- 1Department of Cellular and Translational Physiology and Institute für Forschung und Lehre (IFL), Institute of Physiology, Molecular and Experimental Cardiology, Ruhr-University Bochum, Bochum, Germany
- 2Department of Cardiology and Angiology, Bergmannsheil University Hospital, Ruhr University of Bochum, Bochum, Germany
- 3Department of Cardiology, Angiology, Haemostaseology and Medical Intensive Care, University Medical Center Mannheim, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
- 4Department of Cardiology, Heart Centre Niederrhein, Helios Clinic Krefeld, Krefeld, Germany
- 5Faculty of Health, School of Medicine, University Witten/Herdecke, Witten, Germany
- 6Department of Cardiology and Rhythmology, University Hospital St. Josef-Hospital Bochum, Cardiology and Rhythmology, Ruhr University Bochum, Bochum, Germany
- 7Department of Cardiology, Angiology and Internal Intensive-Care Medicine, Klinikum St. Georg GGmbH Leipzig, Leipzig, Germany
- 8Department of Internal Medicine II, Heart Center Bonn, University Hospital Bonn, Bonn, German
- 9Department of Cardiology, Marienhospital Gelsenkirchen, Gelsenkirchen, Germany
- 10Department of Cardiology and Angiology, Clinic Saarbrücken GGmbH, Saarbrücken, Germany
- 11Department of Cardiology, University Heart Center, University Hospital Zurich, Zurich, Switzerland
- 12Department of Cardiology, Frankfurt University Hospital, Goethe University, Frankfurt am Main, Germany
- 13HCEMM-Cardiovascular Research Group, Department of Pharmacology and Pharmacotherapy, University of Budapest, Budapest, Hungary
- 14Department of Physiology, Cardiovascular Research Institute Maastricht, University Maastricht, Maastricht, Netherlands
Background: Data on the use of the wearable cardioverter defibrillator in patients suffering from inherited and congenital heart disease are limited. Consequently, evidence for guideline recommendations in this patient population is lacking.
Methods: In total 1,675 patients were included in a multicenter registry of eight European centers. In the present cohort, we included 18 patients suffering from congenital and inherited heart disease.
Results: Nine patients (50%) were male with a mean age of 41.3 ± 16.4 years. Four patients suffered from hypertrophic cardiomyopathy (HCM), four patients suffered from non-compaction cardiomyopathy (NCCM), two patients were diagnosed with arrhythmogenic right ventricular cardiomyopathy (ARVC) and one patient suffered from muscular dystrophy of the limb-girdle type with cardiac involvement, secondary cardiomyopathy. Three patients presented with Brugada syndrome (BrS). One patient suffered from long-QT syndrome type 1 (LQTS1). Furthermore, two patients had congenital heart defects and one patient suffered from cardiac sarcoidosis (CS). There were no appropriate/inappropriate shocks with the WCD in this cohort. One patient had recurrent self-limiting sustained ventricular tachycardia during the wear time, but actively inhibited a shock and was hospitalized. The compliance rate in this cohort was 77.8% with a mean wear time of 45.3 ± 26.9 days with a mean follow-up time of 570 ± 734 days. 55.6% (10/18) of the patients received an ICD after WCD wear time.
Conclusions: This retrospective study of patients with inherited and congenital heart disease shows that WCD use is not beneficial in the majority of patients with inherited and congenital heart disease.
1 Introduction
Inherited channelopathies and cardiomyopathies such as Long-QT syndrome (LQTS), Brugada syndrome (BrS), non-compaction cardiomyopathy (NCCM) and hypertrophic (non-) obstructive cardiomyopathy (HCM), as well as congenital heart defects are at high risk for ventricular tachyarrhythmias and sudden cardiac death (SCD) (1–4).
Implantable Cardioverter-Defibrillators (ICD) have been used for a long time in primary or secondary prevention of sudden cardiac death (SCD). However, ICDs might be related to several complications e.g., infection, lead failure or dysfunction in patients suffering from inherited cardiovascular diseases (5–7). Patients may refuse ICD implantation or there may be other reasons to postpone ICD implantation for the time being. Respectively, patients with suspected inherited heart disease might still have to undergo genetic testing or further risk stratification before ICD implantation. In addition, patients who already received an ICD might suffer from an infection and need a temporary ICD explantation and subsequently antibiotic therapy (8), or patients cannot receive an immediate ICD implantation because of a new medication with still insufficient dosage.
For these temporary high-risk situations, the wearable cardioverter-defibrillator (WCD) has been suggested and is used already in different patient groups and settings (9).
Studies regarding the use of the WCD in inherited and congenital heart disease are sparce and therefore currently not recommended by any international guideline. The 2022 ESC guideline for the management of patients with ventricular arrhythmias and the prevention of sudden death suggest WCD use in patients with a secondary prevention ICD indication, who are temporarily not candidates for ICD implantation and for patients in the early phase after myocardial infarction (4). Recently published data showed that the use of WCD could be beneficial in patients suffering from myocarditis and preserved systolic left ventricular ejection fraction (10).
Therefore, we sought to assess the utility of WCD in this cohort of patients with inherited and congenital heart disease.
2 Methods
2.1 Patient recruitment
In this multicenter, retrospective study, patients were recruited between April 2012 and December 2021. 1,675 patients with an indication and prescription of a WCD (LifeVest; ZOLL; Pittsburgh, PA) from nine hospitals (Bergmannsheil University Hospital, University Hospital Mannheim, Helios Clinic Krefeld, University Hospital St. Josef-Hospital Bochum, Klinikum St. Georg Leipzig, University Hospital Bonn, Clinic Saarbrücken, University Hospital Zurich, Frankfurt University Hospital) were included. The study was approved by the local ethics committee. All procedures, analyses and statistical evaluations were performed in accordance with guidelines and regulations of the institutional research committee and conform to the 1975 declaration of Helsinki.
2.2 Data collection from the wearable cardioverter defibrillator
Data from the WCD (wear time, wear days, arrhythmic events) was extracted from ZOLL LifeVest NetworkTM and hospital patient records. The WCD was programmed as recently described (11). The ventricular fibrillation (VF) zone was programmed at a heart rate of 200–220 bpm with a response time of 25 s.
The events were classified and reviewed by at least two independent physicians. Ventricular tachycardia events were divided in sustained ventricular tachycardia (VT, lasting ≥30 s or causing hemodynamic compromise) and non-sustained VT (< 30 s). Shocks were sorted as appropriate shocks (the cause was VT or VF) or inappropriate shocks (the cause was a non-sustained VT or any other reason other than VT or VF).
A sufficient compliance was defined as an average of ≥20 wear hours per day.
2.3 Clinical data collection
All data was collected retrospectively using the electronic patient records and reports. For follow-up data, treating hospital archives were screened and treating physicians or patients were contacted.
Characterizing parameters of the patients such as gender, age, indication for WCD use and underlying heart condition and comorbidities were extracted.
Beside baseline characteristics at the initial hospital stay such as baseline left ventricular ejection fraction (LVEF), the New York Heart Association (NYHA) classification, ECG parameters (PR, QRS and QTc- interval) and medication at discharge, follow-up data was collected at the times of three-month (short-term) and six to twelve-month (long-term) follow-up. LVEF was measured by the biplane Simpson's method using echocardiography and/or cardiac magnetic resonance imaging (MRI). An improvement of LVEF was defined as ≥35% if baseline LVEF was below 35%. At the end of the WCD wear time, the reason for stopping WCD use was documented. Additionally, any cardiac implantable electronic devices (CIEDs) that might have been implanted after WCD use, were monitored for documented arrhythmic events if possible. During follow-up, data were collected on rehospitalizations or death.
2.4 Statistical analysis
Continuous variables were analyzed for normal distribution using the Shapiro-Wilk normality test. Data are presented as mean ± standard deviation if normally and as median with 25th and 75th percentile if non-normally distributed. Categorical variables are displayed as frequencies and percentages.
3 Results
3.1 Baseline characteristics
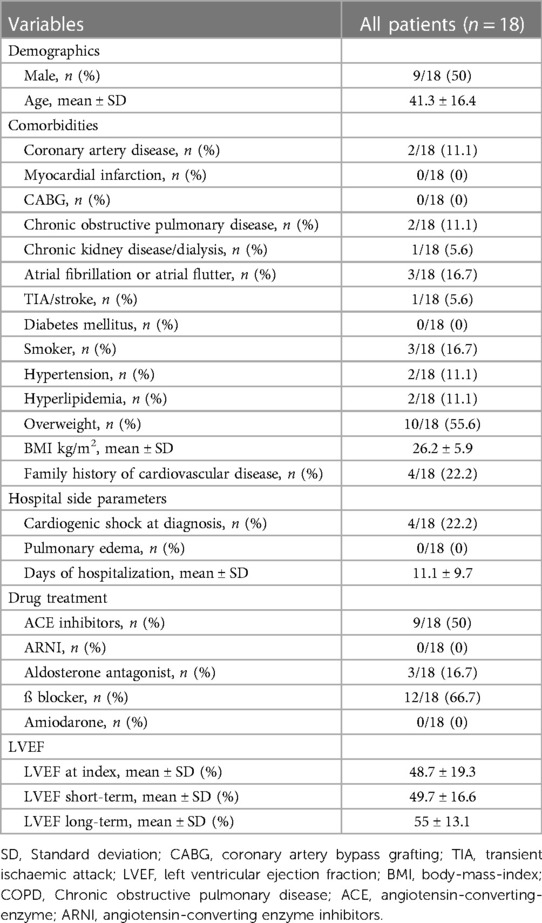
In the present cohort, we included 18 patients suffering from congenital and inherited heart disease. Nine patients (50%) were male with a mean age of 41.3 ± 16.4 years, Table 1.
At the initial hospital stay, the mean LVEF of the whole cohort was 48.7 ± 19.3%, with a LVEF≤35% in four patients. Three of those had the diagnosis of non-compaction cardiomyopathy (NCCM) and one patient suffered from muscular dystrophy of the limb-girdle type with cardiac involvement. None of these patients had an improvement of their LVEF over the first 12-months, Table 1. Detailed baseline characteristics are shown in Table 1.
3.2 Wearable cardioverter defibrillator data and indications for WCD use
Primary preventive protection by WCD was recommended in eleven patients, seven patients received the WCD as secondary prevention due to ventricular tachyarrhythmia prior to WCD use.
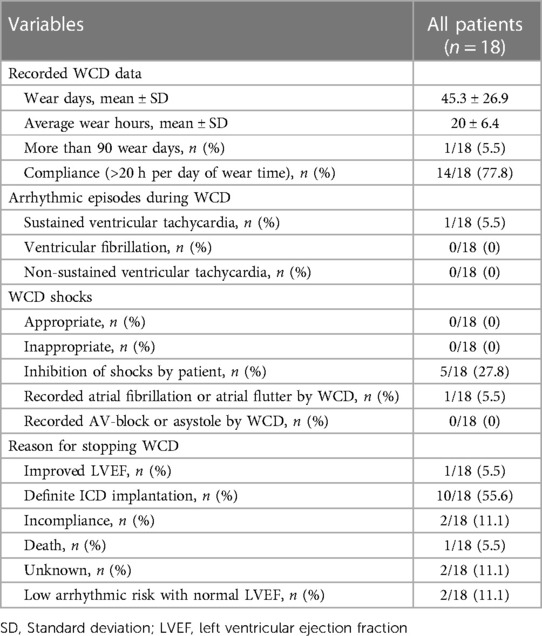
The average wear hours of the WCD in this cohort were 20 ± 6.4 h per day, with a compliance rate of 77.8%. The mean wear time of the WCD was 45.3 ± 26.9 days with only one patient (patient nr. 17) wearing the WCD for more than 90 days, Table 2.
27.8% of the cohort actively inhibited shocks due to false alarms, Table 2. Artefacts, including those caused by movement, led to warning signals that can be followed by shocks if not inhibited by the patient. All of these false alarms were inhibited actively by the patients with the response-button. All other artefacts or supraventricular tachycardias were correctly detected by the WCD and did not result in a warning signal, nor an inappropriate WCD shock. No appropriate WCD shocks were documented in this cohort during follow-up.
3.2.1 Hypertrophic cardiomyopathy
In our cohort, four patients suffered from hypertrophic cardiomyopathy (HCM), one of them with an obstructed left ventricular outflow tract (patient nr. 2) and three patients without (patients nr. 1, 3 and 4). Three out of four patients used the WCD as secondary prevention due to prior sustained VT/VF, their mean LVEF was 59.1%. Patient nr. 1 had an infection of her ICD and was bridged during antibiotic therapy with the WCD. Patient nr. 2 received an event recorder two years prior, due to recurrent presyncope. He was then admitted to the hospital in 2015 due to a tumor excision and the event recorder showed multiple nsVTs, which led to an ICD indication. The tumor excision delayed ICD implantation by a few weeks, which was bridged by the WCD.
Patient nr. 3 and 4 were newly diagnosed with HCM and received the WCD after survived SCD in times of further diagnostic measures. Individual patient data regarding definite ICD implantation and follow-up is provided in Table 3.
3.2.2 Non-compaction cardiomyopathy
Another four patients suffered from non-compaction cardiomyopathy (NCCM), patients nr. 11, 12, 13 and 14. All four patients had severe LV dysfunction, with an LVEF≤35% in three patients and an LVEF≤40% in one patient with a mean LVEF of 25.4% in this population. Only patient nr. 14 had a prior sustained ventricular tachyarrhythmias.
3.2.3 Arrhythmogenic right ventricular cardiomyopathy
Two patients (nr. 5 and 6) were diagnosed with arrhythmogenic right ventricular cardiomyopathy (ARVC) and subsequently wore the WCD. One patient (patient nr. 5) had further diagnostic measures and wore the WCD as a bridging option, and one patient (patient nr. 6), had cardiac arrest and was incompliant, but declined the use of the WCD after only two hours due to discomfort.
3.2.4 Brugada syndrome
Three patients presented with Brugada syndrome (BrS) (patients nr. 7, 8 and 9):
All three patients used the WCD as primary prevention for SCD. Patient nr. 7 had a previous dysfunction of the ICD but was not sure whether he wanted another ICD and the WCD was used for bridging during this decision period. This patient previously had a syncope, and VF was induced during the electrophysiological study in 2007. Patients nr. 8 and 9 both received their WCD, as diagnostics for BrS and differential diagnosis were still going on. Patient nr. 8 presented with a syncope and patient nr. 9 with an inducible VF in EPS. Besides 19 false alarms in patient nr. 8, there were no conspicuities during the WCD wear time.
3.2.5 Long-QT syndrome
Patient nr. 10 suffered from long-QT syndrome type 1 (LQTS1). The patient received the WCD as a primary prevention method for SCD and monitoring option while being dosed up on methylphenidate for her previously diagnosed “Attention Deficit Hyperactivity Disorder” (ADHD). This patient did not have an ICD indication and did not receive an ICD after the WCD wear time, but physicians worried about possible QT-time prolongation due to the new drug, which can prolong the QTc interval, and prescribed the WCD as a monitoring device. After titration of methylphenidate the QTc interval was 470 ms at maximum with no clinical symptoms. The WCD and monitoring were terminated without any further ECG abnormalities after a wear time of 60 days.
3.2.6 Congenital heart defect
Two patients (patients nr. 16 and 17) had congenital heart defects. Patient nr. 16 had a corrected transposition of the great arteries, had a suspected shunt defect and was at the hospital for reprogramming of his CRT-D and deactivating his tachyarrhythmia absoluta. Patient nr. 17 suffered from pulmonary atresia with an intact ventricular septum and used the WCD as secondary prevention due to prior sustained VT/VF.
3.2.7 Cardiac sarcoidosis
Patient nr. 18 was diagnosed with cardiac sarcoidosis (CS) but unfortunately stopped the WCD treatment after only five hours due to incompliance and concerns regarding the device.
3.2.8 Secondary cardiomyopathy
Lastly, patient nr. 15 suffered from muscular dystrophy of the limb-girdle type with cardiac involvement, secondary cardiomyopathy, Table 3.
The patient received the WCD as a bridging therapy after ICD-system explantation due to device malfunction and received a temporary CRT-P due to no intrinsic rhythm. After explantation of the ICD, the patient suffered from an episode of ventricular fibrillation in the hospital, whose termination was unsuccessful by multiple defibrillations and amiodarone administrations and was ultimately achieved by lowering the heart rate due to overstimulation with the temporary pacemaker. This arrhythmia was followed by episodes of sustained VTs. The WCD was used as a bridging therapy. There were no shocks delivered by the WCD. Unfortunately, this patient was admitted one month later with recurrent self-limiting sustained VTs, also documented by the WCD. The patient died shortly after admission due to cardiogenic shock, despite extensive attempts of resuscitation. Data regarding definite ICD implantation and follow-up is provided in Table 4.
The main reason for stopping WCD use was definite ICD implantation in 10 out of 18 patients (55.6%). Patient nr. 12 had an LVEF improvement large enough to discontinue WCD use. Two patients (patient nr. 3 and 18) stopped WCD treatment due to incompliance and two patients stopped WCD use due to a normal LVEF from the start and no arrhythmias during the wear time (patient nr. 10 and 16), Table 2. Unfortunately, in two patients the reason for stopping the WCD use was unknown.
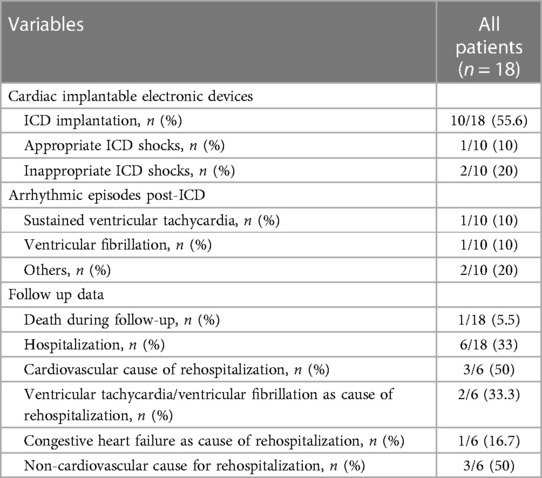
3.3 ICD implantation and follow-up
The mean follow-up time of the cohort was 570 ± 734 days. 55.6% (10/18) of the patients received an ICD after WCD wear time as shown in Tables 3, 4. Three of these patients received an appropriate (n = 1) /inappropriate (n = 2) shock during follow-up, Tables 3, 4. Patient nr. 13 with NCCM suffered from sustained VT7VF resulting in an appropriate shock 126 days after stopping the WCD treatment and during the use of the ICD. This patient had not had any form of ventricular arrhythmia before and received the WCD as primary prevention due to an LVEF of 20% at baseline. Due to persistent LVEF≤35% an ICD was implanted. The other two patients received an inappropriate shock (patient nr. 9 and 14), Tables 3, 4.
Six patients were hospitalized, three out of six due to a cardiovascular cause. Two of these patients were hospitalized due to sustained VT/VF and one patient suffered from congestive heart failure. The remaining three patients had a non-cardiovascular cause of hospitalization, Table 4.
4 Discussion
The European Society of Cardiology (ESC) recommends WCD use in adult patients with a secondary prevention ICD indication, who temporarily cannot be implanted with an ICD (class IIa recommendation) (4). The American Heart Association (AHA) states in the 2016 WCD Science Advisory that the use of the WCD may be reasonable when there is concern about an increased risk of SCD that may resolve over time (class IIb recommendation) and recommends the use of WCD in situations associated with increased risk of death in which ICDs have been shown to reduce SCD but not overall survival (class IIb recommendation) (12).
In our multicenter WCD-registry with 1,675 patients, we identified 18 patients with an inherited or congenital heart disease. 61.1% received the WCD as primary prevention and in 38.9% the WCD was recommended for secondary prevention due to sustained ventricular tachyarrhythmia prior to WCD use.
None of these patients received an appropriate WCD shock. Only one patient (patient nr. 15) had self-limiting sustained VTs which were correctly identified by the WCD but a shock was avoided by the patient. In five patients there were warning signals due to artefacts, but WCD shock delivery was stopped with the response button by the patients.
This shock rate of 0% was previously described in other studies about inherited and congenital heart diseases. E.g., Sarubbi et al. described eight patients with congenital heart disease, also without WCD shocks (13).
Nevertheless, Rao et al. described a cohort of 43 patients with congenital and 119 patients with inherited heart disease from the USA with two appropriate shocks and four inappropriate shocks, resulting in an event rate of 27 appropriate shocks per 100 patient-years. All shocks were received by patients with an inherited heart disease (14).
Likewise, Owen et al. described a cohort of nine LQTS patients with one appropriate shock after two days of wear time in a patient after multiple syncopal episodes and seizures (15).
Spar et al. described a cohort in pediatric patients with 167 patients with cardiomyopathy, 90 patients with congenital heart disease, 47 patients with channelopathy and 36 patients with cardiac arrest without diagnosis, where two appropriate shocks were documented, both in the congenital heart disease group (16).
Therefore, we may argue that the WCD is not beneficial for all patient groups with inherited and congenital heart disease. Even though there still is a lack of data for the use of WCD in these patients, in the published studies there are often no shocks or a low shock rate, similar to our study (13, 14, 16, 17). Most appropriate shocks that are documented occurred in patients with inherited heart disease.
However, as Rao et al. (14) shows, there might also be a considerable inappropriate shock rate, with an event rate of 63 inappropriate shocks per 100 patient-years in these patients. In our study, there were no inappropriate shocks with the WCD, but two inappropriate shocks during follow-up with the ICD (patients nr. 9 and 16), leading to an inappropriate shock rate of 20%. Of note, 27.8% of patients avoided an inappropriate WCD shock after being alarmed by the WCD by pressing the response-button.
In this cohort, there are many different individual reasons and clinical scenarios for WCD use.
The LQTS1 patient (patient nr. 10), received the WCD as a monitoring option while being dosed up on methylphenidate for their previously diagnosed ADHD. The 2022 ESC guidelines for management of patients with ventricular arrhythmias and prevention of SCD (4) states that drugs, that are known to prolong of the QTc time should be avoided for the prevention of SCD in the LGTS. In the case of our patient, the LQTS had been diagnosed a few years prior the current starting of medication with methylphenidate. After an initial prolongation of the QTc up to 520 ms years ago, LQTS was diagnosed, and the medication stopped. Now, after a few years, the patient again needed to be dosed up with methylphenidate and received the WCD as a way of bridging this time to an established dose and high risk for SCD.
Long-QT syndrome has an absolute SCD risk of 4.9%–13% (18) and has a heightened risk for Torsade de Pointes tachycardia (2). In 13% of patients, a sudden cardiac arrhythmia or death occurs before age 40. The magnitude of the QT interval prolongation remains the most powerful risk factor for SCD with a QTc>500 ms as a described predictor for life-threatening events (19). In patient nr. 10, after a wear time of 60 days and a stable maximum QTc time of 470 ms with no clinical symptoms, the WCD and monitoring were terminated, without any further ECG abnormalities. No permanent ICD was implanted, and the treatment of this patient was continued with betablockers.
Another example of individual indications and benefits from the WCD are the two patients (nr. 5 and 6) suffering from ARVC. One patient (patient nr. 5) had further diagnostic tests for ARVC and wore the WCD as a bridging option, and the other patient (patient nr. 6) had cardiac arrest but declined the use of the WCD after only two hours due to discomfort. This patient was implanted with an ICD, with whom he experienced an inappropriate shock during follow-up. Risk factors described in the literature for SCD in ARVC patients, were not met in patient nr. 5 (20–24). In patient nr. 6, the main risk factor was the previous cardiac arrest.
Consequently, these were two patients with very different presentations of ARVC. On the one hand, patient nr. 5 had no prior arrhythmic events and was implanted with a primary prevention ICD without any ICD-related complications, the WCD solely being the bridge until the diagnostic tests were completed and the diagnosis of ARVC confirmed. She wore the WCD as a safety measure until ICD implantation but had no apparent benefit from it.
Patient nr. 6 on the other hand, was at a very high risk for SCD, because of the previous cardiac arrest. She declined WCD use after two hours and was implanted with an ICD but had an inappropriate shock shortly after. This patient might have benefited from the WCD. Moreover, with the WCD she could have manually aborted the inappropriate shock with the response button, which may have a led to appropriate measures to avoid an inappropriate shock with the definite ICD.
A pooled annualized incidence rate of both aborted SCD and SCD without ICD treatment by Agbaedeng et al., describes a rate of 12.66 per 1,000 patients with ARVC. Appropriate ICD shocks are described at an annual rate of 84.7 per 1,000 patients (21).
Kutyifa et al. describes a congenital/inherited subgroup of their prospective study with WCD use which also included ARVC patients. Patients with ischemic and congenital/inherited heart disease were shown to have significantly higher probabilities of VT/VF than those with nonischemic cardiomyopathy with a rate of sustained VT of 3% after 3 months (25).
In this study, we also looked at patients with non-compaction cardiomyopathy (NCCM) which presents itself with an alteration of trabeculation (26–27). VTs are reported in 38%–47% of adult patients with major risk factors, these being syncope, low LVEF, previous VT/VF and family history of SCD (26).
Patient nr. 13 did not have multiple risk factors for ventricular tachyarrhythmias, except a low EF of 20% which improved to 32% over the first year. This patient received an appropriate ICD shock 126 days after ICD-implantation, due to recorded VT and VF. This may speculate, that the risk for SCD might also be high in some patients with NCCM, with their only risk factor being a low LVEF, where an ICD implantation should be considered early.
Patient nr. 15 with muscular dystrophy of the limb-girdle type with cardiac involvement and secondary cardiomyopathy, received their WCD as a bridge after ICD-system explantation. This patient had a history of VT/VF and suffered from ventricular fibrillation after explantation and from self-limiting VTs while wearing the WCD. After hospitalization, the patient sadly died shortly after with cardiogenic shock. To our knowledge, there is no data available on the rate of annual SCD in patients with muscular dystrophy of the limb-girdle type with cardiac involvement. These cases are associated with an alteration of different ion channel currents, which may enhance the risk of SCD (28). It is known that there is a heightened risk of atrial arrhythmias, conduction disease, bradycardia, ventricular arrhythmias, and sudden cardiac death, with ventricular arrhythmias and conduction disease often occurring after the development of a dilated cardiomyopathy (29–31). Therefore, ICD indication is usually guided by guidelines for non-ischemic cardiomyopathy (NICM) patients (29). Risk factors for SCD in the muscular dystrophy group are, nsVT, male sex and LVEF<45% (29). Patient nr. 15 had multiple risk factors for SCD e.g., an EF of 13% and benefited from the WCD in that she was alarmed for sustained VT and went to the hospital.
Overall compliance in our cohort was 77.8%, with an average wear time of 20 ± 6.4 h. Nevertheless, when removing patients nr. 18 and 6 due to immediate incompliance, compliance rate was at 87.5% with an average wear time of 22.1 ± 2.1 h. This condensed group wore the WCD 51 ± 22.7 days on average.
Our compliance rate can be compared to previously published studies, with Spar et al. (16) reporting a median wear time of 20.6 h per day, Sarubbi et al. (13) a wear time of 21.2 ± 1 h/d, Rao at al. (14). a compliance for daily use of 91% and Kutyifa et al. (25) a median daily use of 22.5 h of the whole cohort.
Olic et al. also reports patients with inherited and congenital heart disease stopping WCD use due to incompliance and discomfort, but that may be influenced by the fact that their patients were pregnant at the time and consequently of younger age (32). In our previous study regarding age differences in WCD use, the wear hours in younger patients (age <45 years) tended to be lower (20.11 ± 4.88 h) and predictors for worse compliance were younger age and non-ischemic cardiomyopathy (33). Therefore, we may argue, that the present cohort with an average age of 41 years may be less compliant to WCD use due to inherited and congenital heart diseases mainly being present in a younger age group.
5 Study limitations
Due to the limited size of this cohort, the retrospective data collection and the heterogeneity of the underlying disease, this study has limitations regarding direct applicability to clinical scenarios. Nevertheless, the aim of this study was to present more data on these rare indications and diseases for WCD in a systematic fashion.
6 Conclusion
This retrospective study of patients with inherited and congenital heart disease shows, that WCD use is not beneficial in the majority of patients with inherited and congenital heart disease. The appropriate WCD shock rate seems to be relatively low, especially in patients with congenital heart disease. Nevertheless, indications and reasons in this patient group varied individually and the WCD might be a good way to bridge some patients at high risk for SCD until definite ICD implantation, completion of further work-up and optimal drug therapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics commission of the medical faculty of the Ruhr University Bochum, head of commission: Prof. Dr. rer. nat. Hans-Joachim Trampisch. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
KK: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. TD: Data curation, Writing – review & editing. CB: Data curation, Writing – review & editing. MG: Data curation, Writing – review & editing. NK: Data curation, Writing – review & editing. TK: Data curation, Writing – review & editing. D-IS: Data curation, Writing – review & editing. HL: Data curation, Writing – review & editing. FS: Data curation, Writing – review & editing. MA: Data curation, Writing – review & editing. TB: Data curation, Writing – review & editing. CW: Data curation, Writing – review & editing. BK: Data curation, Writing – review & editing. SR: Data curation, Writing – review & editing. JK: Data curation, Writing – review & editing. AS: Data curation, Writing – review & editing. JE: Data curation, Writing – review & editing. FD: Data curation, Writing – review & editing. AM: Writing – review & editing. IA: Data curation, Writing – review & editing. AA: Data curation, Writing – review & editing. NH: Data curation, Writing – review & editing. IE-B: Data curation, Methodology, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
We acknowledge support by the Open Access Publication Funds of the Ruhr-Universität Bochum. We thank the financial support from the Else-Kröner-foundation (2022 EKES.48) for this research to IEl, the German-Heart-Foundation/German Foundation of Heart Research for this research to IEl, the Hector-Stiftung for this Research to IEL, IA and NH. This research was funded by EU's Horizon 2020 research and innovation program under grant agreement No. 739593 to NH; DFG (Deutsche Forschungsgemeinschaft) HA 7512/2-4 and HA 7512/2-1 to NH. Funders had no role in paper design, data collection, data analysis, interpretation, writing of the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were not an editorial board member of Frontiers, at the time of submission. AS received educational grants through his institution from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, BMS/Pfizer, and Medtronic; and speaker/advisory board/consulting fees from Bayer Healthcare, Biotronik, Daiichi-Sankyo, Medtronic, Novartis, Pfizer, Stride Bio Inc. and Zoll.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stewart MH, Macicek SL, Morin DP. Implantable cardioverter-defibrillators in adult congenital heart disease. J Innov Card Rhythm Manag. (2018) 9(6):3172–81. doi: 10.19102/icrm.2018.090601
2. Moss AJ, Zareba W, Hall WJ, Schwartz PJ, Crampton RS, Benhorin J, et al. Effectiveness and limitations of beta-blocker therapy in congenital long-QT syndrome. Circulation. (2000) 101(6):616–23. doi: 10.1161/01.CIR.101.6.616
3. Oechslin EN, Harrison DA, Connelly MS, Webb GD, Siu SC. Mode of death in adults with congenital heart disease. Am J Cardiol. (2000) 86(10):1111–6. doi: 10.1016/S0002-9149(00)01169-3
4. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NA, et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by the association for European paediatric and congenital cardiology (AEPC). Eur Heart J. (2022) 43(40):3997–4126. doi: 10.1093/eurheartj/ehac262
5. Siskin M, Cerrone M, Shokr M, Aizer A, Barbhaiya C, Dai M, et al. ICD Shocks and complications in patients with inherited arrhythmia syndromes. Int J Cardiol Heart Vasc. (2021) 37:100908. doi: 10.1016/j.ijcha.2021.100908
6. El-Battrawy I, Roterberg G, Liebe V, Ansari U, Lang S, Zhou X, et al. Implantable cardioverter-defibrillator in brugada syndrome: long-term follow-up. Clin Cardiol. (2019) 42(10):958–65. doi: 10.1002/clc.23247
7. El-Battrawy I, Besler J, Ansari U, Liebe V, Schimpf R, Tülümen E, et al. Long-term follow-up of implantable cardioverter-defibrillators in short QT syndrome. Clin Res Cardiol. (2019) 108(10):1140–6. doi: 10.1007/s00392-019-01449-3
8. Bongiorni MG, Burri H, Deharo JC, Starck C, Kennergren C, Saghy L, et al. 2018 EHRA expert consensus statement on lead extraction: recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: endorsed by APHRS/HRS/LAHRS. Europace. (2018) 20(7):1217. doi: 10.1093/europace/euy050
9. Israel C, Staudacher I, Leclercq C, Botto GL, Scherr D, Fach A, et al. Sudden cardiac death while waiting: do we need the wearable cardioverter-defibrillator? Clin Res Cardiol. (2022) 111(11):1189–97. doi: 10.1007/s00392-022-02003-4
10. El-Battrawy I, Koepsel K, Tenbrink D, Kovacs B, Dreher TC, Blockhaus C, et al. Use of the wearable cardioverter-defibrillator among patients with myocarditis and reduced ejection fraction or ventricular tachyarrhythmia: data from a multicenter registry. J Am Heart Assoc. (2023) 12(18):e030615. doi: 10.1161/JAHA.123.030615
11. El-Battrawy I, Kovacs B, Dreher TC, Klein N, Rosenkaimer S, Röger S, et al. Real life experience with the wearable cardioverter-defibrillator in an international multicenter registry. Sci Rep. (2022) 12(1):3203. doi: 10.1038/s41598-022-06007-y
12. Piccini JP S, Allen LA, Kudenchuk PJ, Page RL, Patel MR, Turakhia MP. Wearable cardioverter-defibrillator therapy for the prevention of sudden cardiac death: a science advisory from the American heart association. Circulation. (2016) 133(17):1715–27. doi: 10.1161/CIR.0000000000000394
13. Sarubbi B, Palma M, Merola A, Fusco F, Correra A, Colonna D, et al. Wearable cardioverter-defibrillator (life-vest): a feasible bridging treatment in adult congenital heart disease. Indian Pacing Electrophysiol J. (2022) 22(5):217–22. doi: 10.1016/j.ipej.2022.06.005
14. Rao M, Goldenberg I, Moss AJ, Klein H, Huang DT, Bianco NR, et al. Wearable defibrillator in congenital structural heart disease and inherited arrhythmias. Am J Cardiol. (2011) 108(11):1632–8. doi: 10.1016/j.amjcard.2011.07.021
15. Owen HJ, Bos JM, Ackerman MJ. Wearable cardioverter defibrillators for patients with long QT syndrome. Int J Cardiol. (2018) 268:132–6. doi: 10.1016/j.ijcard.2018.04.002
16. Spar DS, Bianco NR, Knilans TK, Czosek RJ, Anderson JB. The US experience of the wearable cardioverter-defibrillator in pediatric patients. Circ Arrhythm Electrophysiol. (2018) 11(7):e006163. doi: 10.1161/CIRCEP.117.006163
17. Kovacs B, Reek S, Sticherling C, Schaer B, Linka A, Ammann P, et al. Use of the wearable cardioverter-defibrillator - the Swiss experience. Swiss Med Wkly. (2020) 150:w20343. doi: 10.4414/smw.2020.20343
18. Khan HM, Leslie SJ. Risk factors for sudden cardiac death to determine high risk patients in specific patient populations that may benefit from a wearable defibrillator. World J Cardiol. (2019) 11(3):103–19. doi: 10.4330/wjc.v11.i3.103
19. Singh M, Morin DP, Link MS. Sudden cardiac death in long QT syndrome (LQTS), brugada syndrome, and catecholaminergic polymorphic ventricular tachycardia (CPVT). Prog Cardiovasc Dis. (2019) 62(3):227–34. doi: 10.1016/j.pcad.2019.05.006
20. Krahn AD, Wilde AAM, Calkins H, La Gerche A, Cadrin-Tourigny J, Roberts JD, et al. Arrhythmogenic right ventricular cardiomyopathy. JACC Clin Electrophysiol. (2022) 8(4):533–53. doi: 10.1016/j.jacep.2021.12.002
21. Agbaedeng TA, Roberts KA, Colley L, Noubiap JJ, Oxborough D. Incidence and predictors of sudden cardiac death in arrhythmogenic right ventricular cardiomyopathy: a pooled analysis. Europace. (2022) 24(10):1665–74. doi: 10.1093/europace/euac014
22. Gasperetti A, Carrick RT, Costa S, Compagnucci P, Bosman LP, Chivulescu M, et al. Programmed ventricular stimulation as an additional primary prevention risk stratification tool in arrhythmogenic right ventricular cardiomyopathy: a multinational study. Circulation. (2022) 146(19):1434–43. doi: 10.1161/CIRCULATIONAHA.122.060866
23. Cadrin-Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. (2022) 43(32):e1–9. doi: 10.1093/eurheartj/ehac180
24. Fan X, Yang G, Duru F, Grilli M, Akin I, Zhou X, et al. Arrhythmogenic cardiomyopathy: from preclinical models to genotype-phenotype correlation and pathophysiology. Stem Cell Rev Rep. (2023) 19(8):2683–708. doi: 10.1007/s12015-023-10615-0
25. Kutyifa V, Moss AJ, Klein H, Biton Y, McNitt S, MacKecknie B, et al. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: data from the prospective registry of patients using the wearable cardioverter defibrillator (WEARIT-II registry). Circulation. (2015) 132(17):1613–9. doi: 10.1161/CIRCULATIONAHA.115.015677
26. Towbin JA, Lorts A, Jefferies JL. Left ventricular non-compaction cardiomyopathy. Lancet. (2015) 386(9995):813–25. doi: 10.1016/S0140-6736(14)61282-4
27. Srivastava S, Yavari M, Al-Abcha A, Banga S, Abela G. Ventricular non-compaction review. Heart Fail Rev. (2022) 27(4):1063–76. doi: 10.1007/s10741-021-10128-3
28. El-Battrawy I, Zhao Z, Lan H, Li X, Yücel G, Lang S, et al. Ion channel dysfunctions in dilated cardiomyopathy in limb-girdle muscular dystrophy. Circ Genom Precis Med. (2018) 11:e001893. doi: 10.1161/CIRCGEN.117.001893
29. Groh WJ. Arrhythmias in the muscular dystrophies. Heart Rhythm. (2012) 9(11):1890–5. doi: 10.1016/j.hrthm.2012.06.038
30. van Rijsingen IA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, van der Kooi AJ, et al. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers a European cohort study. J Am Coll Cardiol. (2012) 59(5):493–500. doi: 10.1016/j.jacc.2011.08.078
31. Groh WJ, Bhakta D, Tomaselli GF, Aleong RG, Teixeira RA, Amato A, et al. 2022 HRS expert consensus statement on evaluation and management of arrhythmic risk in neuromuscular disorders. Heart Rhythm. (2022) 19(10):e61–e120. doi: 10.1016/j.hrthm.2022.04.022
32. Olic JJ, Stöllberger C, Schukro C, Odening KE, Reuschel E, Fischer M, et al. Usage of the wearable cardioverter-defibrillator during pregnancy. Int J Cardiol Heart Vasc. (2022) 41:101066. doi: 10.1016/j.ijcha.2022.101066
Keywords: inherited channelopathies, sudden cardiac death, wearable-cardioverter defibrillator, congenital heart diasease, ventricular arrhythmia
Citation: Koepsel K, Dreher TC, Blockhaus C, Gotzmann M, Klein N, Kuntz T, Shin D-I, Lapp H, Schiedat F, Abumayyaleh M, Beiert T, Weth C, Kovacs B, Rosenkaimer S, Kowitz J, Saguner AM, Erath JW, Duru F, Mügge A, Akin I, Aweimer A, Hamdani N and El-Battrawy I (2024) No beneficial use of the wearable cardioverter defibrillator among patients suffering from inherited and congenital heart disease: data from a European multicenter registry. Front. Cardiovasc. Med. 11:1384736. doi: 10.3389/fcvm.2024.1384736
Received: 10 February 2024; Accepted: 18 June 2024;
Published: 10 July 2024.
Edited by:
Michiaki Nagai, University of Oklahoma Health Science Center, United StatesReviewed by:
Brahim Kichou, NEDIR Mohamed Hospital, AlgeriaEmanuele Micaglio, Unit of Clinical Arrhythmology and Electrophysiology, IRCCS San Donato Polyclinic, Italy
© 2024 Koepsel, Dreher, Blockhaus, Gotzmann, Klein, Kuntz, Shin, Lapp, Schiedat, Abumayyaleh, Beiert, Weth, Kovacs, Rosenkaimer, Kowitz, Saguner, Erath, Duru, Mügge, Akin, Aweimer, Hamdani and El-Battrawy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ibrahim El-Battrawy, aWJyYWhpbS5lbGJhdHRyYXd5MjAwNkBnbWFpbC5jb20=
 Katharina Koepsel
Katharina Koepsel Tobias C. Dreher3
Tobias C. Dreher3 Michael Gotzmann
Michael Gotzmann Thomas Kuntz
Thomas Kuntz Mohammad Abumayyaleh
Mohammad Abumayyaleh Thomas Beiert
Thomas Beiert Boldizsar Kovacs
Boldizsar Kovacs Firat Duru
Firat Duru Ibrahim Akin
Ibrahim Akin Nazha Hamdani
Nazha Hamdani Ibrahim El-Battrawy
Ibrahim El-Battrawy