- 1Department of Pharmacy, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Critical Care Medicine, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
Background: Angiotensin II receptor blockers (ARBs) are utilized for the management of hypertension and diabetes. Previous meta-analyses suggested that azilsartan medoxomil (AZL-M) improved blood pressure (BP) reduction, but there were no safety findings or suggestions for patients with hypertension or diabetes.
Methods: We performed an efficacy and safety meta-analysis of randomized controlled trials (RCTs) evaluating AZL-M therapy for reducing BP in patients with hypertension. Patients with hypertension complicated by diabetes were analyzed. The relevant literature was searched in English and Chinese databases for RCTs involving AZL-M in hypertension. Efficacy variables included the change from baseline in the 24-h mean systolic/diastolic BP measured by ambulatory BP monitoring, the change from baseline in clinic systolic/diastolic BP, and responder rates. Safety variables included total adverse events (AEs), serious AEs, AEs leading to discontinuation, and AEs related to the study drug. The raw data from the included studies were utilized to calculate the odds ratio (OR) for dichotomous data and the mean difference (MD) for continuous data, accompanied by 95% confidence intervals (CIs). Statistical analysis was performed using R software.
Results: A total of 11 RCTs met the inclusion criteria, representing 7,608 patients, 5 of whom had diabetes. Pooled analysis suggested a reduction in BP among patients randomized to 40 mg of AZL-M vs. control therapy [24-h ambulatory blood pressure monitoring (ABPM) mean systolic blood pressure (SBP) (MD: −2.85 mmHg), clinic SBP (MD: −3.48 mmHg), and clinic diastolic blood pressure (DBP) (MD: −1.96 mmHg)] and for 80 mg of AZL-M vs. control therapy [24-h ABPM mean SBP (MD: −3.59 mmHg), 24-h ABPM mean DBP (MD: −2.62 mmHg), clinic SBP (MD: −4.42 mmHg), clinic DBP (MD: −3.09 mmHg), and responder rate (OR: 1.46)]. There was no difference in the reduction of risks, except for dizziness (OR: 1.56) in the 80-mg AZL-M group or urinary tract infection (OR: 1.82) in the 40-mg AZL-M group. Analysis of patients with diabetes revealed that AZL-M can provide superior management, while safety and tolerability were similar to those of control therapy.
Conclusions: AZL-M appears to reduce BP to a greater extent than dose-control therapy and does not increase the risk of adverse events in patients with hypertension and diabetes compared with placebo.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=464284, identifier PROSPERO CRD42023464284.
1 Introduction
In the last three decades, despite a stable global age-standardized prevalence, there has been a consistent year-on-year increase in the number of patients diagnosed with hypertension, primarily due to population growth (1). The prevalence of hypertension in China continues to rise due to an aging population. Despite progress, the control rate of hypertension remains low, increasing from 2.8% in 1991 to only 16.8% in 2015. Given the close causal relationship between blood pressure (BP) levels and cardiovascular disease morbidity and mortality, which account for over 40% of all deaths, it is crucial to prioritize blood pressure control (2).
Angiotensin-converting enzyme inhibitors (ACEI) and angiotensin II receptor blockers (ARBs) have been recognized as an effective approach to managing hypertension and are recommended as first-line treatment by various guidelines (3–5). ACEI/ARB agents are particularly recommended for patients with comorbidities such as diabetes (6), heart failure (7, 8), or renal insufficiency (9, 10). Azilsartan medoxomil (AZL-M), the eighth ARB agent approved in China for treating hypertension in 2021, acts as a prodrug that rapidly converts into azilsartan within the body and exhibits a long half-life of approximately 11 h. Based on dose-ranging studies and pharmacokinetic/pharmacodynamic analyses, daily doses of either 40 or 80 mg of AZL-M demonstrate superior efficacy in controlling blood pressure among most patients (11, 12). Previous meta-analyses (13) suggested that AZL-M is more effective in the treatment of hypertension than the other hypertension drugs, but there were no safety findings or suggestions for patients with hypertension and diabetes. To provide clinicians with guidance regarding drug selection and safer usage, we conducted a meta-analysis evaluating both efficacy and safety outcomes from randomized controlled trials (RCTs).
2 Methods
2.1 Registration of systematic review
This study has been registered in the online platform International Prospective Register of Systematic Reviews (PROSPERO). The protocol of this systematic review and meta-analysis is available in PROSPERO (CRD42023464284). https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=464284.
2.2 Search strategy
This study followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) protocol (14). The MEDLINE (via PubMed), Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), WANFANG, and China Biology Medicine disc (CBMdisc) databases were systematically searched from the beginning of the records through 14 September 2023. The search strategy included medical subject heading terms and keywords related to “hypertension,” “high blood pressure,” “azilsartan medoxomil,” and “TAK-491”; two authors independently performed the search. We assessed all relevant English and Chinese articles for eligibility.
2.3 Eligibility criteria and data extraction
Studies with the following characteristics were included: (1) adult patients aged >18 years with diagnosed hypertension, with clinic SBP between 150 and 180 mmHg or less; (2) the study design was a prospective randomized controlled clinical trial; and (3) patients were randomly assigned to receive AZL-M vs. any control therapy or placebo.
The exclusion criteria were as follows: (1) non-human studies; (2) non-comparative studies; (3) known secondary hypertension; (4) severe diastolic hypertension (seated DBP at least 114 mmHg); (5) stage IV chronic kidney disease [glomerular filtration rate (GFR) 30 ml/min per 1.73 m2]; and (6) type 1 or poorly controlled T2DM (HbA1c < 8%).
Two authors independently reviewed the titles and abstracts to identify potentially relevant studies. The extracted data included study characteristics, patient characteristics, interventions, outcomes, and other relevant findings. A third author cross-checked the extracted data.
2.4 Quality assessment and risk of bias
Two independent authors assessed the risk of bias and the quality of all RCTs using the Cochrane Handbook of Systematic Reviews of Interventions (15, 16).
2.5 Outcomes and statistical analysis
The primary outcome measures included the change from baseline in the 24-h mean systolic blood pressure (SBP) measured by ambulatory blood pressure monitoring (ABPM) (24-h ABPM mean SBP), change from baseline in clinic SBP, responder rates (RRs), total adverse events (AEs), serious AEs, AEs leading to discontinuation, and AEs related to the study drug. Secondary outcomes included the change from baseline in the 24-h mean diastolic blood pressure (DBP) measured by ambulatory blood pressure monitoring (24-h ABPM mean DBP), change from baseline in clinic DBP, and adverse events such as headache, dizziness, hyperlipidemia, urinary tract infection, hypotension, and nasopharyngitis.
AZL-M (40 or 80 mg) was chosen as the comparator for control therapy in this meta-analysis. Statistical analysis was performed using R software 4.3. The raw data from the included studies were utilized to calculate the odds ratio (OR) for dichotomous data and the mean difference (MD) for continuous data, accompanied by 95% confidence intervals (CIs). These measures were pooled using a random-effects model. The findings of the pooled studies were presented through forest plots. Egger's (17) test and funnel plots were employed to assess publication bias for effectiveness outcomes and adverse events. Heterogeneity was evaluated and categorized as low (<25%), moderate (25%–75%), or high (>75%) using Higgin's I2 tests. A P-value of 0.05 was considered significant for all analyses.
3 Results
3.1 Baseline characteristics
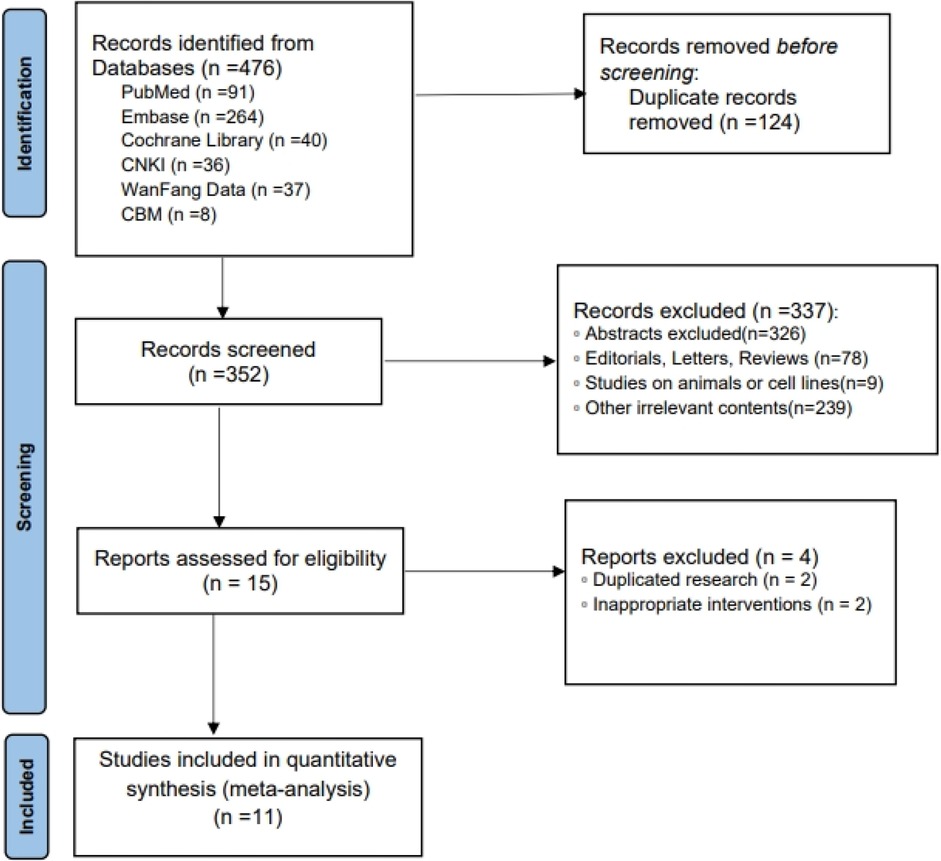
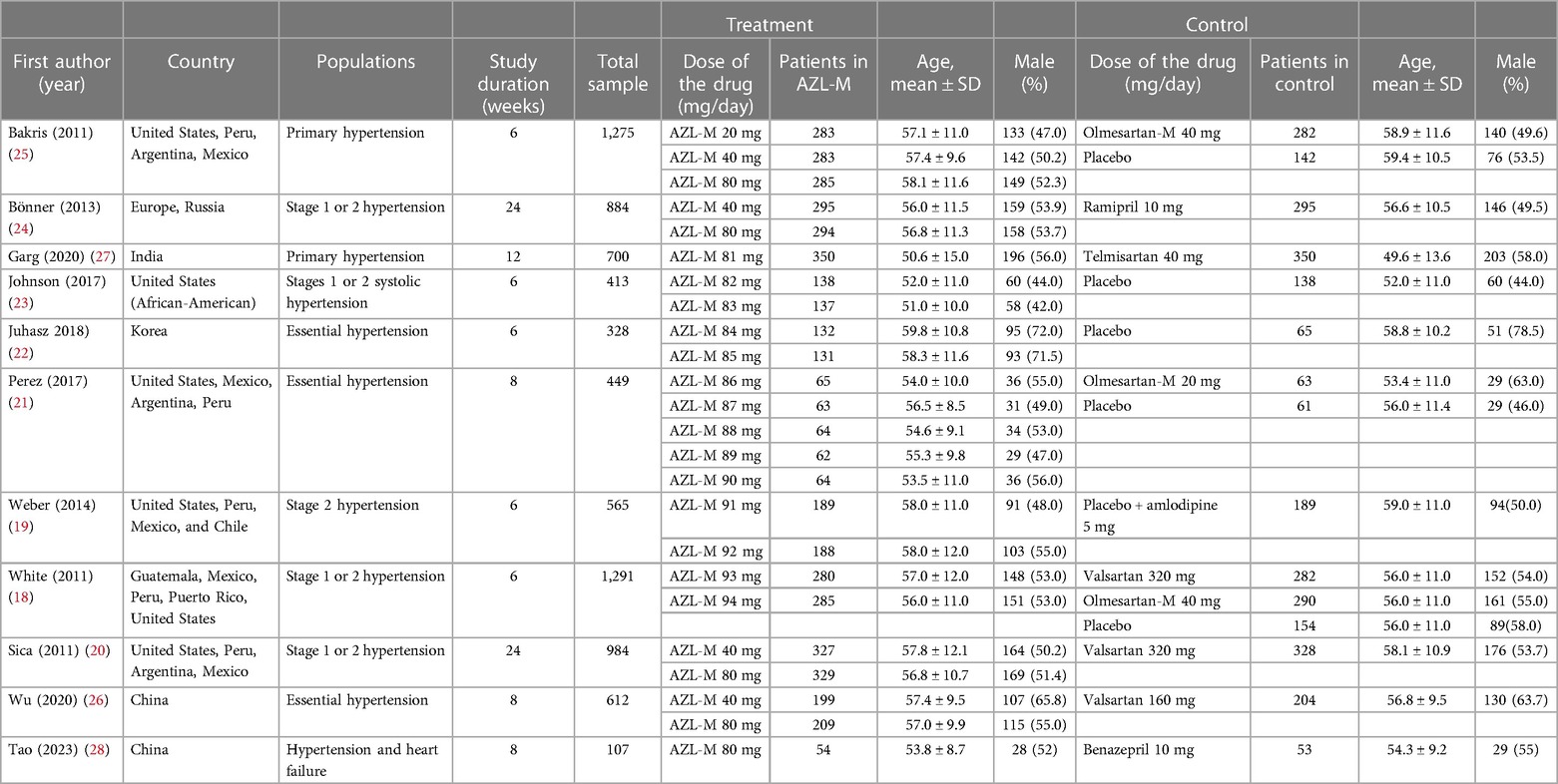
A total of 11 RCTs (18–28) met the inclusion criteria, representing 7,608 patients (Figure 1). The quality assessment for the included studies is presented in Figure 2. Among the included trials, six were ARB-controlled trials (18, 20, 21, 25–27) (olmesartan, telmisartan, valsartan), two were ACEI-controlled trials (24, 28) (ramipril, benazepril), one amlodipine plus placebo-controlled trial (19), and four were placebo trials (18, 21–23). Almost all the studies included intervention groups with 40 and 80 mg doses of AZL-M, while one study had two different ARB control therapies. Follow-up ranged from 6 to 24 weeks. Despite the noted heterogeneity in design between the trials, there was sufficient similarity between the populations and the hypotheses to merit the inclusion of all 11 trials in the quantitative meta-analysis. Except for Peng et al. (28), which had a population of hypertension and heart failure, they all have the same population of hypertension (Table 1).
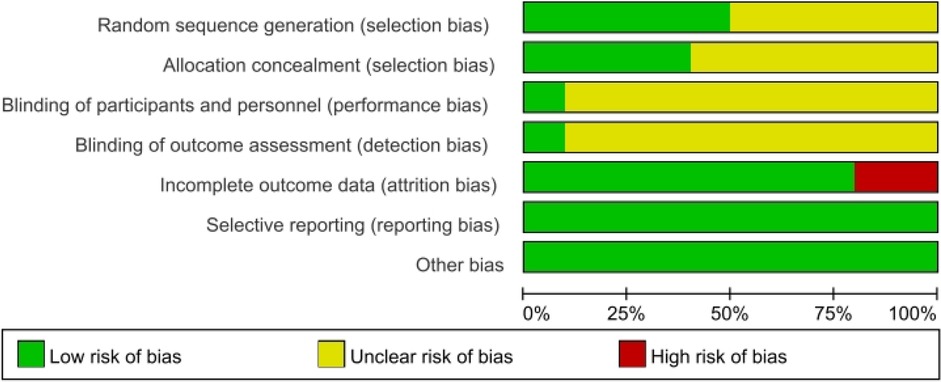
Figure 2 Methodological quality graph: author's judgments about each methodological quality item are presented as a percentage across all included studies.
3.2 Efficacy meta-analysis
Changes from baseline in 24-h ABPM mean SBP were significantly greater with 40 mg of AZL-M (MD: −2.85 mmHg, 95% CI: −3.97 to −1.73 mmHg, p < 0.05) and 80 mg of AZL-M (MD: −3.59 mmHg, 95% CI: −4.57 to −2.61 mmHg, p < 0.05) than with control therapy. When compared with 24-h ABPM mean DBP, there was a statistically significant difference in the 80-mg AZL-M group (MD: −2.62 mmHg, 95% CI: −3.62 to −1.62 mmHg, p < 0.05), whereas 40 mg of AZL-M was non-inferior to control therapy (MD: −1.03 mmHg, 95% CI: −3.70 to 1.64 mmHg, p = 0.57) (Figure 3).
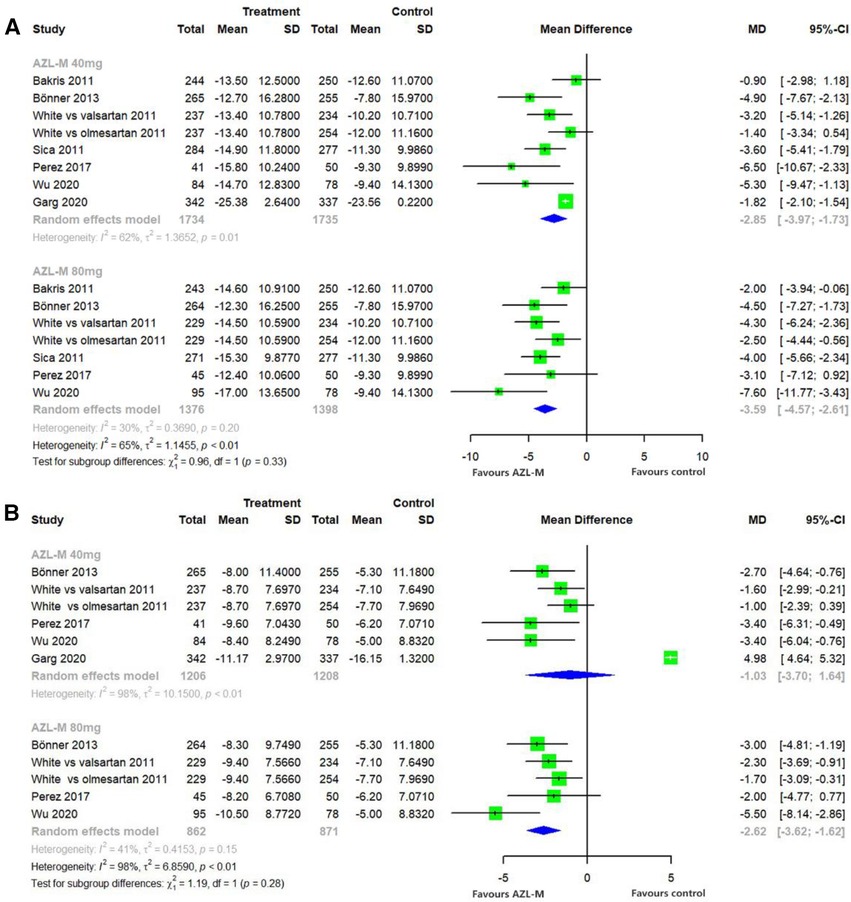
Figure 3 Forest plot of 24-h ABPM mean SBP (A) and 24-h ABPM mean DBP (B) among hypertensive patients randomized to azilsartan medoxomil vs. control therapy.
Changes from baseline in the clinic SBP compared with control therapy demonstrated a statistically significant difference in the 40-mg AZL-M group (MD: −3.48 mmHg, 95% CI: −5.26 to −1.70 mmHg, p < 0.05) and the 80-mg AZL-M group (MD: −4.42 mmHg, 95% CI: −6.38 to −2.47 mmHg, p < 0.05). In contrast, the clinic DBP also showed a statistically significant difference in the 40-mg AZL-M group (MD: −1.96 mmHg, 95% CI: −3.49 to −0.43 mmHg, p < 0.05) and the 80-mg AZL-M group (MD: −3.09 mmHg, 95% CI: −4.58 to −1.61 mmHg, p < 0.05) compared to the control therapy (Figure 4).
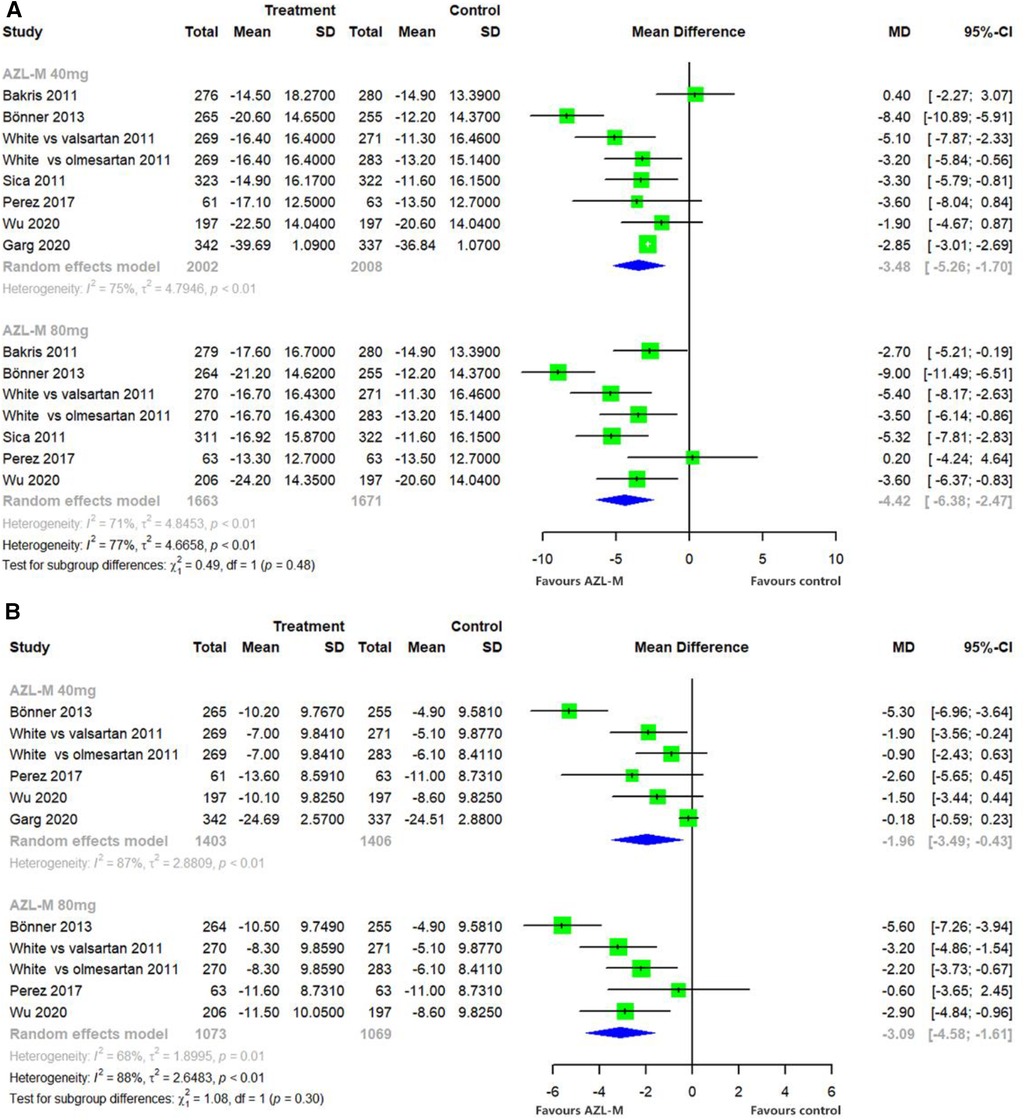
Figure 4 Forest plot of clinic SBP (A) and clinic DBP (B) among hypertensive patients randomized to azilsartan medoxomil vs. control therapy.
The proportion of patients who achieved a reduction of clinic SBP to <140 mmHg or a reduction of >20 mmHg was significantly higher in the 80-mg AZL-M group (OR: 1.46, 95% CI: 1.11–1.91, p = 0.256) compared with control therapy. Similarly, 40 mg of AZL-M was non-inferior to control therapy (OR: 1.29, 95% CI: 0.83–2.01, p < 0.05) (Figure 5).
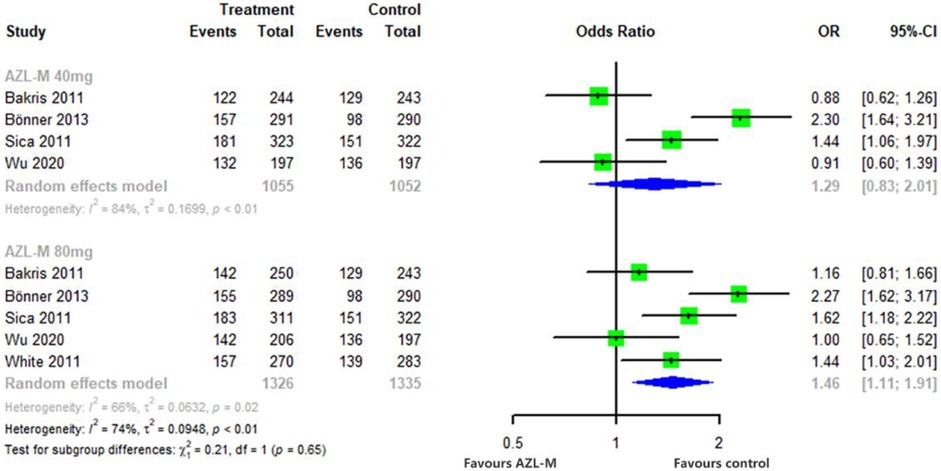
Figure 5 Forest plot of responder rates among hypertensive patients randomized to azilsartan medoxomil vs. control therapy.
3.3 Safety meta-analysis
In the safety analysis set, all the pooled data were compered in two groups, namely, control therapy and placebo, if available. The safety meta-analysis is presented in Table 2. The results revealed that there was no difference in the reduction of risks for total adverse events, AEs leading to discontinuation, serious AEs, and AEs related to the study drug. However, there was a higher risk of dizziness (OR: 1.56, 95% CI: 1.08–2.26, p < 0.05) in the 80-mg AZL-M group and more risks of urinary tract infection (OR: 1.82, 95% CI: 1.14–2.90, p < 0.05) in the 40-mg AZL-M group. Nevertheless, there was no difference in the risk of headache, hyperlipidemia, hypotension, or nasopharyngitis.
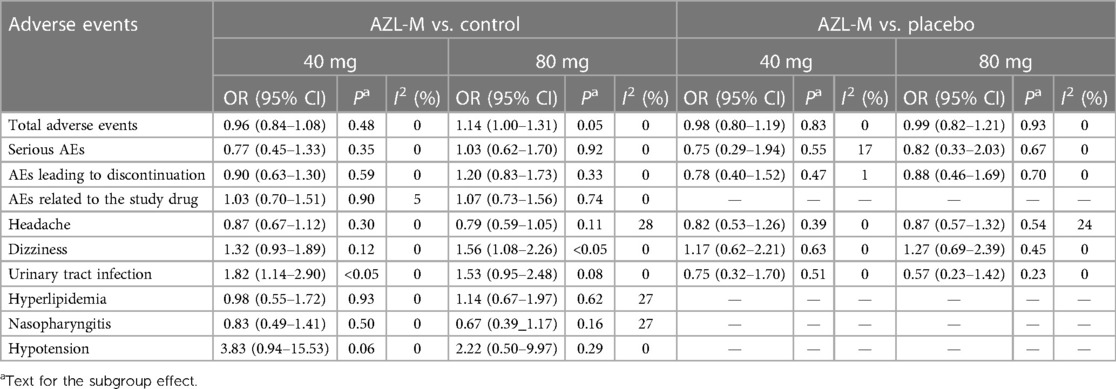
Table 2 Results of the safety meta-analysis of azilsartan medoxomil vs. control therapy and placebo.
3.4 Hypertension with diabetes
We conducted an analysis on patients with hypertension combined with diabetes. Among the included studies, five (18, 20, 22, 23, 25) involved patients with diabetes. However, studies by Johnson et al. (23) and Juhasz et al. (22) were compared to a placebo, and comparable data from the others was unavailable. Nevertheless, one article (29) just included outcomes from the three RCTs (18, 20, 25), comparing the effects of AZL-M with olmesartan and valsartan on ambulatory and clinic blood pressure in patients with type 2 diabetes and prediabetes. The analyses indicate that AZL-M at the approved dose of 80 mg provides superior management, with safety and tolerability similar to the control therapy (29).
3.5 Publication bias and sensitivity analysis
Publication bias tests were performed with >10 studies according to the guidelines, but our included studies were fewer than 10. The outcomes of the efficacy analyses had several heterogeneous results. We performed several sensitivity analyses, and excluding any single trial from the analysis did not substantially alter the overall results, except for 40 mg of AZL-M for 24-h ABPM mean DBP; when we excluded the trial by Garg et al. (27), it showed a statistically significant result favoring 40-mg AZL-M therapy (MD: −1.97 mmHg, 95% CI: −2.87 to −1.06 mmHg, p < 0.01) (Figure 6).
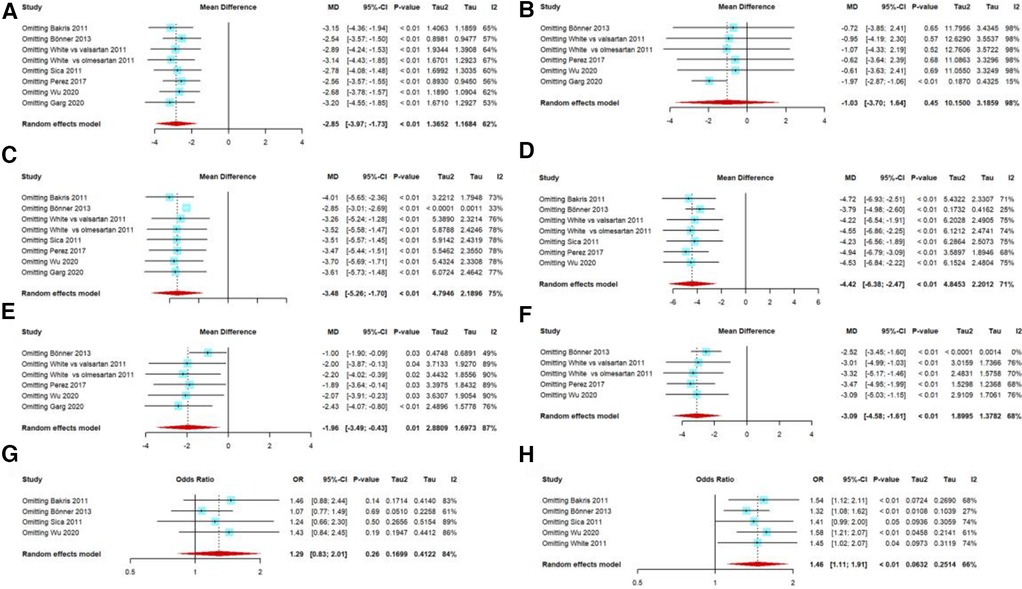
Figure 6 Sensitivity analysis: 40 mg AZL-M vs. control therapy in 24-h ABPM mean SBP (A)/DBP (B), 40 mg AZL-M vs. control therapy in clinic SBP (C)/DBP (E), 80 mg AZL-M vs. control therapy in clinic SBP (D)/DBP (F), and responder rate on 40 mg (G)/80 mg (H) AZL-M vs. control therapy.
4 Discussion
We conducted a meta-analysis on a randomized controlled trial of 40 and 80 mg of AZL-M, which are approved dosages for hypertension treatment in China. The analysis compared these dosages with control therapy and placebo, revealing that AZL-M demonstrated superior reductions in mean SBP and DBP measured by 24-h ABPM, as well as clinic SBP, clinic DBP, and responder rate. These efficacy results are consistent with previous research (13) and remained robust in sensitivity analyses except for the study by Garg et al. (27), which impacted the overall outcome. We attribute this to differences in patient selection criteria and blinding methods between Garg et al. and other studies. The study by Garg et al. included a patient with a clinic SBP of ≥150 to ≤180 mmHg (stage 2), while the other studies included stage 1 patients. The study by Garg et al. was an open-label, assessor-blinded trial, which introduced systematic bias because investigators or trial participants were aware of the treatment assignment.
ARBs are typically well tolerated (30), and the side effect profile is generally similar to that seen with ACE inhibitors, although hypotensive symptoms appear to be more common with ARBs (31). The most commonly reported adverse events in AZL-M include headache, dyslipidemia, dizziness, and hyperlipidemia. The incidence of hypotension appears to be low, but there is a higher incidence of dizziness and a lower incidence of urinary tract infection based on this analysis. The pooled studies had varying durations ranging from 6 to 24 weeks; however, longer follow-up studies have indicated similar results. The observational study by Gitt et al. (32) showed improvements in BP control, while the study by Bakris et al. (33) demonstrated tolerable profiles over 52 weeks.
The efficacy analysis consisted of 24-h mean ABPM SBP/DBP and clinic SBP/DBP. Blood pressure measured by ABPM can differentiate between white-coat hypertension and masked hypertension (34)and can predict all-cause mortality and cardiovascular events (35). Patients with hypertension can benefit from treatment with AZL-M in reducing cardiovascular events (28, 36). Hypertension increases the risk for a variety of cardiovascular diseases (37); for each 20/10 mmHg increase in systolic/diastolic blood pressure, there is a doubling of coronary heart- and stroke-related mortality (38, 39).
AZL-M is a prodrug that is rapidly hydrolyzed to the active moiety, azilsartan, with a half-life of approximately 11 h. Azilsartan inhibits angiotensin II's vasoconstrictor and aldosterone-secreting effects by selectively blocking the binding of angiotensin II to the AT1 receptor in vascular smooth muscle and adrenal gland tissues (azilsartan has a stronger affinity for the AT1 receptor than the AT2 receptor) (40). The action is independent of the angiotensin II synthesis pathways. Beyond BP control, azilsartan has potential effects that include amelioration of the deleterious effects of angiotensin II such as cardiac hypertrophy, fibrosis, insulin resistance, and stabilization of coronary plaques (41); as also, it causes positive changes in leptin, C-reactive protein, IL-6, adiponectin levels (42). In healthy individuals, no AZL-M dose adjustments are required based on age, sex, or race (black/white) (43).
Furthermore, ARBs are extensively utilized for the management of hypertension, chronic kidney disease, heart failure, and diabetes. We analyzed the data of patients with hypertension and diabetes; one article compared the effects of AZL-M with olmesartan and valsartan and indicated that 80 mg of AZL-M provides superior management. Fixed-dose combinations of AZL-M and chlorthalidone have shown significant reductions in systolic blood pressure along with good tolerability among hypertensive participants with stage 3 chronic kidney disease (33). In patients with heart failure with preserved ejection fraction (HFpEF), azilsartan improved the diastolic function parameters of the left ventricle (44). In patients with hypertension who are overweight or obese, AZL-M also provided good BP control (45).
However, our analysis has several limitations. First, considerable heterogeneity was observed in the results of the efficacy meta-analysis, which may be attributed to factors such as race, treatment duration, and study methodologies. Second, because the duration of treatment was relatively short whereas hypertension requires lifelong management, this study could not adequately capture long-term benefits or side effects. Third, we relied on data from randomized controlled trials where enrolled patients may not represent those typically encountered in clinical practice. Hypertension is often accompanied by multiple complications, yet we included only one study related to heart failure.
5 Conclusion
In conclusion, AZL-M appears to provide a greater reduction in BP than control therapy in patients with hypertension and has no greater risk of adverse events than control therapy or placebo in patients with hypertension and diabetes. Nonetheless, more evidence is still needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
LZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. G-CW: Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. QX: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft. Q-LC: Data curation, Investigation, Writing – original draft. QZ: Formal Analysis, Investigation, Writing – original draft. X-xL: Validation, Writing – review & editing. L-aP: Conceptualization, Visualization, Writing – review & editing, Methodology. XX: Conceptualization, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398(10304):957–80. doi: 10.1016/s0140-6736(21)01330-1
2. Writing Group of 2018 Chinese Guidelines for the Management of Hypertension, Chinese Hypertension League, Chinese Society of Cardiology, Chinese Medical Doctor Association Hypertension Committee, Hypertension Branch of China International Exchange and Promotive Association for Medical and Health Care, and Hypertension Branch of Chinese Geriatric Medical Association. 2018 Chinese guidelines for the management of hypertension. Chin J Cardiovasc Med. (2019) 24(01):24–56.
3. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. (2018) 71(19):e127–248. doi: 10.1016/j.jacc.2017.11.006
4. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. (2020) 75(6):1334–57. doi: 10.1161/hypertensionaha.120.15026
5. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39(33):3021–104. doi: 10.1093/eurheartj/ehy339
6. Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. (2003) 138(7):593–602. doi: 10.7326/0003-4819-138-7-200304010-00018
7. Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-alternative trial. Lancet. (2003) 362(9386):772–6. doi: 10.1016/s0140-6736(03)14284-5
8. Konstam MA, Neaton JD, Dickstein K, Drexler H, Komajda M, Martinez FA, et al. Effects of high-dose versus low-dose losartan on clinical outcomes in patients with heart failure (HEAAL study): a randomised, double-blind trial. Lancet. (2009) 374(9704):1840–8. doi: 10.1016/s0140-6736(09)61913-9
9. Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, Motolese M, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The angiotensin-converting-enzyme inhibition in progressive renal insufficiency study group. N Engl J Med. (1996) 334(15):939–45. doi: 10.1056/nejm199604113341502
10. Ninomiya T, Perkovic V, Turnbull F, Neal B, Barzi F, Cass A, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta-analysis of randomised controlled trials. Br Med J. (2013) 347:f5680. doi: 10.1136/bmj.f5680
11. Wang JG, Zhang M, Feng YQ, Ma CS, Wang TD, Zhu ZM, et al. Is the newest angiotensin-receptor blocker azilsartan medoxomil more efficacious in lowering blood pressure than the older ones? A systematic review and network meta-analysis. J Clin Hypertens. (2021) 23(5):901–14. doi: 10.1111/jch.14227
12. Ehlken B, Shlaen M, de Torres MDPLF, Hisada M, Bennett D. Use of azilsartan medoxomil in the primary-care setting in Germany: a real-world evidence study. Int J Clin Pharm Ther. (2019) 57(6):275–83. doi: 10.5414/CP203359
13. Takagi H, Mizuno Y, Niwa M, Goto SN, Umemoto T. A meta-analysis of randomized controlled trials of azilsartan therapy for blood pressure reduction. Hypertens Res. (2014) 37(5):432–7. doi: 10.1038/hr.2013.142
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. (2021) 134:103–12. doi: 10.1016/j.jclinepi.2021.02.003
15. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
16. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10(10):Ed000142. doi: 10.1002/14651858.Ed000142
17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
18. White WB, Weber MA, Sica D, Bakris GL, Perez A, Cao C, et al. Effects of the angiotensin receptor blocker azilsartan medoxomil versus olmesartan and valsartan on ambulatory and clinic blood pressure in patients with stages 1 and 2 hypertension. Hypertension. (2011) 57(3):413–20. doi: 10.1161/HYPERTENSIONAHA.110.163402
19. Weber MA, White WB, Sica D, Bakris GL, Cao C, Roberts A, et al. Effects of combining azilsartan medoxomil with amlodipine in patients with stage 2 hypertension. Blood Press Monit. (2014) 19(2):90–7. doi: 10.1097/MBP.0000000000000027
20. Sica D, White WB, Weber MA, Bakris GL, Perez A, Cao C, et al. Comparison of the novel angiotensin II receptor blocker azilsartan medoxomil vs valsartan by ambulatory blood pressure monitoring. J Clin Hypertens (Greenwich). (2011) 13(7):467–72. doi: 10.1111/j.1751-7176.2011.00482.x
21. Perez A, Cao C. The impact of azilsartan medoxomil treatment (capsule formulation) at doses ranging from 10 to 80mg: significant, rapid reductions in clinic diastolic and systolic blood pressure. J Clin Hypertens (Greenwich). (2017) 19(3):312–21. doi: 10.1111/jch.12895
22. Juhasz A, Wu J, Hisada M, Tsukada T, Jeong MH. Efficacy and safety of azilsartan medoxomil, an angiotensin receptor blocker, in Korean patients with essential hypertension. Clin Hypertens. (2018) 24:1. doi: 10.1186/s40885-018-0086-4
23. Johnson W, White WB, Sica D, Bakris GL, Weber MA, Handley A, et al. Evaluation of the angiotensin II receptor blocker azilsartan medoxomil in African-American patients with hypertension. J Clin Hypertens (Greenwich). (2017) 19(7):695–701. doi: 10.1111/jch.12993
24. Bönner G, Bakris GL, Sica D, Weber MA, White WB, Perez A, et al. Antihypertensive efficacy of the angiotensin receptor blocker azilsartan medoxomil compared with the angiotensin-converting enzyme inhibitor ramipril. J Hum Hypertens. (2013) 27(8):479–86. doi: 10.1038/jhh.2013.6
25. Bakris GL, Sica D, Weber M, White WB, Roberts A, Perez A, et al. The comparative effects of azilsartan medoxomil and olmesartan on ambulatory and clinic blood pressure. J Clin Hypertens (Greenwich). (2011) 13(2):81–8. doi: 10.1111/j.1751-7176.2010.00425.x
26. Wu J, Du X, Lv Q, Li Z, Zheng Z, Xia Y, et al. A phase 3 double-blind randomized (CONSORT-compliant) study of azilsartan medoxomil compared to valsartan in Chinese patients with essential hypertension. Medicine (United States). (2020) 99(32):E21465. doi: 10.1097/MD.0000000000021465
27. Garg M, Manik G, Singhal A, Singh V, Varshney R, Sethi A. Efficacy and safety of azilsartan medoxomil and telmisartan in hypertensive patients: a randomized, assessor-blinded study. Saudi J Med Med Sci. (2020) 8(2):87–94. doi: 10.4103/sjmms.sjmms-19-19
28. Peng T, LIi Xq, Long Cm, Wang R, LIi Xl. Clinical trial of mesarartan potassium in the treatment of patients with hypertension and heart failure. Chin J Clin Pharmacol. (2023) 39(14):1992–6. doi: 10.13699/j.cnki.1001-6821.2023.14.004
29. White WB, Cuadra RH, Lloyd E, Bakris GL, Kupfer S. Effects of azilsartan medoxomil compared with olmesartan and valsartan on ambulatory and clinic blood pressure in patients with type 2 diabetes and prediabetes. J Hypertens. (2016) 34(4):788–97. doi: 10.1097/HJH.0000000000000839
30. Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. (2000) 355(9204):637–45. doi: 10.1016/s0140-6736(99)10365-9
31. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. (2008) 358(15):1547–59. doi: 10.1056/NEJMoa0801317
32. Gitt AK, Bramlage P, Potthoff SA, Baumgart P, Mahfoud F, Buhck H, et al. Azilsartan compared to ACE inhibitors in anti-hypertensive therapy: one-year outcomes of the observational EARLY registry. BMC Cardiovasc Disord. (2016) 16(1):56. doi: 10.1186/s12872-016-0222-6
33. Bakris GL, Zhao L, Kupfer S, Juhasz A, Hisada M, Lloyd E, et al. Long-term efficacy and tolerability of azilsartan medoxomil/chlorthalidone vs olmesartan medoxomil/hydrochlorothiazide in chronic kidney disease. J Clin Hypertens (Greenwich). (2018) 20(4):694–702. doi: 10.1111/jch.13230
34. Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the American Society of Hypertension and the International Society of Hypertension. J Hypertens. (2014) 32(1):3–15. doi: 10.1097/hjh.0000000000000065
35. Piper MA, Evans CV, Burda BU, Margolis KL, O'Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. preventive services task force. Ann Intern Med. (2015) 162(3):192–204. doi: 10.7326/m14-1539
36. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. (2002) 360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8
37. Flint AC, Conell C, Ren X, Banki NM, Chan SL, Rao VA, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. (2019) 381(3):243–51. doi: 10.1056/NEJMoa1803180
38. Son JS, Choi S, Kim K, Kim SM, Choi D, Lee G, et al. Association of blood pressure classification in Korean young adults according to the 2017 American College of Cardiology/American Heart Association guidelines with subsequent cardiovascular disease events. JAMA. (2018) 320(17):1783–92. doi: 10.1001/jama.2018.16501
39. Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, et al. Association of blood pressure classification in young adults using the 2017 American College of Cardiology/American Heart Association blood pressure guideline with cardiovascular events later in life. JAMA. (2018) 320(17):1774–82. doi: 10.1001/jama.2018.13551
40. Hjermitslev M, Grimm DG, Wehland M, Simonsen U, Krüger M. Azilsartan medoxomil, an angiotensin II receptor antagonist for the treatment of hypertension. Basic Clin Pharmacol Toxicol. (2017) 121(4):225–33. doi: 10.1111/bcpt.12800
41. Arumugam S, Sreedhar R, Thandavarayan RA, Karuppagounder V, Krishnamurthy P, Suzuki K, et al. Angiotensin receptor blockers: focus on cardiac and renal injury. Trends Cardiovasc Med. (2016) 26(3):221–8. doi: 10.1016/j.tcm.2015.06.004
42. Nedogoda SV, Chumachek EV, Tsoma VV, Salasyuk AS, Smirnova VO, Popova EA. Effectiveness of in insulin resistance correction and the adipokines level reduction in patients with arterial hypertension in comparison with other ARBs. Russ J Cardiol. (2019) 24(1):70–9. doi: 10.15829/1560-4071-2019-1-70-79
43. Harrell RE, Karim A, Zhang W, Dudkowski C. Effects of age, sex, and race on the safety and pharmacokinetics of single and multiple doses of azilsartan medoxomil in healthy subjects. Clin Pharmacokinet. (2016) 55(5):595–604. doi: 10.1007/s40262-015-0333-8
44. Sakamoto M, Asakura M, Nakano A, Kanzaki H, Sugano Y, Amaki M, et al. Azilsartan, but not candesartan improves left ventricular diastolic function in patients with hypertension and heart failure. Int J Gerontol. (2015) 9(4):201–5. doi: 10.1016/j.ijge.2015.06.003
Keywords: azilsartan medoxomil, hypertension, diabetes, meta-analysis, angiotensin II receptor blockers (ARBs)
Citation: Zhu L, Wei G-C, Xiao Q, Chen Q-L, Zhao Q, Li X-x, Pan L-a and Xiong X (2024) Efficacy and safety of azilsartan medoxomil in the treatment of hypertension: a systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1383217. doi: 10.3389/fcvm.2024.1383217
Received: 7 April 2024; Accepted: 19 June 2024;
Published: 4 July 2024.
Edited by:
Maria Lorenza Muiesan, University of Brescia, ItalyReviewed by:
Raffaella Dell'Oro, University Milano Bicocca, ItalyDavide Agnoletti, University of Bologna, Italy
© 2024 Zhu, Wei, Xiao, Chen, Zhao, Li, Pan and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling-ai Pan, cGFubGluZ2FpMjAwNEAxNjMuY29t; Xuan Xiong, eGlvbmd4dWFuQG1lZC51ZXN0Yy5lZHUuY24=
†These authors have contributed equally to this work
 Ling Zhu1,†
Ling Zhu1,† Guo-Cui Wei
Guo-Cui Wei Xiu-xia Li
Xiu-xia Li Ling-ai Pan
Ling-ai Pan Xuan Xiong
Xuan Xiong