- 1Department of Medicine, Smt. Kashibai Navale Medical College and General Hospital, Pune, India
- 2Department of Medicine, Royal Glamorgan Hospital, Pontyclun, United Kingdom
- 3Department of Internal Medicine, East Carolina University, Greenville, NC, United States
- 4Department of Medicine, Mansoura University, Mansoura, Egypt
- 5Department of Medicine, Liaquat National Medical College, Karachi, Pakistan
- 6Department of Haematology-Oncology, National University Hospital (NUH), Singapore, Singapore
- 7Department of Radiology and Medical Imaging, Jinnah Hospital, Lahore, Pakistan
- 8Department of Cardiology, King Edward Medical University, Lahore, Pakistan
- 9Department of Internal Medicine, Mass General Brigham-Salem Hospital, Salem, MA, United States
- 10Department of Internal Medicine, United Health Services Hospital, Johnson, NY, United States
- 11Hamad Medical Corporation, Doha, Qatar
- 12National Heart & Lung Institute, Imperial College London, London, United Kingdom
- 13Department of Cardiology, Royal Brompton Hospital, London, United Kingdom
- 14Cardiology Division, Department of Internal Medicine, Temple University Hospital, Philadelphia, PA, United States
- 15Department of Cardiovascular Disease, Adena Regional Medical Center, Chillicothe, OH, United States
- 16Department of Clinical Biochemistry, King’s College Hospital NHS Foundation Trust, London, United Kingdom
- 17Honorary Senior Lecturer, Faculty of Life Sciences & Medicine, King’s College London, London, United Kingdom
Background: Optical coherence tomography (OCT) and intravascular ultrasound (IVUS) are superior to coronary angiography for guiding percutaneous coronary intervention (PCI). However, whether one technique is superior to the other is inconclusive.
Methods: We searched PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov from inception to November 2023 for randomized controlled trials (RCTs) comparing OCT and IVUS in patients undergoing PCI. RevMan 5.4 was used to pool outcomes with risk ratio (RR) as the effect measure.
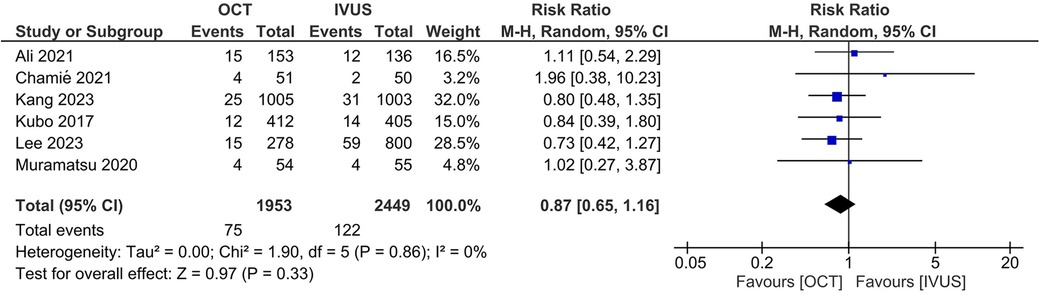
Results: Six RCTs (4,402 patients) were included in this meta-analysis. There was no significant difference between the OCT- and IVUS-guided PCI groups in the risk of major adverse cardiovascular events (RR 0.87, 95% CI: 0.65, 1.16; I2 = 0%) and cardiac mortality (RR 0.73, 95% CI: 0.24, 2.21; I2 = 0%). The results were consistent across the subgroups of the presence or absence of left main disease (Pinteraction >0.1). There were no significant differences between OCT and IVUS in the risk of target lesion revascularization (RR 0.78, 95% CI: 0.47, 1.30; I2 = 0%), target vessel revascularization (RR 1.06, 95% CI: 0.69, 1.62; I2 = 0%), target-vessel myocardial infarction (RR 0.79, 95% CI: 0.40, 1.53; I2 = 0%), stent thrombosis (RR 0.59, 95% CI: 0.12, 2.97; I2 = 0%), and all-cause mortality (RR 1.01, 95% CI: 0.53, 1.90; I2 = 0%).
Conclusions: Our meta-analysis demonstrated similar clinical outcomes in OCT- and IVUS-guided PCI. New large-scale multicenter RCTs with long-term follow-up are required to confirm or refute our findings and provide more reliable results.
Systematic Review Registration: PROSPERO, identifier, CRD42023486933
Introduction
Despite its known limitations, coronary angiography has long been considered the gold standard for diagnosing coronary artery disease and guiding percutaneous coronary intervention (PCI) (1). More specifically, its reliance on a 2-dimensional projection falls short of fully capturing the 3-dimensional nature of the coronary lumen (2).
Recently, optical coherence tomography (OCT) and intravascular ultrasound (IVUS) have emerged as valuable tools capable of overcoming several limitations associated with coronary angiography (1). Multiple studies have indicated that IVUS- and OCT-guided PCI yield better clinical outcomes, including reduced cardiac mortality and major adverse cardiac events (MACE), compared to coronary angiography-guided PCI (3–5). IVUS optimizes and guides stent placement by providing enhanced information regarding vessel lumen dimensions, plaque characteristics, overall plaque burden, and the extent of calcification (6). OCT offers higher resolution than IVUS and can be particularly helpful in guiding PCI, especially in lipid-rich plaque and severely calcified lesions (6, 7).
While multiple trials have focused on establishing the superiority of IVUS and OCT compared to coronary angiography alone, only a limited number of studies have compared OCT directly to IVUS. Previous meta-analyses have largely focused on indirect comparisons to determine which imaging modality is superior to the other (3, 4) and, in some cases, have also included observational studies that provide a poorer quality of evidence (4, 5). Recently, the results of the largest trial to date addressing this question, the OCTIVUS trial (2,008 patients), have been published (8). Therefore, we sought to conduct this meta-analysis to compare the outcomes of OCT-guided PCI to IVUS-guided PCI using data from randomized controlled trials (RCTs).
Methods
This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement and the Cochrane Collaboration guidelines (9, 10). The protocol has been registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the following identifier (CRD42023486933). No form of ethical approval was required for our study as only publicly available data was used.
Data sources and searches
The following databases were searched from inception to November 2023: MEDLINE (via PubMed), Embase, the Cochrane Central Register of Controlled Trials (CENTRAL, via the Cochrane Library), and ClinicalTrials.gov. A search strategy comprising relevant medical subject headings (MeSH) and keywords was utilized and has been reported in detail in Supplementary Table S1. In addition, a partial grey literature search (via Google Scholar) and backward citation tracking using relevant medical literature were also conducted.
Eligibility criteria
Studies meeting the following criteria were included: (1) study design: RCTs only; (2) population: patients undergoing PCI regardless of indication; (3) intervention: OCT-guided PCI; (4) comparator: IVUS-guided PCI; (5) outcomes: reporting of any outcome of interest. For multi-arm trials, only data for the IVUS and OCT arms were obtained.
The exclusion criteria included the following: (1) all study designs other than RCTs, such as quasi-randomized trials and observational studies; (2) studies conducted on animals; and (3) single-arm trials.
Study selection and data extraction
All literature retrieved from our search was imported into Mendeley Desktop 1.19.8, where all duplicates were removed; studies were then transferred to Rayyan to begin the screening process. Two reviewers independently screened the title and abstract of all relevant papers, followed by a full-text screening. The two authors resorted to discussion and consultation with a third author to resolve conflicts.
Data regarding study characteristics (including authors, trial name, and study location), patient population (including age and gender), cardiac disease (acute coronary syndrome, left main disease, multi-vessel disease, as well as lesion type and type of stent), study follow-up, and primary and secondary outcomes were extracted into a pre-piloted Excel spreadsheet.
Outcomes
Our primary outcomes were the incidence of MACE and cardiac mortality. Our secondary outcomes included target lesion revascularization (TLR), target vessel revascularization (TVR), target vessel myocardial infarction (MI), stent thrombosis, and all-cause mortality.
Risk of bias assessment
In order to assess the internal validity of the included RCTs, two authors independently applied the revised Cochrane “Risk of Bias” tool (RoB 2.0) (11). RoB 2.0 assesses the risk of bias using the following five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of outcome, and selective outcome reporting. The studies were assigned a rating of low risk of bias, some concerns, or a high risk of bias. Any disagreement was resolved by consulting a third reviewer.
Data synthesis
The meta-analysis was carried out using Review Manager (RevMan, Version 5.4; The Cochrane Collaboration, Copenhagen, Denmark) under a random-effects model. Risk ratio (RR) with the corresponding 95% confidence interval (CI) was utilized as the effect measure. We used the I2 and Chi2 statistics to report statistical heterogeneity (I2 = 25%–50% was considered mild, 50%–75% moderate, and >75% severe heterogeneity). Additionally, a subgroup analysis based on including or excluding patients with left main disease in the studies was undertaken for our primary outcomes. A P-value of <0.1 was considered critical for the test for subgroup differences (12). Furthermore, a sensitivity analysis was conducted by excluding studies at a high risk of bias. It is not recommended to assess publication bias when the number of included studies is less than 10; nevertheless, for our primary outcomes, we constructed funnel plots and ran Egger's regression test to evaluate for publication bias.
Results
Study selection and characteristics
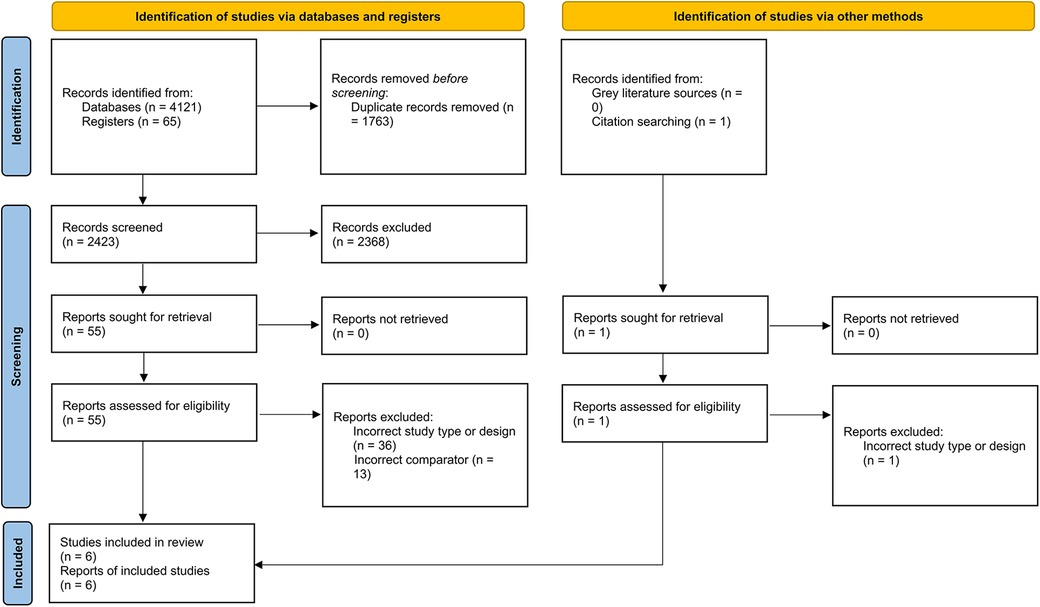
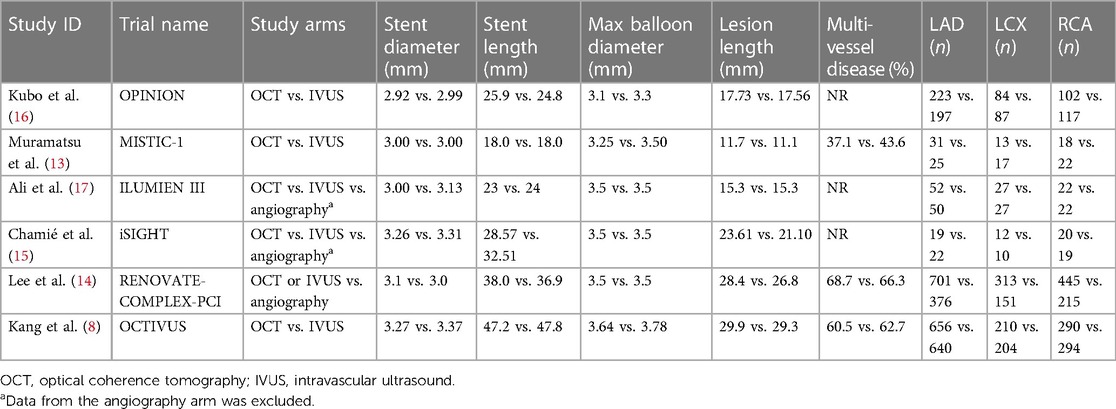
A total of 6 RCTs (4,402 patients) were included in this meta-analysis after a thorough systemic search (Figure 1) (13–17). Two of these studies were from Japan (13, 16), two from South Korea (8, 14), and one from Brazil (15); the remaining study was conducted in 8 countries (17). Three RCTs included patients with left main disease (8, 14, 16). The types of lesions differed between the trials, including thrombotic lesions, calcifications, and bifurcation lesions. Detailed information about each study is provided in Tables 1, 2.
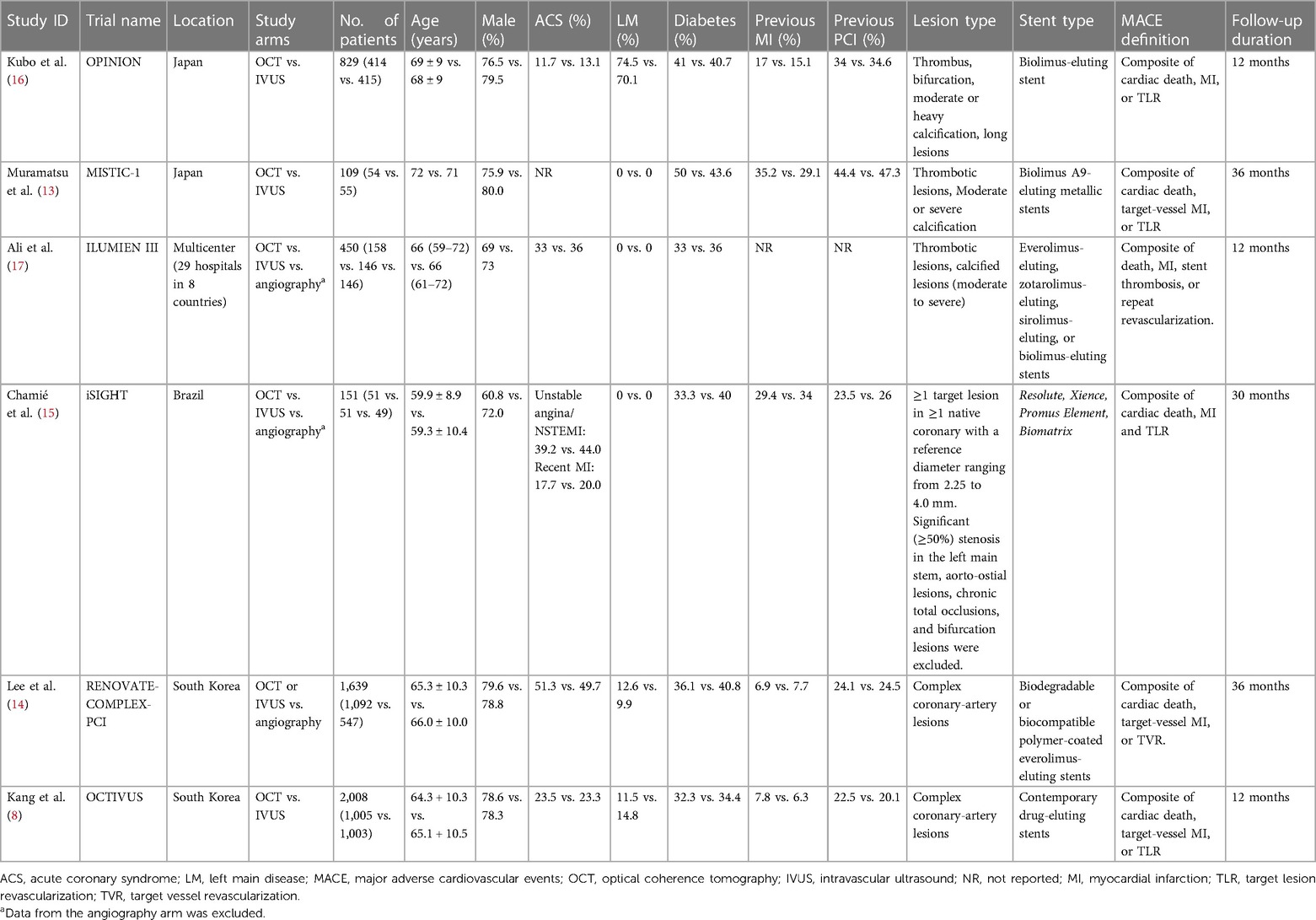
Table 1. Characteristics of included studies and main baseline clinical characteristics of included patients.
Risk of bias assessment
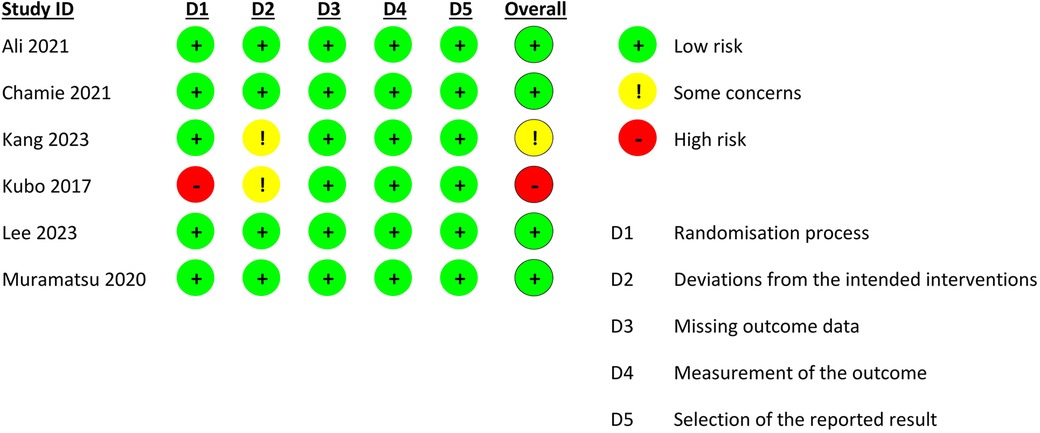
Four studies were deemed to be at a low risk of bias (13–15, 17), one study had some concerns due to deviations from intended interventions (8), and one study had a high risk of bias due to issues in the domain of randomization (Figure 2) (16).
Results of the meta-analysis
Primary outcomes
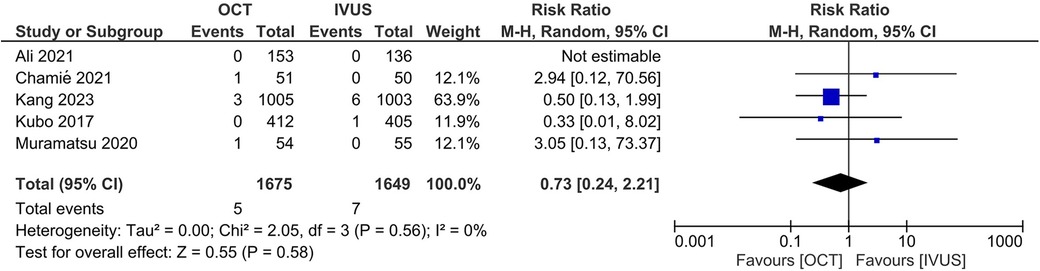
There was no significant difference between the OCT- and IVUS-guided PCI groups in the risk of MACE (RR 0.87, 95% CI: 0.65, 1.16; I2 = 0%; Figure 3) and cardiac mortality (RR 0.73, 95% CI: 0.24, 2.21; I2 = 0%; Figure 4). The results were consistent across the subgroups of the presence or absence of left main disease (Pinteraction >0.1; Supplementary Figures S1, S2). A sensitivity analysis excluding the trial with a high risk of bias demonstrated similar findings. There was no indication of publication bias in either of the two primary outcomes (Egger's P-value >0.05; Supplementary Figures S3, S4).
Secondary outcomes
There were no significant differences between OCT and IVUS in the risk of TLR (RR 0.78, 95% CI: 0.47, 1.30; I2 = 0%; Supplementary Figure S5), TVR (RR 1.06, 95% CI: 0.69, 1.62; I2 = 0%; Supplementary Figure S6), target-vessel MI (RR 0.79, 95% CI: 0.40, 1.53; I2 = 0%; Supplementary Figure S7), stent thrombosis (RR 0.59, 95% CI: 0.12, 2.97; I2 = 0%; Supplementary Figure S8), and all-cause mortality (RR 1.01, 95% CI: 0.53, 1.90; I2 = 0%; Supplementary Figure S9). The results did not change substantially upon exclusion of the trial with a high risk of bias.
Discussion
To the best of our knowledge, this is the most comprehensive meta-analysis on this topic to date. Our analysis comparing OCT with IVUS guidance demonstrated no difference between the two imaging modalities regarding the risk of MACE, cardiac mortality, TLR, TVR, target-vessel MI, stent thrombosis, and all-cause mortality. The results were consistent regardless of the presence or absence of left main disease in the pooled patient analysis.
These findings align with previous analyses comparing the same outcomes between the two modalities (3–5, 18), although there has been some indication that IVUS might be the better imaging modality (3). Nevertheless, the prior meta-analyses suffered from many limitations, including indirect comparisons, the incorporation of observational studies (which confer the risk of confounding bias), and the inclusion of only a few small RCTs, which provided low statistical power. Our analysis focused only on randomized trials that directly compared OCT and IVUS and had increased power due to the inclusion of recent large-scale RCTs, therefore providing more reliable results.
The finer resolution and image quality of both OCT and IVUS allow for a better understanding of luminal anatomy, plaque location, and precise vessel dimensions, which allow for improved stent sizing and positioning (19, 20). Their improved ability to discern stent malpositioning, under-expansion, and edge dissection elucidates the improved clinical outcomes compared to conventional angiographic guidance (3, 4). However, when compared to each other, our findings show no evidence of superior clinical benefit of either OCT or IVUS. These results further consolidate the guidelines of the American Heart Association/American College of Cardiology/Society of Cardiovascular Angiography & Interventions, which state that OCT and IVUS are justifiable alternatives to each other, with the sole exception of ostial left main disease, in which case IVUS is preferred (21). Nevertheless, it is important to note that due to the low incidence of some outcomes, future large trials and subsequent meta-analyses will be needed to attain adequate statistical power to elucidate whether either of these two techniques is superior.
The majority of studies indicate that OCT guidance at the time of PCI leads to the use of larger stent diameters than would have been chosen based on angiography alone. However, when compared to IVUS, OCT has been shown to result in a smaller minimal stent area (MSA) (22, 23). Although the use of infrared light-based technology behind OCT allows the production of detailed cross-sectional imaging of the luminal wall with a 10-fold higher resolution compared to IVUS, its relative inability to traverse through the entire vessel wall limits the complete assessment of the full vessel dimension (24, 25). The ultrasound-guided approach in IVUS allows for deeper transmittance along with much better and consistent visualization of the external elastic lamina, elucidating the entire vessel wall thickness (5). Nevertheless, these differences between the two techniques did not translate to any differences in relevant clinical outcomes in our analysis.
The repeated need to clear the blood columns by saline or contrast to generate precise imaging in OCT-guided PCI adds to its procedural complexity, questioning its application in contrast-sensitive patients with compromised renal function and potentially limiting its widespread use (26). A recent report showed that the application of OCT and IVUS guidance is limited to only 0.6% and 8.7% of PCIs for MI in the US, respectively (26); factors restricting their extensive use include limited operator expertise, higher financial burden, and the lack of necessary technology in some hospitals (27, 28).
There are some limitations to our meta-analysis. Although all of our outcomes had low statistical heterogeneity, some residual heterogeneity likely exists due to differences in anatomical and procedural characteristics between the trials. Additionally, since we did not have access to individual patient data, we could not extensively investigate potential effect modifiers in our study-level analysis. Furthermore, despite our meta-analysis being the largest one to date, it may still be underpowered for some outcomes. Lastly, the impact of OCT vs. IVUS on long-term outcomes is uncertain due to a lack of longer follow-ups; further large-scale RCTs with more extensive follow-ups are required to confirm our findings and provide conclusive proof.
Conclusions
Our meta-analysis comparing OCT-guided PCI with IVUS-guided PCI demonstrated no significant difference between the two modalities regarding the incidence of MACE, cardiac death, TLR, TVR, target-vessel MI, stent thrombosis, and all-cause mortality. The choice of the imaging modality will depend on the availability of necessary technology and resources, and operator expertise. New large-scale multicenter RCTs with long-term follow-up are required to confirm or refute our findings and provide more reliable results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
VV: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. AE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. SH: Data curation, Investigation, Methodology, Writing – original draft. REA: Data curation, Formal Analysis, Methodology, Writing – original draft. FM: Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. JP: Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft. MA: Investigation, Validation, Visualization, Writing – original draft. HC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AA: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. WR: Data curation, Validation, Visualization, Writing – review & editing. AN: Resources, Supervision, Writing – review & editing. RA: Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing. VL: Investigation, Resources, Supervision, Validation, Visualization, Writing – review & editing. HV: Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. RV: Supervision, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1395606/full#supplementary-material
References
1. Sattar Y, Abdul Razzack A, Kompella R, Alhajri N, Arshad J, Ullah W, et al. Outcomes of intravascular ultrasound versus optical coherence tomography guided percutaneous coronary angiography: a meta regression-based analysis. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. (2022) 99:E1–E11. doi: 10.1002/ccd.29976
2. Lee CH, Hur SH. Optimization of percutaneous coronary intervention using optical coherence tomography. Korean Circ J. (2019) 49:771–93. doi: 10.4070/kcj.2019.0198
3. Park DY, An S, Jolly N, Attanasio S, Yadav N, Gutierrez JA, et al. Comparison of intravascular ultrasound, optical coherence tomography, and conventional angiography-guided percutaneous coronary interventions: a systematic review, network meta-analysis, and meta-regression. Catheter Cardiovasc Interv. (2023) 102:440–50. doi: 10.1002/ccd.30784
4. Şaylık F, Hayıroglu Mİ, Akbulut T, Çınar T. Comparison of long-term outcomes between intravascular ultrasound-, optical coherence tomography- and angiography-guided stent implantation: a meta-analysis. Angiology. (2023):00033197231198674. doi: 10.1177/00033197231198674
5. Siddiqi TJ, Khan MS, Karimi Galougahi K, Shlofmitz E, Moses JW, Rao S, et al. Optical coherence tomography versus angiography and intravascular ultrasound to guide coronary stent implantation: a systematic review and meta-analysis. Catheter Cardiovasc Interv. (2022) 100:S44–56. doi: 10.1002/ccd.30416
6. Gao X-F, Kong X-Q, Zuo G-F, Wang Z-M, Ge Z, Zhang J-J. Intravascular ultrasound-guided versus angiography-guided percutaneous coronary intervention: evidence from observational studies and randomized controlled trials. US Cardiol Rev. (2020) 14:e03. doi: 10.15420/usc.2020.03
7. Ramasamy A, Chen Y, Zanchin T, Jones DA, Rathod K, Jin C, et al. Optical coherence tomography enables more accurate detection of functionally significant intermediate non-left main coronary artery stenoses than intravascular ultrasound: a meta-analysis of 6919 patients and 7537 lesions. Int J Cardiol. (2020) 301:226–34. doi: 10.1016/j.ijcard.2019.09.067
8. Kang D-Y, Ahn J-M, Yun S-C, Hur S-H, Cho Y-K, Lee CH, et al. Optical coherence tomography–guided or intravascular ultrasound–guided percutaneous coronary intervention: the OCTIVUS randomized clinical trial. Circulation. (2023) 148:1195–206. doi: 10.1161/CIRCULATIONAHA.123.066429
9. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71–n71. doi: 10.1136/bmj.n71
10. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Hoboken, New Jersey: Wiley Blackwell (2019). doi: 10.1002/9781119536604
11. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898–l4898. doi: 10.1136/bmj.l4898
12. Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob Health. (2019) 7:192–8. doi: 10.1016/j.cegh.2018.05.005
13. Muramatsu T, Ozaki Y, Nanasato M, Ishikawa M, Nagasaka R, Ohota M, et al. Comparison between optical frequency domain imaging and intravascular ultrasound for percutaneous coronary intervention guidance in biolimus A9-eluting stent implantation: a randomized MISTIC-1 non-inferiority trial. Circ Cardiovasc Interv. (2020) 13:e009314. doi: 10.1161/CIRCINTERVENTIONS.120.009314
14. Lee JM, Choi KH, Song YB, Lee J-Y, Lee S-J, Lee SY, et al. Intravascular imaging-guided or angiography-guided complex PCI. N Engl J Med. (2023) 388:1668–79. doi: 10.1056/NEJMoa2216607
15. Chamié D, Costa JR, Damiani LP, Siqueira D, Braga S, Costa R, et al. Optical coherence tomography versus intravascular ultrasound and angiography to guide percutaneous coronary interventions. Circ Cardiovasc Interv. (2021) 14:e009452. doi: 10.1161/CIRCINTERVENTIONS.120.009452
16. Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J. (2017) 38:3139–47. doi: 10.1093/eurheartj/ehx351
17. Ali Z, Karimi Galougahi K, Maehara A, Shlofmitz R, Fabbiocchi F, Guagliumi G, et al. Outcomes of optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation: one-year results from the ILUMIEN III: oPTIMIZE PCI trial. EuroIntervention. (2021) 16:1085–91. doi: 10.4244/EIJ-D-20-00498
18. Saleh Y, Al-abcha A, Abdelkarim O, Abdelfattah OM, Abela GS, Hashim H, et al. Meta-analysis investigating the role of optical coherence tomography versus intravascular ultrasound in low-risk percutaneous coronary intervention. Am J Cardiol. (2022) 164:136–8. doi: 10.1016/j.amjcard.2021.10.016
19. Mintz GS, Matsumura M, Ali Z, Maehara A. Clinical utility of intravascular imaging: past, present, and future. JACC Cardiovasc Imaging. (2022) 15:1799–820. doi: 10.1016/j.jcmg.2022.04.026
20. Truesdell AG, Alasnag MA, Kaul P, Rab ST, Riley RF, Young MN, et al. Intravascular imaging during percutaneous coronary intervention: JACC state-of-the-art review. J Am Coll Cardiol. (2023) 81:590–605. doi: 10.1016/j.jacc.2022.11.045
21. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145:e4–17. doi: 10.1161/CIR.0000000000001039
22. Maehara A, Ben-Yehuda O, Ali Z, Wijns W, Bezerra HG, Shite J, et al. Comparison of stent expansion guided by optical coherence tomography versus intravascular ultrasound. JACC Cardiovasc Interv. (2015) 8:1704–14. doi: 10.1016/j.jcin.2015.07.024
23. Habara M, Nasu K, Terashima M, Kaneda H, Yokota D, Ko E, et al. Impact of frequency-domain optical coherence tomography guidance for optimal coronary stent implantation in comparison with intravascular ultrasound guidance. Circ Cardiovasc Interv. (2012) 5:193–201. doi: 10.1161/CIRCINTERVENTIONS.111.965111
24. Kubo T, Akasaka T, Shite J, Suzuki T, Uemura S, Yu B, et al. OCT Compared with IVUS in a coronary lesion assessment. JACC Cardiovasc Imaging. (2013) 6:1095–104. doi: 10.1016/j.jcmg.2013.04.014
25. Bezerra HG, Attizzani GF, Sirbu V, Musumeci G, Lortkipanidze N, Fujino Y, et al. Optical coherence tomography versus intravascular ultrasound to evaluate coronary artery disease and percutaneous coronary intervention. JACC Cardiovasc Interv. (2013) 6:228–36. doi: 10.1016/j.jcin.2012.09.017
26. Park DY, Vemmou E, An S, Nikolakopoulos I, Regan CJ, Cambi BC, et al. Trends and impact of intravascular ultrasound and optical coherence tomography on percutaneous coronary intervention for myocardial infarction. IJC Heart Vasc. (2023) 45:101186. doi: 10.1016/j.ijcha.2023.101186
27. Vallabhajosyula S, El Hajj SC, Bell MR, Prasad A, Lerman A, Rihal CS, et al. Intravascular ultrasound, optical coherence tomography, and fractional flow reserve use in acute myocardial infarction. Catheter Cardiovasc Interv. (2020) 96:E59–66. doi: 10.1002/ccd.28543
Keywords: optical coherence tomography, intravascular ultrasound, percutaneous coronary intervention, OCT, IVUS
Citation: Vats V, Elahi A, Hidri S, Abdelkader RE, Munaf F, Prince JM, Asif MA, Cheema HA, Ahmad A, Rehman WU, Nashwan AJ, Ahmed R, Lakhter V, Virk HUH and Vincent RP (2024) Optical coherence tomography-guided vs. intravascular ultrasound-guided percutaneous coronary intervention: a systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 11:1395606. doi: 10.3389/fcvm.2024.1395606
Received: 18 March 2024; Accepted: 20 May 2024;
Published: 31 May 2024.
Edited by:
Gianluca Caiazzo, Azienda Sanitaria Locale Caserta, ItalyReviewed by:
Michela Faggioni, University of Pennsylvania, United StatesIsmail Dogu Kilic, Pamukkale University, Türkiye
© 2024 Vats, Elahi, Hidri, Abdelkader, Munaf, Prince, Asif, Cheema, Ahmad, Rehman, Nashwan, Ahmed, Lakhter, Virk and Vincent. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence:
Royce P. Vincent, cm95Y2UudmluY2VudEBuaHMubmV0
Huzaifa Ahmad Cheema, aHV6YWlmYWFobWFkY2hlZW1hQGdtYWlsLmNvbQ==
 Vaibhav Vats1
Vaibhav Vats1 Sinda Hidri
Sinda Hidri Rem Ehab Abdelkader
Rem Ehab Abdelkader Farhan Munaf
Farhan Munaf Muhammad Ahsan Asif
Muhammad Ahsan Asif Huzaifa Ahmad Cheema
Huzaifa Ahmad Cheema Wajeeh Ur Rehman
Wajeeh Ur Rehman Abdulqadir J. Nashwan
Abdulqadir J. Nashwan Raheel Ahmed
Raheel Ahmed Royce P. Vincent
Royce P. Vincent