
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Cardiovasc. Med. , 13 March 2024
Sec. Cardiovascular Epidemiology and Prevention
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1380506
This article is part of the Research Topic Addressing insulin resistance and hyperinsulinemia for cardiovascular disease prevention View all 7 articles
Cardiovascular mortality is still excessively high, despite the considerable progress made in the prevention and treatment of cardiovascular diseases. Although many cardiovascular risk factors (such as arterial hypertension, hypercholesterolemia, diabetes, etc.), identified in the general population, are being promptly treated, to date little consideration is given to a cardiovascular risk factor which we believe has largely demonstrated in the scientific literature of the last three decades that, if neglected, can produce a series of relevant negative effects on the cardiovascular system: insulin resistance (IR)/hyperinsulinemia (Hyperins). This risk factor is still not sufficently sought in the general population and, consequently, is not treated promptly, as it should be, to avoid its negative impact on the cardiovascular system. IR's prevalence is constantly growing worldwide, and it is estimated to have reached a prevalence of 51% of the general population in developed and developing countries, and Hyperins is a constant and strong feature of IR. This article aims to stimulate the scientific community towards IR/Hyperins as relevant cardiovascular risk factor, since it is still neglected. The scientific literature analyzed and used to for this article was found on PubMed, Scopus, Science Direct, etc, using the following keywords: insulin, insulin signaling, insulin resistance, hyperinsulinemia, cardiovascular risk factors, cardiovascular system, cardiovascular diseases. We selected studies that explored the association between IR/Hyperins and the cardiovascular system, and those that discussed the possibilities of screening and treatment of IR/Hyperins.
It is clear to everyone that, despite the significant progress made in the prevention and treatment of cardiovascular diseases, cardiovrascular mortality is still excessively high. It is estimated that every year in Europe there are 2 million deaths from cardiovascular causes (1), and deaths from cardiovascular causes are still in first place among the various causes of death. Indeed, there is a cardiovascular risk factor, which is pathophysiologically connected with the other well-recognized cardiovascular risk factors, treated according to the latest guidelines, which is still not fully taken into consideration: insulin resistance/hyperinsulinemia (IR/Hyperins). This important cardiovascular risk factor is not screened in the general population and, consequently, is not treated, as it is done, instead, with the other recognized cardiovascular risk factors.
It is estimated that IR's prevalence is constantly growing worldwide, reaching up to 51% of the general population, with the highest values for developed and/or developing countries (2). Hyperins is a constant and prominent feature of IR (3, 4). Insulin is a hormone that, in addition to the regulation of glucose metabolism, has important actions in several systems and organs, with the cardiovascular system as one of its main targets, and like all hormones it can cause damage if its levels are outside the normal range (5). Unfortunately, Hyperins that accompanies IR is mostly asymptomatic or paucisyntomatic, making its early diagnosis very difficult. Hence, Hyperins associated with IR lasts, in most cases, for many years before showing itself to have caused some damage or, more often, to have resulted in overt type 2 diabetes (3, 4). In this article we aim at analyzing the evidence in the scientific literature for considering IR/Hyperins as an important CV risk factor, like or more than other well-recognized CV risk factors, and verifying if there are possibilities of mass screening and, consequently, of timely treatment. The scientific literature consulted and used to write the article was found in Pub med, Scopus, Science Direct, etc. using the following keywords: insulin, insulin signaling, insulin resistance, hyperinsulinemia, cardiovascular risk factors, cardiovascular diseases, cardiovascular system. We selected the studies that explored the association between IR/Hyperins and the cardiovascular system, and those that discussed the possibilities of screening and treatment of IR/Hyperins.
IR is characterized by a decrease in sensitivity of the body's cells to insulin. Consequently, the action of insulin in regulating blood sugar is reduced compared to normal and, as an effect of this alteration, a condition of compensatory Hyperins (the increase in circulating insulin levels) is generated: the cells do not internalize glucose because they do not respond normally to insulin and, thus, the pancreas secretes more insulin to maintain normal blood sugar levels (3, 4). Therefore, Hyperins is a constant and relevant feature of the IR. Over time (years), the pancreas will no longer succeed in its compensatory maneuver of increasing insulin secretion, therefore subjects with IR will degenerate towards prediabetes and, subsequently, towards overt type 2 diabetes (3, 4).
The causes of IR are various and not entirely underdstood: overweight and obesity (particularly visceral); sedentary lifestyle; unbalanced diet in favor of excessive carbohydrate intake; chronic stress; prolonged use of diabetogenic drugs; genetic causes; etc. Muscle tissue, adipose tissue, and the liver are the main targets of insulin resistance. The symptoms and signs of IR are scarce and non-specific (drowsiness and tiredness, increased appetite, concentration difficulties, tendency to gain weight, predominantly abdominal adiposity, high levels of LDL cholesterol, high fasting triglyceride levels, tendency to arterial hypertension, etc.) (3, 4, 6). For this reason, IR/Hyperins lasts, often unrecognized, for many years. Diagnosis is easier in subjects with metabolic syndrome and in infertile women with micro polycystic ovary syndrome, but IR can also be present in subjects that are difficult to suspect, such as in normal weight and thin subjects (7).
For this reason, this condition remains unrecognized for many years, being discovered only when prediabetes or overt diabetes appear, or when a cardiovascular complication occurs. At the basis of IR/Hyperins there may be a reduced number of insulin receptors or, more often, a defect in post-receptorial signal transduction of insulin receptor. The binding of insulin to its receptor in target tissues leads to the activation of complex pathways, which regulate the transcription of target genes. Two major signaling pathways have been identified: (A) The phosphoinositide-3 kinase-dependent pathway (PI3-K), which primarily mediates insulin metabolic pathways, including the regulation of glucose metabolism in muscle, adipose tissue, and liver, and the regulation of nitric oxide (NO) production by the endothelium and vascular smooth muscle cells (VSMC). (B) The mitogen-activated protein kinase (MAPK)-dependent pathway, which mediates the nonmetabolic, but equally important, actions of insulin, including its mitogenic and proliferative actions, and the secretion of endothelin-1 (ET-1) by of the endothelial cells.
Under IR conditions, these pathways, which contribute to normal metabolic and cardiovascular homeostasis, are unbalanced by a specifically prevalent alteration at the level of the PI3-K dependent metabolic pathway, so that compensatory Hyperins ends up overstimulating the MAPK dependent pathway. This produces severe cell growth and vasoconstriction due to the prevalence of ET-1 secretion over NO secretion, which is mediated by the altered PI3-K pathway, resulting in significant endothelial dysfunction, which, as it is well known, underlies the development and progression of the atherosclerosis (8–12).
Moreover, Hyperins produces hyperactivation of the sympathetic system and stimulates the reabsorption of sodium in the renal tubules, thus facilitating the onset of arterial hypertension (13, 14). All this, if not counteracted, can determine important structural and functional alterations of the cardiovascular system overtime. For this reason, it would be extremely important, as for the other cardiovascular risk factors, to carry out an early screening of the IR/Hyperins, in order to promptly intervene with a preventive treatment.
The gold standard for the diagnosis of IR is the euglycemic-hyperinsulinemic clamp, which, however, being complex and expensive, is used only for scientific research purposes. However, for screening purposes, two surrogate indices that have been shown to correlate with clamp could be used: 1. The Homeostatic Model Evaluation Index (HOMA-IR); 2. The triglyceride glucose index (TyG). The former is based on the simultaneous dosage of fasting insulin and blood glucose [HOMA-IR = (glucose mmol/L × insulin mU/L)/22.5], and its normal value in adults is between 0.23 and 2.5, while in children it reaches up to 3.6. TyG is calculated by first taking the logarithm of the product of triglycerides and fasting glucose, and then dividing by 2 [TyG = log (triglycerides × glucose)/2]. The normal value is less than 4.5 (15, 16).
Globally, in recent decades, there have been significant improvements in mortality rates in all continents, even if critical situations remain in some poor countries. In the European Union (EU), in 2016, such rates were more comforting than the world average, and Italy was one of the most virtuous countries along with France and Spain. Cardiovascular diseases, tumors and respiratory diseases are the main causes of death in Italy and in Europe, cumulatively accounting for about 70% of total deaths (17). Despite the considerable progress made in the prevention and treatment, cardiovascular diseases are still the first cause of death in Europe with a percentage of 33% of total deaths in 2020 (18). For this reason, we must try to do more in the preventive field of cardiovascular diseases. In particular, we believe that the risk factor IR/Hyperins has been considered a marginal risk factor for too many years, while important preventive efforts are implemented only when this turns into overt diabetes. Unfortunately, it could be too late, because IR/Hyperins, preceding diabetes by many years (even up to 15 years) (4), most likely, could have already caused important cardiovascular alterations, so that, according to prevention guidelines, the diabetes is treated in secondary prevention, that is, as if a first cardiovascular event has already occurred in that patient. Indeed, when we carry out an initial instrumental verification in a patient with recent onset of type 2 diabetes, we easily realize that there are already evident cardiovascular alterations, such as left ventricular remodeling and atherosclerosis at various explorable vascular districts (19) (Figure 1).
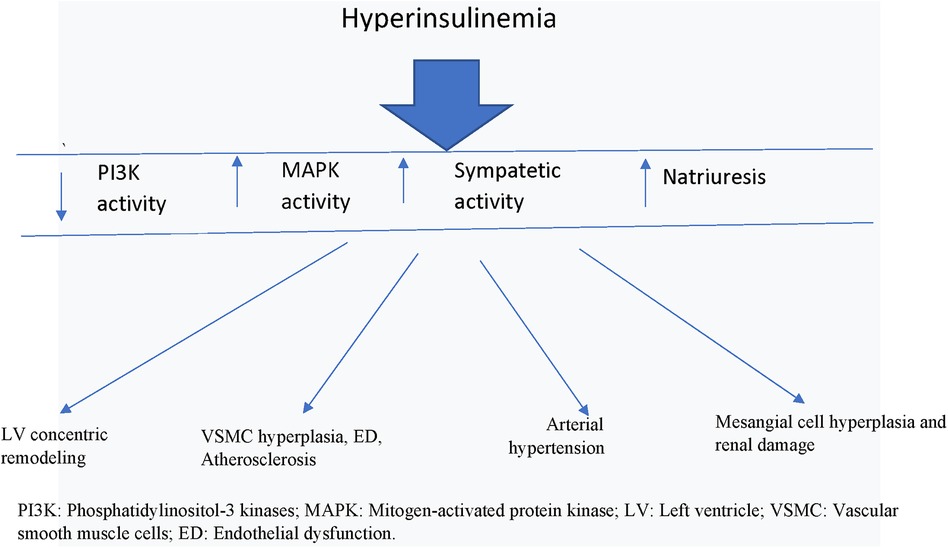
Figure 1. Actions of hyperinsulinemia on cardiovascular system. PI3K, Phosphatidylinositol-3 kinases; MAPK, mitogen-activated protein kinase; LV, left ventricle; VSMC, vascular smooth muscle cells; ED, endothelial dysfunction.
There is a vast scientific literature supporting a negative action of IR/Hyperins on the cardiovascular system (20–25). On the other hand, insulin is a hormone, even a rather important one, and, like all the hormones that regulate our body functions, it will cause damage both if the circulating levels are low and if, as in the case of IR, they are high for many years. This is a rather simplistic interpretation of the thing, however, if we examine the problem more in detail, we find considerable scientific literature to support negative actions of IR/Hyperins on the CV system. It is well known that endothelial dysfunction (ED) appears early in the development of the atherosclerotic process. One of the main mechanisms through which IR/Hyperins determines ED is due to the malfunction of the PI3-K dependent signaling pathway. This produces an imbalance between the reduced production of NO, linked to the bad functioning of this pathway, and the increased secretion of ET-1 by the endothelial cells, linked to the signaling of the MAPK dependent pathway, which remains practically unchanged and subject to the action of greater quantities of insulin. This causes vasoconstriction with alteration of the district flows (26).
Furthermore, increased levels of circulating insulin via common insulin-like growth factor-1 (IGF-1) receptors and the MAPK-dependent signaling pathway stimulates VSMC and endothelial cell proliferation, resulting in increased wall thickness of the vessels, loss of vascular elasticity, and parietal calcifications, so that the process of atherosclerosis is triggered (27) (Figure 1).
Therefore, under IR/Hyperins conditions, the excessive stimulation of the MAPK cascade, which has vasoconstrictive (↥ ET-1) and growth-promoting effects at various cellular levels, ends up determining part of the deleterious effects at the cardiovascular level. At this point there is also solid evidence to support the fact that Hyperins may also be a determinant of arterial hypertension, in fact it has been demonstrated that insulin causes retention of sodium and water in the kidney, and that both endogenous and exogenous Hyperins were found related to increases in blood pressure (28) (Figure 1). In fact, insulin receptors have been detected in the renal tubules, and their stimulation by insulin has been shown to produce increased sodium reabsorption. In addition to the mechanisms described above, there is a clear and demonstrable relationship between insulin levels and sympathetic nervous system activity (Figure 1). Increased blood pressure has been shown to be associated with both insulin levels and sympathetic nervous system activity, a relationship that was noted both in the general population and after adjustment for indexed body mass and body fat distribution. Furthermore, discontinuation of Hyperins in obese subjects resulted in a reduction in plasma norepinephrine levels and blood pressure (14).
Sympathetic hyperactivation characterizes IR/Hyperins conditions and may contribute to the increased cardiovascular morbidity and, in turn, to the reduced insulin sensitivity of these patients (29) (Figure 1). During euglycemic clamp with two different increased doses of insulin it was verified that the increase in insulin levels produced significant increases in circulating norepinephrine levels (from 119 ± 19 pg/ml to 258 ± 25 p < 0.02 and 285 ± 95 p < 0.01, respectively) (30, 31).
As already mentioned, insulin is also a growth factor and, as such, the conditions of IR/Hyperins have been found to be associated with an increased prevalence of chronic kidney disease, and there are numerous studies supporting the existence of numerous mechanisms that link IR/Hyperins with kidney damage. Insulin, by itself, promotes the proliferation of renal mesangial cells and stimulates the production of other important growth factors such as insulin-like growth factor-1 and transforming growth factor beta. Insulin also upregulates the expression of angiotensin II type 1 receptor in the mesangial cells, thereby increasing the damaging effects of angiotensin II in the kidney, and stimulates the production and action of ET-1 in the kidneys, while reducing the renal vascular production of NO. These mechanisms are implicated in the development and progression of diabetic nephropathy (32).
The actions of insulin on the mass and remodeling of the left ventricle are also extremely important. Indeed, it is known that left ventricular mass (LVM) is associated with an increased risk of cardiovascular events (33). Furthermore, increased left ventricular mass, documented by electrocardiography or echocardiography, has been shown to be an independent risk factor for the development of heart failure (HF) (34). An additional study also found that LVM index is an independent predictor of sudden cardiac death, and may help to improve sudden death risk prediction along with other conventional cardiovascular risk factors (35) (Figure 1).
A recent study demonstrated, in 88 black sub-Saharan African hypertensive patients with left ventricular hypertrophy (LVH), that obesity and IR/Hyperins were the primary predictors of LVH, and suggested that, among various risk factors, particular emphasis had to be placed on the correction of obesity and IR (36). In another study carried out in Japan it was shown, by echocardiography in 210 normotensive subjects and 180 mildly or moderately hypertensive subjects, that the sum of glucose levels (or hemoglobin A1c levels) in all subjects, and the sum of insulin levels (or 2-hour post-load glucose insulin), in subjects without diabetes mellitus, correlated significantly with LV relative wall thickness (RWT) independent of age, systolic blood pressure, and body mass index. The authors concluded that hyperglycemia and Hyperins could promote concentric LV changes in normotensive and mildly/moderately hypertensive subjects (37). Other investigators have demonstrated that IR/Hyperins and waist-to-hip ratio are closely associated with concentric LV remodeling, independent of BMI, and suggest that IR/Hyperins treatment could give an important boost to regression of the concentric remodeling of the LV (38). In studies of our group we verified, in 59 pts with metabolic syndrome and IR/Hyperins, the presence of increased LVMI and RWT associated with LV diastolic dysfunction (39, 40).
IR/Hyperins has also been shown to be highly prevalent (67%) in non-diabetic patients with chronic heart failure (HF). In these patients it has been verified that the fasting IR indices progressively increase with the worsening of the New York Heart Association class. Furthermore, patients with HF and IR had significantly lower exercise capacity and peak O2 achieved than patients with HF but no evidence of IR (41). It is well known that LVH is a constant feature of diabetic cardiomyopathy, and is thought to be a strong predictor of adverse cardiovascular events, in particular, of HF with preserved ejection fraction (HFpEF). In fact, LVH can determine an alteration of the filling function of the LV and, consequently, diastolic HF (42). For this reason, it was hypothesized that the treatment of IR/Hyperins in patients with HF could lead to a slowing down of the negative evolution of HF.
In addition, it has been shown that inflammation may be the common pathway between insulin resistance, hypertension and other cardiovascular risk factors. Inflammation is involved in both hyperinsulinemia, hypertension and other cardiovascular conditions (43–45). In conditions of IR there is the production of pro inflammatory cytokines which determine a state of IR in the target tissues of insulin's actions (46, 47). An underlying inflammatory state that can cause IR/Hyperins, which in turn can cause inflammation creating a self-regenerating short circuit. Many studies have highlighted how chronic inflammation through the production of cytokines can be the basis of atherosclerosis and its progression (48, 49).
The first thing to do to improve the condition of IR/Hyperins is a radical change in lifestyle by increasing physical activity and reducing daily caloric intake through a balanced diet not rich in carboidrates. However, clinical practice shows that only a low percentage of subjects consistently implement these lifestyle changes. For this reason, the majority of the insulin resistant population needs to be helped by adding to their diet substances that increase insulin sensitivity and reduce circulating levels of insulin. There are numerous substances, both drugs and natural substances, that act positively in this sense, but, even today, no substance is authorized for this purpose outside of the diagnosis of diabetes. Among these substances, certainly, Sodium-Glucose cotrasporter2-inhibitors (SGLT2-i), Metformine, Berberine, Glucagon Peptide -1 receptor agonists (GLP-1 Ras) and L-arginine deserve particular attention.
Sodium-glucose cotransporter2 inhibitors (SGLT2-i) are a relatively recent class of oral drugs, approved for the treatment of adults with type 2 diabetes and, recently, also for the treatment of HF (50, 51). They include canaglifozin, dapaglifozin and empaglifozin. A vast scientific literature is now available demonstrating how treatment with SGLT2-i reduces the risk of hospitalization, death from cardiovascular events and all causes of death in patients with HF (52). A recent study on 6,263 patients >40 years old with EF > 40%, assigned to receive dapaglifozin (10 mg once daily) or placebo in addition to standard therapy, demonstrated a significant reduction in the risk of worsening of HF and death from cardiovascular events (53). Another recent study of metaregression-analysis of vaste proportion, aimed at assessing the effects of these drugs on all-cause mortality, has shown that SGLT2-i reduce all-cause mortality in randomized trials (54).
The exact mechanisms by which these drugs determine their beneficial effects are not exactly known. However, the most likely hypothesized mechanisms are: regulation of blood volume, cardiorenal mechanisms, direct effects on contractility and sodium homeostasis, metabolic effects, improved cardiac remodeling, reduction of inflammation and oxidative stress, etc. (55). Administration of SGLT2-i to subjects with IR/Hyperins reduces the amount of blood glucose, due to the increased excretion of urinary glucose. For this reason, the amount of insulin required is reduced and, as consequence, the daily average levels of circulating insulin will be reduced compared to pretreatment (56). SGLT2-i reduce IR and insulin levels, consequently reducing their deleterious cardiovascular effects.
Metformin is a long-standing oral antidiabetic, belonging to biguanide class of drugs, which has many scientific demonstrations of effectiveness in reducing IR/Hyperins (57). A literature review study investigated the effects of Metformin on all-cause mortality and the incidence of ageing diseases in diabetic subjects compared to a non-diabetic population, and diabetic subjects treated with therapies different from Metformin. The results of the study highlighted that mortality was significantly reduced in diabetic subjects treated with Metformin, both compared to non-diabetics and diabetics not treated with Metformin. Furthermore, the group of patients treated with Metformin also had a reduction in cancer, compared to non-diabetics, and in cardiovascular diseases, compared to diabetics not taking Metformin (58).
Metformin treatment has also demonstrated notable benefits in patients with HFpEF. In fact, the results of a fairly recent study, carried out using meta-regression analysis on observational and randomized studies, shows that treatment with Metformin significantly reduces (p < 0.003) mortality in patients with HFpEF (59). Metformin has been shown to have numerous favorable effects on the heart of subjects with HF. In fact, it improves the energy status of the myocardium as a consequence of the modulation of glucose and lipid metabolism, the reduction of oxidative stress and inflammation, and the reduction of pathological cardiac remodeling (60). A few years ago the results of a meta-analysis were published demonstrating how metformin therapy has favorable effects on left ventricular mass (LVM) and EF both in subjects with and without pre-existig cardiovascular disease (61).
Berberine is a plant alkaloid that has been in use for over 2000 years in Chinese and Indian Ayurvedic medicine, and has proven over time to have many beneficial effects on human health. It is contained in many plants including Coptis Chinensis. There is a large amount of scientific literature that supports the fact that berberine has favorable effects on cardiovascular risk factors such as hypercholesterolemia, hyperglycemia, arterial hypertension and insulin resistance (62–65).
Berberine, in addition to having many experimental studies to support its positive action on glucose metabolism and IR/Hyperins, also has many clinical studies that support its role in cardiovascular prevention. A review and meta-analysis of randomized controlled trials on berberine has highlighted how it can be a valid alternative, without causing serious adverse reactions, to drugs currently used for cardiovascular prevention (66). In a randomized, placebo -controlled study lasting 4 months, 59 patients with metabolic syndrome were treated with a combination of nutraceuticals, containing berberine 500 mg, or placebo. The results of this study demonstrated that pts treated with berberine had a significant reduction in IR/Hyperins, as documented by reductions in HOMA-IR and fastig insulin levels (39). Furthermore, in these pts, using Doppler-ecocardiography, was documented a significant reduction in LVM and relative wall thickness (RWT), and an improvement in LV diastolic function (40). Another randomized and controlled study of berberine 500 mg per day compared to placebo, carried out in 145 subjects with metabolic syndrome and left ventricular hypertrophy, demonstrated how treatment with berberine for 6 months resulted in a significant reduction (p < 0.001) in LVM. For this reason, the authors conclude that this treatment could represent an effective strategy to reduce an important risk factor such as LVM (67).
Also important is that, in a study carried out in 2 groups of pts with HF, 79 pts treated with berberine in addition to standard therapy and 76 pts treated with standard therapy plus placebo, during a 2-year follow-up, 7 pts died in the berberine group and 13 pts in the placebo group (p < 0.02) (68).
Glucagon-like peptide 1 receptor agonists (GLP-1 Ras) are a class of drugs not long ago authorized for the treatment of type 2 diabetes and obesity. They are administered by subcutaneous injection and are quite expensive drugs. This class of drugs includes semaglutide, albiglutide, delaglutide, exenatide, liraglutide, and lixiglutide. It seems that they act not only by reducing body weight, but also by acting on mechanisms involved in the determinism of IR/Hyperins, such as, for example, increasing the expression of glucose transporters in insulin-dependent tissues, reducing inflammation and oxidative stress, and modulating lipid metabolism (69, 70).
However, although GLP-Ras have actually been shown to improve cardiovascular risk factors such as body weight, blood pressure, LDL cholesterol and triglycerides, and glycemic control, they have been shown to reduce all-cause mortality in patients with type 2 diabetes at high risk of cardiovascular events, but have not reduced cardiovascular mortality, non-fatal myocardial infarction, non-fatal stroke and hospitalizations for HF (71, 72). Another recent meta-analysis carried out on 54,092 pts from 7 randomized controlled trials on the use of GLP-1 Ras in subjects with type 2 diabetes, of whom 16% also had a history of HF, demonstrated that these drugs appear to protect the diabetic population from the development of HF, but, in subjects with pre-existing HF, they do not reduce the onset of episodes of HF exacerbation with consequent hospitalization, nor mortality (73). A further recent meta-analysis carried out to verify whether treatment with GLP-1 Ras in subjects with HF, with or without type 2 diabetes, could lead to a reduction in morbidity and mortality compared to placebo, also demonstrated that this therapy did not reduce the number of major adverse cardiovascular events, including cardiovascular mortality or reduction in hospital admissions for HF, and did not lead to an improvement in HF or six-minute walking test (74).
In addition, recently, numerous reports of serious adverse events have been published, such as cases of severe pancreatitis. In fact, patients taking either semaglutide or liraglutide had nine times an elevated risk for pancreatitis but, also, they had a very high risk to develop bowel obstruction, and to experience gastroparesis (75). Furthermore, European Medicine Agency published a statement on ongoing review of GLP-1 receptor agonists about the possibility that liraglutide and semaglutide can stimulate suicidal thoughts and self-injury, made by the Icelandic medicines agency (76).
L-arginine is an amino acid defined as conditionally essential. This is because it is essential for growing subjects and malnourished adults suffering from serious illnesses or who have suffered major trauma or burns, but not for healthy adult subjects, because it can be synthesized by the body and is regularly taken in with the diet in a quantity that varies between 3.5 and 5.0 grams per day. Once in the body, L-arginine is converted into nitric oxide by nitric oxide synthases (NOS), stimulating vessel dilation and improving blood flow to the tissues. Among many things, it has been shown that L-arginine supplementation improves insulin sensitivity and endothelial function in obese patients with type 2 diabetes (77). It is also well known that IR is strongly associated with a reduction in NO availability and endothelial dysfunction. Importantly, ED secondary to reduced NO bioavailability is a very early and prominent feature of IR. It has also been shown that exogenously delivered NO can stimulate insulin transport across endothelial cells and can improve impaired endothelial insulin transport, caused by experimental IR, across endothelial cells (78). Some authors have demonstrated that supplementation with L-arginine, in rats subjected to a high-fat diet, produced a significant improvement in insulin sensitivity, as documented by the reduction in circulating insulin levels and HOMA-IR, and this resulted at least in part related to an increase in adiponectin concentration (79).
In another study, Lucotti et al. demonstrated that treatment with L-arginine, for 21 days, associated with a low-calorie diet and exercise training, in obese patients with type 2 diabetes produced a clear and significant improvement in anthropometric parameters, such as fat mass and waist circumference, and a clear improvement in IR, as documented by the significant reduction in fasting insulinemia and HOMA-IR. The improvement of many other parameters was also documented, including, particularly interesting, the increase in adiponectin and flow mediated dilation, an index of endothelial function (80). So, L-arginine is a very interesting substance, having many benefits for the body and, among these, the action of improving IR should absolutely not be overlooked when we want to implement the prevention of cardiovascular diseases.
As already underlined, despite the considerable progress made in their prevention and treatment, cardiovascular diseases still are the leading cause of death worldwide. IR/Hyperins, despite the extensive scientific literature that demonstrates the deleterious effects on cardiovascular system, has never been taken into consideration, with propre screening and treatment as an independent risk factor for the development of cardiovascular disease. Instead, as well highlighted by wide scientific literature, the effects of a chronically increased insulin levels have proved to be particularly harmful at the level of the cardiovascular system, causing important damages to the heart, brain, kidneys, eyes and peripheral vessels. IR/Hyperins determines the development and progression of atherosclerosis in all vascular districts, and concentric remodeling of the LV which can result in HF and sudden death. For these reasons, we believe that it is extremely important to test for IR/Hyperins subjects suspected of having it, in order to be able to start preventive treatments with the means currently available, as it is done for other recognized cardiovascular risk factors. We are clearly behind schedule.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
SF: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. VM: Data curation, Methodology, Supervision, Writing – review & editing. LT: Data curation, Methodology, Supervision, Validation, Writing – review & editing. VF: Data curation, Methodology, Validation, Writing – review & editing, Conceptualization. FA: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Istituto Superiore di Sanità. Le Statistiche Delle Malattie Cardiovascolari in Europa per il 2008. Roma, Italy: Istituto Superiore di Sanità-EpiCentro (2008).
2. Bermudez V, Salazar J, Martinez MS, Chavez-Castillo M, Olivar LC, Calvo MJ, et al. Prevalence and associated factors of insulin resistance in adults from maracaibo city, Venezuela. Adv Prev Med. (2016) 2016:9405105. doi: 10.1155/2016/9405105
3. Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. (2001) 109(Suppl. 2):S135–48. doi: 10.1055/s-2001-18576
4. Freeman AM, Acevedo LA, Pennings N. Insulin Resistance. In: StatPearls [Internet]. Treasure Island, (FL): StatPearls Publishing (2023). PMID: 29939616
5. Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev. (2007) 28(5):463–91. doi: 10.1210/er/2007-0006
6. Diabetes.co.uk. the global diabetes community. Insulin resistance. September 8 (2022). Available online at:https://www.diabetes.co.uk/insulin-resistance.html (accessed March 7, 2024).
7. Zhu Y, Sidell MA, Arterburn D, Daley MF, Desai J, Fitzpatrick SL, et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI: patient outcomes research to advance learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care. (2019) 42:2211–9. doi: 10.2337/dc19-0532
8. Rahman MS, Hossain KS, Das S, Kundu S, Adegoke EO, Rahman MA, et al. Role of insulin in health and disease: an update. Int J Mol Sci. (2021) 22:6403. doi: 10.3390/ijms22126403
9. Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. (2007) 100:328–41. doi: 10.1161/01/RES.0000256090.42690.05
10. Chopra I, Li HF, Wang H, Webster KA. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia. (2012) 55:783–94. doi: 10.1007/s00125-011-2407-y
11. Kashyap SR, Roman LJ, Lamont J, Masters BS, Bajaj M, Suraamornkul S, et al. Insulin resistance is associated with impaired nitric oxide synthase activity in skeletal muscle of type 2 diabetic subjects. J Clin Endocrinol Metab. (2005) 90:1100–5. doi: 10.1210/jc.2004-0745
12. Sarafidis PA, Bakris GL. Review: insulin and endothelin: an interplay contributing to hypertension development? J Clin Endocrinol Metab. (2007) 92:379–85. doi: 10.1210/jc.2006-1819
13. Bachmann KN, Deger SM, Alsouqi A, Huang S, Xu M, Ferguson JF, et al. Acute effects of insulin on circulating natriuretic peptide levels in humans. PLoS One. (2018) 13:e0196869. doi: 10.1371/journal.pone.0196869
14. Landsberg L. Insulin and the sympathetic nervous system in the pathophysiology of hypertension. Blood Press Suppl. (1996) 1:25–9. PIMD: 9162434
15. Ziaee A, Esmailzadehha N, Oveisi S, Ghorbani A, Ghanei L. The threshold value of homeostasis model assessment for insulin resistance in qazvin metabolic diseases study (QMDS): assessment of metabolic syndrome. J Res Health Sci. (2015) 15:94–100. PMID: 26175291
16. Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1240/jc.2010-0288
17. Eurostat, statistic explained. Causes of Death Statistic. Data Extracted in March (2023). Available online at: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Causes_of_death_statistic (accessed March 7, 2024).
18. World Health Organization. The Global Health Observatory: Explore a World of Health Data. World Health Statistics (2023): Access the Report. Available online at: https://www.who.int/data/gho (accessed March 7, 2024).
19. Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American heart association and the American diabetes association. Circulation. (2007) 115:114–26. doi: 10.1161/CIRCULATIONAHA.106.179294
20. Ormazabal V, Naif S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. (2018) 17(1):122. doi: 10.1186/12933-018-0762-4
21. Kosmas CE, Bousvarou MD, Kostara CE, Papakonstantinou EJ, Salamou E, Guzman E. Insulin resistance and cardiovascular disease. J Int Med Res. (2023) 51(3):3000605231164548. doi: 10.1177/03000605231164548
22. Janssen JAMJL. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, cardiovascular disease and cancer. Int J Mol Sci. (2021) 22(15):7797. doi: 10.3390/ijms22157797
23. Hill MA, Yang Y, Zhang L, Sun Z, Mia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metab Clin Exp. (2021) 119:154766. doi: 10.1016/j.metabol.2021.154766
24. Stout RW. Hyperinsulinemia and atherosclerosis. Diabetes. (1996) 45(Suppl 3):S45–6. doi: 10.2337/diab.45.3.s45
25. Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinemia in diabetic cardiomyopathy. Nat Rev Endocrinol. (2016) 12(3):144–53. doi: 10.1038/nrendo.2015.216
26. Nishiyama SK, Zhao J, Wray DW, Richardson RS. Vascular function and endothelin-1: tipping the balance between vasodilation and vasocostiction. J Appl Physiol. (2017) 122(2):354–60. doi: 10.1152/japplphysiol.00772.2016
27. Adeva-Andany MM, Ameneiros-Rodrìguez E, Fernández-Fernández C, Dominguez-Montero A, Funcasta-Calderón R. Insulin resistance is associated with subclinical vascular disease in humans. World J Diabetes. (2019) 10:63–77. doi: 10.4239/wjd.v10.i2.63
28. Brosolo G, Da Porto A, Bulfora L, Vacca A, Bertin N, Scandolin L, et al. Insulin resistance and high blood pressure: mechanistic insight on the role of kidney. Biomedicines. (2022) 10(10):2374. doi: 10.3390/biomedicines10102374
29. Scherrer U, Sartori C. Insulin as a vascular and sympathoexcitatory hormone. Circulation. (1997) 96:4104–13. doi: 10.1161/01.CIR.96.11.4104
30. Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. (1991) 87(6):2246–52. doi: 10.1172/LCI115260
31. Arauz-Pacheco C, Lender D, Snell PG, Huet B, Ramirez LC, Breen L, et al. Relationship between insulin sensitivity, hyperinsulinemia, and insulin-mediated sympathetic activation in normotensive and hypertensive subjects. Am J Hypertens. (1996) 9(12 Pt1):1172–8. doi: 10.1016/S0895-7061(96)00256-7
32. Serafidis PA, Ruilope LM. Insulin resistance, hyperinsulinemia, and renal injury: mechanisms and implications. Am J Nephrol. (2006) 26(3):232–44. doi: 10.1159/000093632
33. Hoang K, Zhoo Y, Gardin JM, Carnethon M, Makunal K, Yanez D, et al. Left ventricular mass ad predictor of cardiovascular disease events in older adults with and without metabolic syndrome and diabetes. JACC Crdiovasc Imaging. (2015) 8(9):1007–15. doi: 10.1016/j.jcmg.2015.04.019
34. Drazner MH, Rame JE, Marino EK, Gottdiener JJ, Kitzman DW, Gordon JM, et al. Increased left ventricular mass id a risk factor of a depressed left ventricular ejection fraction within five years: the cardiovascular health study. J Am Coll Cardiol. (2004) 43(12):2207–15. doi: 10.1016/j.jacc.2003.11.064
35. Laukkanen JA, Kan H, Knel S, Willeit P, Karppi J, Ronkainen K, et al. Left ventricular mass and the risk of sudden cardiac death: a population based study. J Am Heart Assoc. (2014) 3(6):e001285. doi: 10.1161/JAHA.114.001285
36. Kianu Phanzu B, Nkodila Natuhoyila AN, Kintaki Vita E, M'Buyamba Kabangu JR, Longo-Mbenza B. Association between insulin resistance and left ventricular hypertrophy in asymptomatic, black, sub-saharan African, hypertensive patients: a case- control study. BMC Cardiovasc Disord. (2021) 21(1):1. doi: 10.1186/s12872-020-01829-y
37. Ohya Y, Abeb I, Fujii K, Ohmori S, Onaka U, Kobayashi K, et al. Hyperinsulinemia and left ventricular geometry in a work-site population in Japan. Hypertension. (1996) 27:729–34. doi: 10.1161/01.hyp.27.3.729
38. Shah RV, Abbasi SA, Heydari B, Rickers C, Jacobs DR Jr, Wang L, et al. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. (2013) 61(16):1698–706. doi: 10.1016/j.jacc.2013.01.053
39. Affuso F, Ruvolo A, Micillo F, Saccà L, Fazio S. Effects of a nutraceutical combination (berberine, red yeast rice and polycosanols) on lipid levels and endothelial function: randomized, double-blind placebo-controlled study. Nutr Metab Cardiovasc Dis. (2010) 20(9):656–61. doi: 10.1016/j.numecd.2009.05.017
40. Carlomagno G, Pirozzi C, Mercurio V, Ruvolo A, Fazio S. Effects of a nutraceutical combination on left ventricular remodeling and vasoreactivity in subjects with the metabolic syndrome. Nutr Metab Cardiovasc Dis. (2012) 22(5):e13–4. doi: 10.1016/j.numecd.2011.12.0
41. AlZadjali MA, Godfrey V, Khan F, Choy A, Doney AS, Wong AK, et al. Insulin resistance is highly prevalent and is associated with reduced exercise tolerance in nondiabetic patients with heart failure. J Am Coll Cardiol. (2009) 53(9):747–53. doi: 10.1016/j.jacc.2008.08.081
42. Palmieri V, Russo C, Bella JN. Treatment of isolated left ventricular diastolic dysfunction in hypertension: reaching blood pressure target matters. Hypertension. (2010) 55(2):224–5. doi: 10.1161//HYPERTENSIONAHA.109.144717
43. Aktas G, Alcelik A, Ozlu T, Tosun M, Tekce BK, Savli H, et al. Association between omentin levels and insulin resistance in pregnancy. Exp Clin Endocrinol Diabetes. (2014) 122(3):163–6. doi: 10.1055/s-0034-1370917
44. Aktas G, Khalid A, Kurtkulagi O, Duman TT, Bilgin S, Kahveci G, et al. Poorly controlled hypertension is associated with elevated serum uric acid to HDL-cholesterol ratio: a cross-sectional cohort study. Postgrad Med. (2022) 134(3):297–302. doi: 10.1080/00325481.2022.2039007
45. Sorriento D, Iaccarino G. Inflammation and cardiovascular diseases: the most recent findings. Int J Mol Sci. (2019) 20(16):3879. doi: 10.3390/ijms20163879
46. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. (2010) 72:219–46. doi: 10.1146/annurev-physiol-021909-135846
47. Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. (2014) 105(2):141–50. doi: 10.1016/j.diabres.2014.04.006
48. Alfaddagh A, Martin SS, Leucker TM, Michos ED, Blaha MJ, Lowenstein CJ, et al. Inflammation and cardiovascular disease: from mechanisms to therapeutics. Am J Prev Cardiol. (2020) 4:100130. doi: 10.1016/j.ajpc.2020.100130
49. Liberale L, Badimon L, Montecucco F, Lüscher TF, Libby P, Camici GG. Inflammation, aging, and cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. (2022) 79(8):837–47. doi: 10.1016/j.jacc.2021.12.017
50. Kelsey MD, Nelson AJ, Green JB, Granger CB, Peterson ED, McGuire DK, et al. Guidelines for cardiovascular risk reduction in patients with type2 diabetes: JACC guideline comparison. J Am Coll Cardiol. (2022) 79(18):1849–57. doi: 10.1016/j.jacc.2022.02.046
51. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 41(36):3599–726. doi: 10.1093/eurheartj/ehab368
52. Butler J, Usman MS, Khan MS, Greene SJ, Friede T, Vaduganathan M, et al. Efficacy and safety of SGLT2 inhibitors in heart failure: systematic review and meta-analysis. ESC Heart Fail. (2020) 7(6):3298–309. doi: 10.1002/ehf2.13169
53. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387(12):1089–98. doi: 10.1056/NEJMoa2206286
54. Silveri GA, Monami M, Mannucci E. Sodium-glucose co-transporter-2 inhibitors: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. (2021) 23(4):1052–6. doi: 10.1111/dom.14286
55. Pabel S, Hamdani N, Luedde M, Sossalla S. SGLT2 inhibitors and their mode of action in heart failure-has the mystery been unravelled? Curr Heart Fail Rep. (2021) 18(5):315–28. doi: 10.1007/s11897-021-00526-8
56. Hosokawa Y, Ogawa W. SGLT2 inhibitors for genetic and acquired insulin resistance: considerations for clinical use. J Diabetes Investig. (2020) 11(6):1431–3. doi: 10.1111/jdi.13309
57. Herman R, Kravos NA, Jensterle M, Janež A, Dolžan V. Metformin and insulin resistance: a review of underlying mechanisms behind changes in GLUT4-mediated glucose transport. Int J Mol Sci. (2022) 23(3):1264. doi: 10.3390/ijms23031264
58. Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and disorders of aging independent of ita effect on diabetes control. A systematic review. Aging Res Rev. (2017) 40:31–44. doi: 10.1016/j.arr.2017.08.003
59. Halabi A, Sen J, Huynh Q, Marwick TH. Metformin treatment in heart failure with preserved ejection fraction: a systematic review and meta-regression analysis. Cardiovasc Diabetol. (2020) 19(1):124. doi: 10.1186/s12933-020-01100-w
60. Salvatore T, Galiero R, Caturano A, Vetrano E, Rinaldi L, Coviello F, et al. Effects of metformin in heart failure: from pathophysiological rationale to clinical evidence. Biomolecules. (2021) 11(12):1834. doi: 10.3390/biom11121834
61. Kamel AM, Sabry N, Farid S. Effect of metformin on left ventricular mass and functional parameters in non-diabetic patients: a meta-analysis of randomized clinical trials. BMC Cardiovasc Disord. (2022) 22(1):405. doi: 10.1186/s12872-022-02845-w
62. Kong W-J, Zhang H, Song D-Q, Xue R, Zhao W, Wej J, et al. Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metab Clin Exp. (2009) 58(1):109–19. doi: 10.1016/j.metabol.2008.08.013t19059538
63. Cao C, Su M. Effects of berberine on glucose-lipid metabolism, inflammatory factors and insulin resistance in patients with metabolic syndrome. Exp Ther Med. (2019) 17(4):3009–14. doi: 10.3892/etm.2019.7295
64. Affuso F, Mercurio V, Fazio V, Fazio S. Cardiovascular and metabolic effects of berberine. World J Cardiol. (2010) 2(4):71–7. doi: 10.4330/wjc.v2.i4.71
65. Bellavite P, Fazio S, Affuso F. A descriptive review of the action mechanisms of berberine, quercetin and silymarin on insulin resistance/hyperinsulinemia and cardiovascular prevention. Molecules. (2023) 28(11):4491. doi: 10.3390/molecules28114491
66. Yang L, Zhu W, Zhang X, Zhan X, Su W, Shen T. Efficacy and safety of berberine for several cardiovascolar diseases: a systematic review and meta-analysis of randomized controlled trials. Phytomedicine. (2023) 112:154716. doi: 10.1016/j.phymed.2023.154716
67. Mercurio V, Pucci G, Bosso G, Fazio V, Battista F, Iannuzzi A, et al. A nutraceutical combination reduces left ventricular mass in subjects with metabolic syndrome and left ventricular hypertrophy: a multicenter, randomized, double-blind, placebo-controller trial. Clin Nutr. (2020) 39(5):1379–84. doi: 10.1016/j.clnu.2019.06.026
68. Zeng XH, Zeng XJ, Li YY. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. (2003) 92(2):173–6. doi: 10.1016/S0002-9149(03)00533-2
69. Bednarz K, Kowalczyk K, Cwynar M, Czapla D, Czarkowski W, Imita D, et al. The role of GLP-1 receptor agonists in insulin resistance with concomitant obesity treatment in polycystic ovary syndrome. Int J Miol Sci. (2022) 23(8):4334. doi: 10.103390/ijms23084334
70. Jiang Y, Zhihong W, Ma B, Fan L, Ti N, Lu B, et al. GLP-1 improves adipocyte insulin sensitivity following induction of endoplasmic reticulum stress. Front Pharmacol. (2018) 9:1168. doi: 10.3389/fphar.2018.01168
71. Piccini S, Favacchio G, Panico C, Morenghi E, Folli F, Mazziotti G, et al. Time-dependent effect of GLP-1 receptor agonists on cardiovascular benefits: a real world study. Cardiovasc Diabetol. (2023) 22:69. doi: 10.1186/s12933.023.01800.z
72. Peterson SC, Barry AR. Effect of glucagon-like peptide-1 receptor agonists on all-cause mortality and cardiovascular outcomes: a meta-analysis. Cure Diabetes Rev. (2018) 14(3):273–9. doi: 10.2174/1573399813666170414101450
73. Ferreira JP, Saraiva F, Dharma A, Vasquez-Nòvoa F, Angélico-Gonçalves A, Leite AR, et al. Glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes with and without chronic heart failure: a meta-analysis of randomized placebo-controlled trials. Diabetes Obes Metab. (2023) 25(6):1495–502. doi: 10.1111/dom.14997
74. Merza N, Akram M, Mengal A, Rashid AM, Mahboob A, Faryad M, et al. The safety and efficacy of GLP-1 receptor agonists in heart failure patients: a systematic review and meta-analysis. Curr Probl Cardiol. (2023) 48(5):101602. doi: 10.1016/j.cpcardiol.2023.101602
75. Sodhi M, Rezaeianzadeh R, Kezouh A, Etminan M. Gastrointestinal adverse events associated with glucagon-like peptide-1 receptor agonists for weight loss. JAMA. (2023) 330(18):1795–7. doi: 10.1001/JAMA.2023.19574
76. European medicines agency. Science Medicines Health. EMA Statement on Ongoing Review of GLP-1 Receptor Agonists. News 11/07/2023 (2023). Available online at: https://www.ema.europa.eu>news (accessed March 7, 2024).
77. Piatti PM, Monti LD, Valsecchi G, Magni F, Setola E, Marchesi F, et al. Long -term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care. (2001) 24(5):875–80. doi: 10.2337/diacare.24.5.875
78. Wang H, Wang AX, Aylor K, Barrett EJ. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes. (2013) 62(12):4030–42. doi: 10.2337/db13-0627
79. Miczke A, Sulisburska J, Pupek-Musialik D, Ostrowska A, Jablecka A, Krejpcio Z, et al. Effect of L-arginine supplementation on insulin resistance and serum adiponectin concentration in rats with far diet. Int J Clin Exp Med. (2015) 8(7):10358–66. PIMD: 26379826
80. Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, et al. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab. (2006) 291(5):E906–912. doi: 10.1152/ajpendo.00002.2006
Keywords: insulin signaling, insulin resistance, hyperinsulinemia, cardiovascular risk factors, cardiovascular diseases
Citation: Fazio S, Mercurio V, Tibullo L, Fazio V and Affuso F (2024) Insulin resistance/hyperinsulinemia: an important cardiovascular risk factor that has long been underestimated. Front. Cardiovasc. Med. 11:1380506. doi: 10.3389/fcvm.2024.1380506
Received: 1 February 2024; Accepted: 4 March 2024;
Published: 13 March 2024.
Edited by:
Harry H. X. Wang, Sun Yat-sen University, ChinaReviewed by:
Fabrizio Salvucci, Ticinello Cardiovascular and Metabolic Centre, Italy© 2024 Fazio, Mercurio, Tibullo, Fazio and Affuso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serafino Fazio fazio0502@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.