic characteristic (Model 2), this association remained significant (OR = 12.082, 95% CI: 2.689–54.290, P = 0.001). After further adjustment for biochemical indicators (Model 3), the positive correlation between NLR and all-cause death remained significant (OR = 10.571, 95% CI: 2.307–48.442, P = 0.002). Ultimately, the model we constructed through stepwise logistic regression analysis is: logit [P(Death)] = −7.690 + 0.060⋅Age—1.090 Hypertension + 0.001 AST + 1.768⋅NLR class1 + 2.358⋅NLR class2. However, this association was not found when PLR and MLR were considered as categorical variables (Tables 2–4).
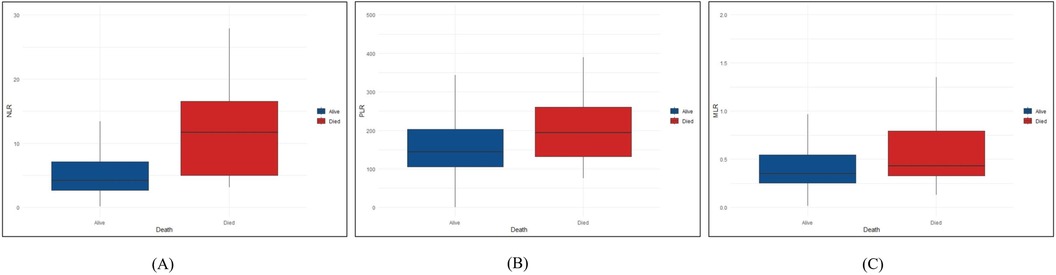
Figure 2 Differences in NLR, PLR and MLR between the died and alive groups. We described the distribution differences of NLR, PLR and MLR between died and alive groups. (A) The distribution differences of NLR between died and alive groups. (B) The distribution differences of PLR between died and alive groups. (C) The distribution differences of MLR between died and alive groups. NLR, neutrophil-to-lymphocyte ratio; PLR, platelet to lymphocyte ratio; MLR, monocyte to lymphocyte ratio.
3.3 The ROC curve between composite inflammation ratios and all-cause death
The AUC of NLR (0.764, 95% CI: 0.681–0.847) was significantly higher than that of other inflammation markers (Figure 3). The cut-off value was 11.434, with a specificity of 0.904, sensitivity of 0.581, Positive predictive value (PPV) of 0.4289, and Negative predictive value (NPV) of 0.946. The AUC of PLR and MLR were 0.619, 95% CI: 0.526–0.712 and 0.648, 95%CI: 0.537–0.758. The cut-off value of PLR was 163.478, with a specificity of 0.564, sensitivity of 0.677, PPV of 0.162, and NPV of 0.934. The cut-off value for MLR is 0.598, with a specificity of 0.804, sensitivity of 0.484, PPV of 0.234, and NPV of 0.926.
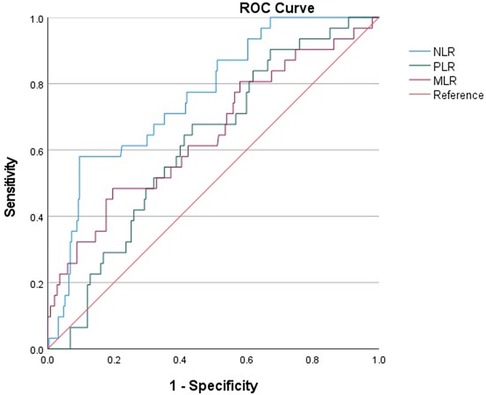
Figure 3 ROC curves for predicting all-cause death in all patients. ROC curves for predicting all-cause mortality plotted by the NLR, PLR and MLR in AMI patients combined with COPD. ROC, receiver operating characteristic; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet to lymphocyte ratio; MLR, monocyte to lymphocyte ratio.
4 Discussion
In this study, a total of 281 AMI patients combined with COPD were included, among which 31 patients (11.0%) experienced in-hospital mortality. Patients with higher NLR values were found to experience a significantly increased rate of all-cause mortality compared to those with lower NLR, and the similar association was found in the MLR and PLR. After adjusting for potential confounding factors, NLR were identified as independent predictor of all-cause mortality. Notably, NLR showed superior prognostic value compared to PLR and MLR. To our knowledge, this is the first study to evaluate the predictive value of inflammatory markers for poor prognosis in AMI patients combined with COPD.
Previous studies have found the correlation between NLR, PLR and MLR with the severity of various diseases, including sepsis, pulmonary diseases and coronary artery disease (11). Our research has found that NLR was a significant predictor of poor prognosis in AMI patients combined with COPD. Although the prognostic value of inflammation for COPD patients with concomitant AMI has not been previously explored, previous studies have already identified NLR as predictor for AMI and COPD, respectively. NLR has been identified as a significant biomarker for both inflammation monitoring and prognosis in COPD patients. Shaghayegh Rahimirad et al. conducted a retrospective study on 315 COPD patients and found that the NLR values were higher in patients who died in the hospital compared to those who were discharged alive. Additionally, patients with higher NLR levels had a higher mortality rate than those with lower NLR levels (12). Paliogiannis et al. also found that NLR plays a significant role in predicting exacerbation and mortality in COPD patients (13). Ahmed Gouda El-Gazzar et al. found that NLR serves as a direct and valuable marker for predicting in-hospital mortality in COPD patients (14). A meta-analysis on the prognosis of COPD patients suggests that NLR may be an independent predictor of acute exacerbations in COPD patients. Furthermore, a high NLR may be associated with increased mortality in COPD patients, especially among Asians (15).
The potential mechanisms by which NLR is associated with poor prognosis in COPD patients may include: during the inflammatory process of COPD, neutrophils levels notably increase during severe acute exacerbations, particularly those induced by bacterial infections, though this increase is not exclusively linked to bacterial causes (16). Neutrophils release a substantial quantity of inflammatory mediators, contributing to the acute inflammatory response at the site of tissue injury (17). Neutrophils adhere to endothelial cells and cause lung tissue damage by discharging oxygen free radicals and proteolytic enzymes, ultimately leading to the destruction of alveoli and emphysema. Chronic immune stimulation has been observed to lead to an increase in lymphocyte levels. A low lymphocyte count is correlated with a poor prognosis in patients with acute diseases and chronic conditions, such as CVD and COPD. The reduction in lymphocytes, signaling a lower survival rate, is associated with factors characteristic of COPD, including being older and having a poor nutritional status (18).
In recent years, studies have observed that changes in NLR are a more reliable indicator of CVD than any other white blood cell subtype. Many studies have reported a significant association between elevated NLR levels and both cardiovascular mortality and all-cause mortality during hospital stay or long-term period in ST-elevation myocardial infarction (STEMI) patients (19). Zhenjun Ji et al. conducted a study involving 2,618 AMI patients and found that, compared to other common blood routine examinations (BRE), NLR was a better predictor of in-hospital mortality in AMI patients (20). Another study involving AMI patients found that high NLR levels were associated with a higher incidence of major adverse cardiovascular event (MACE) compared to lower NLR levels (21). Jingyu He et al. found that when AMI patients were stratified into tertiles based on NLR, patients in the T3 group had a 4.621 times higher risk of mortality compared to those in the T1 group (22). G-K Lee et al. found that NLR measured at admission, 24 h and 72 h after admission, and before discharge can all predict mortality in STEMI patients (23). This is consistent with our findings, the NLR was higher in the mortality group of AMI patients with COPD.
With inflammatory stimulation, the excessive production and release of neutrophils can promote the formation and progression of atherosclerosis. Also, neutrophils play an important role in the instability of atherosclerotic plaques, and they are the first subtype of white blood cells to infiltrate damaged myocardium, subsequently leaving the myocardial tissue after phagocytosing debris. During the onset and progression of AMI, the body experiences heightened inflammatory activation and increased cortisol levels. Elevated cortisol is known to induce apoptosis, resulting in lymphocyte reduction and an inversion of the CD4+/CD8+ T lymphocyte ratio. A significant decrease in CD4+ lymphocytes has been identified as a predictor of poor prognosis in AMI patients (24). Under the stress condition of myocardial infarction, the activation of the neuroendocrine system promotes lymphocyte apoptosis and inhibits the proliferation and differentiation of lymphocytes, leading to a reduction in peripheral blood lymphocyte count. Therefore, the decrease in lymphocyte count is closely related to the severity of myocardial infarction (25).
Comorbidities are recognized as a critical aspect of COPD, with CVD being one of the most common prevalent systemic manifestations that significantly impact these patients (26). Previous studies have demonstrated a strong association between COPD and CVD, particularly AMI. This association is caused not only by common risk factors but also by COPD-specific factors such as systemic inflammation and hypoxia, which contribute to the pathophysiological interplay between COPD and AMI. Although the exact mechanism by which COPD increases the risk of CVD remains unclear, previous studies have found that patients with COPD often exhibit elevated levels of systemic inflammatory biomarkers, such as C-reactive protein, IL-6, and fibrinogen, which can predict the risk of CVD in both the general population and patients with COPD (27). Maria-Elpida Christopoulou et al. thought that factors such as systemic inflammation, thrombosis, and oxidative stress associated with COPD make AMI closely related to COPD (28). The mechanisms underlying this association also include the unique physiological crosstalk between the lungs and the heart. This physiological interaction means that inflammation in the lungs can significantly impact the heart. Furthermore, systemic inflammation and hypoxia resulting from COPD have been identified as independent risk factors for AMI (29). CVD and COPD are both characterized by inflammation (30), and it's believed that systemic inflammatory response could significantly contribute to the pulmonary and systemic endothelial dysfunction (31). This highlights the importance of inflammatory markers in AMI patients with COPD. However, there are still few studies in this area.
Moreover, in patients with COPD, systemic inflammation may induce a procoagulant state, evidenced by elevated levels of tissue factor procoagulant activity and thrombin-antithrombin complexes. These changes lead to both pro-thrombotic and pro-inflammatory states (32). In CVD, the production of cytokines caused by activation of immune cells within atherosclerotic plaques, such as interferon-γ, tumor necrosis factor-α and acute-phase inflammatory proteins, including fibrinogen, amyloid and C-reactive protein, is observed (33). The mediators are observed in the inflammatory reactions in the bronchi of COPD patients. Previous studies have shown that chronic pulmonary inflammation can accelerate the progression of atherosclerotic plaques and that acute inflammatory episodes, such as acute exacerbations of COPD, can induce plaque rupture, potentially leading to cardiovascular events and worsening the prognosis (34). This is consistent with our findings that higher NLR levels were associated with increased mortality in AMI patients combined with COPD. NLR can systematically and accurately reflect the degree of inflammation and stress in these patients.
Notably, CVD is responsible for up to 1/3 of deaths in COPD patients (26). Laurien Goedemans et al. found the prognosis for COPD patients combined with AMI is notably poorer compared to those without COPD, including all-cause death and major adverse cardiac and cerebrovascular events (MACCE) during both short-term and long-term follow-up (5). By using the UK Myocardial Ischemia National Audit Program database, Kieran J Rothnie et al. found that patients with COPD had higher in-hospital and 180-day mortality rates compared to those without COPD (35). Previous studies have proposed that the increased mortality in these patients may be since they always present with atypical symptoms such as chest pain or dyspnea during acute phases. This can result in diagnostic and treatment delays (36), potentially leading to delayed reperfusion and an increase in infarct size, thereby worsening the prognosis (35). Our research considered that inflammatory responses play a significant role in this adverse prognosis.
Therefore, monitoring blood levels of systemic inflammatory biomarkers, particularly NLR, may provide an additional level of risk stratification for AMI patients combined with COPD. By analyzing the level of NLR, clinicians can gain valuable insights into the inflammatory status of these patients, which is crucial for identifying those at higher risk of adverse outcomes. Combining our findings with previous research, we propose that NLR is a valuable tool for accurately assessing the severity and prognosis of AMI patients with COPD. For high-risk patients identified through elevated NLR levels, several strategies can be implemented to decrease NLR and subsequently reduce mortality risk. These strategies include anti-inflammatory treatment (37), lifestyle improvements such as quitting smoking (38), maintaining a balanced diet, and engaging in regular physical activity (39), optimized management of comorbidities like hypertension and diabetes, early and aggressive intervention for patients with elevated NLR, and personalized treatment plans tailored to individual patient needs. By integrating these approaches, clinicians can more effectively manage inflammation and improve the overall prognosis for COPD patients with AMI, reducing their mortality risk.
Some limitations should be recognized. First, this is a single-center study with a small sample size. Second, due to the retrospective characteristic of the study, some clinical information could not be obtained. Third, the study was conducted in Northwest China, which may limit the generalizability of the findings to other populations. Future studies should include diverse geographical regions to validate and extend these findings. Finally, our findings need to be validated with external datasets to ensure the robustness and applicability of the results. Despite these limitations, this study can provide a basis for future research on the relationship between inflammation and adverse outcomes in AMI patients with COPD, promoting risk stratification and management for these patients.
5 Conclusion
In summary, NLR was independent risk factor for in-hospital death in AMI patients combined with COPD. Moreover, patients with higher NLR associated with a higher risk of in-hospital death compared to those with lower values. Clinicians should be more vigilant with patients who have higher inflammatory indicators, recognizing the heavier burden of adverse prognosis in such patients. Early identification and appropriate treatment are very important for the management of these patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (No: XJTU1AF2023LSK-515). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study does not involve additional biological samples, and only retrospectively collects medical record information of hospitalized patients, as well as laboratory, radiological, and ultrasonographic examination results conducted during hospitalization; it will not produce additional interventions and will not adversely affect the rights and health of the patients.
Author contributions
PD: Conceptualization, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. FW: Conceptualization, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. HY: Investigation, Supervision, Visualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research was funded by the Shaanxi Provincial Key R&D Program General Project (2022SF-058; No. 2022SF-239), the Natural Science Foundation of Shaanxi Province (2021JM-038), the Innovation Capacity Support Plan Project of Shaanxi Province (2022KJXX-49) and the Key Research and Development Program of Shaanxi Province (No. 2022SF-239).
Acknowledgments
We are grateful to the Biobank of The First Affiliated Hospital of Xi'an Jiaotong University for providing clinical data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. (2017) 389(10082):1931–40. doi: 10.1016/S0140-6736(17)31222-9
2. Sayed AAE-A, Ahmed MM, Salah H. Pattern of chronic obstructive pulmonary disease patients. QJM Int J Med. (2023) 116(Supplement_1). doi: 10.1093/qjmed/hcad069.166
3. Anderson JL, Morrow DA. Acute myocardial infarction. N Engl J Med. (2017) 376(21):2053–64. doi: 10.1056/NEJMra1606915
4. Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, et al. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol. (2018) 72(17):2071–81. doi: 10.1016/j.jacc.2018.08.1043
5. Goedemans L, Bax JJ, Delgado V. COPD and acute myocardial infarction. Eur Respir Rev. (2020) 29(156):190139. doi: 10.1183/16000617.0139-2019
6. Hua Y, Sun JY, Lou YX, Sun W, Kong XQ. Monocyte-to-lymphocyte ratio predicts mortality and cardiovascular mortality in the general population. Int J Cardiol. (2023) 379:118–26. doi: 10.1016/j.ijcard.2023.03.016
7. Delcea C, Buzea CA, Vîjan AE, Bădilă E, Dan GA. The platelet to lymphocyte ratio in heart failure: a comprehensive review. Rom J Intern Med. (2023) 61(2):84–97. doi: 10.2478/rjim-2023-0006
8. Ball MK, Waypa GB, Mungai PT, Nielsen JM, Czech L, Dudley VJ, et al. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am J Respir Crit Care Med. (2014) 189(3):314–24. doi: 10.1164/rccm.201302-0302OC
9. Balta S, Ozturk C. The platelet-lymphocyte ratio: a simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets. (2015) 26(7):680–1. doi: 10.3109/09537104.2014.979340
10. Rothnie KJ, Yan R, Smeeth L, Quint JK. Risk of myocardial infarction (MI) and death following MI in people with chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open. (2015) 5(9):e007824. doi: 10.1136/bmjopen-2015-007824
11. Mirna M, Schmutzler L, Topf A, Hoppe UC, Lichtenauer M. Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio predict length of hospital stay in myocarditis. Sci Rep. (2021) 11(1):18101. doi: 10.1038/s41598-021-97678-6
12. Rahimirad S, Ghaffary MR, Rahimirad MH, Rashidi F. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute exacerbation of chronic obstructive pulmonary disease. Tuberk Toraks. (2017) 65(1):25–31. doi: 10.5578/tt.27626
13. Paliogiannis P, Fois AG, Sotgia S, Mangoni AA, Zinellu E, Pirina P, et al. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: recent evidence and future perspectives. Eur Respir Rev. (2018) 27(147):170113. doi: 10.1183/16000617.0113-2017
14. El-Gazzar AG, Kamel MH, Elbahnasy OKM, El-Naggar ME. Prognostic value of platelet and neutrophil to lymphocyte ratio in COPD patients. Expert Rev Respir Med. (2020) 14(1):111–6. doi: 10.1080/17476348.2019.1675517
15. Ye Z, Ai X, Liao Z, You C, Cheng Y. The prognostic values of neutrophil to lymphocyte ratio for outcomes in chronic obstructive pulmonary disease. Medicine (Baltimore). (2019) 98(28):e16371. doi: 10.1097/MD.0000000000016371
16. Bathoorn E, Kerstjens H, Postma D, Timens W, MacNee W. Airways inflammation and treatment during acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. (2008) 3(2):217–29. doi: 10.2147/COPD.S1210
17. Jala VR, Haribabu B. Leukotrienes and atherosclerosis: new roles for old mediators. Trends Immunol. (2004) 25(6):315–22. doi: 10.1016/j.it.2004.04.003
18. Rovina N, Koutsoukou A, Koulouris NG. Inflammation and immune response in COPD: where do we stand? Mediators Inflamm. 2013 2013:413735. doi: 10.1155/2013/413735
19. Zhao N, Mi L, Liu X, Pan S, Xu J, Xia D, et al. Combined value of red blood cell distribution width and global registry of acute coronary events risk score for predicting cardiovascular events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. PLoS One. (2015) 10(10):e0140532. doi: 10.1371/journal.pone.0140532
20. Ji Z, Liu G, Guo J, Zhang R, Su Y, Carvalho A, et al. The neutrophil-to-lymphocyte ratio is an important indicator predicting in-hospital death in AMI patients. Front Cardiovasc Med. (2021) 8(706852). doi: 10.3389/fcvm.2021.706852
21. Chen B, Yuan L, Chen X, Li J, Tao J, Li W, et al. Correlations and prognostic roles of the nutritional status and neutrophil-to-lymphocyte ratio in elderly patients with acute myocardial infarction undergoing primary coronary intervention. Int Heart J. (2020) 61(6):1114–20. doi: 10.1536/ihj.20-138
22. He J, Li J, Wang Y, Hao P, Hua Q. Neutrophil-to-lymphocyte ratio (NLR) predicts mortality and adverse-outcomes after ST-segment elevation myocardial infarction in Chinese people. Int J Clin Exp Pathol. (2014) 7(7):4045–56.25120783
23. Lee GK, Lee LC, Chong E, Lee CH, Teo SG, Chia BL, et al. The long-term predictive value of the neutrophil-to-lymphocyte ratio in type 2 diabetic patients presenting with acute myocardial infarction. QJM. (2012) 105(11):1075–82. doi: 10.1093/qjmed/hcs123
24. Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. Eur Heart J. (2006) 27(21):2530–7. doi: 10.1093/eurheartj/ehl222
25. Mooren FC, Blöming D, Lechtermann A, Lerch MM, Völker K. Lymphocyte apoptosis after exhaustive and moderate exercise. J Appl Physiol (1985). 2002 93(1):147–53. doi: 10.1152/japplphysiol.01262.2001
26. Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. (2015) 3(8):631–9. doi: 10.1016/S2213-2600(15)00241-6
27. Kunisaki KM, Dransfield MT, Anderson JA, Brook RD, Calverley PMA, Celli BR, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med. (2018) 198(1):51–7. doi: 10.1164/rccm.201711-2239OC
28. Christopoulou ME, Papakonstantinou E, Stolz D. Matrix metalloproteinases in chronic obstructive pulmonary disease. Int J Mol Sci. (2023) 24(4):3786. doi: 10.3390/ijms24043786
29. Zinellu A, Zinellu E, Mangoni AA, Pau MC, Carru C, Pirina P, et al. Clinical significance of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute exacerbations of COPD: present and future. Eur Respir Rev. (2022) 31(166):220095. doi: 10.1183/16000617.0095-2022
30. Cavaillès A, Brinchault-Rabin G, Dixmier A, Goupil F, Gut-Gobert C, Marchand-Adam S, et al. Comorbidities of COPD. Eur Respir Rev. (2013) 22(130):454–75. doi: 10.1183/09059180.00008612
31. Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, Reeves AP, et al. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the emphysema and cancer action project (EMCAP) study. Am J Respir Crit Care Med. (2007) 176(12):1200–7. doi: 10.1164/rccm.200707-980OC
32. Vaidyula VR, Criner GJ, Grabianowski C, Rao AK. Circulating tissue factor procoagulant activity is elevated in stable moderate to severe chronic obstructive pulmonary disease. Thromb Res. (2009) 124(3):259–61. doi: 10.1016/j.thromres.2008.12.030
33. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. (2005) 352(16):1685–95. doi: 10.1056/NEJMra043430
34. Man SF, Van Eeden S, Sin DD. Vascular risk in chronic obstructive pulmonary disease: role of inflammation and other mediators. Can J Cardiol. (2012) 28(6):653–61. doi: 10.1016/j.cjca.2012.06.013
35. Rothnie KJ, Smeeth L, Herrett E, Pearce N, Hemingway H, Wedzicha J, et al. Closing the mortality gap after a myocardial infarction in people with and without chronic obstructive pulmonary disease. Heart. (2015) 101(14):1103–10. doi: 10.1136/heartjnl-2014-307251
36. Andell P, Koul S, Martinsson A, Sundström J, Jernberg T, Smith JG, et al. Impact of chronic obstructive pulmonary disease on morbidity and mortality after myocardial infarction. Open Heart. (2014) 1(1):e000002. doi: 10.1136/openhrt-2013-000002
37. Szymanska P, Rozalski M, Wilczynski M, Golanski J. Systemic immune-inflammation index (SII) and neutrophil to lymphocyte ratio (NLR) are useful markers for assessing effects of anti-inflammatory diet in patients before coronary artery bypass grafting. Rocz Panstw Zakl Hig. (2021) 72(3):327–35. doi: 10.32394/rpzh.2021.0170
38. Komiyama M, Ozaki Y, Miyazaki Y, Katanasaka Y, Sunagawa Y, Funamoto M, et al. Neutrophil/lymphocyte ratio is correlated with levels of inflammatory markers and is significantly reduced by smoking cessation. J Int Med Res. (2021) 49(6):3000605211019223. doi: 10.1177/03000605211019223
Keywords: acute myocardial infarction, chronic obstructive pulmonary disease, neutrophil-to-lymphocyte ratio, platelet to lymphocyte ratio, monocyte to lymphocyte ratio, prognosis
Citation: Dang P, Wang F and Yu H (2024) Prognostic potential of neutrophil-to-lymphocyte ratio, platelet to lymphocyte ratio, and monocyte to lymphocyte ratio in acute myocardial infarction patients combined with chronic obstructive pulmonary disease. Front. Cardiovasc. Med. 11: 1401634. doi: 10.3389/fcvm.2024.1401634
Received: 15 March 2024; Accepted: 26 June 2024;
Published: 12 July 2024.
Edited by:
Nicola Mumoli, ASST Ovest Milanese, ItalyReviewed by:
Josef Yayan, University of Witten/Herdecke, GermanyEmanuel Moisa, Carol Davila University of Medicine and Pharmacy, Romania
Fen Jiang, University of South China, China
Feitian Li, Fudan University, China
© 2024 Dang, Wang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hang Yu, eXVoYW5nQHhqdHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Peizhu Dang
Peizhu Dang Feiyang Wang
Feiyang Wang Hang Yu
Hang Yu