
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 25 July 2024
Sec. Atherosclerosis and Vascular Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1383032
Background: Numerous observational studies have suggested an association between psychiatric traits and carotid intima-media thickness (cIMT). However, whether these associations have a causal relationship remains unknown, largely due to issues of reverse causality and potential confounders. This study aims to elucidate the potential causal role of psychiatric traits in the risk of arterial injury as measured by cIMT.
Methods: We utilized instrumental variables for attention deficit/hyperactivity disorder (ADHD, n = 226,534), bipolar disorder (n = 353,899), major depressive disorder (n = 142,646), post-traumatic stress disorder (n = 174,494), obsessive-compulsive disorder (n = 9,725), autism spectrum disorder (n = 173,773), and anxiety disease (n = 17,310), derived from the largest corresponding genome-wide association studies (GWAS). Summary statistics for cIMT associations were obtained from a meta-analysis combining GWAS data from the Cohorts for Heart and Aging Research in Genomic Epidemiology consortia (n = 71,128) and the UK Biobank study (n = 45,185). The inverse-variance weighted method served as the primary analytical tool, supplemented by additional statistical methods in the secondary analyses to corroborate the findings. Adjustments were made according to the Bonferroni correction threshold.
Results: The Mendelian randomization analyses indicated a suggestive causal link between genetically predicted ADHD and cIMT (beta = 0.05; 95% confidence interval, 0.01–0.09; p = 0.018). Sensitivity analyses largely concurred with this finding. However, no significant associations were found between other psychiatric traits and cIMT.
Conclusions: This study provides insights into the risk effect of ADHD on cIMT, suggesting that arteriopathy and potential associated complications should be considered during the treatment and monitoring of patients with ADHD.
Cardiovascular diseases (CVD) are the primary global mortality cause (1). Given that thickening of the arterial wall is an early indicator of subsequent plaque development, and because ultrasound can non-invasively measure carotid intima-media thickness (cIMT) (2, 3), cIMT is commonly utilized as a surrogate marker to study how early risk factors correlate with cardiovascular health.
Patients with psychiatric disorders have been observed to have an increased risk of premature all-cause mortality, especially from CVDs (4). Several studies have explored the association between mental characteristics and cIMT. However, these findings often suffer from inadequate adjustment for confounding factors and inconsistent definitions of mental health, thereby limiting causal inference. For instance, A meta-analysis of 19 prospective studies (4,490 cases vs. 27,583 controls) suggested that individuals with depressive symptoms had obviously thicker cIMT compared to the normal group without depressive symptoms (standard mean differences of 0.137; 95% CI, 0.047–0.227; p = 0.003) (5). However, the Young Finns Study did not observe a prospective relationship between depressive symptoms and subsequent cIMT (6). Furthermore, The Etude sur le Vieillissement Artériel study, a four-year prospective study investigation involving 726 middle-aged subjects, revealed a significant association between anxiety and an increase in cIMT in both males and females. Notably, this association remained independent of traditional cardiovascular risk factors (7). While a 2-year follow-up study of 518 postmenopausal women found that anxiety was associated with atherogenic lipid levels, but not with subclinical atherosclerosis (8). Overall, these findings suggest that the causal relationship between psychological traits and cIMT remains unclear.
Mendelian Randomization (MR) serves as a crucial method of instrumental variable analysis, improving the strength of causal inferences in observational epidemiological studies. MR analysis uses single nucleotide polymorphisms (SNPs) associated with specific exposures as instrumental variables (IVs) to assess if the relationship between an exposure and an outcome is causal (9). The underlying strength of MR lies in the random allocation of SNPs at conception, significantly reducing susceptibility to confounding biases. Additionally, since the genotype is not influenced by the phenotype, MR effectively minimizes confounding factors and the risk of reverse causality, thereby enhancing the validity of causal inferences in epidemiological research (10).
In recent years, a growing number of genome-wide association studies (GWAS) have been published (11–17), aiming to identify genetic risk loci associated with psychosocial factors. These studies lay a hopeful groundwork for evaluating the impact of mental health on cIMT from a genetic perspective. In this study, we utilize a two-sample MR design to explore the causality between psychiatric traits and arterial injury (18) as determined by cIMT.
This study employed two-sample MR analyses, utilizing genetic summary-level genetic statistics from comprehensive and recent GWASs, to explore the causal relationship between seven psychiatric traits and cIMT. The IVs in this analysis were based on three rigorous assumptions (Figure 1): (a) Relevance assumption: IVs must demonstrate a strong correlation with the psychiatric traits; (b) Independence assumption: IVs should not be associated with potential confounding factors; (c) Exclusion restriction: IVs must influence cIMT exclusively through the psychiatric traits (19). All original studies adhered to ethical standards, including informed consent from participants and approval from relevant ethics committees.
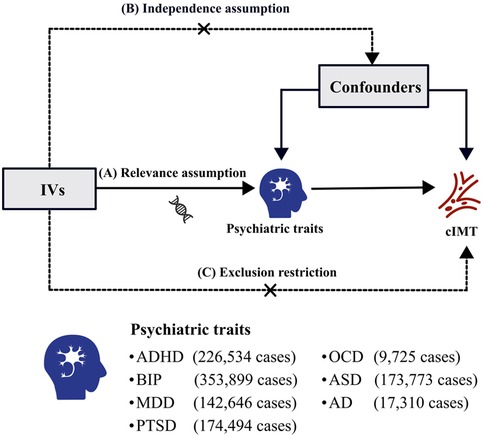
Figure 1 Three pivotal assumptions of MR analysis: (A) relevance assumption: the IVs must exhibit a strong correlation with the psychiatric traits; (B) independence assumption: IVs should not be associated with potential confounding factors; (C) exclusion restriction: IVs must influence the cIMT only via the psychiatric traits. IVs, instrumental variables; cIMT, carotid intima-media thickness; ADHD, attention deficit/hyperactivity disorder; BIP, bipolar disorder; MDD, major depressive disorder; PTSD, post-traumatic stress disorder; OCD, obsessive–compulsive disorder; ASD, autism spectrum disorder; AD, anxiety disease.
Estimates of SNP-exposure associations for seven psychiatric traits were sourced from the Psychiatric Genomics Consortium (PGC) (20), which includes attention deficit hyperactivity disorder (ADHD), bipolar disorder (BIP), major depressive disorder (MDD), post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD), autism spectrum disorder (ASD), and anxiety disorders (AD). As the largest global consortium in psychiatric research, PGC has significantly advanced the meta- and mega-analyses of genome-wide genetic data on psychiatric disorders (20). Detailed definitions for these seven psychiatric traits are outlined in Supplementary Table 1. SNPs strongly associated with exposure (BIP, ADHD) were identified at the genome-wide significance level (p < 5 × 10−8). For other psychiatric traits (MDD, PTSD, OCD, AD, and ASD), SNPs were selected using a less stringent threshold of 1 × 10−5. Linkage disequilibrium (LD) testing, based on the European 1,000 Genomes Project reference panel (r2 < 0.01 and clump distance > 10,000 kb) (21), was conducted to ensure the independence of selected SNPs (21). In cases of LD, SNP with the higher p-value was excluded. Ultimately, 26 independent SNPs were identified for ADHD, 29 for BIP, 69 for MDD, 38 for PTSD, 25 for OCD, 58 for ASD, and 20 for AD. To reduce the risk of weak instrument bias, we applied an F statistic threshold of >10 to filter SNPs associated with the exposures (22).
The outcome-related SNPs for this study were sourced from a meta-analysis that combined data from the UK Biobank and the most extensive previous GWAS meta-analysis of cIMT conducted by the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) consortia (Supplementary Table 2). This meta-analysis was performed to assess cIMTmax using the Multi-Trait Analysis of GWAS (MTAG) approach (23, 24). MTAG, a statistical instrument utilized for the analysis of aggregated data from multiple GWAS, implemented a generalized inverse variance-weighted (IVW) meta-analysis to mitigate biases stemming from sample overlap.
The CHARGE consortia's GWAS meta-analysis included 31 studies on cIMT, encompassing a total of 71,128 individuals (24), all of European descent. These participants were evaluated for cIMT parameters using high-resolution B-mode ultrasound. The UK Biobank, a prospective cohort based on the UK population, collected extensive genetic, physical, and health data from approximately 500,000 individuals aged 40–69 between 2006 and 2010. In the UK Biobank's genetic analysis of cIMT (involving 45,185 individuals), adjustments were made for gender, genotyping array, and the top 30 principal components to control for population stratification (23).
Genetic IVs identified as psychiatric traits were queried for matching SNPs in the GWAS outcome. If specific SNPs were not present in the outcome data, proxy SNPs (r2 > 0.9) from the European population were used as substitutes, accessed via an online resource (https://ldlink.nci.nih.gov/?tab=ldproxy). The count of valid IVs for each exposure-outcome pairs is detailed in Table 1 (11–17).
We employed IVW methods as the primary analytical approach to assess the effect of genetically predicted psychiatric traits on the risk of cIMT thickening (25). Using Wald estimation, we derived causal assessments for each SNP and generated corresponding standard errors through the delta method. These estimates were then integrated into a fixed-effects IVW meta-analysis to provide a comprehensive evaluation (26). Supplementary methods including the weighted median (WM), maximum likelihood (ML), MR-Egger regression, and leave-one-out sensitivity analysis were utilized to verify the robustness of our findings. The WM method allows for up to 50% of the IVs to be invalid while still yielding consistent estimates (27). The significance of the WM method value lies in its robustness to invalid instruments. Maximum likelihood, a standard approach for parameter estimation in probability distributions, maximizes the likelihood function to achieve lower standard errors (28). The MR-Egger method provides unbiased estimates even in the presence of horizontal pleiotropy (29). Additionally, leave-one-out sensitivity analysis was conducted to assess the stability of the results, sequentially excluding each SNP to test the reproducibility of the primary findings and ascertain if any single SNP significantly influenced the outcomes.
To detect potential horizontal pleiotropy, we used the MR-Egger intercept test (p < 0.05 indicating SNPs impact the outcome through pathways other than the exposure). The MR pleiotropy residual sum and outlier (MR-PRESSO) method was also employed for evaluating horizontal pleiotropy (30). This method, based on the IVW regression framework, identifies IVs with horizontal pleiotropy as outliers in the regression analysis. Specifically, MR-PRESSO detects SNPs with horizontal pleiotropy using a global test based on the leave-one-out procedure and an outlier test (31, 32). Cochran's Q statistics were used to examine heterogeneity among individual SNPs (33). If significant heterogeneity was identified (p < 0.05), a multiplicative random effects IVW analysis was performed. Following the Bonferroni correction standard, a p-value < 0.007 (0.05/7 tests) was considered indicative of a significant causal relationship between exposures and outcomes. p-values between 0.007 and 0.05 were deemed suggestive of associations.
All analyses were conducted using R version 4.3.1 and relevant R packages (i.e., “TwoSample MR” and “MR-PRESSO”) (21).
The IVs used for the MR analysis of seven psychiatric traits on cIMT are detailed in Supplementary Table 3–S9. The F-statistics of all IVs exceed the threshold of 10, indicating their strong predictive power for psychiatric traits in the MR analysis.
Using the IVW method, the results indicate a potential risk effect of ADHD on cIMT thickening, with a beta of 0.05 (95% CI = 0.01–0.09, p = 0.018). This suggests that with each unit increase in the log-odds of ADHD, the cIMT increases by 1.05 millimeters. Although not all methods used to evaluate the ADHD-cIMT association reached statistical significance, the direction of the association was consistent across most statistical models (Supplementary Figure 1). A stable relationship was also observed in the maximum likelihood analysis (beta = 0.05; 95% CI = 0.01–0.09; p = 0.016) (Figure 2). Sensitivity analysis of ADHD and cIMT using the Cochran's Q test, based on the IVW method, revealed no evidence of heterogeneity in the ADHD IVs (p = 0.054). Moreover, the intercept of the MR-Egger test was not statistically significant (p = 0.220), as well as the MR-PRESSO test did not find any outliers, indicating an absence of horizontal pleiotropy (Supplementary Table 10). The leave-one-out sensitivity analysis also demonstrated that no single instrumental SNP significantly affected the ADHD-cIMT causality (Supplementary Figure 1).
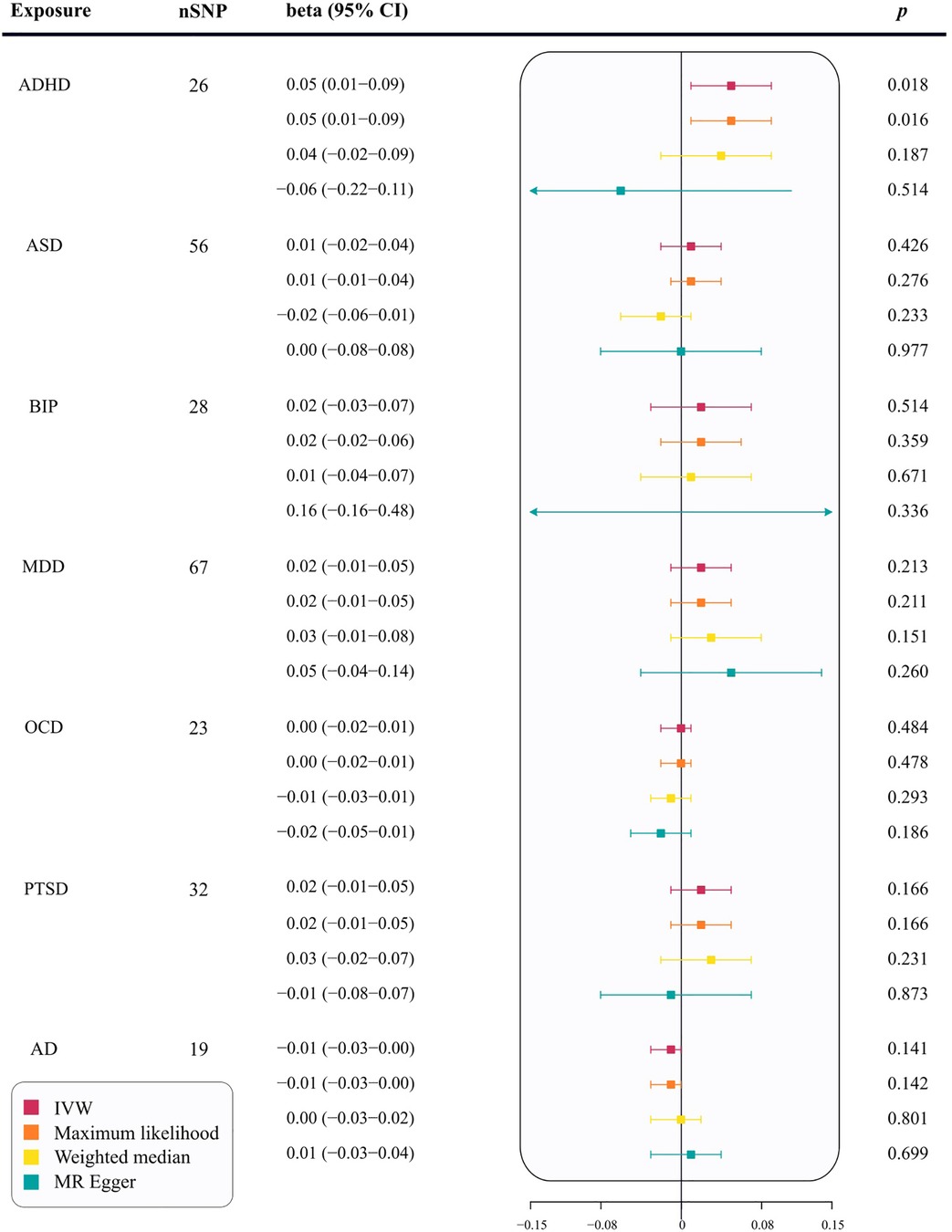
Figure 2 The correlation between seven psychiatric traits and cIMT in MR analysis. nSNP, number of single nucleotide polymorphisms; ADHD, attention deficit/hyperactivity disorder; BIP, bipolar disorder; MDD, major depressive disorder; PTSD, post-traumatic stress disorder; OCD, obsessive–compulsive disorder; ASD, autism spectrum disorder; AD, anxiety disease; SNPs, single-nucleotide polymorphisms; IVW, inverse-variance weighted; MR−Egger, Mendelian Randomization-Egger regression; CI, confidence interval; cIMT, carotid intima-media thickness; MR, Mendelian Randomization.
In the primary analysis, no causal relationships were found between ASD (beta = 0.01; 95% CI = −0.02–0.04; p = 0.426), BIP (beta = 0.02; 95% CI = −0.03–0.07; p = 0.514), MDD (beta = 0.02; 95% CI = −0.01–0.05; p = 0.213), OCD (beta = 0.00; 95% CI = −0.02–0.0; p = 0.484), PTSD (beta = 0.02; 95% CI = −0.01–0.05; p = 0.166), or AD (beta = −0.01; 95% CI = −0.03–0.00; p = 0.141) and cIMT thickening (Figure 2). Supplementary analyses, including maximum likelihood, weighted median, and MR-Egger, supported these findings (p > 0.05, Figure 2). The MR-Egger regression showed no evidence of horizontal pleiotropy (p for intercept < 0.05). MR-PRESSO analysis identified two outliers for ASD and one for BIP. However, the relationships remained stable after outlier exclusion, with no significant changes in estimates before and after outlier removal (p for distortion test >0.05) (Supplementary Table 10).
Utilizing the largest published GWAS, we applied a comprehensive framework to examine the genetic relationship between seven psychiatric traits and cIMT. Our analysis provides suggestive evidence for the potential causal effect of ADHD on the increased risk of cIMT thickening. Supplementary analyses also show directionally consistent association patterns across most statistical models.
Increased cIMT is a recognized marker of arterial injury and serves as an indicator of cardiovascular risk factors. Assessing a patient's cIMT, regardless of the presence of carotid plaques, can provide information on the potential occurrence of cardiovascular events in asymptomatic patients (34) and on cardiovascular outcomes in patients with known atherosclerotic disease (35). A meta-analysis incorporating 8 clinical studies with a total of over 37,000 patients and an average follow-up period of 5.5 years demonstrated a positive correlation between increased cIMT and the occurrence of cardiovascular events. This study suggested that for every 0.1 mm increase in cIMT, the future risk of myocardial infarction (MI) increases by ≤15% (36). Similarly, another meta-analysis concluded that combining the traditional Framingham Risk Score with cIMT may provide better predictive performance for stroke and MI (37). cIMT was also widely used as an efficacy endpoint for cardiovascular therapeutic drugs, such as statins, antihypertensives, and antidiabetics (38–41). Using cIMT measurements as a biomarker for atherosclerosis progression can facilitate the evaluation of treatment effectiveness before endpoints such as MI, stroke, and death occur. This approach can accelerate drug development and guide medication strategies earlier.
Previous studies had suggested that several psychiatric traits were associated with cIMT. In a study by Hakan et al. (42), 42 children with ADHD and 42 age- and sex- matched healthy controls were evaluated to assess the relationship between cIMT and ADHD symptom severity. Findings revealed significantly higher median cIMT values in the ADHD group compared to controls, with a notable correlation between cIMT and ADHD severity. Similar conclusions were echoed in subsequent research (43). However, these studies had limitations, including small sample sizes, making individual bias correction challenging, and limited follow-up periods, potentially leading to underestimation of incidence rates. Our research overcomes these constraints of traditional studies, and our findings on the ADHD-cIMT association are consistent with existing clinical research.
The link between ADHD and systemic inflammation has been extensively investigated. Nikola's (44) study found elevated markers of inflammation, including platelet distribution width, interleukins [interleukin -1β, interleukin-6 (IL-6)], Tumor Necrosis Factor-alpha (TNF-α), and the M1 proinflammatory profile, in adolescents with ADHD compared to healthy controls. This was further supported by a comprehensive meta-analysis showing generally higher IL-6 and lower TNF-α levels in individuals with ADHD (45). Chronic inflammation, known for its adverse impact on endothelial functions and its role in accelerating subclinical atherosclerosis via proinflammatory cytokines, is an emerging risk factor for CVD. It has also been independently linked to higher cIMT (46). Consequently, the collective evidence strongly suggests that chronic inflammation may be a key mechanism driving the development of arterial injury in ADHD populations.
Discrepancies exist in clinical research regarding the effects of depression on cIMT. In the Bogalusa study, Azad et al. conducted a cross-sectional study (47) enrolling 996 individuals aged 24–44 years to examine the association between depression symptoms and arterial injury, as determined by cIMT. The results indicated significant associations between depression scores and cIMT, particularly in individuals with a higher TC/HDL ratio. Similar findings were reported in a study focusing on police officers (48). However, the Young Finns study (6), involving 996 adults aged 30–45, found no association between cumulative depression index and cIMT, either before or after adjusting for traditional risk factors. Our findings suggest a lack of evidence for a causal relationship between MDD and cIMT, potentially elucidating contradictions observed in clinical trials.
Regarding anxiety and cIMT, evidence for a causal relationship is mixed. Itamar et al. (49) observed that individuals with higher symptoms of anxiety and/or depression had increased cIMT values, a finding echoed in subsequent studies (7, 50). However, a study targeting postmenopausal women in China found no significant association between perceived stress, anxiety, and cIMT (8), indicating that the causal relationship between anxiety and cIMT may vary by race and region. The disparities between these studies and ours could be attributed to potential biases and reverse causal relationships inherent in observational studies. Nevertheless, given the consistent results of the majority of prospective studies, the potential causal effects of anxiety on cIMT cannot be conclusively dismissed.
In this study, we utilized the largest GWAS meta-analysis to investigate the causal relationships between seven psychiatric traits and the risk of increased cIMT through MR analyses. Our research has several strengths. Firstly, we employed two-sample MR analyses to ascertain the causality between the seven psychiatric traits and cIMT (51). This method uses genetically assigned variations as IVs, effectively reducing confounding factors and reverse causality common in observational studies. Secondly, we sourced summary-level data from the most extensive GWAS dataset, enhancing the precision of SNP selection and the statistical robustness of our analyses. Finally, our robustness assessment, including multiple statistical models and leave-one-out analyses, strengthened the reliability of our primary findings.
Nevertheless, it is essential to recognize certain inherent limitations. Firstly, the GWAS dataset predominantly consisted of individuals of European ancestry, which may limit the generalizability of our findings to other ethnic groups. Additional studies are necessary to validate our results in non-European populations. Secondly, while we cannot entirely rule out the possibility of horizontal pleiotropy and its impact on our findings, it is noteworthy that our MR-PRESSO analysis did not indicate any significant evidence to this issue. Lastly, the lack of patient-level data, such as gender and socioeconomic status, constrained our capacity to explore the complex causal relationships between the seven psychiatric disorders and cIMT in relation to specific genders and social statuses. For instance, previous research has suggested that men with lower socioeconomic status might be more susceptible to increased cIMT compared to women (52).
In summary, our study provides insights into the risk effect of ADHD on cIMT, primarily within the European population. When treating and monitoring patients with ADHD, consideration should be given to arteriopathy and potential related complications. Additional research is needed to elucidate the mechanisms and biological pathways linking psychiatric traits to cIMT.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
KH: Conceptualization, Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. JY: Conceptualization, Data curation, Writing – review & editing. FY: Conceptualization, Writing – review & editing. TH: Data curation, Writing – review & editing. YD: Conceptualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1383032/full#supplementary-material
1. Timmis A, Vardas P, Townsend N, Torbica A, Katus H, De Smedt D, et al. European Society of Cardiology: cardiovascular disease statistics 2021. Eur Heart J. (2022) 43(8):716–99. doi: 10.1093/eurheartj/ehab892
2. de Groot E, Hovingh GK, Wiegman A, Duriez P, Smit AJ, Fruchart JC, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. (2004) 109(23 Suppl 1):III33–38. doi: 10.1161/01.CIR.0000131516.65699.ba
3. Dalla Pozza R, Ehringer-Schetitska D, Fritsch P, Jokinen E, Petropoulos A, Oberhoffer R, et al. Intima media thickness measurement in children: A statement from the Association for European Paediatric Cardiology (AEPC) Working Group on Cardiovascular Prevention endorsed by the Association for European Paediatric Cardiology. Atherosclerosis. (2015) 238(2):380–7. doi: 10.1016/j.atherosclerosis.2014.12.029
4. Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. (2015) 14:339–47. doi: 10.1002/wps.20252
5. Wu Y, Sun D, Wang B, Li Y, Ma Y. The relationship of depressive symptoms and functional and structural markers of subclinical atherosclerosis: a systematic review and meta-analysis. Eur J Prev Cardiol. (2018) 25:706–16. doi: 10.1177/2047487318764158
6. Keltikangas-Järvinen L, Savelieva K, Josefsson K, Elovainio M, Pulkki-Råback L, Juonala M, et al. Accumulation of depressive symptoms and carotid intima-Media thickness: the cardiovascular risk in young Finns study. Ann Behav Med. (2017) 51:620–8. doi: 10.1007/s12160-017-9884-2
7. Paterniti S, Zureik M, Ducimetière P, Touboul PJ, Fève JM, Alpérovitch A. Sustained anxiety and 4-year progression of carotid atherosclerosis. Arterioscler Thromb Vasc Biol. (2001) 21:136–41. doi: 10.1161/01.ATV.21.1.136
8. Yu RHY, Ho SC, Lam CWK, Woo JLF, Ho SSY. Psychological factors and subclinical atherosclerosis in postmenopausal Chinese women in Hong Kong. Maturitas. (2010) 67:186–91. doi: 10.1016/j.maturitas.2010.06.014
9. Gupta V, Walia GK, Sachdeva MP. ‘Mendelian randomization’: an approach for exploring causal relations in epidemiology. Public Health. (2017) 145:113–9. doi: 10.1016/j.puhe.2016.12.033
10. Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
11. Demontis D, Walters GB, Athanasiadis G, Walters R, Therrien K, Nielsen TT, et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat Genet. (2023) 55:198–208. doi: 10.1038/s41588-022-01285-8
12. Mullins N, Forstner AJ, O'Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. (2021) 53:817–29. doi: 10.1038/s41588-021-00857-4
13. Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. (2018) 50:668–81. doi: 10.1038/s41588-018-0090-3
14. Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. (2019) 10:4558. doi: 10.1038/s41467-019-12576-w
15. International Obsessive Compulsive Disorder Foundation Genetics Collaborative (IOCDF-GC) and OCD Collaborative Genetics Association Studies (OCGAS). Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. (2018) 23:1181–8. doi: 10.1038/mp.2017.154
16. Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. (2019) 51:431–44. doi: 10.1038/s41588-019-0344-8
17. Otowa T, Hek K, Lee M, Byrne EM, Mirza SS, Nivard MG, et al. Meta-analysis of genome-wide association studies of anxiety disorders. Mol Psychiatry. (2016) 21:1391–9. doi: 10.1038/mp.2015.197
18. Raggi P, Stein JH. Carotid intima-media thickness should not be referred to as subclinical atherosclerosis: a recommended update to the editorial policy at atherosclerosis. Atherosclerosis. (2020) 312:119–20. doi: 10.1016/j.atherosclerosis.2020.09.015
20. Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Børglum AD, Breen G, et al. Psychiatric genomics: an update and an agenda. Am J Psychiatry. (2018) 175:15–27. doi: 10.1176/appi.ajp.2017.17030283
21. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. (2018) 7:e34408. doi: 10.7554/eLife.34408
22. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. (2011) 40:740–52. doi: 10.1093/ije/dyq151
23. Yeung MW, Wang S, van de Vegte YJ, Borisov O, van Setten J, Snieder H, et al. Twenty-five novel loci for carotid intima-media thickness: a genome-wide association study in >45000 individuals and meta-analysis of >100000 individuals. Arterioscler Thromb Vasc Biol. (2022) 42:484–501. doi: 10.1161/ATVBAHA.121.317007
24. Franceschini N, Giambartolomei C, de Vries PS, Finan C, Bis JC, Huntley RP, et al. GWAS and colocalization analyses implicate carotid intima-media thickness and carotid plaque loci in cardiovascular outcomes. Nat Commun. (2018) 9:5141. doi: 10.1038/s41467-018-07340-5
25. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. (2017) 36(11):1783–802. doi: 10.1002/sim.7221
26. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. (2013) 37:658–65. doi: 10.1002/gepi.21758
27. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
28. Milligan BG. Maximum-likelihood estimation of relatedness. Genetics. (2003) 163(3):1153–67. doi: 10.1093/genetics/163.3.1153
29. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32(5):377–89. doi: 10.1007/s10654-017-0255-x
30. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
31. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology. (2017) 28(1):30–42. doi: 10.1097/EDE.0000000000000559
32. Ong JS, MacGregor S. Implementing MR-PRESSO and GCTA-GSMR for pleiotropy assessment in Mendelian randomization studies from a practitioner's perspective. Genet Epidemiol. (2019) 43(6):609–16. doi: 10.1002/gepi.22207
33. Greco MFD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. (2015) 34(21):2926–40. doi: 10.1002/sim.6522
34. Bots ML, Evans GW, Tegeler CH, Meijer R. Carotid intima-media thickness measurements: relations with atherosclerosis, risk of cardiovascular disease and application in randomized controlled trials. Chin Med J (Engl). (2016) 129:215–26. doi: 10.4103/0366-6999.173500
35. Kablak-Ziembicka A, Przewlocki T, Sokołowski A, Tracz W, Podolec P. Carotid intima-media thickness, hs-CRP and TNF-α are independently associated with cardiovascular event risk in patients with atherosclerotic occlusive disease. Atherosclerosis. (2011) 214:185–90. doi: 10.1016/j.atherosclerosis.2010.10.017
36. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. (2007) 115(4):459–67. doi: 10.1161/CIRCULATIONAHA.106.628875
37. Den Ruijter HM, Peters SAE, Anderson TJ, Britton AR, Dekker JM, Eijkemans MJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. (2012) 308:796–803. doi: 10.1001/jama.2012.9630
38. Crouse JR, Raichlen JS, Riley WA, Evans GW, Palmer MK, O'Leary DH, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR trial. JAMA. (2007) 297:1344–53. doi: 10.1001/jama.297.12.1344
39. Fleg JL, Mete M, Howard BV, Umans JG, Roman MJ, Ratner RE, et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (stop atherosclerosis in native diabetics study) trial. J Am Coll Cardiol. (2008) 52:2198–205. doi: 10.1016/j.jacc.2008.10.031
40. Baguet JP, Asmar R, Valensi P, Nisse-Durgeat S, Mallion JM. Effects of candesartan cilexetil on carotid remodeling in hypertensive diabetic patients: the MITEC study. Vasc Health Risk Manag. (2009) 5:175–83. doi: 10.2147/VHRM.S3409
41. Lonn EM, Gerstein HC, Sheridan P, Smith S, Diaz R, Mohan V, et al. Effect of ramipril and of rosiglitazone on carotid intima-media thickness in people with impaired glucose tolerance or impaired fasting glucose: STARR (STudy of atherosclerosis with ramipril and rosiglitazone). J Am Coll Cardiol. (2009) 53:2028–35. doi: 10.1016/j.jacc.2008.12.072
42. Öğütlü H, Taydas O, Karadag M, Çalışgan B, Kantarci M. Is common carotid artery intima-media thickness (cIMT) a risk assessment marker in children with attention deficit/hyperactivity disorder? Int J Psychiatry Clin Pract. (2021) 25:325–30. doi: 10.1080/13651501.2021.1933043
43. Uzun N, Akıncı MA, Alp H. Cardiovascular disease risk in children and adolescents with attention deficit/hyperactivity disorder. Clin Psychopharmacol Neurosci. (2023) 21:77–87. doi: 10.9758/cpn.2023.21.1.77
44. Ferencova N, Visnovcova Z, Ondrejka I, Hrtanek I, Bujnakova I, Kovacova V, et al. Peripheral inflammatory markers in autism Spectrum disorder and attention deficit/hyperactivity disorder at adolescent age. Int J Mol Sci. (2023) 24(14):11710. doi: 10.3390/ijms241411710
45. Misiak B, Wójta-Kempa M, Samochowiec J, Schiweck C, Aichholzer M, Reif A, et al. Peripheral blood inflammatory markers in patients with attention deficit/hyperactivity disorder (ADHD): a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. (2022) 118:110581. doi: 10.1016/j.pnpbp.2022.110581
46. Willeit P, Thompson SG, Agewall S, Bergström G, Bickel H, Catapano AL, et al. Inflammatory markers and extent and progression of early atherosclerosis: meta-analysis of individual-participant-data from 20 prospective studies of the PROG-IMT collaboration. Eur J Prev Cardiol. (2016) 23:194–205. doi: 10.1177/2047487314560664
47. Kabir AA, Srinivasan SR, Sultana A, Chen W, Wei CY, Berenson GS. Association between depression and intima-media thickness of carotid bulb in asymptomatic young adults. Am J Med. (2009) 122(12):1151.e1–8. doi: 10.1016/j.amjmed.2009.07.016
48. Violanti JM, Charles LE, Gu JK, Burchfiel CM, Andrew ME, Nedra Joseph P, et al. Depressive symptoms and carotid artery intima-media thickness in police officers. Int Arch Occup Environ Health. (2013) 86(8):931–42. doi: 10.1007/s00420-012-0829-6
49. Santos IS, Goulart AC, Brunoni AR, Kemp AH, Lotufo PA, Bensenor IM. Anxiety and depressive symptoms are associated with higher carotid intima-media thickness. Cross-sectional analysis from ELSA-brasil baseline data. Atherosclerosis. (2015) 240:529–34. doi: 10.1016/j.atherosclerosis.2015.04.800
50. Seldenrijk A, van Hout HPJ, van Marwijk HWJ, de Groot E, Gort J, Rustemeijer C, et al. Sensitivity to depression or anxiety and subclinical cardiovascular disease. J Affect Disord. (2013) 146:126–31. doi: 10.1016/j.jad.2012.06.026
51. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. (2023) 4:186. doi: 10.12688/wellcomeopenres.15555.3
Keywords: psychiatric trait, attention deficit/hyperactivity disorder, carotid intima-media thickness, arterial injury, Mendelian randomization
Citation: He K, Ying J, Yang F, Hu T and Du Y (2024) Seven psychiatric traits and the risk of increased carotid intima-media thickness: a Mendelian randomization study. Front. Cardiovasc. Med. 11: 1383032. doi: 10.3389/fcvm.2024.1383032
Received: 6 February 2024; Accepted: 16 July 2024;
Published: 25 July 2024.
Edited by:
Masanori Aikawa, Brigham and Women's Hospital and Harvard Medical School, United StatesReviewed by:
Mingzhu Wang, Shanghai University of Traditional Chinese Medicine, China© 2024 He, Ying, Yang, Hu and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuewu Du, eXdkdV9saGx5eUBvdXRsb29rLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.