
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 24 July 2024
Sec. Coronary Artery Disease
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1385318
Introduction: The objective of this research was to evaluate the risk of major adverse cardiovascular events (MACEs) associated with the use of various proton pump inhibitors (PPIs) in combination with clopidogrel in patients who underwent percutaneous coronary intervention (PCI).
Methods: To accomplish this, we analyzed data from randomized controlled trials and retrospective cohort studies sourced from key electronic databases. These studies specifically examined the effects of different PPIs, such as lansoprazole, esomeprazole, omeprazole, rabeprazole, and pantoprazole, when used in conjunction with clopidogrel on MACEs. The primary focus was on the differential impact of these PPIs, while the secondary focus was on the comparison of gastrointestinal (GI) bleeding events in groups receiving different PPIs with clopidogrel vs. a placebo group. This study's protocol was officially registered with INPLASY (INPLASY2024-2-0009).
Results: We conducted a network meta-analysis involving 16 studies with a total of 145,999 patients. Our findings indicated that rabeprazole when combined with clopidogrel, had the lowest increase in MACE risk (effect size, 1.05, 95% CI: 0.66–1.66), while lansoprazole was associated with the highest risk increase (effect size, 1.48, 95% CI: 1.22–1.80). Esomeprazole (effect size, 1.28, 95% CI: 1.09–1.51), omeprazole (effect size, 1.23, 95% CI: 1.07–1.43), and pantoprazole (effect size, 1.38, 95% CI: 1.18–1.60) also significantly increased MACE risk. For the secondary outcome, esomeprazole (effect size, 0.30, 95% CI: 0.09–0.94), omeprazole (effect size, 0.34, 95% CI: 0.14–0.81), and pantoprazole (effect size, 0.33, 95% CI: 0.13–0.84) demonstrated an increased potential for GI bleeding prevention.
Conclusions: In conclusion, the combination of lansoprazole and clopidogrel was found to significantly elevate the risk of MACEs without offering GI protection in post-PCI patients. This study is the first network meta-analysis to identify the most effective regimen for the concurrent use of clopidogrel with individual PPIs.
Systematic Review Registration: https://inplasy.com/inplasy-2024-2-0009/, identifier (INPLASY2024-2-0009).
Antiplatelet therapy is now the standard treatment for patients who have percutaneous coronary intervention (PCI). After a balloon injury and the insertion of a stent, the inner layer of the blood vessel at that location becomes damaged. This leads to the activation of both the coagulation cascade and platelets (1, 2). After PCI, a key focus is on preventing blood clots in the newly widened arteries, which is where medications such as clopidogrel come into play. Clopidogrel is an antiplatelet drug, essential in post-PCI management. It works by inhibiting platelets in the blood from clumping together to form clots. This action is particularly important in patients who have had stents placed during PCI, as stents increase the risk of clot formation within the artery (3).
Clopidogrel, usually taken in combination with aspirin, significantly reduces the risk of stent thrombosis, a serious complication where a blood clot forms on the stent, potentially leading to major cardiovascular events (MACEs) (4). The duration of clopidogrel therapy can vary based on the type of stent used and the patient's overall risk profile. Recent advancements in drug-eluting stents have influenced the recommended length of clopidogrel therapy (5). Continuous monitoring and follow-up are essential to manage any side effects of clopidogrel, such as bleeding risk, and to adjust treatment plans as needed for the individual patient. The goal is to balance the prevention of clotting with the risk of excessive bleeding, optimizing patient outcomes post-PCI (6).
To alleviate these side effects, PPIs such as esomeprazole, lansoprazole, pantoprazole, omeprazole, and rabeprazole have been introduced. PPIs are a class of medications widely used to treat conditions caused by excessive stomach acid production. They work by irreversibly blocking the hydrogen/potassium ATPase enzyme system of the gastric parietal cells, effectively reducing gastric acid secretion. PPIs are primarily used to manage gastroesophageal reflux disease (GERD), peptic ulcers, and Zollinger–Ellison syndrome (7). They are also prescribed to prevent and treat gastric ulcers induced by nonsteroidal anti-inflammatory drugs (NSAIDs) and to eradicate Helicobacter pylori infections in combination with antibiotics.
Combining PPIs with clopidogrel is beneficial for reducing the risk of GI bleeding in patients undergoing antiplatelet therapy. PPIs effectively decrease stomach acid, protecting the GI lining from damage, while clopidogrel prevents blood clots. The coadministration of antiplatelet agents and PPI is often a clinical choice for physical due to a decrease in the risk of GI bleeding (8). However, there are concerns about the potential interaction between clopidogrel and PPIs. The main concern is that both drugs use the same metabolic enzyme CYP2C19. PPIs might impede the conversion of clopidogrel into its active form through CYP2C19. This could result in lower blood levels of the active metabolite and possibly diminish clopidogrel's effectiveness in preventing platelet aggregation (9). It is noteworthy that different PPIs utilize different liver enzymes for metabolism. Due to the drug–drug interaction (DDI) concerns, the choice of PPI and clopidogrel should always be cautious. To figure out the effect of MACEs on coadministration in different PPIs with clopidogrel is important. Our study aims to determine which specific PPIs, when coadministered with clopidogrel, might have the lowest MACEs and GI bleeding risk.
We executed this study following the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension for network meta-analysis (PRISMA NMA) (10). The study has been registered on INPLASY under the registration number INPLASY2024-2-0009 (11). The approval from the ethical review board or securing informed consent from participants was unnecessary.
Two authors (M-YA and W-LC) independently conducted electronic searches across the following databases, including PubMed, Cochrane Reviews, Cochrane Central, Web of Science, and ClinicalTrials.gov. The search utilized the following keywords: (“Clopidogrel”) AND (“Percutaneous coronary intervention”) AND (“Lansoprazole” OR “Omeprazole” OR “Esomeprazole” OR “Pantoprazole” OR “Rabeprazole” OR “Dexlansoprazole” OR “Proton pump inhibitors”). The systematic review and network meta-analysis search spanned from the earliest entry in each database up to the most recent search date (24 January 2024). First, the two authors systematically assessed the titles and abstracts of identified studies for eligibility through a consensus process. In instances where consensus could not be reached by the initial two reviewers, a third author was consulted. There were no language restrictions imposed on this search.
The network meta-analysis was structured according to the PICO (population, intervention, comparison, and outcome), encompassing the following criteria: (1) P, human participants with post-PCI using clopidogrel; (2) I, coadministrated PPI; (3) C, placebo group without intervention; and (4) O, MACE risk. The study applied the subsequent inclusion criteria: (1) the randomized controlled trials and retrospective cohort studies that enrolled patients after PCI using clopidogrel with or without individual PPIs; (2) the randomized controlled trials and retrospective studies that enrolled patients after PCI using clopidogrel with or without individual PPIs longer than a month; (3) the placebo group receiving no PPIs; and (4) studies providing available data on MACEs.
The exclusion criteria applied in this review and network meta-analysis comprised the following: (1) patients without PCI intervention, (2) studies lacking individual PPI analysis of MACEs risk, (3) studies without antiplatelet agent clopidogrel, (4) studies without placebo/control groups, (5) data that are either incomplete or not accessible despite efforts to reach the authors through email, and (6) research involving participants who were also part of a trial previously incorporated into our analysis.
While performing our network meta-analysis, we followed certain guidelines in building our model to ensure uniformity and minimize variability. Our paired comparisons were restricted to either clopidogrel plus PPI vs. placebo or control group. We deliberately excluded comparisons between clopidogrel plus PPI and other antiplatelet agents combined with PPI, such as prasugrel. This choice was based on the understanding that incorporating other antiplatelet agents could create diverse network structures because of the distinct characteristics of these treatments, potentially leading to inconsistent results (12).
For assessing the methodological soundness of the studies included in our analysis, we used the Cochrane risk of bias tool for randomized control trials (version 2, RoB 2, based in London, UK) (13) and Newcastle-Ottawa scale (NOS) assessment for non-randomized control studies (14). The Cochrane risk of bias tool instrument evaluates six crucial aspects to determine the quality of a study, including the process of randomization, compliance with the intervention, management of missing outcome data, measurement of outcomes, selective outcome reporting, and the overall likelihood of bias. The NOS comprises three domains, namely, selection of study groups, comparability of study groups, and assessment of outcome to determine the quality of a study.
In our study, the primary outcome focused on assessing the effect size of various PPIs on the incidence of MACEs, when coadministered with clopidogrel. We analyzed lansoprazole, esomeprazole, omeprazole, pantoprazole, and rabeprazole, comparing their impact on MACEs without PPI usage.
In our study, the secondary outcome focused on evaluating the impact of individual PPIs coadministered with clopidogrel on the risk of GI bleeding. This analysis aimed to understand the size effect of each PPI had on reducing GI bleeding risks in comparison to a placebo group. The specific PPIs examined were esomeprazole, omeprazole, pantoprazole, rabeprazole, and lansoprazole.
The data extraction was independently carried out by two authors, M-YA and W-LC. This process involved collecting various types of information from the studies, such as demographic details, study design, specifics of the PPI, and both primary and secondary outcomes. The procedures for extracting data, converting, and merging the results were performed in line with the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions, as well as other pertinent medical literature (15–19).
Given the diversity of the included studies in our article, we employed a random-effects model for the network meta-analysis. The analysis was executed using the MetaInsight software (version 4.0.2, Complex Reviews Support Unit, National Institute for Health Research, London, UK), operating within a frequentist statistical framework. MetaInsight, a web-based tool for network meta-analysis, utilizes the netmeta package in R software to perform frequentist statistical analyses.
In the initial stage of our analysis, we created forest plots and network plots to visually represent the pairwise comparisons from each study. Additionally, we generated forest plots to assess the relative risk of MACEs and GI bleeding compared to a placebo group. These plots offered a detailed view of the outcomes, showing effect sizes as point estimates accompanied by 95% confidence intervals (95% CI). We ranked the different types of PPIs based on their efficacy and presented numerical data for both direct and indirect comparisons in tables. To check for any inconsistencies in the data, we carried out inconsistency tests. The threshold for statistical significance was set at a two-tailed p-value of <0.05.
To improve the trustworthiness of our study's results, we employed the one-study-removed sensitivity analysis method, which involves sequentially excluding each study. This approach helped ensure that the effect estimates from any individual study did not disproportionately influence the overall findings. By systematically removing each study one by one from the analysis of MACEs and GI bleeding, we were able to determine whether the ultimate conclusions and the rankings of the studies remained stable.
We evaluated possible publication bias in accordance with the procedures outlined in the Cochrane Handbook for Systematic Reviews of Interventions (15). To illustrate this, we generated a funnel plot using the Comprehensive Meta-Analysis Software, version 4 (BioStat, Englewood, NJ, USA), specifically examining the comparisons with the placebo group. Additionally, Egger's regression test was utilized to quantitatively ascertain the extent and significance of any potential publication bias.
Figure 1 displays the PRISMA flowchart illustrating the process of our literature search. The checklist for the PRISMA NMA extension can be found in Supplementary Table S1. Our selection process involved filtering out duplicate articles and discarding those irrelevant to our research, based on screening of their titles and abstracts, which resulted in the inclusion of 16 studies (20–35). These were either randomized controlled trials or retrospective or prospective cohort studies.
In total, our analysis encompassed 16 studies involving 145,999 individuals. The proton pump inhibitors (PPIs) evaluated in these studies included lansoprazole, esomeprazole, omeprazole, pantoprazole, and rabeprazole. The network model illustrating the interaction with clopidogrel co-treatment with individual PPI is shown in Figure 2. For additional information regarding the inclusion criteria and details about the studies, please refer to Table 1.
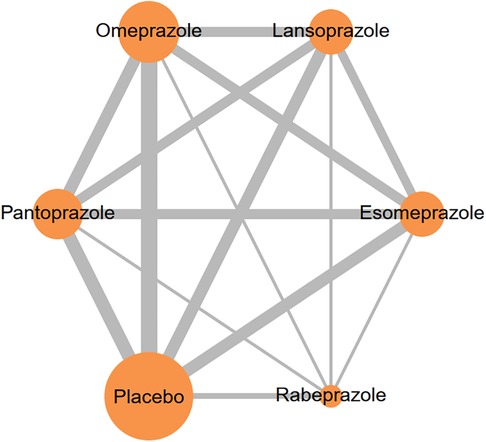
Figure 2 Network plots illustrate the effects of different PPIs concurrent with clopidogrel on MACE risk. The size of each node and thickness of each line represent the number of trials included in the analysis.
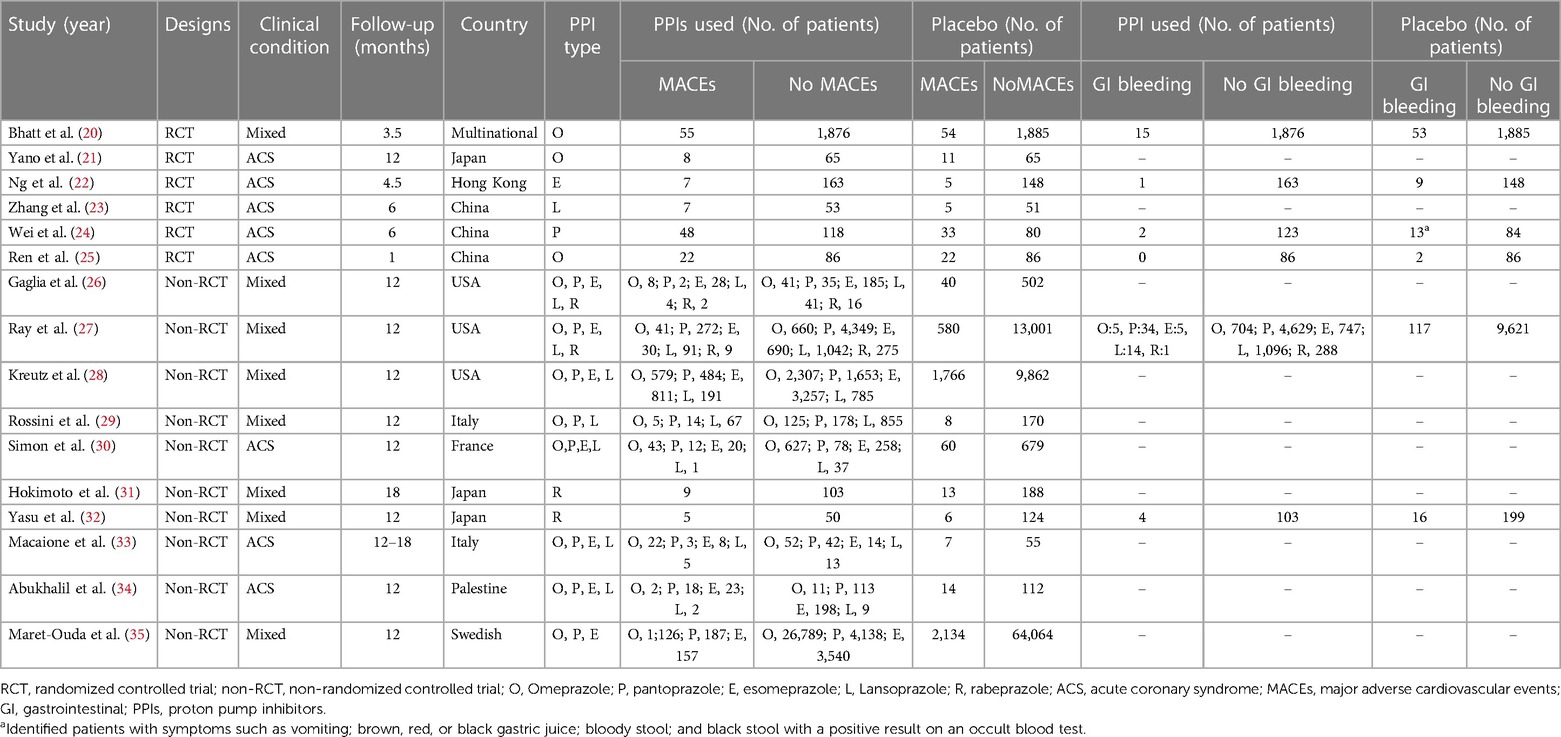
Table 1 Summary of the included studies in concurrent PPIs and clopidogrel resulted in MACEs and GI bleeding.
The evaluation of the methodological quality of the studies is shown in Supplementary Table S2–S3 and Figure S1. The studies identified with a potential bias risk demonstrated protocol variations among different study groups, potentially influencing adherence and the results of the interventions. Comprehensive information on the risk of bias evaluation is available in Supplementary Table S2–S3.
In our study, lansoprazole (effect size, 1.48, 95% CI: 1.22–1.80), esomeprazole (effect size, 1.28, 95% CI: 1.09–1.51), omeprazole (effect size, 1.23, 95% CI: 1.07–1.43), and pantoprazole (effect size, 1.38, 95% CI: 1.18–1.60) showed significant increasing MACEs compared without PPI usage. However, rabeprazole (effect size, 1.05, 95% CI: 0.66–1.66) did not show a significant difference compared with the placebo group (see Figure 3). Please see Supplementary Figure S2 for a comprehensive overview of the direct comparisons between different study groups, as detailed in each study.
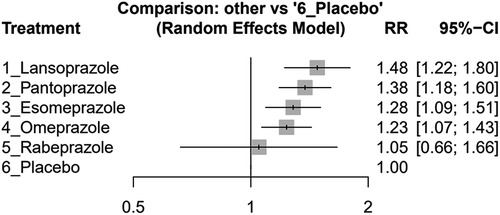
Figure 3 Forest plots illustrating the risk ratio (RR) of MACEs in clopidogrel concurrent with different PPIs and placebo groups among post-PCI patients.
We have also analyzed the individual outcomes and total mortality. Total mortality included four studies, myocardial infarction included eight studies, stent thrombosis included three studies, stroke included two studies, and cardiac death included four studies. The results are shown in Supplementary Figure S3. Our analysis revealed no significant differences in total mortality, stroke, or cardiac death between individual PPIs combined with clopidogrel. However, esomeprazole (effect size, 4.41, 95% CI: 1.63–11.92) and omeprazole (effect size, 3.7, 95% CI: 1.39–9.87) were found to significantly increase the incidence of stent thrombosis. Additionally, these two PPIs were also found to significantly increase the risk of myocardial infarction (esomeprazole (effect size, 1.59, 95% CI: 1.07–2.34) and omeprazole (effect size, 1.41, 95% CI: 1.00–1.97).
The individual PPI coadministrated with clopidogrel influenced the MACE risk on their effect size, as shown in Table 2. The result indicated that rabeprazole with clopidogrel had a similar risk to MACEs in the group with clopidogrel without any PPI. The lansoprazole group showed the highest risk of MACEs, followed by esomeprazole, omeprazole, and pantoprazole. The detailed comparison and ranking are shown in Table 2.
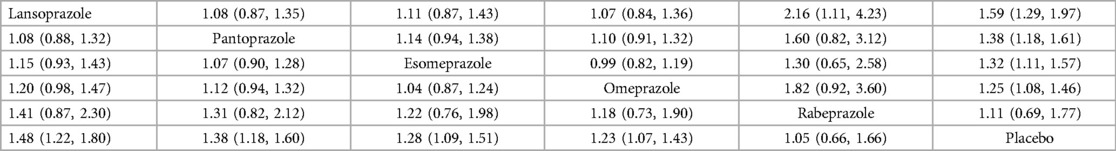
Table 2 Pairwise comparison and ranking the risk of MACEs in different PPIs concurrent with clopidogrel.
The individual PPI coadministrated with clopidogrel influenced the GI bleeding risk on their size, as shown in Figure 4. The result indicated that esomeprazole (effect size, 0.30, 95% CI: 0.09–0.94), omeprazole (effect size, 0.34, 95% CI: 0.14–0.81), and pantoprazole (effect size, 0.33, 95% CI: 0.13–0.84) significantly reduced the GI bleeding risk compared with placebo. However, rabeprazole (effect size, 0.36, 95% CI: 0.10–1.25) and lansoprazole (effect size, 0.74, 95% CI: 0.24–2.26) did not show significant difference compared with the placebo group. The detailed comparison and ranking are shown in Supplementary Table S4.
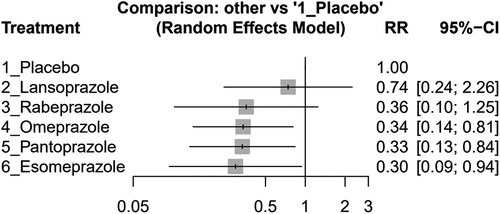
Figure 4 Forest plots of the risk ratio (RR) of GI bleeding in clopidogrel concurrent with different PPIs and placebo groups among post-PCI patients.
The construction of the network involved the establishment of nodes and the execution of both direct and indirect comparisons to assess consistency. Supplementary Table S5 presents the outcomes of the coadministrated clopidogrel with individual PPI evaluations, and Supplementary Table S6 contains the findings related to GI bleeding risk. With all conducted comparisons yielding p-values >0.05, there was no indication of inconsistency between the direct and indirect comparisons.
The one-study removal analysis yielded consistent rankings and maintained the clinical importance of each type of PPI. Lansoprazole, pantoprazole, and esomeprazole were found to significantly increase the incidence of MACEs, while omeprazole remained at borderline significance. Rabeprazole was unique in showing no significant impact on the rise in MACE risk, as illustrated in Supplementary Figure S5a–p. These findings affirm the robustness of our study's results, demonstrating that they remain stable regardless of whether individual studies are included or excluded, and are not affected by adjustments to the assumed values in the calculations.
Refer to Supplementary Figure S6 to view the funnel plot. The results from Egger's test, with a p-value of 0.302, suggest the absence of significant publication bias.
Our study results showed that the coadministration of esomeprazole, lansoprazole, pantoprazole, and omeprazole with clopidogrel significantly increased the risk of MACEs compared to using clopidogrel alone. However, when rabeprazole was coadministered with clopidogrel, there was no significant difference in MACEs compared to the placebo group. In the secondary analysis, the combination of esomeprazole, omeprazole, and pantoprazole with clopidogrel significantly reduced the risk of GI bleeding compared to clopidogrel alone. In contrast, the combinations of lansoprazole and rabeprazole with clopidogrel did not show a significant reduction in GI bleeding risk compared to the placebo group.
Clopidogrel, commonly combined with aspirin, has been a standard treatment post-PCI. Prior research has indicated that clopidogrel, when used as a post-PCI medication, is effective in preventing cardiovascular (CV) events, death, or thrombosis. However, the use of dual antiplatelet therapy or prolonged antiplatelet treatment significantly increases the risk of GI bleeding (36–38). To address this, PPIs have been recommended as a co-treatment with clopidogrel to reduce GI bleeding risk (39). However, it is widely known that clopidogrel and PPIs are both metabolized by the same liver enzyme, resulting in a significant DDI. Clopidogrel is a prodrug that initially needs to be metabolized by liver enzymes CYP1A2, CYP2C19, and CYP2B6 to form the intermediate compound 2-oxo-clopidogrel. In the second metabolic phase, 2-oxo-clopidogrel is further processed by enzymes CYP3A4/5, CYP2C9, CYP2C19, and CYP2B6 to become its active form, inhibiting platelet aggregation (40). The CYP2C19 plays a critical role in both phases of forming the active compound of clopidogrel, and then PPIs inhibit the CYP2C19 enzyme. Unlike clopidogrel, prasugrel requires only one hepatic CYP450-dependent metabolism step to convert to its active metabolite, involving CYP2B6, CYP2C9, CYP2C19, CYP2D6, and CYP3A4. Therefore, its anti-aggregation effect is less likely to be affected by CYP2C19 inhibitors. Ticagrelor binds to the ADP receptor at a different site than ADP, acting as an allosteric antagonist. It inhibits ADP-induced P2Y12 receptor signaling non-competitively and does not require metabolic activation to produce its active metabolite (41). A previous in vivo RCT study also demonstrated that for patients with AMI undergoing primary PCI, omeprazole reduced the incidence of gastrointestinal bleeding without diminishing ticagrelor's antiplatelet aggregation effect or increasing the risk of MACEs (42).
The extent of interaction between specific PPIs and clopidogrel remains a topic of debate (43). Our study aims to use network meta-analysis to identify the specific PPI least associated with MACEs and also had GI bleeding prevention.
PPIs are mainly metabolized by CYP2C19 and can inhibit this enzyme. The degree and duration of CYP2C19 inhibition vary among individual PPIs (44). Studies have shown distinct mechanisms among different PPIs. Lansoprazole and pantoprazole, being direct-acting inhibitors, can directly inhibit CYP2C19 and have a short elimination half-life, possibly having a less significant impact on clinical MACE risk. However, lansoprazole has been shown to strongly inhibit CYP2C19 in vitro, leading to controversy in findings. Omeprazole and esomeprazole irreversibly inhibit CYP2C19 at low concentrations through NADPH coenzyme assistance, known as metabolism-dependent inhibitors (MDIs) (45). Both of them were thought to be strong CYP2C19 inhibitors to interfere with clopidogrel’s turn to active form. Rabeprazole, which is believed to be metabolized not only through CYP enzyme-mediated pathways but also via non-enzymatic routes, is considered to have a lesser impact on clopidogrel (46). Ilaprazole, a new PPI, is believed to have a limited effect on the pharmacodynamics of clopidogrel, suggesting it may not be clinically relevant according to in vitro studies (47). Our study observed that only the combination of rabeprazole and clopidogrel did not significantly increase the risk of MACEs compared to placebo. In contrast, omeprazole, esomeprazole, pantoprazole, and lansoprazole all showed a significant increase in MACE risk. Notably, the combination of lansoprazole and clopidogrel was associated with the highest increase in MACE risk.
In recent years, attention has turned to how genetic differences can influence drug metabolism. Clopidogrel is a notable example, with its metabolism varying across races. Studies have revealed significant racial differences in clopidogrel metabolism. Genetic mutations, such as the single nucleotide variations in CYP2C19*2 and CYP2C19*3 compared to the CYP2C19*1, result in poor metabolism of clopidogrel due to reduced enzyme activity (48). Asian populations (12%–100%) more frequently exhibit these poor metabolizers (PM) of CYP2C19 genotypes compared to Caucasians and Africans (3%–5%) (49). Patients with these mutations have significantly reduced clopidogrel antiplatelet efficacy and a higher risk of CV events than those with normal genotypes (50). Interestingly, while there have been studies on CYP2C19 variations across races, there is a few of research on the pharmacokinetics and pharmacodynamics of clopidogrel–PPI co-metabolism across different racial groups and the effect of different CYP2C19 genotype impacted (51, 52). Recent meta-analyses found no significantly increased MACEs in groups without CYP2C19 variant alleles when any PPI was coadministrated with clopidogrel. In contrast, those with CYP2C19 variant alleles showed a significant increase in MACEs (53). To summarize, while some studies recommend PPIs with clopidogrel, especially for high bleeding risk populations, this may be controversial for Asian populations with a higher prevalence of variant alleles, potentially leading to more frequent MACEs.
Clopidogrel is a crucial drug used for patients post-PCI treatment. While clopidogrel reduces the risk of MACEs, it carries the potential side effect of GI bleeding, with patients at high risk of GI bleeding requiring careful consideration as guideline recommendations (9). Long-term use of antiplatelet agents can notably increase the risk of GI bleeding, particularly with dual antiplatelet therapy. In patients with a history of prior GI bleeding, Helicobacter pylori infection, or concurrent use of other drugs such as antiplatelets, anticoagulants, corticosteroids, or NSAIDs, the risk of GI bleeding may be further heightened. For these populations, combining PPIs with antiplatelet agents could be a strategy to mitigate GI bleeding risk (9). In our analysis of secondary outcomes, it was observed that lansoprazole and rabeprazole, when coadministered with clopidogrel, did not significantly enhance GI bleeding prevention compared to the group receiving clopidogrel alone. On the other hand, omeprazole, esomeprazole, and pantoprazole demonstrated a significant reduction in GI bleeding incidents compared to the placebo group. The esomeprazole combined with clopidogrel could reduce the most GI bleeding risk.
Another factor we should though about is the influence of the PPI dosage. Although our study and previous studies had shown that omeprazole and esomeprazole coadministrated with clopidogrel increased MACEs, studies by Yano et al. (omeprazole 10 mg) and Ng et al. (esomeprazole 20 mg) suggested that lower doses of PPIs might not increase the risk of MACEs while still providing bleeding protection. In our study, although the combination of esomeprazole or omeprazole with clopidogrel appeared to increase the risk of MACEs, both PPIs significantly reduced GI bleeding. Future research should aim to determine whether lower doses of omeprazole and esomeprazole can effectively prevent GI bleeding without increasing the risk of MACEs.
Our study has some limitations. Firstly, our primary objective was to assess the risk of MACEs, but there was variability in the definition of MACEs across the studies we analyzed. While most included myocardial infarction (MI), stroke, and/or cardiovascular death, some also considered other conditions (such as thrombosis). Secondly, the duration of follow-up in our studies varied widely, ranging from a minimum of one month to several years. Different follow-up durations post-PCI can indicate varying levels of MACE risk. Additional limitations included as follows: (1) The included studies varied in patient characteristics, such as a history of previous GI bleeding, Helicobacter pylori infection, and so on, which could increase bleeding risk when using clopidogrel. (2) The absence of data on the CYP2C19 genotypes. There is a need for further randomized, high-quality studies in this area.
In our network meta-analysis, we discovered that the combination of lansoprazole, pantoprazole, esomeprazole, and omeprazole with clopidogrel might increase the risk of major adverse cardiac events (MACEs). However, coadministration of rabeprazole with clopidogrel did not appear to significantly impact MACE risk. We also noted that using omeprazole, esomeprazole, and pantoprazole concurrently with clopidogrel could significantly reduce the risk of gastrointestinal (GI) bleeding compared to using clopidogrel alone. However, using rabeprazole and lansoprazole did not reduce GI bleeding risk in post-PCI patients. Lansoprazole combined with clopidogrel significantly increased MACEs and without GI bleeding protection function in post-PCI patients. Our study was the first network meta-analysis study to figure out the best regiment when concurrent clopidogrel with individual PPI. However, further randomized and high-quality studies are necessary to substantiate this conclusion.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
M-YA: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. Y-ZC: Data curation, Writing – review & editing. C-LK: Data curation, Writing – review & editing. W-LC: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1385318/full#supplementary-material
1. Pant S, Neupane P, Ramesh K, Barakoti M. Post percutaneous coronary intervention antiplatelet therapy: current perceptions, prospects and perplexity. Cardiol J. (2011) 18:712–7. doi: 10.5603/cj.2011.0042
2. Natsuaki M, Kimura T. Antiplatelet therapy after percutaneous coronary intervention—past, current and future perspectives—. Circ J. (2022) 86:741–7. doi: 10.1253/circj.CJ-21-0751
3. Fanaroff A, Rao S. Antiplatelet therapy in PCI. Interv Cardiol Clin. (2016) 5:221.383. doi: 10.1016/j.iccl.2015.12.007
4. Cho S, Kang D-Y, Kim J-S, Park D-W, Kim I-S, Kang TS, et al. Dual antiplatelet therapy after percutaneous coronary intervention for left main coronary artery disease. Rev Esp Cardiol (Engl Ed). (2023) 76:245–52. doi: 10.1016/j.rec.2022.07.007
5. Faxon DP, Lawler E, Young M, Gaziano M, Kinlay S. Prolonged clopidogrel use after bare metal and drug-eluting stent placement: the veterans administration drug-eluting stent study. Circ Cardiovasc Interventions. (2012) 5:372–80. doi: 10.1161/CIRCINTERVENTIONS.111.967257
6. Aronow HD, Steinhubl SR, Brennan DM, Berger PB, Topol EJ, Investigators C, et al. Bleeding risk associated with 1 year of dual antiplatelet therapy after percutaneous coronary intervention: insights from the clopidogrel for the reduction of events during observation (credo) trial. Am Heart J. (2009) 157:369–74. doi: 10.1016/j.ahj.2008.09.011
7. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. (2008) 10:528–34. doi: 10.1007/s11894-008-0098-4
8. Saven H, Zhong L, McFarlane IM, McFarlane I. Co-prescription of dual-antiplatelet therapy and proton pump inhibitors: current guidelines. Cureus. (2022) 14:e21885. doi: 10.7759/cureus.21885
9. Members WC, Abraham NS, Hlatky MA, Antman EM, Bhatt DL, Bjorkman DJ, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology foundation task force on expert consensus documents. Circulation. (2010) 122:2619–33. doi: 10.1161/CIR.0b013e318202f701
10. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
11. Ai MY, Chang W. Evaluating the efficacy and safety of concurrent proton pump inhibitors and clopidogrel therapy in post-PCI patients: A comprehensive systematic review and network meta-analysis (2024). Available online at: https://inplasy.com/inplasy-2024-0-0009/ (accessed February 02, 2024).
12. Zhang J, Yuan Y, Chu H. The impact of excluding trials from network meta-analyses–an empirical study. PLoS One. (2016) 11:e0165889. doi: 10.1371/journal.pone.0165889
13. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
14. Bae J-M. A suggestion for quality assessment in systematic reviews of observational studies in nutritional epidemiology. Epidemiol Health. (2016) 38:e2016014. doi: 10.4178/epih.e2016014
15. Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (updated August 2023). Chichester: John Wiley & Sons Ltd (2023). p. 285–320. doi: 10.1002/9781119536604.ch11
16. Higgins JP, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Chichester: John Wiley & Sons Ltd (2023). p. 143–76. doi: 10.1002/9781119536604.ch6
17. Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Chichester: John Wiley & Sons Ltd (2023). p. 241–84. doi: 10.1002/9781119536604.ch10
18. Page MJ, Higgins JP, Sterne JA. Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Chichester: John Wiley & Sons Ltd (2023). p. 349–74. doi: 10.1002/9781119536604.ch13
19. Higgins JPT, Eldridge S, Li T. Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Chichester: John Wiley & Sons Ltd (2023). p. 569–93. doi: 10.1002/9781119536604.ch23
20. Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. (2010) 363:1909–17. doi: 10.1056/NEJMoa1007964
21. Yano H, Tsukahara K, Morita S, Endo T, Sugano T, Hibi K, et al. Influence of omeprazole and famotidine on the antiplatelet effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes–a prospective, randomized, multicenter study–. Circ J. (2012) 76:2673–80. doi: 10.1253/circj.cj-12-0511
22. Ng F-H, Tunggal P, Chu W-M, Lam K-F, Li A, Chan K, et al. Esomeprazole compared with famotidine in the prevention of upper gastrointestinal bleeding in patients with acute coronary syndrome or myocardial infarction. Am J Gastroenterol. (2012) 107:389–96. doi: 10.1038/ajg.2011.385
23. Zhang J-R, Wang D-Q, Du J, Qu G-S, Du J-L, Deng S-B, et al. Efficacy of clopidogrel and clinical outcome when clopidogrel is coadministered with atorvastatin and lansoprazole: a prospective, randomized, controlled trial. Medicine (Baltimore). (2015) 94:e2262. doi: 10.1097/MD.0000000000002262
24. Wei P, Zhang Y-G, Ling L, Tao Z-Q, Ji L-Y, Bai J, et al. Effects of the short-term application of pantoprazole combined with aspirin and clopidogrel in the treatment of acute STEMI. Exp Ther Med. (2016) 12:2861–4. doi: 10.3892/etm.2016.3693
25. Ren Y-H, Zhao M, Chen Y-D, Chen L, Liu H-B, Wang Y, et al. Omeprazole affects clopidogrel efficacy but not ischemic events in patients with acute coronary syndrome undergoing elective percutaneous coronary intervention. Chin Med J. (2011) 124:856–61. doi: 10.3760/cma.j.issn.0366-6999.2011.06.010
26. Gaglia MA Jr, Torguson R, Hanna N, Gonzalez MA, Collins SD, Syed AI, et al. Relation of proton pump inhibitor use after percutaneous coronary intervention with drug-eluting stents to outcomes. Am J Cardiol. (2010) 105:833–8. doi: 10.1016/j.amjcard
27. Ray WA, Murray KT, Griffin MR, Chung CP, Smalley WE, Hall K, et al. Outcomes with concurrent use of clopidogrel and proton-pump inhibitors: a cohort study. Ann Intern Med. (2010) 152:337–45. doi: 10.7326/0003-4819-152-6-201003160-00003
28. Kreutz RP, Stanek EJ, Aubert R, Yao J, Breall JA, Desta Z, et al. Impact of proton pump inhibitors on the effectiveness of clopidogrel after coronary stent placement: the clopidogrel Medco outcomes study. Pharmacotherapy. (2010) 30:787–96. doi: 10.1592/phco.30.8.787
29. Rossini R, Capodanno D, Musumeci G, Lettieri C, Lortkipanidze N, Romano M, et al. Safety of clopidogrel and proton pump inhibitors in patients undergoing drug-eluting stent implantation. Coron Artery Dis. (2011) 22:199–205. doi: 10.1097/MCA.0b013e328343b03a
30. Simon T, Steg PG, Gilard M, Blanchard D, Bonello L, Hanssen M, et al. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome p450 2c19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French registry of acute ST-elevation and non–STelevation myocardial infarction (FAST-MI) registry. Circulation. (2011) 123:474–82. doi: 10.1161/CIRCULATIONAHA.110.965640
31. Hokimoto S, Mizobe M, Akasaka T, Arima Y, Kaikita K, Nakagawa K, et al. Impact of CYP2C19 polymorphism and proton pump inhibitors on platelet reactivity to clopidogrel and clinical outcomes following stent implantation. Thromb Res. (2014) 133:599–605. doi: 10.1016/j.thromres.2014.01.003
32. Yasu T, Ikee R, Miyasaka Y, Chubachi H, Saito S. Efficacy and safety of concomitant use of rabeprazole during dual-antiplatelet therapy with clopidogrel and aspirin after drug-eluting stent implantation: a retrospective cohort study. Yakugaku Zasshi. (2010) 130:1743–50. doi: 10.1248/yakushi.130.1743
33. Macaione F, Montaina C, Evola S, Novo G, Novo S. Impact of dual antiplatelet therapy with proton pump inhibitors on the outcome of patients with acute coronary syndrome undergoing drug-eluting stent implantation. Int Sch Res Notices. (2012) 2012:692761. doi: 10.5402/2012/692761
34. Abukhalil AD, Al Sheikh T, Muallem S, Al-Shami N, Naseef HA. Prevalence and safety of prescribing PPIs with clopidogrel in Palestine. Patient Prefer Adherence. (2023) 17:749–59. doi: 10.2147/PPA.S404139
35. Maret-Ouda J, Santoni G, Xie S, Rosengren A, Lagergren J. Proton pump inhibitor and clopidogrel use after percutaneous coronary intervention and risk of major cardiovascular events. Cardiovasc Drugs Ther. (2021) 6:1121–8. doi: 10.1007/s10557-021-07219-6
36. Zhang X, Qi L, Liu Y. Aspirin in combination with clopidogrel in the treatment of acute myocardial infarction patients undergoing percutaneous coronary intervention. Pak J Med Sci. (2019) 35:348. doi: 10.12669/pjms.35.2.87
37. Squizzato A, Bellesini M, Takeda A, Middeldorp S, Donadini MP. Clopidogrel plus aspirin versus aspirin alone for preventing cardiovascular events. Cochrane Database Syst Rev. (2017) 12(12):CD005158. doi: 10.1002/14651858.CD005158.pub4
38. Vallurupalli NG, Goldhaber SZ. Gastrointestinal complications of dual antiplatelet therapy. Circulation. (2006) 113:e655–8. doi: 10.1161/CIRCULATIONAHA.105.590612
39. Yasuda H, Matsuo Y, Sato Y, Ozawa S-I, Ishigooka S, Yamashita M, et al. Treatment and prevention of gastrointestinal bleeding in patients receiving antiplatelet therapy. World J Crit Care Med. (2015) 4:40. doi: 10.5492/wjccm.v4.i1.40
40. Polasek TM, Doogue MP, Miners JO. Metabolic activation of clopidogrel: in vitro data provide conflicting evidence for the contributions of CYP2C19 and pon1. Ther Adv Drug Saf. (2011) 2:253–61. doi: 10.1177/2042098611422559
41. Scott SA, Owusu Obeng A, Hulot J-S. Antiplatelet drug interactions with proton pump inhibitors. Expert Opin Drug Metab Toxicol. (2014) 10:175–89. doi: 10.1517/17425255.2014.856883
42. Zhang F, Su S, Hou Y, Zhao L, Wang Z, Liu F, et al. Effects (mace and bleeding events) of ticagrelor combined with omeprazole on patients with acute myocardial infarction undergoing primary PCI. Hellenic J Cardiol. (2020) 61:306–10. doi: 10.1016/j.hjc.2019.06.001
43. Bouziana SD, Tziomalos K. Clinical relevance of clopidogrel-proton pump inhibitors interaction. World J Gastrointest Pharmacol Ther. (2015) 6:17. doi: 10.4292/wjgpt.v6.i2.17
44. Rouby E, Lima N, and Johnson JJ, A J. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol. (2018) 14:447–60. doi: 10.1080/17425255.2018.1461835
45. Ogilvie BW, Yerino P, Kazmi F, Buckley DB, Rostami-Hodjegan A, Paris BL, et al. The proton pump inhibitor, omeprazole, but not lansoprazole or pantoprazole, is a metabolism-dependent inhibitor of CYP2C19: implications for coadministration with clopidogrel. Drug Metab Dispos. (2011) 39:2020–33. doi: 10.1124/dmd.111.041293
46. Ishizaki T, Horai Y. Review article: cytochrome p450 and the metabolism of proton pump inhibitors—emphasis on rabeprazole. Aliment Pharmacol Ther. (1999) 13:27–36. doi: 10.1046/j.1365-2036.1999.00022.x
47. Ye Z, Chen P, Tan C, Gong X, Li R, Dong Z, et al. Effects of ilaprazole on the steady-state pharmacodynamics of clopidogrel in healthy volunteers: an open-label randomized crossover study. Front Pharmacol. (2022) 13:952804. doi: 10.3389/fphar.2022.952804
48. Brown S-A, Pereira N. Pharmacogenomic impact of CYP2C19 variation on clopidogrel therapy in precision cardiovascular medicine. J Pers Med. (2018) 8:8. doi: 10.3390/jpm8010008
49. Goldstein JA. Clinical relevance of genetic polymorphisms in the human cyp2c subfamily. Br J Clin Pharmacol. (2001) 52:349–55. doi: 10.1046/j.0306-5251.2001.01499.x
50. Qian W, Chen L, Zhang L, Gao M, Wang C, Qian X, et al. Comparison of ticagrelor and clopidogrel in the treatment of patients with coronary heart disease carrying CYP2C19 loss of function allele. J Thorac Dis. (2022) 14:2591. doi: 10.21037/jtd-22-740
51. Kenngott S, Olze R, Kollmer M, Bottheim H, Laner A, Holinski-Feder E, et al. Clopidogrel and proton pump inhibitor (ppi) interaction: separate intake and a non-omeprazole ppi the solution? Eur J Med Res. (2010) 15:220–4. doi: 10.1186/2047-783x-15-5-220
52. Furuta T, Iwaki T, Umemura K. Influences of different proton pump inhibitors on the anti-platelet function of clopidogrel in relation to CYP2C19 genotypes. Br J Clin Pharmacol. (2010) 70:383–92. doi: 10.1111/j.1365-2125.2010.03717.x
Keywords: major adverse cardiovascular events (MACEs), gastrointestinal (GI) bleeding, clopidogrel, proton pump inhibitors (PPIs), post-percutaneous coronary intervention (PCI)
Citation: Ai M-Y, Chen Y-Z, Kuo C-L and Chang W-L (2024) A network meta-analysis: evaluating the efficacy and safety of concurrent proton pump inhibitors and clopidogrel therapy in post-PCI patients. Front. Cardiovasc. Med. 11: 1385318. doi: 10.3389/fcvm.2024.1385318
Received: 12 February 2024; Accepted: 1 July 2024;
Published: 24 July 2024.
Edited by:
Gavino Casu, Azienda Ospedaliero Universitaria Sassari, ItalyReviewed by:
Konstantin Schwarz, Karl Landsteiner University of Health Sciences, Austria© 2024 Ai, Chen, Kuo and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Lun Chang, bGl0dGxld2luZEBwaGFybWFjaXN0LnR3
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.