- Department of Geriatrics, The Second Clinical College of Guangzhou University of Chinese Medicine, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, Guangdong, China
Aim: To assess the diagnostic and prognostic performances of the Heart Failure Association Pre-test Assessment, Echocardiography & Natriuretic Peptide, Functional Testing, Final Etiology (HFA-PEFF) score for heart failure with preserved ejection fraction (HFpEF) in a comprehensive manner.
Methods: PubMed, Embase, Cochrane Library, and Web of Science were comprehensively searched from the inception to June 12, 2023. Studies using the “Rule-out” or “Rule-in” approach for diagnosis analysis or studies on cardiovascular events and all-cause death for prognosis analysis were included. The Quality Assessment of Diagnostic Accuracy Studies (QUADAS−2) tool was adopted to assess the quality of diagnostic accuracy studies. The sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and area under the summary receiver operating characteristic (SROC) curve (AUC) were presented with 95% confidence intervals (CIs). For CVEs and all-cause death, the hazard ratio (HR) values were calculated.
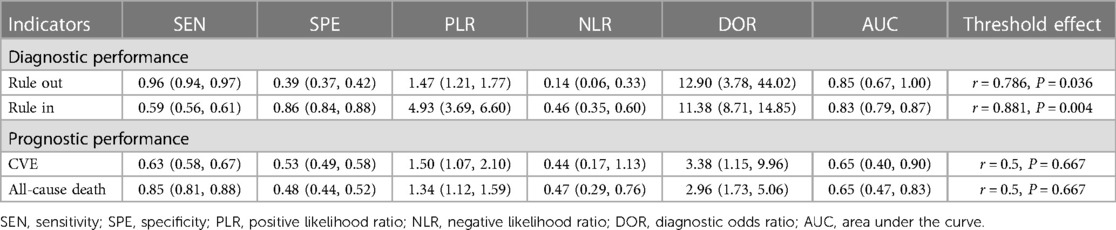
Results: Fifteen studies involving 6420 subjects were included, with 9 for diagnosis analysis, and 7 for prognosis analysis. For the diagnostic performance of the HFA-PEFF score, with the “Rule-out” approach, the pooled SEN was 0.96 (95%CI: 0.94, 0.97), the pooled SPE was 0.39 (95%CI: 0.37, 0.42), and the pooled AUC was 0.85 (95%CI: 0.67, 1.00), and with the “Rule-in” approach, the pooled SEN was 0.59 (95%CI: 0.56, 0.61), the pooled SPE was 0.86 (95%CI: 0.84, 0.88), and the pooled AUC was 0.83 (95%CI: 0.79, 0.87). For the predictive performance of the HFA-PEFF score, regarding CVEs, the pooled SEN was 0.63 (95%CI: 0.58, 0.67), the pooled SPE was 0.53 (95%CI: 0.49, 0.58), and the pooled AUC was 0.65 (95%CI: 0.40, 0.90), and concerning All-cause death, the pooled SEN was 0.85 (95%CI: 0.81, 0.88), the pooled SPE was 0.48 (95%CI: 0.44, 0.52), and the pooled AUC was 0.65 (95%CI: 0.47, 0.83). A higher HFA-PEFF score was associated with a higher risk of all-cause death (HR 1.390, 95%CI 1.240, 1.558, P < 0.001).
Conclusion: The HFA-PEFF score might be applied in HFpEF diagnosis and all-cause death prediction. More studies are required for finding validation.
Introduction
The prognosis of heart failure patients is poor, stratified according to ejection fraction classification (1). Heart failure with preserved ejection fraction (HFpEF) is a common clinical syndrome, influencing half of all heart failure patients globally, with rising prevalence and significant morbidity and mortality (2, 3). Individuals with HFpEF had a 5-year survival rate of 35%–40% after the first hospitalization (4). Although numerous attempts have been made to find an effective targeted therapy for HFpEF, the currently available evidence is inadequate to support specific drug regimens for patients who present with HFpEF (5–8), probably because the fundamental pathophysiology of HFpEF is poorly understood, and a firm diagnosis of HFpEF remains a challenge in real-world practice.
In 2019, the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) proposed the Heart Failure Association Pre-test Assessment, Echocardiography & Natriuretic Peptide, Functional Testing, Final Etiology (HFA-PEFF) algorithm to diagnose HFpEF (9), where the HFA-PEFF score incorporates three domains, functional, morphological, and biomarker, to estimate the likelihood (low, intermediate, or high) of suffering from HFpEF (10). Besides, the strategies for improving outcomes in patients with HFpEF are not well-defined. Better definitions of the population at higher clinical risk may be helpful in adjudicating the intensity of follow-up and optimizing therapies (11). Many clinical, biochemical, and echocardiographic derangements have been linked to worse outcomes, and the HFA-PEFF, which considers several easily available variables, has been shown by the existing studies to be helpful in both diagnosis and prognostic prediction of HFpEF (12–17). At present, Li et al. (4) has comprehensively investigated the diagnostic role of the HFA-PEFF score in HFpEF via a meta-analysis, whereas the prognostic value of the HFA-PEFF score for HFpEF is still unclear.
The objective of this systematic review and meta-analysis was to assess the diagnostic and prognostic performances of the HFA-PEFF score for HFpEF in a comprehensive manner, so as to provide a comprehensive understanding of the HFA-PEFF score and promote the clinical risk management of HFA-PEFF.
Methods
Search strategy
The following four English databases were comprehensively searched by two independent investigators (XM Li and YY Liang) from the inception to June 12, 2023: PubMed, Embase, Cochrane Library, and Web of Science. The English search term was HFA PEFF. Primary screening was carried out by reading titles and abstracts of the retrieved studies with the help of Endnote X9 (Clarivate, Philadelphia, PA, USA). Subsequently, full texts were read to select eligible studies. Discussion was needed when differences arose regarding search results. This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA).
Eligibility criteria
Inclusion criteria included: (1) studies on individuals with suspected HFpEF (for diagnosis analysis) or diagnosed with HFpEF (for prognosis analysis); (2) studies reporting the HFA-PEFF score; (3) studies providing relevant data to calculate sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), area under the curve (AUC), and prognostic HR values; (4) studies using the “Rule-out” or “Rule-in” approach for diagnosis analysis or studies on cardiovascular events (CVEs, including cardiovascular death, hospitalization for HF decompensation, nonfatal myocardial infarction (MI), unstable angina pectoris, coronary revascularization for a new diagnosis of angina or in-stent restenosis after percutaneous coronary intervention, and nonfatal ischemic stroke) and all-cause death for prognosis analysis; (5) English studies.
Exclusion criteria included: (1) animal experiments; (2) studies involving partial HFpEF patients for prognosis analysis; (3) studies with unextractable data; (4) meta-analyses, reviews, abstracts, and errata.
Data extraction and quality assessment
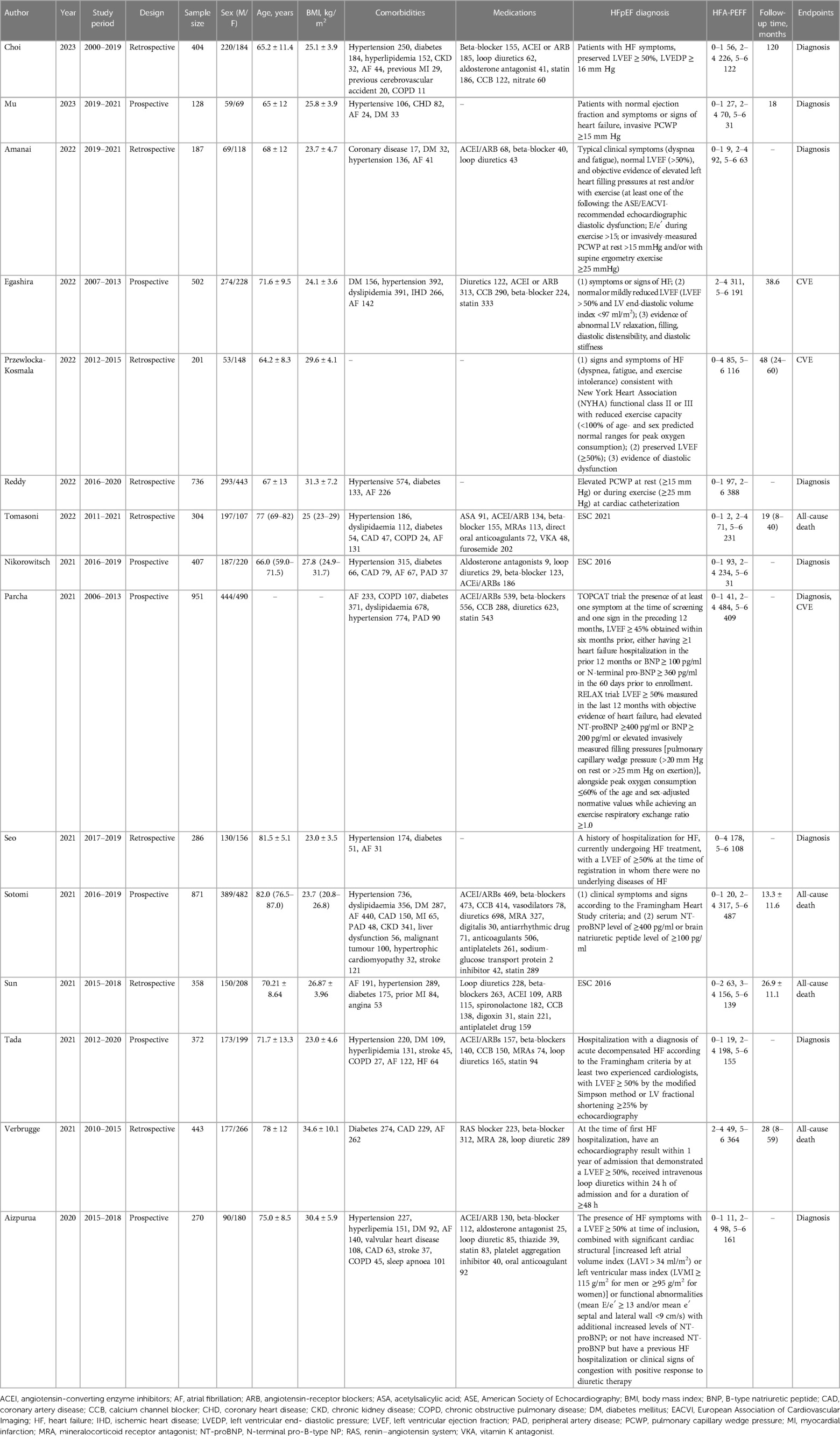
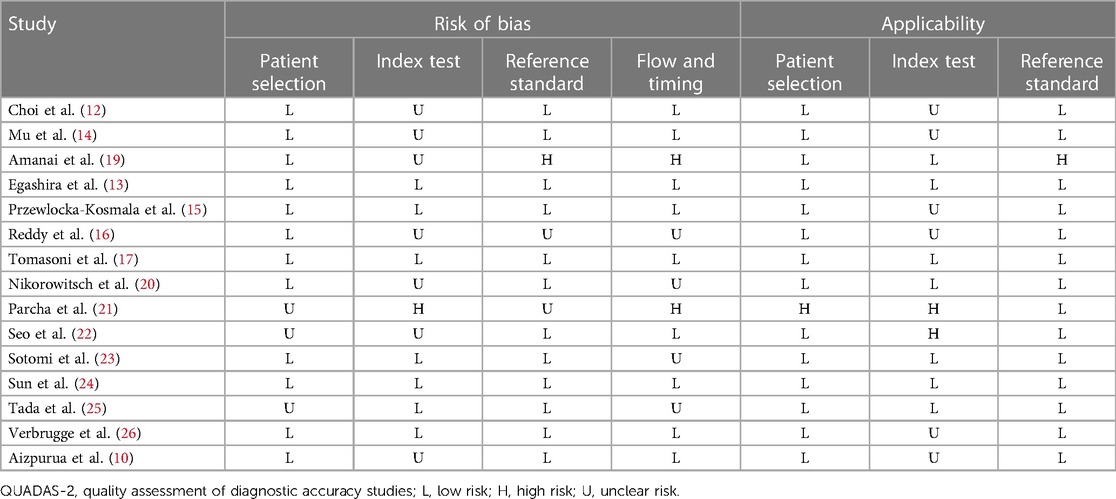
Two investigators (XM Li and YY Liang) independently extracted data from the included studies, including the first author, year of publication, study period, study design, sample size, sex (male/female), age (years), body mass index (BMI, kg/m2), comorbidities, medications, HFpEF diagnosis, HFA-PEFF assessment, follow-up time (months), and endpoints. A third author (XZ Lin) would settle relevant disagreements. The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool was adopted to assess the quality of diagnostic accuracy studies, based on the risk of bias and clinical applicability (18). The risk of bias contained patient selection, index test, reference standard, and flow and timing. Clinical applicability consisted of patient selection, index test, and reference standard. Each item was graded as high (risk), low (risk), or unclear (risk).
Statistical analysis
Statistical analysis was performed using Meta-disc 1.4 (Clinical Biostatistics, Ramony Cajal Hospital, Madrid, Spain), Stata 15.1 (Stata Corporation, College Station, Texas, USA), and Revman 5.4 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Results were obtained via direct extraction or indirect calculation. Meta-disc 1.4 was applied to evaluate whether there was a threshold effect. When the Spearman correlation coefficient between the logarithm of sensitivity and the logarithm of 1-specificity showed a strong positive correlation, it indicated the existence of a threshold effect. To assess the diagnostic and prognostic value of the HFA-PEFF score, the SEN, SPE, PLR, NLR, and DOR as well as 95% confidence intervals (CIs) for clinical outcomes were reported. Summary receiver operating characteristic (SROC) curves were generated, and the AUC was calculated with 95%CIs. Besides, for CVEs and all-cause death, the hazard ratio (HR) values were calculated using Stata 15.1 with the HFA-PEFF as a continuous variable. Revman 5.4 was used to create a quality assessment chart for the included studies. Differences were significant when P values were less than 0.05.
Results
Study characteristics
A total of 275 studies were retrieved from the four databases. After excluding duplicates, and based on the eligibility criteria, 15 studies (10, 12–17, 19–26) involving 6,420 subjects were included for this systematic review and meta-analysis in the end, with 5 studies from Japan, 2 from China, 1 from Germany, 1 from Italy, 1 from Poland, 1 from the Netherlands, 1 from Korea, 1 from USA, and 2 from multiple countries. The flow chart of study selection is demonstrated in Figure 1. The year of publication ranged from 2020 to 2023. Seven studies had prospective designs, and eight studies had retrospective designs. Nine studies were involved in diagnosis analysis, and seven were included for prognosis analysis. Table 1 exhibits the detailed characteristics of the included studies. With the QUADAS-2 for the quality assessment of the included studies, as regards the risk of bias and clinical applicability, most studies exhibited low risks, followed by unclear risks (Table 2).
Diagnostic performance of the HFA-PEFF score
“Rule-out” approach
In the pooled analysis of the “Rule-out” approach, the SROC curve of the HFA-PEFF showed a “shoulder-arm” distribution, and further, the Spearman correlation coefficient for the HFA-PEFF was 0.786 (P = 0.036), which indicated the existence of a threshold effect. The pooled SEN was 0.96 (95%CI: 0.94, 0.97), the pooled SPE was 0.39 (95%CI: 0.37, 0.42), the pooled PLR was 1.47 (95%CI: 1.21, 1.77), the pooled NLR was 0.14 (95%CI: 0.06, 0.33), the pooled DOR was 12.90 (95%CI: 3.78, 44.02), and the pooled AUC was 0.85 (95%CI: 0.67, 1.00) (Table 3; Figure 2).
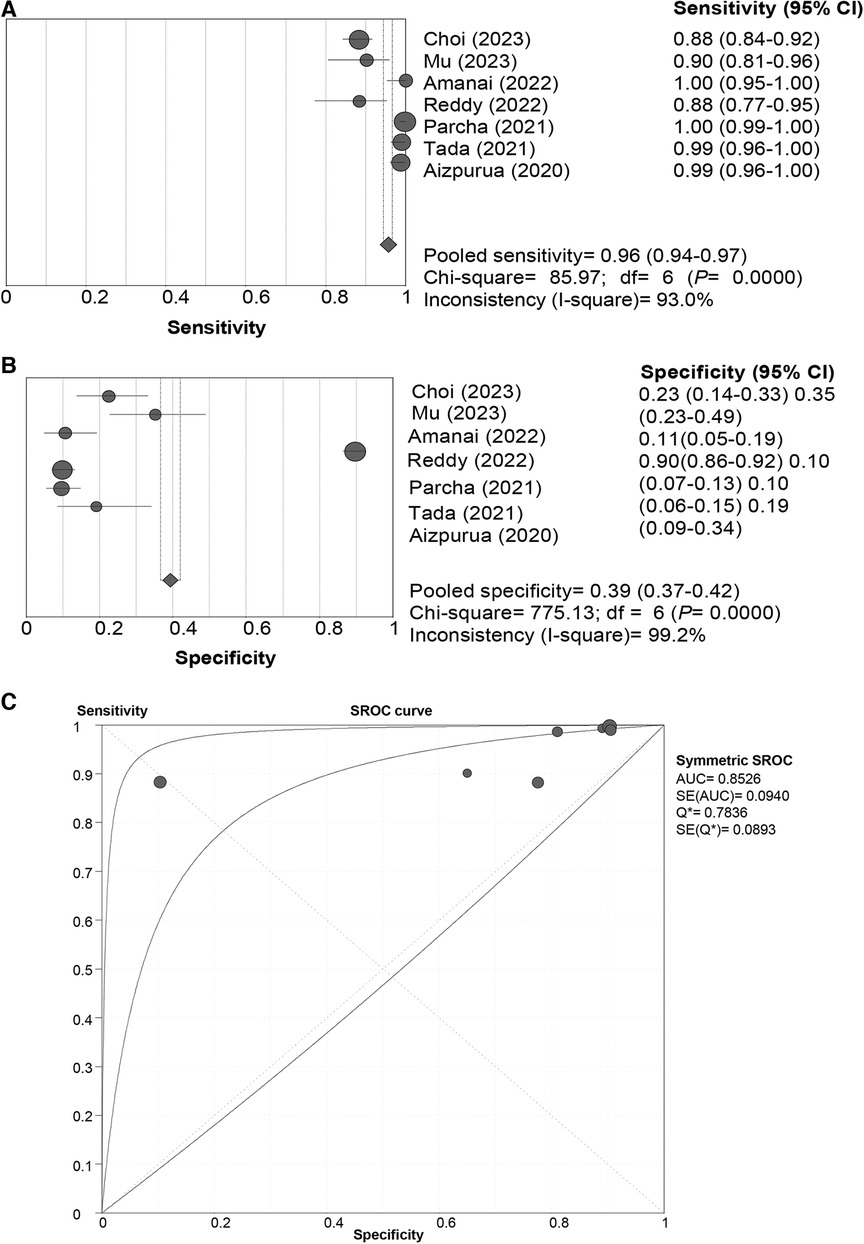
Figure 2 Sensitivity (A), specificity (B) and SROC curve (C) of the HFA-PEFF score for hFpEF diagnosis using the “rule-out” approach. SROC, summary receiver operating characteristic; HFA-PEFF, heart failure association pre-test assessment, echocardiography & natriuretic peptide, functional testing, final etiology; HFpEF, heart failure with preserved ejection fraction; AUC, area under the curve; CI, confidence interval.
“Rule-in” approach
In the pooled analysis of the “Rule-in” approach, a “shoulder-arm” distribution was illustrated by the SROC curve of the HFA-PEFF. The Spearman correlation coefficient for the HFA-PEFF was 0.881 (P = 0.004), suggesting the existence of a threshold effect. The pooled SEN was 0.59 (95%CI: 0.56, 0.61), the pooled SPE was 0.86 (95%CI: 0.84, 0.88), the pooled PLR was 4.93 (95%CI: 95%CI: 3.69, 6.60), the pooled NLR was 0.46 (95%CI: 0.35, 0.60), the pooled DOR was 11.38 (95%CI: 8.71, 14.85), and the pooled AUC was 0.83 (95%CI: 0.79, 0.87) (Table 3; Figure 3).
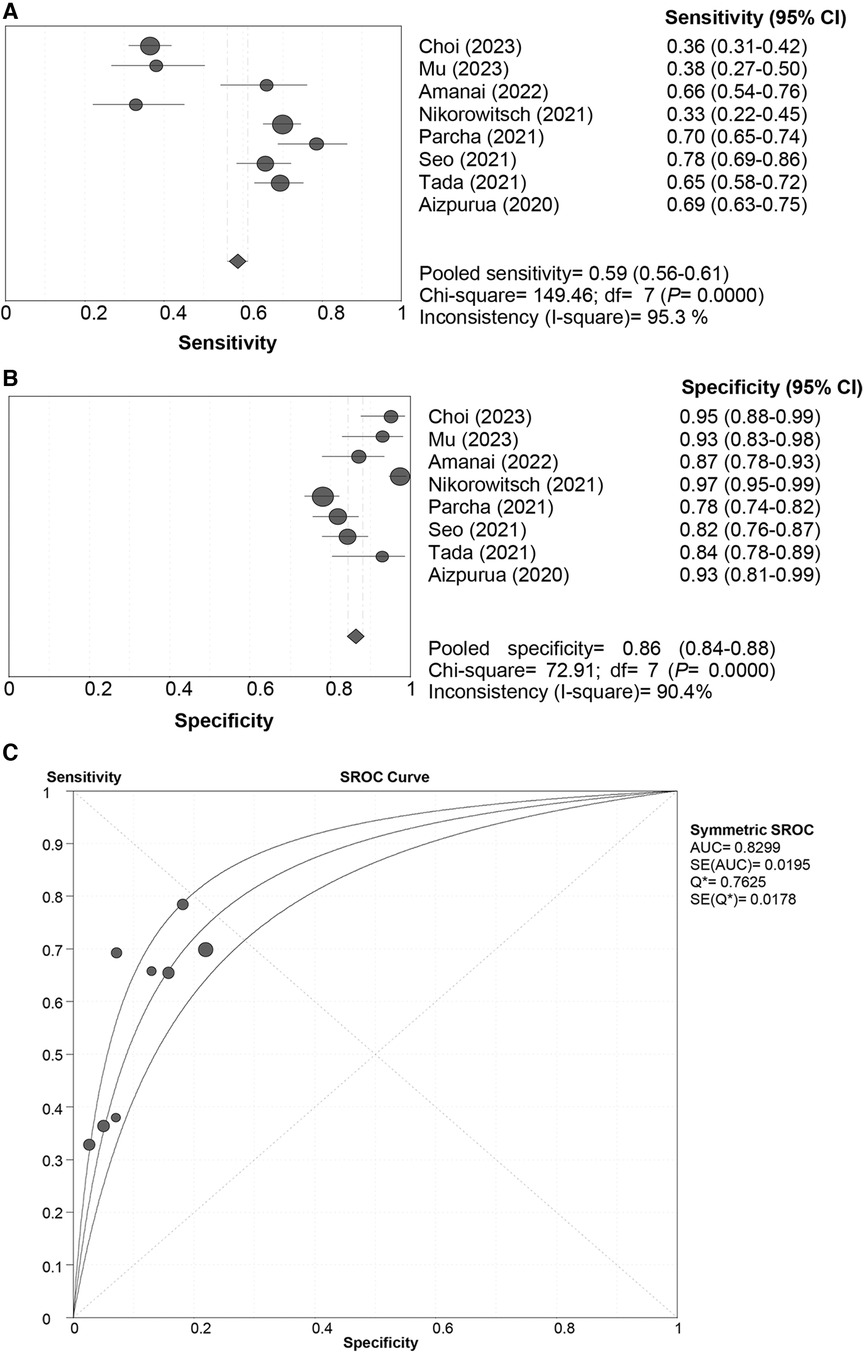
Figure 3 Sensitivity (A), specificity (B) and SROC curve (C) of the HFA-PEFF score for hFpEF diagnosis using the “rule-in” approach. SROC, summary receiver operating characteristic; HFA-PEFF, heart failure association pre-test assessment, echocardiography & natriuretic peptide, functional testing, final etiology; HFpEF, heart failure with preserved ejection fraction; AUC, area under the curve; CI, confidence interval.
Predictive performance of the HFA-PEFF score
CVEs
All the included studies used the “Rule-in” approach for CVE prediction. The SROC curve of the HFA-PEFF did not show a “shoulder-arm” distribution, and further, the Spearman correlation coefficient for the HFA-PEFF was 0.5 (P = 0.667), which indicated the absence of a threshold effect. The pooled SEN was 0.63 (95%CI: 0.58, 0.67), the pooled SPE was 0.53 (95%CI: 0.49, 0.58), the pooled PLR was 1.50 (95%CI: 1.07, 2.10), the pooled NLR was 0.44 (95%CI: 0.17, 1.13), the pooled DOR was 3.38 (95%CI: 1.15, 9.96), and the pooled AUC was 0.65 (95%CI: 0.40, 0.90) (Table 3; Figure 4). The pooled analysis of 2 eligible studies found no significant association between the HFA-PEFF and the risk of CVEs (HR 1.631, 95%CI 0.984, 2.704, P = 0.058).
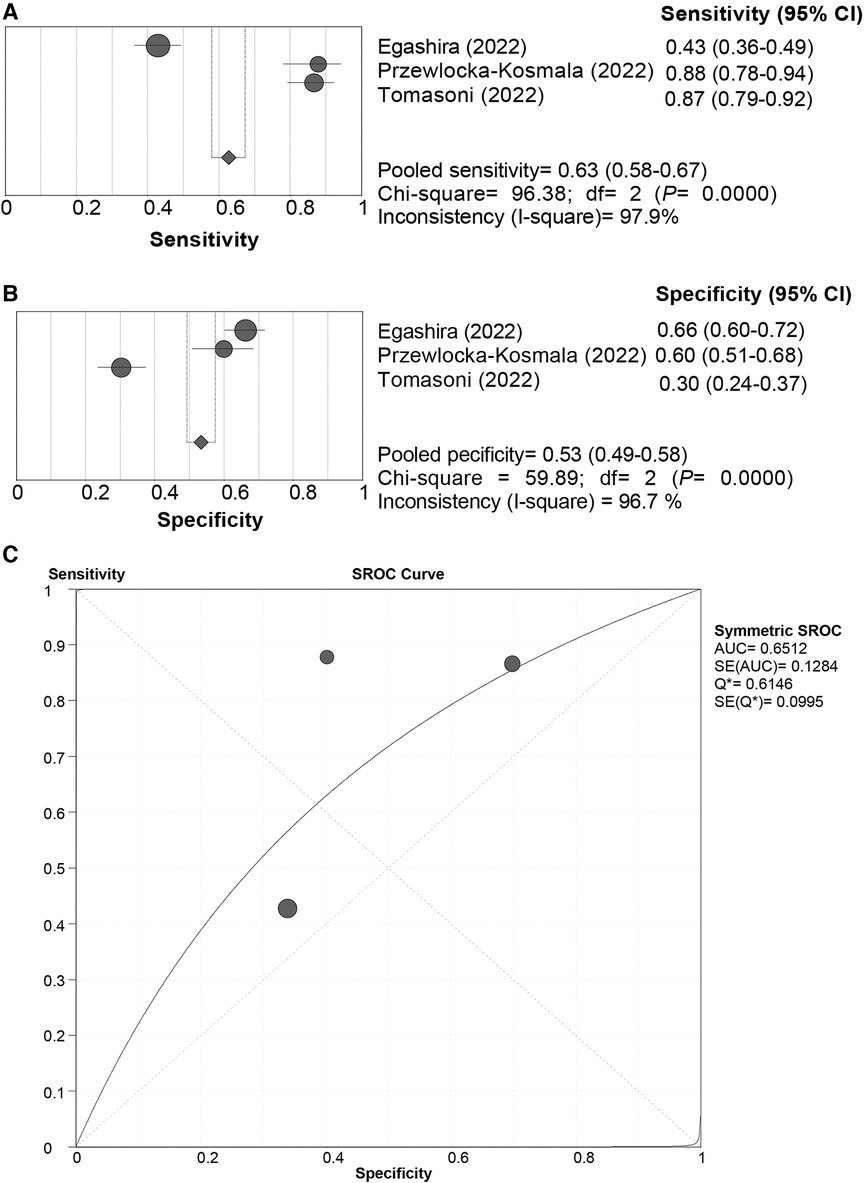
Figure 4 Sensitivity (A), specificity (B) and SROC curve (C) of the HFA-PEFF score for CVE prediction. SROC, summary receiver operating characteristic; HFA-PEFF, heart failure association pre-test assessment, echocardiography & natriuretic peptide, functional testing, final etiology; HFpEF, heart failure with preserved ejection fraction; AUC, area under the curve; CI, confidence interval.
All-cause death
All the included studies used the “Rule-in” approach for all-cause death prediction. The SROC curve of the HFA-PEFF did not display a “shoulder-arm” distribution, and further, the Spearman correlation coefficient for the HFA-PEFF was 0.5 (P = 0.667), suggesting no threshold effect. The pooled SEN was 0.85 (95%CI: 0.81, 0.88), the pooled SPE was 0.48 (95%CI: 0.44, 0.52), the pooled PLR was 1.34 (95%CI: 1.12, 1.59), the pooled NLR was 0.47 (95%CI: 0.29, 0.76), the pooled DOR was 2.96 (95%CI: 1.73, 5.06), and the pooled AUC was 0.65 (95%CI: 0.47, 0.83) (Table 3; Figure 5). The pooled analysis of 3 qualified studies demonstrated that a higher HFA-PEFF score was associated with a higher risk of all-cause death (HR 1.390, 95%CI 1.240, 1.558, P < 0.001).
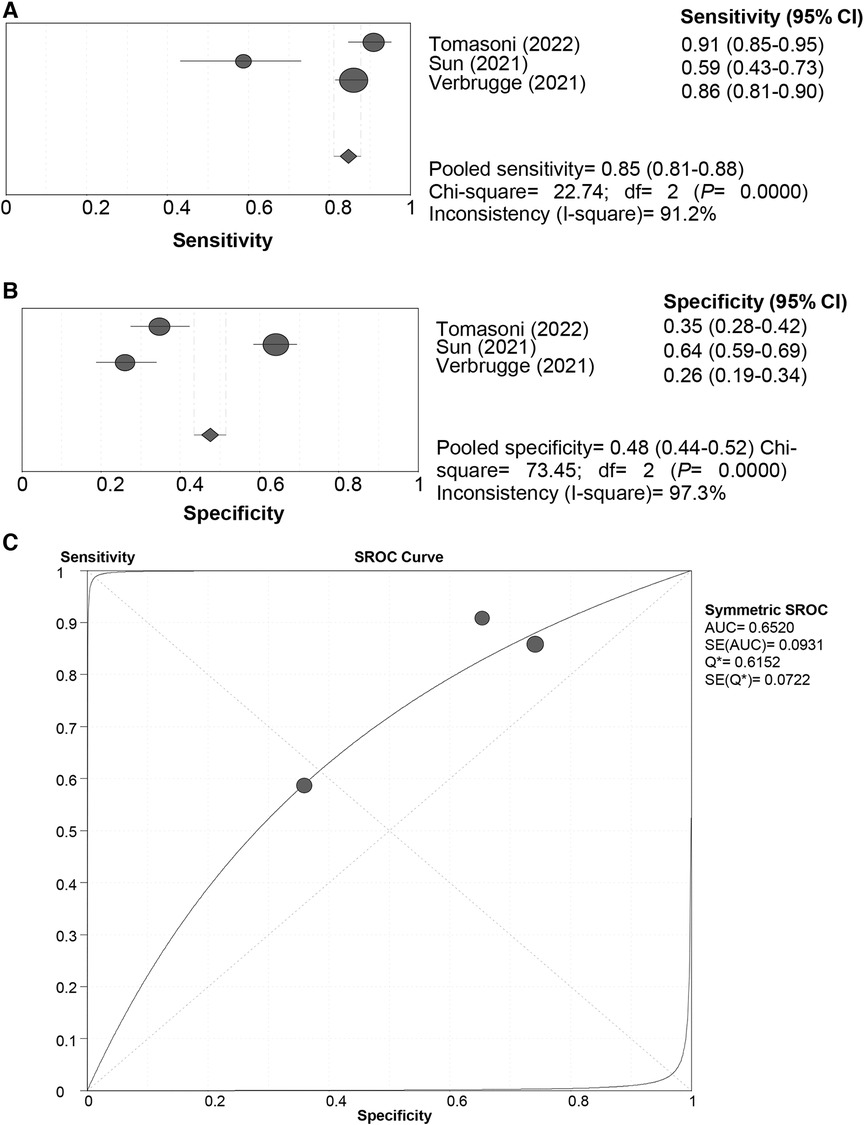
Figure 5 Sensitivity (A), specificity (B) and SROC curve (C) of the HFA-PEFF score for all-cause death prediction. SROC, summary receiver operating characteristic; HFA-PEFF, heart failure association pre-test assessment, echocardiography & natriuretic peptide, functional testing, final etiology; HFpEF, heart failure with preserved ejection fraction; AUC, area under the curve; CI, confidence interval.
Discussion
The current systematic review and meta-analysis comprehensively evaluated the diagnostic and prognostic value of the HFA-PEFF score for HFpEF, and illustrated that with both the “Rule-out” and the “Rule-in” approaches, the HFA-PEFF had a good diagnostic capability for HFpEF based on the pooled AUCs, and the pooled SEN of the “Rule-out” approach was higher than that of the “Rule-in” approach, while the pooled SPE of the “Rule-in” approach was better than that of the “Rule-out” approach; for all-cause death, the HFA-PEFF exhibited a good predictive SEN, which indicated that the HFA-PEFF might be applied in HFpEF diagnosis and all-cause death prediction.
HFpEF diagnosis is usually performed based on three key components. These include symptoms and signs of heart failure (related to pulmonary and systemic congestion), evidence of “preserved ejection fraction”, and the presence of diastolic dysfunction (27). The HFA-PEFF score algorithm was proposed based on a comprehensive diagnostic workup (9, 10). In addition, this score also demonstrated its prognostic significance for individuals with HFA-PEFF in several studies (13, 23, 24). A prior meta-analysis pooled relevant studies to synthetically assess the diagnostic performance of the HFA-PEFF for HFpEF, and it was found that the HFA-PEFF algorithm showed acceptable SPE and SEN for the diagnosis and exclusion of HFpEF (4). This study further conducted an updated comprehensive analysis of HFA-PEFF prognostic value in HFpEF, and the HFA-PEFF was also found to have great performance in HFpEF diagnosis and all-cause death prognostication.
For the diagnosis of HFpEF, the pooled AUC, SEN and SPE of the “Rule-out” approach was 0.85, 0.96 and 0.39, respectively, and the pooled AUC, SEN and SPE of the “Rule-in” approach was 0.83, 0.59 and 0.86, respectively. These suggested that using either the “Rule-out” approach or the “Rule-in” approach, the HFA-PEFF score had a good diagnostic performance; the “Rule-out” approach showed higher SEN, and the “Rule-in” approach had better SPE. To be noted, for patients with intermediate likelihood of HFpEF, exercise induced echocardiography or invasive cardiac hemodynamic measurements would be recommended for the final diagnosis (25). A two-center study has discovered that exercise pulmonary ultrasound exhibits excellent diagnostic value for HFpEF, regardless of the exercise protocol or level of expertise (28). More precise diagnostic modalities are needed, especially in such a serious disease with an incidence close to the incident rate of tuberculosis and other plagues (29–32), and the clinical indications would guide the diagnostic practice in the context of individualized medical management. Future studies are warranted to verify the diagnostic role of the HFA-PEFF score.
However, in a case-control study evaluating outpatient dyspnea of indeterminate cause and HFpEF, the H2FPEF score exhibited better diagnostic performance compared to the HFA-PEFF score (16). A study based on a Japanese patient cohort has found that the H2FPEF score significantly outperforms the HFA-PEFF score in terms of diagnostic accuracy for HFpEF (25). A research investigation into the diagnostic utility of the H2FPEF scoring system and the HFA-PEFF E-level scoring system within the context of HFpEF has revealed that both scores are efficacious in either excluding or establishing a definitive diagnosis of HFpEF (33). These results suggest the need for further research to compare the importance of different scoring systems in diagnosing HFpEF.
With respect to prognosis in HFpEF, the HFA-PEFF presented a pooled SEN of 0.85 in the prediction of all-cause death, although the pooled AUC was 0.65, while for CVEs, the HFA-PEFF did not have a favorable predictive ability. As reported by Seoudy et al. (34), the HFA-PEFF score is associated with all-cause mortality and heart failure rehospitalization in patients with preserved ejection fraction after transcatheter aortic valve implantation. The HFA-PEFF score has three components that each contribute equally to the overall score (9). One component relies upon natriuretic peptide levels, which have been shown to be strongly related to adverse clinical outcomes in HFpEF as well as HFrEF (35, 36). The second component of the score relies on morphological criteria such as left atrial volume index and left ventricular mass. Left atrial volume index in particular has been demonstrated to be among the most powerful echocardiography predictors of future HF events and reflects the risk of mortality as well (37–39). Finally, the third component of the HFA-PEFF score is represented by functional parameters that reflect left ventricular diastolic dysfunction and/or elevated cardiac filling pressures. One of the parameters incorporated is tricuspid valve regurgitation velocity, with high values indicating pulmonary hypertension, which is strongly associated with mortality in HFpEF (40). For every one point increase in the HFA-PEFF score, the risk of all-cause mortality significantly increased by 39%, which showed a quantitative information to facilitate understanding of the association between the HFA-PEFF and the death risk. Corresponding optimized treatments could be provided to high-risk patients with HFpEF to improve their prognosis.
As demonstrated by this study, the HFA-PEFF score may be taken into consideration by clinicians in the diagnosis and all-cause death prognostication of HFpEF, which may help in planning therapeutic methods and improving HFpEF management. Several limitations should be mentioned when interpreting the results. First, there were threshold effects on the diagnostic performance of the HFA-PEFF score for HFpEF using the “Rule-out” and “Rule-in” approaches, which may affect the stability of the results. Second, the availability of a limited number of studies for outcomes such as CVEs may have influenced the reliability of our findings. The small sample size within these studies could have reduced the statistical power to detect significant associations, and the heterogeneity across studies in terms of design, population characteristics, and follow-up duration further complicates the interpretation of the results. Third, an important limitation to consider in the validation of the HFA-PEFF score is the heterogeneity of criteria used by each study to define HFpEF patients. The included studies span a period from 2000 to 2021, during which time diagnostic and classification criteria for HFpEF have evolved. This variability in the definition of HFpEF could introduce bias and affect the comparability of results across studies. Our findings highlight the urgent need for additional studies to evaluate the prognostic value of the HFA-PEFF score in HFpEF, with the aim of providing a more robust evidence base to support its use in clinical practice.
Conclusion
The HFA-PEFF had a good diagnostic capability for HFpEF using both the “Rule-out” and the “Rule-in” approaches based on the pooled AUCs, and it exhibited a good predictive SEN for all-cause death in patients with HFpEF, suggesting that the HFA-PEFF may be considered in HFpEF diagnosis and all-cause death prediction. More studies are needed for finding validation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
XLi: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing. YL: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. XLin: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. (2017) 70(20):2476–86. doi: 10.1016/j.jacc.2017.08.074
2. Mishra S, Kass DA. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2021) 18(6):400–23. doi: 10.1038/s41569-020-00480-6
3. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2017) 14(10):591–602. doi: 10.1038/nrcardio.2017.65
4. Li S, Zhu X, Zhang Y, Li F, Guo S. Validation of heart failure algorithm for diagnosing heart failure with preserved ejection fraction: a meta-analysis. ESC Heart Fail. (2023) 10(4):2225–35. doi: 10.1002/ehf2.14421
5. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. (2014) 370(15):1383–92. doi: 10.1056/NEJMoa1313731
6. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. (2018) 39(1):26–35. doi: 10.1093/eurheartj/ehx564
7. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the charm-preserved trial. Lancet. (2003) 362(9386):777–81. doi: 10.1016/s0140-6736(03)14285-7
8. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. (2019) 381(17):1609–20. doi: 10.1056/NEJMoa1908655
9. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the heart failure association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. (2019) 40(40):3297–317. doi: 10.1093/eurheartj/ehz641
10. Barandiarán Aizpurua A, Sanders-van Wijk S, Brunner-La Rocca HP, Henkens M, Heymans S, Beussink-Nelson L, et al. Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail. (2020) 22(3):413–21. doi: 10.1002/ejhf.1614
11. Kosmala W, Przewlocka-Kosmala M, Rojek A, Mysiak A, Dabrowski A, Marwick TH. Association of abnormal left ventricular functional reserve with outcome in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. (2018) 11(12):1737–46. doi: 10.1016/j.jcmg.2017.07.028
12. Choi KH, Yang JH, Seo JH, Hong D, Youn T, Joh HS, et al. Discriminative role of invasive left heart catheterization in patients suspected of heart failure with preserved ejection fraction. J Am Heart Assoc. (2023) 12(6):e027581. doi: 10.1161/jaha.122.027581
13. Egashira K, Sueta D, Komorita T, Yamamoto E, Usuku H, Tokitsu T, et al. HFA-PEFF scores: prognostic value in heart failure with preserved left ventricular ejection fraction. Korean J Intern Med. (2022) 37(1):96–108. doi: 10.3904/kjim.2021.272
14. Mu G, Wang W, Liu C, Xie J, Zhang H, Zhang X, et al. Combination of svi/s’ and diagnostic scores for heart failure with preserved ejection fraction. Clin Exp Pharmacol Physiol. (2023) 50(8):677–87. doi: 10.1111/1440-1681.13782
15. Przewlocka-Kosmala M, Butler J, Donal E, Ponikowski P, Kosmala W. Prognostic value of the maggic score, H(2)FPEF score, and HFA-PEFF algorithm in patients with exertional dyspnea and the incremental value of exercise echocardiography. J Am Soc Echocardiogr. (2022) 35(9):966–75. doi: 10.1016/j.echo.2022.05.006
16. Reddy YNV, Kaye DM, Handoko ML, van de Bovenkamp AA, Tedford RJ, Keck C, et al. Diagnosis of heart failure with preserved ejection fraction among patients with unexplained dyspnea. JAMA Cardiol. (2022) 7(9):891–9. doi: 10.1001/jamacardio.2022.1916
17. Tomasoni D, Aimo A, Merlo M, Nardi M, Adamo M, Bellicini MG, et al. Value of the HFA-PEFF and H(2) FPEF scores in patients with heart failure and preserved ejection fraction caused by cardiac amyloidosis. Eur J Heart Fail. (2022) 24(12):2374–86. doi: 10.1002/ejhf.2616
18. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. Quadas-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
19. Amanai S, Harada T, Kagami K, Yoshida K, Kato T, Wada N, et al. The H(2)FPEF and HFA-PEFF algorithms for predicting exercise intolerance and abnormal hemodynamics in heart failure with preserved ejection fraction. Sci Rep. (2022) 12(1):13. doi: 10.1038/s41598-021-03974-6
20. Nikorowitsch J, Bei der Kellen R, Kirchhof P, Magnussen C, Jagodzinski A, Schnabel RB, et al. Applying the esc 2016, H(2) FPEF, and HFA-PEFF diagnostic algorithms for heart failure with preserved ejection fraction to the general population. ESC Heart Fail. (2021) 8(5):3603–12. doi: 10.1002/ehf2.13532
21. Parcha V, Malla G, Kalra R, Patel N, Sanders-van Wijk S, Pandey A, et al. Diagnostic and prognostic implications of heart failure with preserved ejection fraction scoring systems. ESC Heart Fail. (2021) 8(3):2089–102. doi: 10.1002/ehf2.13288
22. Seo Y, Ishizu T, Ieda M, Ohte N. Clinical usefulness of the HFA-PEFF diagnostic scoring system in identifying late elderly heart failure with preserved ejection fraction patients. Circ J. (2021) 85(5):604–11. doi: 10.1253/circj.CJ-20-0784
23. Sotomi Y, Iwakura K, Hikoso S, Inoue K, Onishi T, Okada M, et al. Prognostic significance of the HFA-PEFF score in patients with heart failure with preserved ejection fraction. ESC Heart Fail. (2021) 8(3):2154–64. doi: 10.1002/ehf2.13302
24. Sun Y, Si J, Li J, Dai M, King E, Zhang X, et al. Predictive value of HFA-PEFF score in patients with heart failure with preserved ejection fraction. Front Cardiovasc Med. (2021) 8:656536. doi: 10.3389/fcvm.2021.656536
25. Tada A, Nagai T, Omote K, Iwano H, Tsujinaga S, Kamiya K, et al. Performance of the H(2)FPEF and the HFA-PEFF scores for the diagnosis of heart failure with preserved ejection fraction in Japanese patients: a report from the Japanese multicenter registry. Int J Cardiol. (2021) 342:43–8. doi: 10.1016/j.ijcard.2021.08.001
26. Verbrugge FH, Reddy YNV, Sorimachi H, Omote K, Carter RE, Borlaug BA. Diagnostic scores predict morbidity and mortality in patients hospitalized for heart failure with preserved ejection fraction. Eur J Heart Fail. (2021) 23(6):954–63. doi: 10.1002/ejhf.2142
27. Nagueh SF. Heart failure with preserved ejection fraction: insights into diagnosis and pathophysiology. Cardiovasc Res. (2021) 117(4):999–1014. doi: 10.1093/cvr/cvaa228
28. Coiro S, Echivard M, Simonovic D, Duarte K, Santos M, Deljanin-Ilic M, et al. Exercise-induced B-lines for the diagnosis of heart failure with preserved ejection fraction: a two-centre study. Clin Res Cardiol. (2023) 112(8):1129–42. doi: 10.1007/s00392-023-02219-y
29. Tribouilloy C, Rusinaru D, Mahjoub H, Soulière V, Lévy F, Peltier M, et al. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. (2008) 29(3):339–47. doi: 10.1093/eurheartj/ehm554
30. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. (2020) 22(8):1342–56. doi: 10.1002/ejhf.1858
31. Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail. (2019) 21(11):1306–25. doi: 10.1002/ejhf.1594
32. Taylor CJ, Ordóñez-Mena JM, Roalfe AK, Lay-Flurrie S, Jones NR, Marshall T, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. Br Med J. (2019) 364:1223. doi: 10.1136/bmj.l223
33. Wang Z, Fang J, Hong H. Evaluation the value of H(2)FPEF score and HFA-PEFF step E score in the diagnosis of heart failure with preserved ejection fraction. Acta Cardiol. (2023) 78(7):790–5. doi: 10.1080/00015385.2023.2221149
34. Seoudy H, von Eberstein M, Frank J, Thomann M, Puehler T, Lutter G, et al. HFA-PEFF score: prognosis in patients with preserved ejection fraction after transcatheter aortic valve implantation. ESC Heart Fail. (2022) 9(2):1071–9. doi: 10.1002/ehf2.13774
35. van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. (2013) 61(14):1498–506. doi: 10.1016/j.jacc.2012.12.044
36. Bishu K, Deswal A, Chen HH, LeWinter MM, Lewis GD, Semigran MJ, et al. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J. (2012) 164(5):763–70.e3. doi: 10.1016/j.ahj.2012.08.014
37. Hinderliter AL, Blumenthal JA, O'Conner C, Adams KF, Dupree CS, Waugh RA, et al. Independent prognostic value of echocardiography and N-terminal pro-B-type natriuretic peptide in patients with heart failure. Am Heart J. (2008) 156(6):1191–5. doi: 10.1016/j.ahj.2008.07.022
38. Lim TK, Dwivedi G, Hayat S, Majumdar S, Senior R. Independent value of left atrial volume index for the prediction of mortality in patients with suspected heart failure referred from the community. Heart. (2009) 95(14):1172–8. doi: 10.1136/hrt.2008.151043
39. Leung DY, Chi C, Allman C, Boyd A, Ng AC, Kadappu KK, et al. Prognostic implications of left atrial volume index in patients in sinus rhythm. Am J Cardiol. (2010) 105(11):1635–9. doi: 10.1016/j.amjcard.2010.01.027
Keywords: HFA-PEFF, HFpEF, prognosis, diagnosis, meta-analysis
Citation: Li X, Liang Y and Lin X (2024) Diagnostic and prognostic value of the HFA-PEFF score for heart failure with preserved ejection fraction: a systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1389813. doi: 10.3389/fcvm.2024.1389813
Received: 22 February 2024; Accepted: 1 July 2024;
Published: 12 July 2024.
Edited by:
Laura Fusini, Monzino Cardiology Center (IRCCS), ItalyReviewed by:
Kevin Shah, The University of Utah, United StatesStefano Coiro, Hospital of Santa Maria della Misericordia in Perugia, Italy
© 2024 Li, Liang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaozhong Lin, bGlueHpfZ3pob3NwQG91dGxvb2suY29t
 Xinmei Li
Xinmei Li Xiaozhong Lin
Xiaozhong Lin