- 1Department of Internal Medicine, Mayo Clinic, Rochester, MN, United States
- 2Department of Cardiovascular Medicine, Mayo Clinic, Rochester, MN, United States
- 3Department of Radiology, Mayo Clinic, Rochester, MN, United States
Background: Abnormal substrate on invasive electroanatomic mapping (EAM) correlates with areas of myocardial thinning and fibrofatty replacement in Arrhythmogenic Cardiomyopathy (ACM). However, EAM parameters are absent from all sets of diagnostic criteria for ACM.
Case summary: A 41-year-old female with no significant family history was referred for evaluation of frequent premature ventricular complexes (PVCs). Twelve-lead ECG showed diffuse low-voltage QRS complexes. Holter monitor showed 28% burden of PVCs with various morphologies consistent with right ventricular (RV) inflow and outflow tract exits. Transthoracic echocardiogram revealed normal biventricular function and dimension. Cardiac magnetic resonance revealed a mildly increased indexed RV end-diastolic volume with normal RV systolic function and no dyssynchrony, akinesia, dyskinesia, or late gadolinium enhancement. Electrophysiologic study demonstrated 2 predominant PVC morphologies that were targeted with ablation, in addition to extensive abnormality with low-voltage and fractionated electrograms in the peri-tricuspid and right ventricular outflow tract free wall regions with septal sparing, suggestive of RV cardiomyopathy. Subsequent genetic testing revealed two pathogenic variants in the desmoplakin and plakophilin-2 genes, confirming the diagnosis of ACM.
Conclusion: Advanced RV electropathy can precede RV structural changes in ACM. Invasive evaluation of the electroanatomic substrate should be considered in select cases even when imaging findings are not diagnostic. Future iterations of ACM guidelines may need to consider EAM substrate as one of the diagnostic criteria. A high index of diagnostic suspicion for ACM should be maintained in patients with multifocal RV ectopy.
Introduction
Arrhythmogenic cardiomyopathy (ACM) is an inherited condition caused by defects in the genes encoding cardiac desmosomes, characterized by fibrofatty replacement of the right ventricular (RV) myocardium (1), with biventricular and left-ventricular (LV) phenotypes being increasingly recognized (2). The disease typically manifests with ventricular tachyarrhythmias and RV systolic dysfunction (3). The first International Task Force diagnostic criteria for ACM was established in 1994. Subsequent revisions were introduced in 2010 and 2020 in an effort to improve sensitivity and increase earlier diagnosis of the disease (4).
It is well established that areas of abnormal substrate identified by invasive electroanatomic mapping (EAM) correlate anatomically with regions of myocardial thinning and fibrofatty replacement in ACM, and that the use of EAM may increase the sensitivity for detection of the disease (5, 6). However, EAM is absent from all sets of diagnostic criteria.
In this report, we present a patient without major structural cardiac abnormalities by multimodality imaging in whom abnormal findings by EAM led to the diagnosis of ACM with confirmation by genetic testing.
Case presentation
A 41-year-old female with no significant past medical history and no family history of cardiomyopathy or sudden cardiac death was referred for evaluation of persistent frequent premature ventricular contractions (PVCs) following a catheter ablation performed at an outside institution. She was in her usual state of health until a year prior to evaluation, when she developed palpitations, dyspnea on exertion, chest pain, and pre-syncope. The initial evaluation at our institution revealed diffuse low voltage QRS complexes on the 12-lead electrocardiogram, but no inverted T waves or epsilon waves (Figure 1A). A 12-lead Holter monitor showed 28% burden (25,000/24 h) of PVCs with more than 5 different morphologies consistent with RV inflow and outflow tract (RVOT) exits (Figure 1B). A transthoracic echocardiogram revealed normal LV and RV ejection fraction, and normal biventricular dimensions. Cardiac magnetic resonance (CMR) imaging revealed a borderline increased RV chamber size (RV end-diastolic volume indexed for body surface area 107 ml/m2, normal range 51–103 ml/m2) with normal systolic function (RVEF 51%, LVEF 58%) without dyssynchrony, akinesia, or dyskinesia, and no findings suggestive of RV or LV fibrosis, myocarditis, infarction, or late gadolinium enhancement (Figure 2). There were no pertinent findings on physical examination with the exception of occasionally irregular heart sounds due to PVCs.
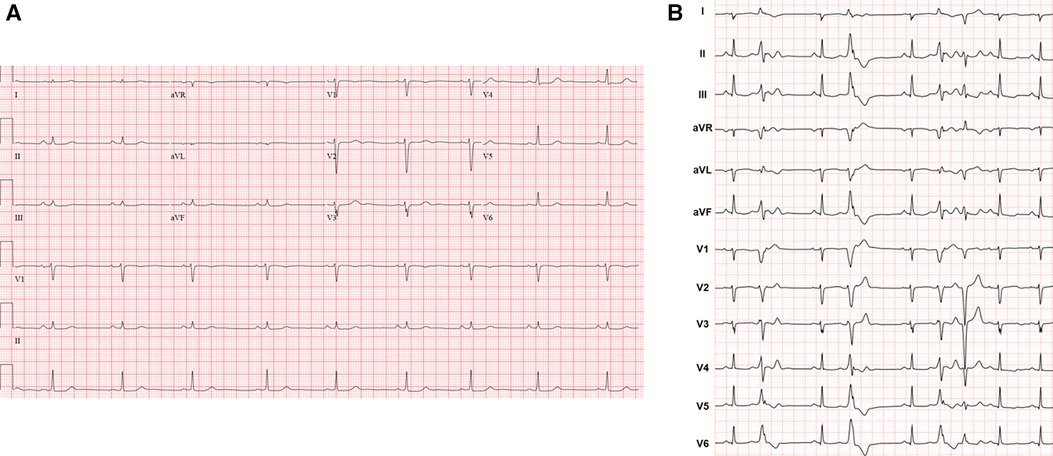
Figure 1. (A) Representative 12-lead electrocardiogram. (B) Twelve-lead Holter monitor tracing. Holter showed more than 5 different RV inflow and outflow tract PVC morphologies with a total burden of 28% over 24 h. Follow-up 24-h Holter 3 months post-ablation demonstrated 6% PVC burden.
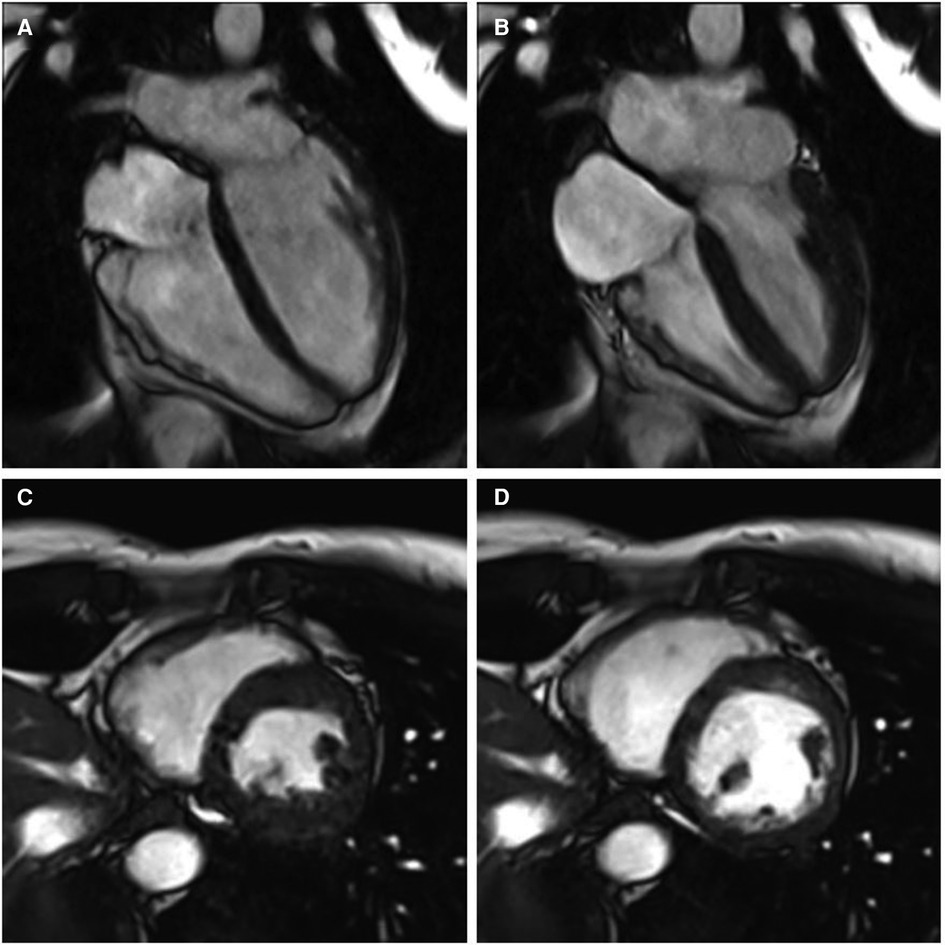
Figure 2. Cine cardiac magnetic resonance balanced steady state free precession cinegraphic imaging (A) 4-chamber end-diastole, (B) 4-chamber end-systole, (C) short axis end-diastole, and (D) short axis end-systole. Imaging demonstrated mild RV enlargement (RV end-diastolic volume index 107 ml/m2, normal range 51–103 ml/m2) without dyssynchrony, akinesia, or dyskinesia; normal biventricular function (RV EF 51%, LV EF 58%), and no late gadolinium enhancement or other structural abnormality.
An electrophysiologic study with catheter ablation was pursued. Endocardial high-density substrate bipolar voltage mapping with a PentaRay multielectrode catheter (Biosense Webster, Diamond Bar, CA) demonstrated extensive abnormality with low-voltage and fractionated electrograms on the free wall peri-tricuspid region and the RVOT free wall with sparing of the septum (Figure 3). Unipolar voltage mapping demonstrated similar area of low-voltage localized mainly to peritricuspid annulus and RVOT. The area of substrate abnormality was also verified with limited mapping with a contact force sensing catheter to ensure that the low voltage was not due to poor contact of the mapping catheter. There were no distinct late potentials and no inducible ventricular tachycardia (VT) with programmed ventricular stimulation with triple extrastimuli. Two predominant PVC morphologies were targeted with acute success at the anterior tricuspid annulus and the anteroseptal supra-pulmonic RVOT (Figure 3). These findings were overall suggestive of a RV-predominant cardiomyopathy despite the absence of definitive imaging findings.
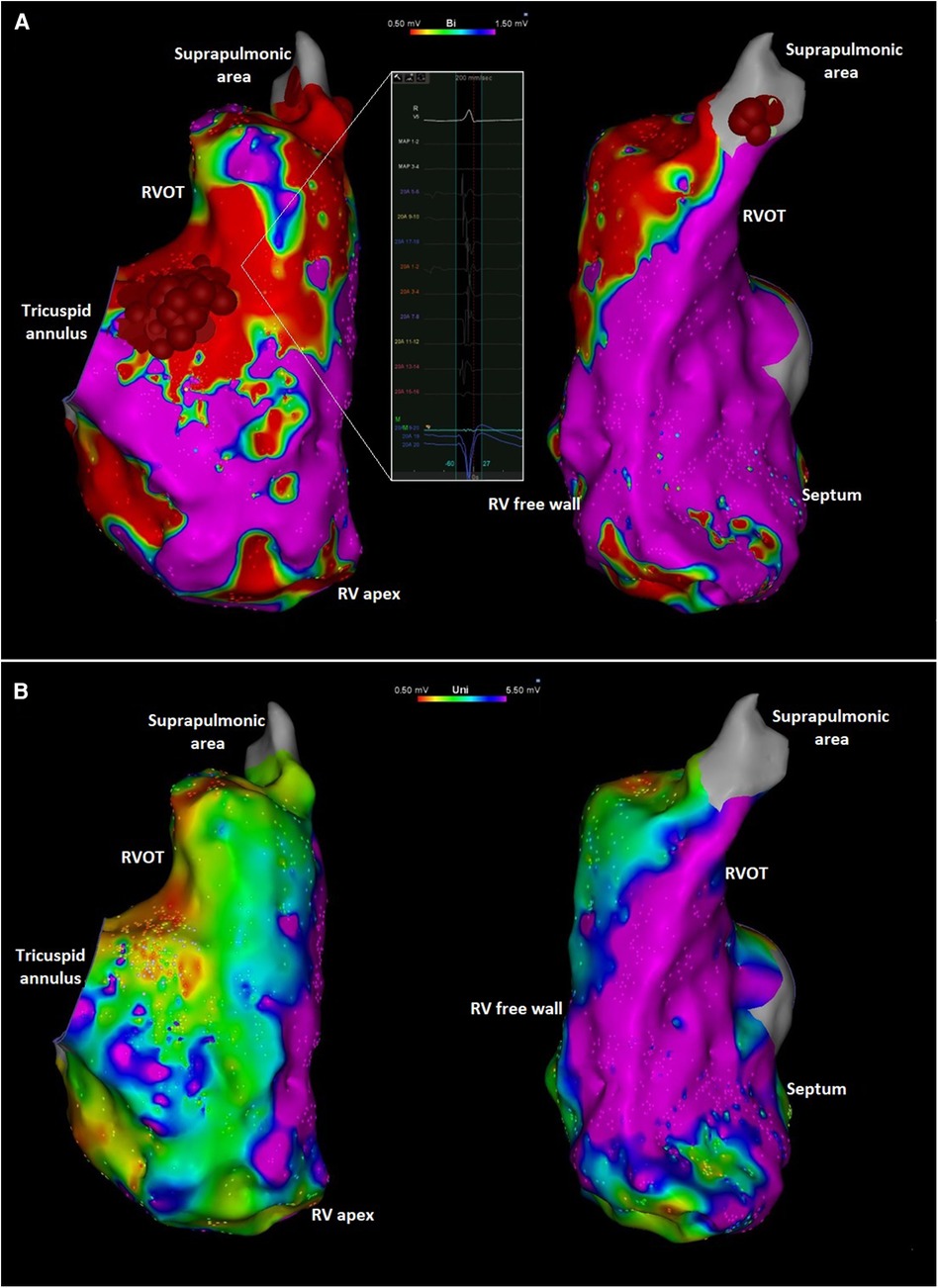
Figure 3. (A) Right anterior oblique (left) and left anterior oblique (right) views of the endocardial right ventricular bipolar electroanatomic maps showing low voltage along the tricuspid annulus and RVOT. The inlet shows an area with low amplitude and fractionated local electrograms. Ablation targets at the peritricuspid and suprapulmonic areas are also shown. The focal abnormality at the mid-distal septum likely reflects an area targeted during PVC ablation procedure previously performed at another institution. (B) Right anterior oblique (left) and left anterior oblique (right) views of the unipolar voltage mapping of the right ventricle showing low voltage in the peritricuspid area and RVOT. Red and purple colors represent low and normal voltage bipolar electrograms, respectively (bipolar: <0.5 mV and >1.5 mV; unipolar: <0.5 mV and >5.5 mV). Intermediate colors represent borderline voltage as shown in the scale in the top center of the figure.
Due to the EAM abnormalities, genetic testing for suspected ACM was recommended. The Invitae Arrhythmia and Comprehensive Cardiomyopathy panel identified two pathogenic variants in the desmoplakin (DSP, c.5028_5031del) and plakophilin-2 (PKP2, c.1912C>T) genes, confirming the underlying genetic cause for her presentation. Her estimated 5-year risk of sustained ventricular arrhythmias was 5.5% based on a recently developed risk stratification model (7). Additionally, because of the presence of two pathogenic ARVC-causing genetic variants, a primary prevention implantable cardioverter-defibrillator was recommended. At 1-year follow-up, the patient had improved symptoms and no interim sustained ventricular arrhythmias.
Discussion
The criteria for the diagnosis of ACM have evolved for nearly three decades. Advances in cardiac imaging, mainly CMR, have allowed the recognition of varying disease phenotypes, which is reflected in the 2020 International Criteria wherein the diagnosis is heavily dependent on the identification of salient RV abnormalities by late gadolinium-enhanced CMR (8). However, the identification of fibrofatty infiltration by CMR, a hallmark characteristic of ACM, presents limitations. The spatial resolution of CMR might have insufficient sensitivity for detection of the structural changes in the early stages of the disease, particularly due to the thinness of the RV free wall (9).
In suspected ACM, invasive EAM can characterize the substrate abnormality corresponding to fibrofatty replacement in early stages of the disease and before these abnormalities are detected by imaging (6). The arrhythmogenic substrate of ACM is typically most pronounced in the epicardium, with endocardial abnormalities absent until later in the disease course (10). Epicardial mapping was not performed in our patient, but it is striking that endocardial EAM abnormality was present well before significant structural RV abnormalities. This is consistent with the notion that advanced RV electropathy can precede RV structural changes. This observation has significant implications in the diagnostic evaluation of ACM such that invasive evaluation of the electroanatomic substrate should be considered in select cases even when imaging findings are underwhelming. Additional benefit of EAM includes the ability to differentiate ACM from mimicking conditions such as myocarditis and cardiac sarcoidosis (11). Further, these patients often have additional indications for electrophysiologic procedures, including risk stratification with programmed ventricular stimulation or catheter ablation of ventricular arrhythmias, as was the case with our patient. The extent of bipolar low-voltage substrate abnormality has also been shown to correlate with noninvasive measures of disease severity (such as ECG and MRI-LGE abnormalities) (12) and may be an independent predictor of arrhythmic events such as sudden cardiac death, appropriate ICD intervention, and sustained VT (13).
The presence of frequent PVCs (>500 per 24 h) is a major diagnostic criterion for ACM (8). Differentiating idiopathic RVOT arrhythmias from those occurring in the setting of an ACM is critical given the marked differences in management and prognosis. The presence of multifocal ectopy with morphology characteristics of RV exit, as seen in our patient, should clue the clinician into the possibility of ACM.
Our case is particularly unique due to the presence of two pathogenic ACM mutations with PKP2 dominantly contributing to the RV abnormality and the DSP mutation contibuting to the LV abnormality, such as diffuse low voltage QRS. Our patient did not meet Modified Task Force or Padua criteria for ACM based on electrocardiographic, imaging, and genetic information available prior to invasive electrophysiologic testing. The value of EAM in strengthening the diagnosis of ACM has been demonstrated in patients who otherwise fulfilled Task Force criteria of ACM prior to invasive mapping (5, 6). Further, Corrado et al. reported abnormal RV EAM findings in a small subset of patients referred for RVOT VT ablation in the absence of echocardiographic evidence of RV myopathy (14). However, CMR imaging was used in only a few patients in that cohort. To our knowledge, the case presented herein is one of the first cases in the literature where invasive EAM findings in a patient with PVCs triggered subsequent genetic testing ultimately confirming the diagnosis of ACM. In another report, Castro et al. demonstrated the diagnosis of ACM in a patient who presented with RVOT VT initially considered to be idiopathic given normal CMR imaging (15). The patient underwent VT ablation where normal bipolar voltage was identified in the endocardium, but abnormal substrate was identified in the RV epicardium where the VT was successfully targeted. Subsequent genetic testing revealed a pathogenic PKP2 mutation leading to a definite ACM diagnosis. These cases add to accumulating evidence suggesting that invasive EAM is a valuable tool for the early detection of ACM.
In summary, the diagnosis of ACM can be challenging. Electrophysiologic substrate abnormalities can precede typical imaging-detected structural abnormalities (Figure 4). Abnormal RV substrate on invasive EAM mapping should be considered among the diagnostic criteria for ACM. A high index of diagnostic suspicion for ACM should be maintained in patients with multifocal RV ectopy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JM: Writing – original draft, Writing – review & editing. SS: Writing – original draft, Writing – review & editing. MZ: Writing – original draft, Writing – review & editing, Conceptualization. JC: Writing – original draft, Writing – review & editing, Conceptualization. KS: Writing – original draft, Writing – review & editing, Conceptualization, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. (1982) 65(2):384–98. doi: 10.1161/01.CIR.65.2.384
2. Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. (1997) 30(6):1512–20. doi: 10.1016/S0735-1097(97)00332-X
3. Groeneweg JA, Bhonsale A, James CA, te Riele AS, Dooijes D, Tichnell C, et al. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet. (2015) 8(3):437–46. doi: 10.1161/CIRCGENETICS.114.001003
4. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. (2010) 121(13):1533–41. doi: 10.1161/CIRCULATIONAHA.108.840827
5. Corrado D, Basso C, Leoni L, Tokajuk B, Bauce B, Frigo G, et al. Three-dimensional electroanatomic voltage mapping increases accuracy of diagnosing arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. (2005) 111(23):3042–50. doi: 10.1161/CIRCULATIONAHA.104.486977
6. Boulos M, Lashevsky I, Reisner S, Gepstein L. Electroanatomic mapping of arrhythmogenic right ventricular dysplasia. J Am Coll Cardiol. (2001) 38(7):2020–7. doi: 10.1016/S0735-1097(01)01625-4
7. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. (2019) 40(23):1850–8. doi: 10.1093/eurheartj/ehz103
8. Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari M, et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol. (2020) 319:106–14. doi: 10.1016/j.ijcard.2020.06.005
9. Etoom Y, Govindapillai S, Hamilton R, Manlhiot C, Yoo SJ, Farhan M, et al. Importance of CMR within the task force criteria for the diagnosis of ARVC in children and adolescents. J Am Coll Cardiol. (2015) 65(10):987–95. doi: 10.1016/j.jacc.2014.12.041
10. Kommata V, Sciaraffia E, Blomström-Lundqvist C. Epicardial conduction abnormalities in patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) and mutation positive healthy family members - A study using electrocardiographic imaging. PLoS One. (2023) 18(1):e0280111. doi: 10.1371/journal.pone.0280111
11. Hoogendoorn JC, Sramko M, Venlet J, Siontis KC, Kumar S, Singh R, et al. Electroanatomical voltage mapping to distinguish right-sided cardiac sarcoidosis from arrhythmogenic right ventricular cardiomyopathy. JACC Clin Electrophysiol. (2020) 6(6):696–707. doi: 10.1016/j.jacep.2020.02.008
12. Santangeli P, Pieroni M, Dello Russo A, Casella M, Pelargonio G, Macchione A, et al. Noninvasive diagnosis of electroanatomic abnormalities in arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. (2010) 3(6):632–8. doi: 10.1161/CIRCEP.110.958116
13. Migliore F, Zorzi A, Silvano M, Bevilacqua M, Leoni L, Marra MP, et al. Prognostic value of endocardial voltage mapping in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Arrhythm Electrophysiol. (2013) 6(1):167–76. doi: 10.1161/CIRCEP.111.974881
14. Corrado D, Basso C, Leoni L, Tokajuk B, Turrini P, Bauce B, et al. Three-dimensional electroanatomical voltage mapping and histologic evaluation of myocardial substrate in right ventricular outflow tract tachycardia. J Am Coll Cardiol. (2008) 51(7):731–9. doi: 10.1016/j.jacc.2007.11.027
15. Castro SA, Pathak RK, Muser D, Santangeli P, Owens A, Marchlinski F, et al. Incremental value of electroanatomical mapping for the diagnosis of arrhythmogenic right ventricular cardiomyopathy in a patient with sustained ventricular tachycardia. HeartRhythm Case Rep. (2016) 2(6):469–72. doi: 10.1016/j.hrcr.2016.06.004
Keywords: arrhythmogenic cardiomyopathy, electroanatomic mapping, premature ventricular contraction, task force criteria, case report
Citation: de Melo JF Jr, Shabtaie SA, van Zyl M, Collins JD and Siontis KC (2024) Case Report: Electroanatomic mapping as an early diagnostic tool in arrhythmogenic cardiomyopathy. Front. Cardiovasc. Med. 11:1392186. doi: 10.3389/fcvm.2024.1392186
Received: 27 February 2024; Accepted: 26 July 2024;
Published: 9 August 2024.
Edited by:
Pietro Enea Lazzerini, University of Siena, ItalyReviewed by:
Konstantinos Vlachos, INSERM Institut de Rythmologie et Modélisation Cardiaque (IHU-Liryc), FranceJavier Eduardo Banchs, Scott & White Memorial Hospital, United States
© 2024 de Melo, Shabtaie, van Zyl, Collins and Siontis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Konstantinos C. Siontis, c2lvbnRpcy5rb25zdGFudGlub3NAbWF5by5lZHU=
 Jose F. de Melo Jr
Jose F. de Melo Jr Samuel A. Shabtaie2
Samuel A. Shabtaie2 Jeremy D. Collins
Jeremy D. Collins Konstantinos C. Siontis
Konstantinos C. Siontis