- Department of Cardiology and Vascular Medicine, Faculty of Medicine, Universitas Padjadjaran—Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
Background: Concerns regarding restenosis after treatment with drug-coated balloons (DCB) remain. We aimed to identify the incidence of target lesion revascularization (TLR) and explore clinical, procedural, and other factors influencing it.
Methods: Single-center retrospective analysis of a prospective cohort PCI registry study included 80 patients (100 lesions) who underwent successful DCB angioplasty between January 2020 and October 2023 and follow-up angiography within 2 years of either planned or unplanned reason. Incidence and factors associated with TLR were analyzed.
Results: Angiographic evaluation was conducted within a median of 151 days (interquartile range: 109 days). During index procedure, 54% were complex lesions. Intravascular imaging (IVI) was performed in 80% of lesions. TLR occurred in 11% of the lesions and was less frequent in the IVI group compared to the angiography-alone group [6.3 vs. 54.5%; odds ratio: 0.156, 95% confidence interval (CI): 0.042–0.580; p = 0.002]. No association was found between baseline and lesion characteristics, lesion complexity, plaque morphology, pre-dilatation procedure balloon type, maximal inflation pressure, or length of DCB between the groups (p > 0.05). Multivariate analysis revealed that IVI utilization was independently associated with a lower TLR rate (adjusted odds ratio: 0.116, 95% CI: 0.020–0.669; p = 0.016).
Conclusion: In DCB angioplasty, only IVI use exhibited a significant difference in the TLR rate among baseline lesion characteristics and lesion preparation and was independently associated with a lower TLR rate.
Introduction
Despite the well-established safety and efficacy of drug-eluting stents (DES), the occurrence of in-stent restenosis (ISR) remains a problem, affecting approximately 5%–10% of all percutaneous coronary interventions (PCIs) (1–3). Certain particular settings are linked to increased rates of restenosis and thrombosis following stenting, such as ISR, small-vessel disease, and bifurcation disease, in which stent placement for treatment may not result in effective long-term and mid-term outcomes on follow-up which remains challenging (2, 4). As a result, using drug-coated balloons (DCB) as a treatment approach for coronary arteries to alleviate the stent burden is an appealing option, as it minimizes the need for permanent stent implants, especially in high-risk lesions (3).
The DCB comprises a semi-compliant balloon responsible for the acute mechanical effect in restoring vessel patency during angioplasty. It is coated with an antiproliferative drug that effectively inhibits smooth muscle cell proliferation and migration and is transferred to the vessel wall during balloon inflation (3, 5). The efficacy and safety of DCB in treating in-stent restenosis (ISR) were classified as class IA in the 2018 ESC/EACTS guidelines, similar to native small-vessel disease in randomized controlled trials (RCTs), and other emerging indications, such as high bleeding risk, large-vessel disease, and complex lesions, have also been studied (3, 5).
Despite its promising potential as an alternative to DES, concerns persist regarding restenosis after DCB implantation, and the incidence and potential influencing factors of target lesion revascularization (TLR) still need to be thoroughly assessed (3). Various clinical scenarios have been explored for DCB angioplasty, yielding promising results. However, the specific lesion characteristics impeding favorable angiography outcomes in DCB angioplasty have yet to be established. Several studies have aimed to predict angiographic outcomes after DCB use in specific lesions (e.g., small vessel coronary disease and ISR), but more studies are needed in actual clinical settings (6–8). We hypothesized that identifying clinical characteristics and procedural techniques as predictors of TLR on follow-up angiography could enhance the optimization of revascularization preparation during DCB utilization. Therefore, we conducted a single-center retrospective analysis of a prospective cohort study to investigate the incidence of TLR and evaluate potential influencing factors associated with it.
Methods
Study design and population
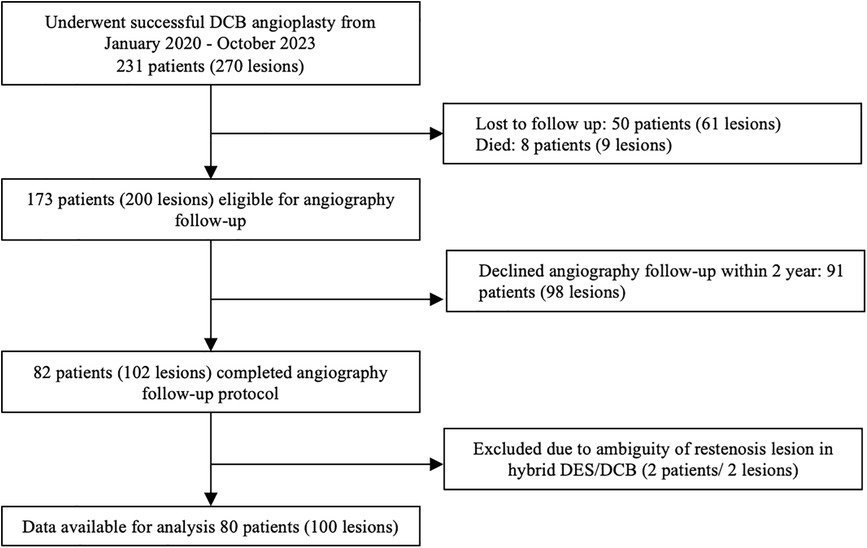
This was a single-center retrospective analysis of a prospective cohort PCI registry of Dr. Hasan Sadikin General Hospital involving patients who underwent successful DCB angioplasty. Each patient was enrolled in the DCB PCI registry of Dr. Hasan Sadikin General Hospital, Bandung. Between January 2020 and October 2023, 231 patients with 270 lesions underwent successful DCB angioplasty. As part of the study protocol, all patients were offered follow-up angiography within three months to two years as a planned procedure if no staging procedure was required. Among them, follow-up angiography was performed in 80 patients (100 lesions) as part of the scheduled angiographic evaluation and staged PCI or in cases where the patient experienced acute coronary syndrome (ACS), necessitating unplanned coronary angiography within two years (Figure 1). The patients were categorized into TLR and non-TLR groups during the follow-up angiography.
The inclusion criteria comprised patients aged ≥ 18 years, with evidence of ischemia, presenting with either stable angina pectoris or ACS and diameter stenosis of coronary vessels ≥70%, and having received successful DCB treatment [defined as patients with TIMI flow >3, with no apparent coronary dissection (i.e., type C or above) and residual stenosis ≤30% post the initial DCB procedure], whom underwent follow-up angiography of either planned or unplanned reason within 2 years. The exclusion criteria comprised patients who did not comply with dual antiplatelet therapy after the first angioplasty and those who were lost to follow-up during the evaluation of major adverse cardiovascular events (MACE) every three months until follow-up angiography. In addition, patients with an indeterminate DCB site on follow-up angiography because of the possibility of stent failure proximal to the DCB segment area (i.e., in the hybrid case) were also excluded.
All procedures involving human participants in this study were approved by the ethical committee of Dr. Hasan Sadikin General Hospital, Bandung, West Java, Indonesia (LB.02.01/X.6.5/19/2023) and were conducted in accordance with the Declaration of Helsinki. The requirement for written informed consent was waived, given the observational retrospective study design by the aforementioned ethical committee.
Devices and intervention protocol
Choice of vascular access, guide catheter, guidewire, balloon, or whether DCB only or hybrid approach with DES combination were left to operator's discretion. The IVI use and administration of glycoprotein IIb/IIIa receptor inhibitors were also left to the operator's discretion. To achieve a stenosis of ≤30%, balloon coronary pre-dilatation was uniformly performed in all patients. The choice of device size and post-dilatation treatment was based on the operator's decision. Balloon pre-dilatation was performed using uncoated balloons to achieve a balloon-to-vessel ratio of 0.8–1.0. The intervention procedure was performed according to the international and Asia-Pacific consensus recommendations for DCB treatment (9). Intravascular imaging was conducted before predilatation, except in cases where the IVI catheter was unable to cross the lesion, predilatation using a small balloon size [2.0 mm semi-compliant balloon (SCB)] was performed before advancing the IVI catheter. In the IVI group, lesion preparation and choice of DCB size were tailored based on morphological assessment and calculation of the reference vessel size using IVI. Patients received standard dual antiplatelet therapy (DAPT) before the procedure. They continued for at least six months in cases where only DCB was used and 12 months in the hybrid approach involving both DCB and DES, especially in ACS subsets. Only paclitaxel-coated balloons (SCB SeQuent Please; B. Braun, Melsungen, Germany) were employed at our center. Intravascular imaging was conducted using optical coherence tomography (OCT; Dragonfly OPTIS, Abbott Vascular) or intravascular ultrasonography (IVUS; Opticross, Boston Scientific).
Angiographic data, definitions, outcomes, and clinical follow-up
Lesion characteristics and complexity were defined by the standard definition outlined by the American Heart Association/American College of Cardiology (AHA/ACC) in 2020 (10). Multivessel disease (MVD) was characterized by more than one major epicardial vessel with >70% stenosis. Lesion length was determined using the length of the DCB or DES or the sum if more than one device was utilized. Vessel diameter measurements included the distal reference diameter, minimum lumen area (MLA), minimum stent area (MSA) in DES, external elastic membrane (EEM) at the target lesion using OCT or IVUS assessment in patients who underwent IVI. In cases without IVI, the optimal diameter of the DCB used was recorded (11, 12). Notably, baseline information and angiographic characteristics were obtained from angiography reports and registries.
The primary objective of this study was to assess the incidence of TLR based on The Academic Research Consortium-2, defined as clinically driven repeat percutaneous intervention of the target lesion or bypass surgery of the target vessel performed for restenosis or other complication of DCB-treated segment restenosis angiographically within the in-segment area, which encompassed the balloon-treated segment and extended 5-mm proximal and distal to the treated area, as determined by visual estimation during follow-up angiography (13). Clinically driven revascularization was determined during follow-up angiography, indicating restenosis ≥ 50% with at least one of the following: symptoms or objective signs of ischemia presumably related to the target vessel; abnormal results on invasive functional diagnostic testing (fractional flow reserve) or stenosis ≥ 70%, even in the absence of ischemic signs or symptoms. This study investigated the incidence and potential associated factors influencing its occurrence. Information regarding TLR was derived from the DCB registry and medical and angiography records. A team of certified interventional cardiologists, blinded to clinical outcomes, meticulously reviewed all angiographic data related to TLR.
All patients in the DCB registry underwent clinical follow-up following the index procedure via telephone interviews every three months and outpatient clinic visits until follow-up angiography. The secondary objectives of this study include the MACE, defined by composite of TLR and nonfatal myocardial infarction (MI) occurring from the initial procedure until the follow-up angiography. MACE was assessed on a patient basis (Table 1) while TLR were evaluated on a lesion basis (Table 2).
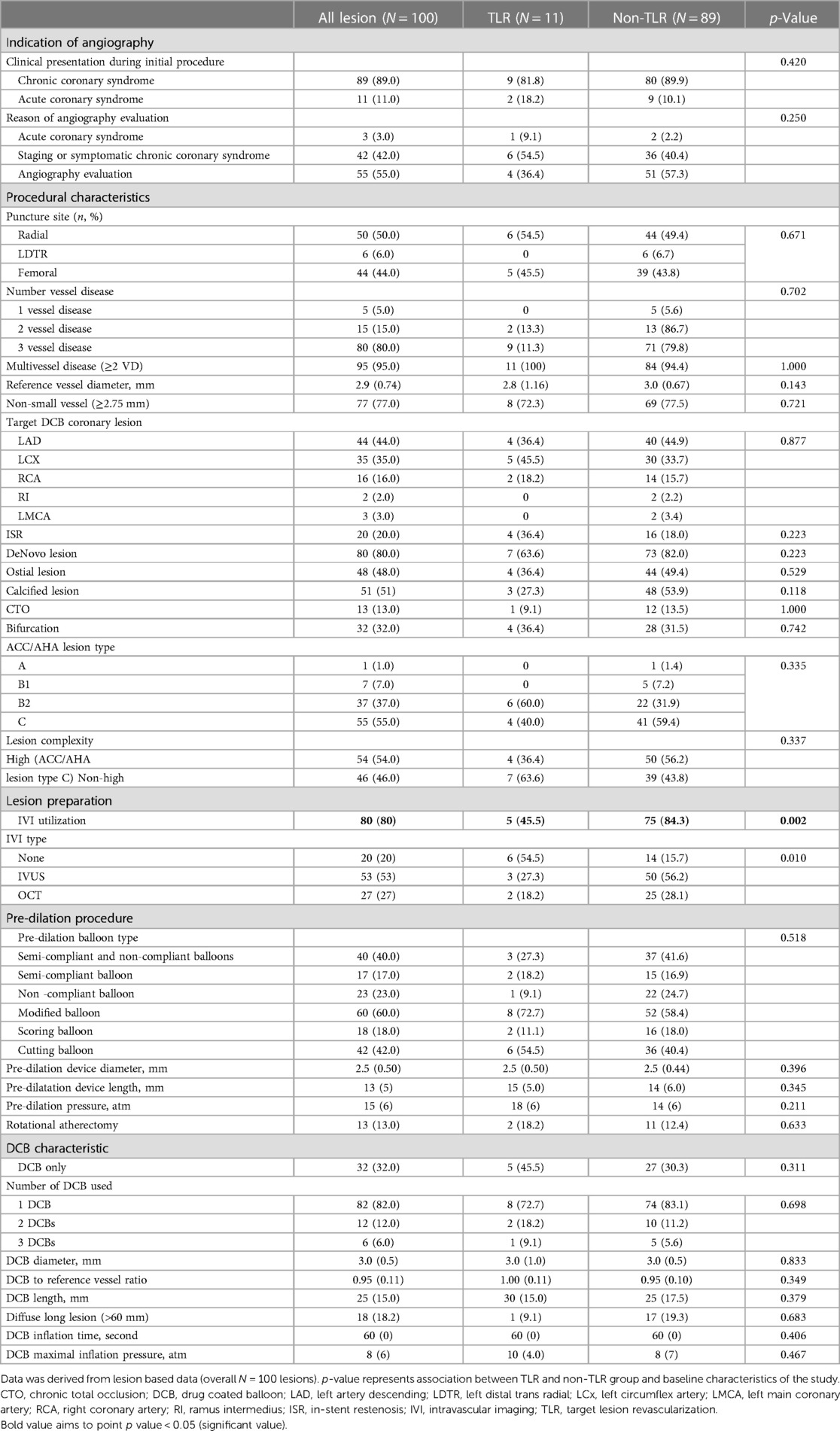
Table 2. Procedural and lesion characteristics, lesion preparation, DCB characteristic and post DCB complication.
Statistical analysis
All statistical analyses were performed using SPSS (version 24.0; SPSS Inc., Chicago, IL, USA). Data were presented as mean ± standard deviation (SD) or median (interquartile range, IQR), and dichotomous variables were represented as counts and percentages. Baseline patient characteristics, lesion features, and procedural details (such as lesion length, reference vessel diameter, pre-dilatation balloon diameter, pre-dilatation inflation pressure, DCB diameter, and DCB length) were analyzed and compared based on the incidence of TLR. Continuous variables were compared using the independent Student's t-test and Mann–Whitney U-test, while categorical variables were assessed using Fisher's exact or chi-squared test, as appropriate. The statistical significance level for the bivariate analysis was set at 0.05. Primary and secondary outcomes were analyzed using Fisher's exact test or the chi-squared test, and the odds ratio (OR) was also calculated. Subgroup analysis was performed to explore lesion characteristics using IVI. Odds ratios (ORs) with 95% confidence intervals (CIs) were computed using a multivariate logistic regression model, incorporating variables with p-values < 0.25 in the univariate analysis to identify the predictors of restenosis.
Results
Baseline characteristics
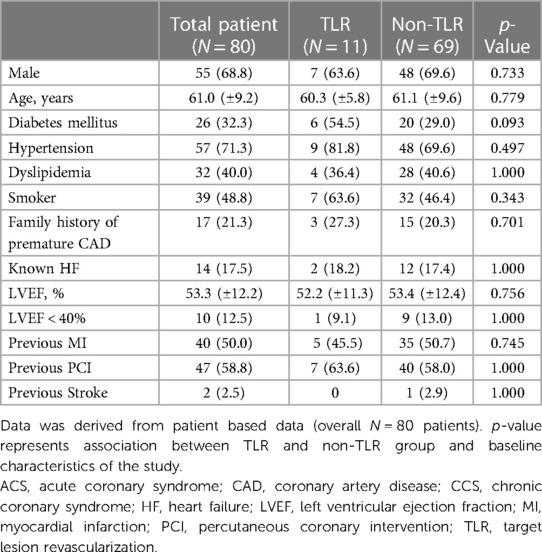
Among the 80 patients who underwent angiographic evaluation, 100 lesions were evaluated for TLR, which was found in 11 cases (11.0% among 100 lesions) and 11 patients (13.8% among 80 patients) and all underwent revascularization of either PCI or bypass surgery. The mean age of the patients was 61.0 (±9.2) years, with 68.8% (n = 55) being male. The angiographic evaluation was conducted at a median of 151 (IQR: 109) days, and there was no significant difference in the number of days to follow-up angiography between the TLR and non-TLR groups (195 [IQR: 114] vs. 147 [IQR: 100]; p = 0.123). Common cardiovascular risk factors included hypertension (71.3%), dyslipidemia (40.0%), smoking (48.8%), diabetes mellitus type II (32.3%), a family history of premature coronary artery disease (CAD) in 21.3%, and a history of MI (50.0%). The mean left ventricular ejection fraction was 53.3% (±12.2). Baseline characteristics for the TLR and non-TLR groups were comparable, as shown in Table 1 (p > 0.05).
Target lesion and procedural characteristics
The baseline target lesions and procedural characteristics are presented in Table 2. The reference vessel diameter was 2.9 (IQR 0.74) mm, with a DCB implanted in non-small vessels (≥2.75 mm) in 77% of the lesions. However, the reference vessel diameter did not significantly correlate with TLR (p = 0.143). ACS was the clinical presentation during the initial procedure in 11.0% (n = 11) of lesions and during follow-up angiography in 3.0% (n = 3). Among the three patients who underwent angiography during follow-up for ACS, all three presented with non-ST-segment elevation MI, and only one patient showed a post-DCB lesion as the culprit lesion.
The proportion of MVD (≥2 VD) did not differ between the TLR and non-TLR groups (p = 1.000); 15% of all lesions had two-vessel disease, and 80% had three-vessel disease. Neither lesion location nor the complexity of the lesion, including chronic total occlusion (CTO), ostial, calcified, and bifurcation lesions, showed an association with TLR on follow-up (p > 0.05). Fifty four (54%) lesions were classified as high-complexity lesions, as defined by the ACC/AHA as type C. The complexity of the lesion (type C vs. type B2 or below) was not associated with TLR events (40.0% vs. 59.4%, p = 0.335). DCBs were employed to treat ISR in 20% of the lesions (n = 20), and 20% developed TLR (n = 4). The TLR rate among the 80 de novo lesions treated with DCB was 8.7% (n = 7).
DCB-only treatment was administered in 32% of the lesions (n = 32), while the remaining involved DES implantation as hybrid approach during the index procedure. There was no observed association between the choice of DCB-only treatment or the hybrid approach involving DES and the occurrence of TLR (p = 0.311) (Table 2). In addition, there were no report of pericardial effusion, cardiac tamponade, or coronary perforation post-DCB implantation.
Lesion preparation, device characteristics
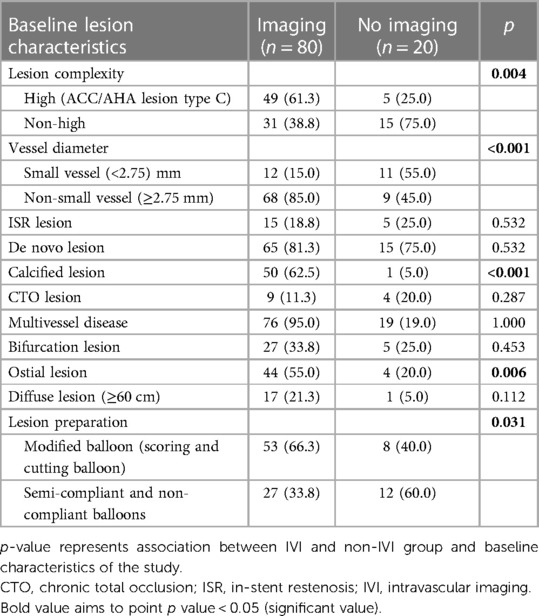
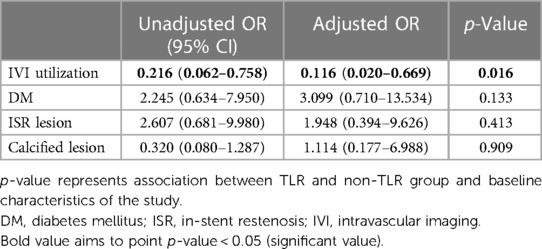
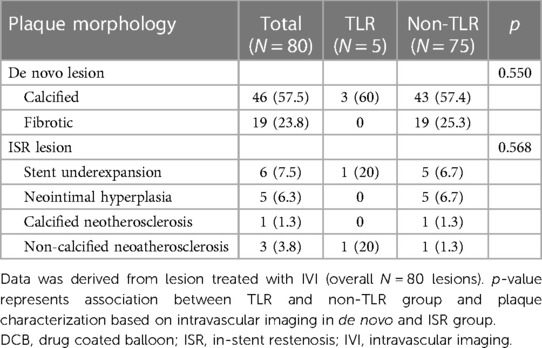
Intravascular imaging, including IVUS and OCT, was conducted for 80 lesions (80%) before DCB angioplasty. Balloon pre-dilatation was uniformly performed in all lesions, in which a modified balloon incorporating a cutting and scoring balloon was used more frequently than a semi-compliant or non-compliant balloon (60% vs. 40%). Subgroup analysis of lesion preparation balloons revealed significantly higher utilization of modified balloons in calcified vs. non-calcified lesions (70.6% vs. 49.0%; OR: 2.5, 95% CI: 1.098–5.691; p = 0.027). No association between the TLR and non-TLR group was found between diameter, length, maximal balloon inflation pressure, and pre-dilatation balloon type (Table 2). In the IVI group, subgroup analysis revealed significantly higher IVI use in highly complex, calcified, ostial, and non-small-vessel lesions (p < 0.05). Other lesion characteristics did not differ substantially between the IVI and non-IVI groups (Table 3). Despite the higher complexity of lesions in the IVI group, subgroup analysis demonstrated that IVI-guided PCI was associated with a lower rate of TLR on follow-up compared to angiography alone (6.3% vs. 54.5%; OR: 0.156, 95% CI: 0.042–0.580; p = 0.002) Figure 2. Multivariate logistic regression model analysis showed that IVI use was independently associated with a lower TLR rate (adjusted OR: 0.116, 95% CI: 0.020–0.669; p = 0.016), as presented in Table 4. No differences in lesion characteristics, as defined by IVI, were observed in either the de novo or ISR groups (Table 5) regarding TLR events.
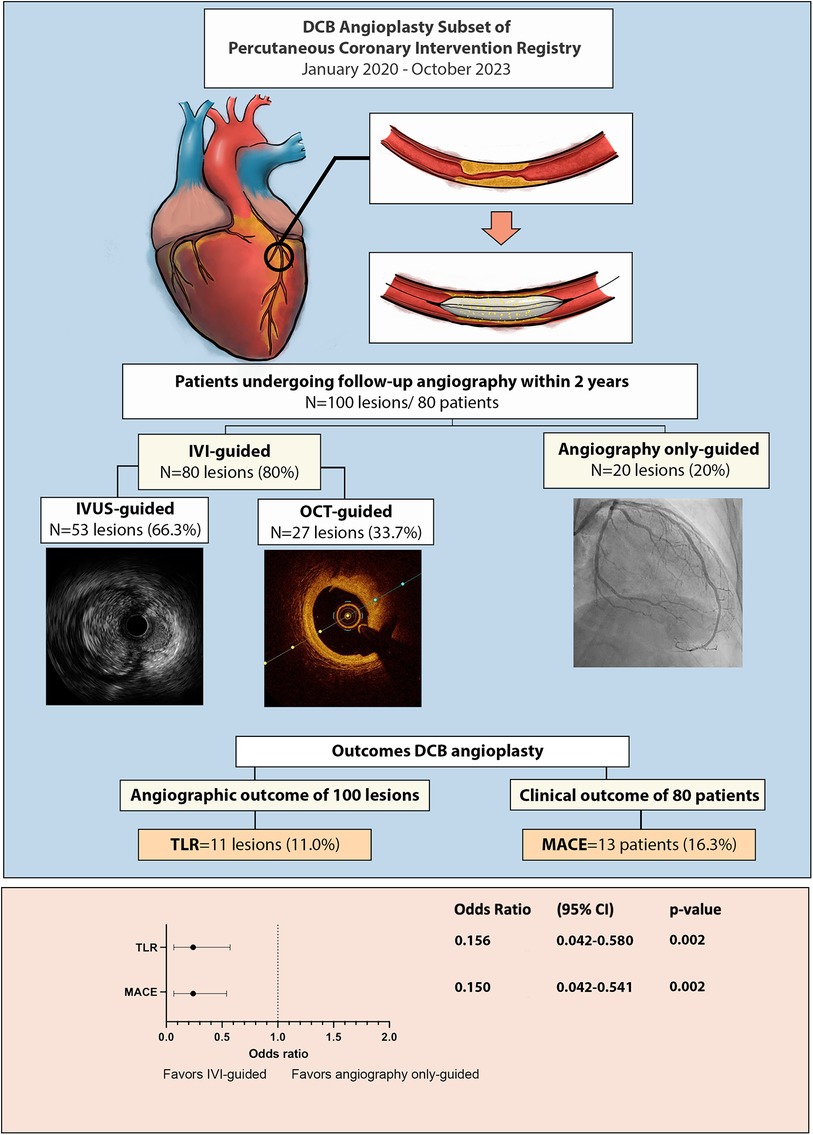
Figure 2. Central illustration: outcome of drug coated balloon angioplasty and the importance of intravascular imaging.
Intravascular imaging usage, clinical and angiography outcome
Not all restenosis cases were clinically significant; 36.4% of all restenosis lesions were found in routine angiography evaluation. MACE, defined as TLR and/or ACS, occurred in 13 (16.3%) patients. Two out of three patients with ACS did not have the culprit lesion related to the post-DCB site. The usage of IVI was also associated with lower clinical outcomes defined by MACE vs. angiography-alone group (9.5% vs. 41.2%; OR: 0.150, 95% CI: 0.042–0.541; p = 0.002).
Discussion
This is a cohort study with a follow-up period of approximately two years (median, five months) of angiography after DCB PCI conducted to capture the real-world scenarios where DCB was employed in various clinical settings, particularly in cases of MVD, either as a treatment for de novo or ISR lesions. Additionally, we also explored the use of DCB-only or the hybrid approach. This study has contributed to the growing trend in DCB usage, highlighting the importance of accumulating more experience in real-world clinical practice. Such experience holds promise for future strategies.
Incidence of TLR in DCB treatment
The observed rates of TLR in this study were higher than the other studies. For instance, in a study by Lee et al., which involved 2,666 coronary artery lesions treated with DCB, TLR occurred in only 5.1% of their patients (14). Our study also demonstrated higher restenosis rates than that in the PEARL registry, a real-world analysis of DCB use in the Netherlands conducted by Vlieger et al. In their study of 513 patients treated with DCB, the incidence of TLR was 11.7% in ISR lesions and 2.9% in de novo lesions (15). We considered that the main reason for this discrepancy was identification of most restenosis cases during follow-up angiography or staging procedures, regardless of the symptoms. Moreover, adopting a more liberal routine angiography follow-up strategy in our center might have resulted in more reintervention cases. Another factor could be the higher proportion of MVD in our study population compared to the Lee et al. registry study, where only 65.2% of patients had MVD. Shin et al. investigated the clinical impact of DCB on MVD and TLR in 3.1% of the DCB group, with follow-up angiography and subsequent revascularization being clinically driven rather than planned, regardless of symptoms. Contrary to their findings, clinically driven angiography due to ACS was observed in only 3% of the lesions in our current study (16). The current study includes all patients undergoing follow-up angiography because of both clinically driven and planned angiographic evaluation strategies for complex lesions, potentially leading to higher angiography findings, including clinically silent lesions. However, there might be unaccounted factors that could have influenced the restenosis rate.
Complexity lesion, lesion preparation, and intravascular imaging usage association with TLR event
The hybrid DES/DCB approach dominated lesion revascularization in our study (68%), with TLR rates similar to those observed in DCB only approach lesion (p = 0.311). So were complexity lesion, as it also did not show difference in TLR rate between complex and non-complex lesion. The lack of a statistically significant association between lesion complexity and TLR in this study suggested that lesion complexity did not impede the utilization of this approach. Costopulous et al. supported the hybrid DES/DCB approach in lesions with a high restenosis risk, demonstrating comparable MACE and TLR at the 2-year follow-up compared to the DES-only strategy (17). This hybrid strategy holds promise in reducing the need for extensive stent implantation, especially in long lesions, thereby minimizing the risk of ISR while maintaining effective antiproliferative action and potentially reducing the need for future reinterventions. A substudy of the HYPER trial further supported the use of DCB as an adjuvant to DES, showcasing its safety and effectiveness in revascularizing coronary bifurcation lesions (18). Even in challenging cases, such as CTO lesions, DCB emerged as a feasible and well-tolerated treatment with a low rate of MACE after successful revascularization with balloon angioplasty (19). Additionally, in the study by Nagai et al. focusing on a stentless strategy for calcified lesions following rotational atherectomy, TLR rates were comparable to those in our study, with no cardiac deaths reported at an average of 196 ± 37 days after the initial PCI (20). Regardless of lesion complexity, achieving optimal angiography results after pre-dilatation (i.e., residual stenosis < 30%, TIMI grade 3, and no flow-limiting dissection) was reported to be crucial before DCB implantation (18). Therefore, emphasizing optimal lesion preparation is essential to reduce future adverse events after DCB intervention, including IVI.
In this study, IVI utilization was significantly higher in the non-TLR group. Eighty percent lesion was treated with IVI during lesion preparation and the TLR was significantly lower in the IVI group (6.3% vs 54.5%; OR: 0.156, 95% CI: 0.042–0.580; p = 0.002). The improvement in coronary blood flow before DCB application was achieved through adequate pre-dilatation to induce dissection and facilitate homogenous drug delivery. Aside from attaining optimal lesion preparation by either non- or semi-compliant balloon or usage of non-compliant or scoring/cutting balloon as predilatation balloon and atherectomy in more complex lesions (i.e., calcified lesions), additional IVI such as IVUS and OCT is recommended (5, 21). Moreover, satisfactory balloon angioplasty pre-DCB implantation results are usually achieved with a vessel ratio of 0.8–1.0, which is more likely to be precise using imaging. This study revealed no significant difference in plaque morphology, as assessed by IVI, between the TLR and non-TLR groups. Thus, optimizing PCI by determining the precise stent sizing and landing zones might be more critical rather than solely focusing on the underlying mechanism of plaque morphology or the type of morphology itself. Intravascular imaging can contribute to a more accurate diagnosis of minor dissection and residual stenosis (22–24). Guidance using IVI has been recommended, particularly for ISR lesions, to identify and correct the morphological causes of lesion failure (Class IIa recommendation) (25). Research has also suggested that additional IVIs should be considered for de novo lesions, especially in more complex cases (9). However, a propensity-matched study by Lv et al. failed to demonstrate a significant difference in target lesion failure between the IVI-guided and angiography-guided groups. Notably, their study included clinical outcomes as endpoints, such as death and MI, in contrast to our study, which specifically focused on the angiographic outcomes of DCB angioplasty, where MI occurred in only 3% (22).
In our study, IVI utilization was higher than in a previous study by Lv et al. (80.0% vs. 7.8%). Intravascular imaging was predominantly employed in 49 out of 54 high-complexity lesions or type C ACC/AHA lesions, which was significantly higher than in 31 out of 46 non-high-complexity lesions (i.e., type B2 or less) (90.7 vs. 67.3%; p = 0.004) (22). The number of calcified and ostial lesions was also significantly higher in the IVI group (Table 3). Intravascular imaging offers a more comprehensive and accurate calcification assessment and provides optimal guidance for lesion preparation in calcified lesions, and it may also help resolve the ambiguity of vessel overlap in ostial lesions (24). Despite the higher complexity of lesions in the IVI group, the TLR rate was lower in the IVI group than in the angiography-alone group. This suggests that using IVI to guide lesion preparation is crucial, particularly for complex lesions.
Other lesion preparation components, such as choice of balloon angioplasty and calcium modification by atherectomy, did not exhibit significant differences between the TLR and non-TLR groups. However, this should not indicate that balloon angioplasty, DCB size selection, or atherectomy planning did not impact lesion preparation before DCB implantation. A sub-study analysis indicated that modified balloon utilization was significantly higher in calcified lesions. Therefore, the lack of a significant association in this study may suggest that the appropriateness of aggressiveness in lesion preparation before DCB implantation, supported by IVI, is crucial to ensure a thorough inspection of lesion characteristics after pre-dilatation. This was consistent with a review by Basavarajaiah et al., who recommended IVI with a modified balloon as the preferred lesion preparation choice for complex calcified lesions (26). Theoretically, the local tissue drug distribution of DCB should be superior after more pronounced neointimal modification with a scoring balloon, as observed in the RCT study by Kufner et al. In that study, DCB pre-dilatation using a scoring balloon in 203 patients with ISR showed a lower rate of angiographic restenosis in the scoring balloon pre-dilatation arm compared to that via standard therapy. However, it is worth noting that the previous study used quantitative coronary angiography for lesion preparation in all subjects, while our study primarily utilized IVI, which could contribute to more optimal lesion preparation regardless of the choice of balloon angioplasty or atherectomy (24). Similar reasoning might also apply to the selection of DCB size and length, which did not significantly impact our study's angiographic outcomes of TLR events. In contrast, Xue et al. investigated factors influencing restenosis in ISR lesions after DCB angioplasty in 199 lesions at nine months of follow-up. They demonstrated that lesion characteristics, such as longer lesions, were associated with restenosis due to increased area exposure to vascular injury and aggravated inflammatory response. However, previous studies did not provide data on IVI use, potentially leading to errors in estimating the degree of stenosis. These studies mainly focused on the ISR population, representing only a small proportion of our study (18.8%) (8). Furthermore, this study's stentless strategy following atherectomy showed results similar to those in the Nagai et al. study, which demonstrated acceptable clinical and angiography outcomes (20).
Limitations
This study had several limitations. Firstly, the small population size and the limited size of the follow-up population were the main limitations of the study. The use of DCB was initially limited to specific lesions such as ISR and small vessel disease, until recently, when its use expanded to include de novo and non-small vessel disease. Thus, it is only recently that enrollment has become faster. Secondly, the inclusion criteria in this study focused only on patients who underwent angiography follow-up without considering in-hospital mortality and death before angiography follow-up. Consequently, this study demonstrated better angiographic outcomes than clinical MACE outcomes after DCB. It is important to note that factors not accounted for in this study, such as drug compliance, lifestyle modifications after initial PCI, and environmental factors, could potentially influence TLR. Thus, further randomized controlled trials are needed to validate whether using IVI improves the outcomes of DCB PCI.
Conclusion
The incidence of TLR in DCB angioplasty was eleven percent. Baseline characteristics such as diabetes mellitus, vessel size, and lesion complexity did not influence the TLR rate. In addition, the utilization of IVI significantly reduced the TLR rate. Therefore, our results suggest that DCB angioplasty can be considered as an option to DES for the treatment of either de novo or ISR in small or non-small coronary lesions, employing a DCB-only approach, or as an adjuvant tool to DES in complex lesions, with no difference in TLR rate as long as lesion preparation is optimized, which might be enhanced by IVI utilization.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics committee of Hasan Sadikin General Hospital, Bandung, West Java, Indonesia. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a registry observational study.
Author contributions
AU: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. AS: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – review & editing. BT: Formal Analysis, Investigation, Supervision, Validation, Writing – review & editing. MA: Formal Analysis, Investigation, Supervision, Writing – review & editing. AY: Conceptualization, Formal Analysis, Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Muramatsu T, Kozuma K, Tanabe K, Morino Y, Ako J, Nakamura S, et al. Clinical expert consensus document on drug-coated balloon for coronary artery disease from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther. (2023) 38:166–76. doi: 10.1007/s12928-023-00921-2
2. Buccheri D, Piraino D, Andolina G, Cortese B. Understanding and managing in-stent restenosis: a review of clinical data, from pathogenesis to treatment. J Thorac Dis. (2016) 8(10):E1150–62. doi: 10.21037/jtd.2016.10.93
3. Buccheri D, Cortese B. Previous mistakes with DCB technology, and how to prevent them in the future. In: Cortese B, editor. Drug-Coated Balloons. Cham: Springer International Publishing (2019). doi: 10.1007/978-3-319-92600-1
4. Giacoppo D, Saucedo J, Scheller B. Coronary drug-coated balloons for De Novo and in-stent restenosis indications. J Soc Cardiovasc Angiogr Interventions. (2023) 2(3):1–17. doi: 10.1016/j.jscai.2023.100625
5. Jeger R V, Eccleshall S, Wan Ahmad WA, Ge J, Poerner TC, Shin ES, et al. Drug-coated balloons for coronary artery disease: third report of the international DCB consensus group. JACC Cardiovasc Interv. (2020) 13:1391–402. doi: 10.1016/j.jcin.2020.02.043
6. Cassese S, Xu B, Habara S, Rittger H, Byrne RA, Waliszewski M, et al. Incidence and predictors of recurrent restenosis after drug-coated balloon angioplasty for restenosis of a drug-eluting stent: the ICARUS cooperation. Rev Esp Cardiol (Eng Ed). (2018) 71:620–27. doi: 10.1016/j.rec.2017.08.005
7. Kang WC, Park SM, Jang AY, Oh PC, Shin ES, Yu CW, et al. Predictors of favorable angiographic outcomes after drug-coated balloon use for de novo small vessel coronary disease (DCB-ONLY). Angiology. (2021) 72:986–93. doi: 10.1177/00033197211015534
8. Xue W, Ma J, Yu X, Ruan Z, Sun Y, Wu T, et al. Analysis of the incidence and influencing factors associated with binary restenosis of target lesions after drug-coated balloon angioplasty for patients with in-stent restenosis. BMC Cardiovasc Disord. (2022) 22:493. doi: 10.1186/s12872-022-02923-z
9. Her AY, Shin ES, Bang LH, Nuruddin AA, Tang Q, Hsieh IC, et al. Drug-coated balloon treatment in coronary artery disease: Recommendations from an Asia-pacific consensus group. Cardiol J. (2021) 28:136–49. doi: 10.5603/CJ.a2019.0093
10. Dehmer GJ, Badhwar V, Bermudez EA, Cleveland JC, Cohen MG, D’Agostino RS, et al. 2020 AHA/ACC key data elements and definitions for coronary revascularization: a report of the American College of Cardiology/American Heart Association Task Force on clinical data standards (writing committee to develop clinical data standards for coronary revascularization). J Am Coll Cardiol. (2020) 75:1975–2088. doi: 10.1016/j.jacc.2020.02.010
11. Gunawardena TD, Corballis N, Merinopoulos I, Wickramarachchi U, Reinhold J, Maart C, et al. Drug-coated balloon vs. drug-eluting stents for de novo unprotected left main stem disease: the SPARTAN-LMS study. J Cardiovasc Dev Dis. (2023) 10:84. doi: 10.3390/jcdd10020084
12. Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. (2018) 39:3281–300. doi: 10.1093/eurheartj/ehy285
13. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized end point definitions for coronary intervention trials: The academic research consortium-2 consensus document. Circulation. (2018) 137:2635–50. doi: 10.1161/CIRCULATIONAHA.117.029289
14. Lee SY, Cho YK, Kim SW, Hong YJ, Koo BK, Bae JW, et al. Clinical results of drug-coated balloon treatment in a large-scale multicenter Korean registry study. Korean Circ J. (2022) 52(6):444–54. doi: 10.4070/kcj.2021.0261
15. Vlieger S, Cheng JM, Oemrawsingh RM, Weevers APJD, Polad J, Gho B, et al. Clinical performance of a paclitaxel drug-coated balloon in real-world percutaneous coronary intervention practice: the PEARL registry. J Invasive Cardiol. (2022) 34:E462–68. PMID: 35652709.35652709
16. Shin ES, Jun EJ, Kim S, Kim B, Kim TH, Sohn CB, et al. Clinical impact of drug-coated balloon-based percutaneous coronary intervention in patients with multivessel coronary artery disease. JACC Cardiovasc Interv. (2023) 16:292–9. doi: 10.1016/j.jcin.2022.1
17. Costopoulos C, Latib A, Naganuma T, Sticchi A, Figini F, Basavarajaiah S, et al. The role of drug-eluting balloons alone or in combination with drug-eluting stents in the treatment of de novo diffuse coronary disease. JACC Cardiovasc Interv. (2013) 6:1153–9. doi: 10.1016/j.jcin.2013.07.005
18. Pellegrini D, Donahue M, Regazzoli D, Tedeschi D, Loffi M, Pellicano M, et al. Drug-coated balloon combined with drug-eluting stent for the treatment of coronary bifurcation lesions: insights from the HYPER study. Eur Heart J Suppl. (2023) 25:C79–83. doi: 10.1093/eurheartjsupp/suad011
19. Jun EJ, Shin ES, Teoh EV, Bhak Y, Yuan SL, Chu CM, et al. Clinical outcomes of drug-coated balloon treatment after successful revascularization of de novo chronic total occlusions. Front Cardiovasc Med. (2022) 9:1–10. doi: 10.3389/fcvm.2022.821380
20. Nagai T, Mizobuchi M, Funatsu A, Kobayashi T, Nakamura S. Acute and mid-term outcomes of drug-coated balloon following rotational atherectomy. Cardiovasc Interv Ther. (2020) 35(3):242–9. doi: 10.1007/s12928-019-00611-y
21. Her AY, Shin ES, Kim S, Kim B, Kim TH, Sohn CB, et al. Drug-coated balloon-based versus drug-eluting stent-only revascularization in patients with diabetes and multivessel coronary artery disease. Cardiovasc Diabetol. (2023) 22(1):120. doi: 10.1186/s12933-023-01853-0
22. Lv S, Ma X, Zhou Y, et al. Intracoronary imaging versus coronary angiography to guide drug-coated balloon intervention in coronary artery disease: a propensity-matched pilot study analysis. Angiology. (2021) 72(10):971–8. doi: 10.1177/00033197211012518
23. Abouelnour A, Gori T. Intravascular imaging in coronary stent restenosis: prevention, characterization, and management. Front Cardiovasc Med. (2022) 9:1–21. doi: 10.3389/fcvm.2022.843734
24. Shlofmitz E, Ali ZA, Maehara A, Mintz GS, Shlofmitz R, Jeremias A. Intravascular imaging-guided percutaneous coronary intervention: a universal approach for optimization of stent implantation. Circ Cardiovasc Interv. (2020) 13(12):E008686. doi: 10.1161/CIRCINTERVENTIONS.120.008686
25. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40(2):87–165. doi: 10.1093/eurheartj/ehy394
Keywords: drug-coated balloon (DCB), intravascular imaging, restenosis, target lesion revascularization, percutaneous coronary intervention
Citation: Undarsa AC, Saboe A, Tiksnadi BB, Akbar MR and Yahya AF (2024) Factors influencing coronary artery target lesion revascularization after drug-coated balloon angioplasty. Front. Cardiovasc. Med. 11:1387074. doi: 10.3389/fcvm.2024.1387074
Received: 16 February 2024; Accepted: 29 April 2024;
Published: 16 May 2024.
Edited by:
Chun Chin Chang, Taipei Veterans General Hospital, TaiwanReviewed by:
Alessandra Laricchia, ASST Fatebenefratelli Sacco, ItalyYuki Katagiri, Sapporo Higashi Tokushukai Hospital, Japan
© 2024 Undarsa, Saboe, Tiksnadi, Akbar and Yahya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Achmad Fauzi Yahya, ZmF1eml5YWh5YTQ2QHlhaG9vLmNvbQ==
 Alberta Claudia Undarsa
Alberta Claudia Undarsa Aninka Saboe
Aninka Saboe Mohammad Rizki Akbar
Mohammad Rizki Akbar