
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 23 May 2024
Sec. Cardiovascular Pharmacology and Drug Discovery
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1379765
Background: This systematic review and meta-analysis aimed to explore the effects of different sodium–glucose cotransporter-2 inhibitors (SGLT2i) on prognosis and cardiac structural remodeling in patients with heart failure (HF).
Methods: Relevant studies published up to 20 March 2024 were retrieved from PubMed, EMBASE, Web of Science, and Cochrane Library CNKI, China Biomedical Literature Service, VIP, and WanFang databases. We included randomized controlled trials of different SGLT2i and pooled the prognosis data of patients with HF. We compared the efficacy of different SGLT2i in patients with HF and conducted a sub-analysis based on left ventricular ejection fraction (LVEF).
Results: We identified 77 randomized controlled trials involving 43,561 patients. The results showed that SGLT2i significantly enhanced outcomes in HF, including a composite of hospitalizations for HF and cardiovascular death, individual hospitalizations for HF, Kansas City Cardiomyopathy Questionnaire (KCCQ) scores, left atrial volume index (LAVi), and LVEF among all HF patients (P < 0.05) compared to a placebo. Sotagliflozin was superior to empagliflozin [RR = 0.88, CI (0.79–0.97)] and dapagliflozin [RR = 0.86, CI (0.77–0.96)] in reducing hospitalizations for HF and CV death. Dapagliflozin significantly reduced hospitalizations [RR = 0.51, CI (0.33–0.80)], CV death [RR = 0.73, CI (0.54–0.97)], and all-cause mortality [RR = 0.69, CI (0.48–0.99)] in patients with HF with reduced ejection fraction (HFrEF). SGLT2i also plays a significant role in improving cardiac remodeling and quality of life (LVMi, LVEDV, KCQQ) (P < 0.05). Among patients with HF with preserved ejection fraction (HFpEF), SGLT2i significantly improved cardiac function in HFpEF patients (P < 0.05). In addition, canagliflozin [RR = 0.09, CI (0.01–0.86)] demonstrated greater safety compared to sotagliflozin in a composite of urinary and reproductive infections of HFpEF patients.
Conclusion: Our systematic review showed that SGLT2i generally enhances the prognosis of patients with HF. Sotagliflozin demonstrated superiority over empagliflozin and dapagliflozin in a composite of hospitalization for HF and CV death in the overall HF patients. Canagliflozin exhibited greater safety compared to sotagliflozin in a composite of urinary and reproductive infections of HFpEF. Overall, the efficacy of SGLT2i was greater in HFrEF patients than in HFpEF patients.
Heart failure (HF) results from either contraction or relaxation dysfunction of the heart, leading to multisystem symptoms and signs. Despite a decrease in the age-standardized prevalence of HF from 1990 to 2019, the reduction is not significant, and HF remains a significant cause of disability and death worldwide (1). Currently, in developed countries (2–4), such as Britain, France, and the United States, the prevalence of HF ranges from 1.5% to 2.0%, while in developing countries (5) and regions, such as Asia and Africa, it spans from 1.3% to 6.7%. According to the latest definitions by the European and American Heart Association, HF is categorized into three main types, namely, HF with mildly reduced ejection fraction (HFrEF) (LVEF, <40%), HF with moderately reduced ejection fraction (LVEF, 40%–50%), and HF with preserved ejection fraction (HFpEF) (EF, ≥50%). However, many previous meta-analyses defined HFpEF as EF ≥ 45%, which contrasts with the current HF classification. Hence, our study adopts the definition of HFpEF ≥ 50%.
SGLT2i is a new class of antidiabetic medications originally developed for managing diabetes. Recent research demonstrated their efficacy in improving outcomes for patients with HF, such as reduction in hospitalizations, cardiovascular mortality, adverse cardiac remodeling, and other associated factors, irrespective of the presence of diabetes. In addition, adverse cardiac remodeling is a critical mechanism in the progression of HF and serves as an independent risk factor for mortality and morbidity in patients with cardiovascular disease (5). Nevertheless, the comprehensive evaluation of SGLT2i effects on adverse cardiac remodeling in HF patients remains limited, with existing studies yielding divergent results. Despite the generally favorable effects of SGLT2i on HF patients, there is a lack of consensus on the most effective SGLT2i variation for HF treatment. Therefore, we conducted a systematic review and meta-analysis of randomized controlled trials (RCT), including a subgroup analysis based on varying ejection fractions to assess the effectiveness and safety profile of six SGLT2i for HF. This study aims to offer valuable evidence to aid clinical decision-making in HF management.
The protocol for this systematic review and meta-analysis was not registered. The data supporting this article are available in the article and its online Supplementary Material.
PubMed, Embase, Web of Science, Cochrane Library, CNKI, China Biomedical Literature Service, VIP, and WanFang databases were systematically searched until 20 March 2024. Additionally, the reference lists of these relevant articles were meticulously reviewed to identify any potentially overlooked trials. The search strategy employed a combination of subject words and free words. The primary search terms included “sodium–glucose cotransporter-2 inhibitors” or “SGLT2i” and “heart failure” and specific drug names such as “empagliflozin” or “dapagliflozin” or “canagliflozin” or “sotagliflozin” or “ipragliflozin” or “ertugliflozin.”
(1) Research type: RCT.
(2) Research subject: All subjects who met the current diagnostic criteria for HF. HFrEF was defined as EF < 50%, and HFpEF was defined as EF ≥ 50%.
(3) Interventions: The experimental group received SGLT2i (dapagliflozin, empagliflozin, sotagliflozin, canagliflozin, ipragliflozin, ertugliflozin), and the control group received a placebo.
(4) Outcome indicators: ① a composite of hospitalization for HF and CV death; ② hospitalization for HF; ③ CV death; ④ all-cause death; ⑤ a composite of uric and reproductive effects; ⑥ 6 min walk distance (6MWT); ⑦ NT-proBNP; ⑧ the Kansas City Cardiomyopathy Questionnaire (KCCQ); ⑨ LAVi; ⑩ E/e'; ⑪ LVMi; ⑫ LVEDV; ⑬; LVESV ⑭ LVEF; ⑮ hematocrit (HCT).
(1) Non-RCT, (2) duplicate publication, (3) meta-analysis studies, (4) ongoing or unpublished studies, (5) studies lacking original data or where data could not be calculated, and (6) observational or cohort studies.
Literature screening involved two researchers who independently reviewed articles based on the established inclusion and exclusion criteria. After individual assessments, they cross-checked their selections to ensure consistency. Key information, such as the first author's name, study design, baseline characteristics, and study endpoints, was systematically extracted from each article.
The quality of the included studies was independently assessed by two researchers using the “risk of bias assessment criteria” from the Cochrane Reviewers’ Handbook (version 5.1.0). The evaluation of the RCTs involved the following components: (1) randomized method, (2) allocation concealment, (3) blinding of participant personnel and outcome assessors, (4) completeness of outcome data, (5) absence of selective outcome reporting, and (6) clarity of reasons for losses to follow-up or discontinuation.
The statistical analysis was conducted using Stata 15.1 software for network meta-analysis. Relative risks or odds ratios were determined for dichotomous variables, while continuous variables were analyzed using the frequentist methodology in network meta-analysis. Heterogeneity was set as I2 < 50% and p > 0.01 for the fixed effect model. Otherwise, the random effects model was applied. Pooled results for continuous variables were expressed as the mean difference (MD). The surface under the cumulative ranking (SUCRA) was employed to indicate the preferred ranking of each treatment. Small sample effects were investigated through a network funnel plot. P < 0.05 was considered statistically significant.
A total of 6,229 relevant literature sources were identified through a comprehensive search across multiple databases. After thorough screening, 77 RCTs (6–82) were included in this study. The study encompassed a cohort of 43,561 patients diagnosed with HF, consisting of 11,734 patients with HFpEF and 31,827 patients with HFrEF. A detailed description of the literature search and screening process is illustrated in Figure 1, while the baseline characteristics are outlined in Table 1. Among the 77 selected articles, the outcome indicators related to HFpEF or HFrEF were simultaneously reported in 5 articles, 22 focused on HFpEF, and the remaining studies were centered on HFrEF. Specifically, 5 studies investigated canagliflozin treatment, which involved a total of 453 patients; 55 studies examined dapagliflozin treatment, with a collective enrollment of 16,201 patients; 16 studies utilized empagliflozin, which included 21,024 patients; 1 study explored the efficacy of ertugliflozin, which enrolled 478 patients; 1 study evaluated ipragliflozin treatment, with a cohort of 68 patients; and 4 studies analyzed the effects of sotagliflozin, which involved a total of 5,537 patients. Notably, except for empagliflozin and dapagliflozin, no studies directly compared the remaining four types of SGLT2i.
The results of the bias risk evaluation are presented in Figure 2 and Supplementary Figure S1. For random sequence generation, 77 studies employing a random number table or a random Excel table were identified as low risk; 33 studies provided detailed descriptions of their allocation concealment procedures; however, the remaining studies lacked such descriptions. Regarding implementation bias, 29 studies were defined as high risk, 15 studies did not provide sufficient details, and the rest were classified as low risk. For the assessment of outcome data, one study was identified as high risk due to insufficient details, one was poorly described, and the others were considered low risk. All studies reported complete data for outcomes. Outcome selection bias indicated that 72 studies were classified as low risk, while 5 required further clarification. The results of other preferences showed that seven studies were defined as high risk, two were poorly described, and the remaining studies were assessed as low risk.

Figure 2. Risk of bias summary of all eligible RCTs evaluating the effect of SGLT2i in HFrEF or HFpEF.
As shown in Table 2, sotagliflozin [RR = 0.69, 95% CI (0.64–0.75)], empagliflozin [RR = 0.79, CI (0.75–0.84)], and dapagliflozin [RR = 0.80, CI:(0.75–0.87)] exhibited significant efficacy in reducing a composite outcome of hospitalization for HF and CV death when compared to placebo. Sotagliflozin demonstrated superiority over empagliflozin [RR = 0.88, CI (0.79–0.97)] and dapagliflozin [RR = 0.86, CI (0.77–0.96)]. The network plot is shown in Figure 3A. The ranking based on SUCRA values is as follows: sotagliflozin (99%), empagliflozin (55%), dapagliflozin (47%), and placebo (0%), as shown in Table 3.
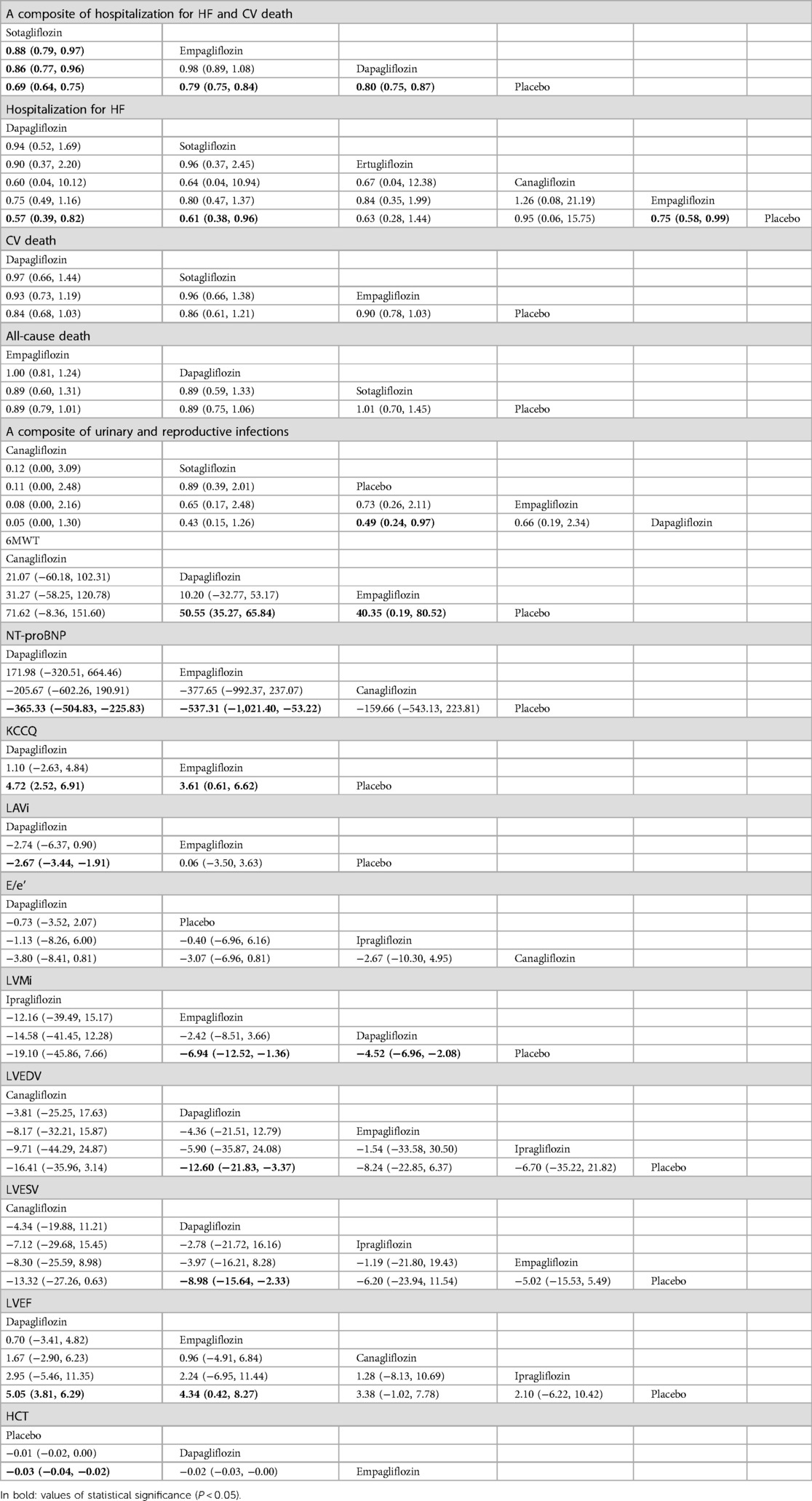
Table 2. Comparison of the efficacy and safety of different SGLT2i in treating HF [RR and mean difference (95% CI)].

Figure 3. A network plot of each comparison in all eligible trials in HFrEF or HFpEF. (A) The network plot of each comparison in terms of a composite of hospitalization for HF and CV death. (B) The network plot of each comparison in terms of hospitalization for HF. (C) The network plot of each comparison in terms of CV death. (D) The network plot of each comparison in terms of all-cause death. (E) The network plot of each comparison in terms of a composite of urinary and reproductive infections. (F) The network plot of each comparison in terms of 6 min walk distance. (G) The network plot of each comparison in terms of NT-proBNP. (H) The network plot of each comparison in terms of KCCQ. (I) The network plot of each comparison in terms of LAVi. (J) The network plot of each comparison in terms of E/e’. (K) The network plot of each comparison in terms of LVMi. (L) The network plot of each comparison in terms of LVEDV. (M) The network plot of each comparison in terms of LVESV. (N) The network plot of each comparison in terms of LVEF. (O) The network plot of each comparison in terms of HCT.
As shown in Table 2, empagliflozin [RR = 0.75, CI (0.58–0.99)], sotagliflozin (RR = 0.61, CI 0.38–0.96), and dapagliflozin [RR = 0.57, CI (0.39–0.82)] significantly reduced hospitalization for HF compared to placebo. However, no significant differences were observed between ertugliflozin and canagliflozin or among the different SGLT2i treatments. The network plot is shown in Figure 3B. The ranking based on SUCRA values is as follows: dapagliflozin (74%), sotagliflozin (67%), erugliflozin (59%), empagliflozin (43%), canagliflozin (41%), and placebo (13%).
As shown in Table 2, empagliflozin, dapagliflozin, and sotagliflozin showed no difference in reducing all-cause mortality and cardiovascular mortality compared to placebo. The network plot is shown in Figure 3D, while the ranking based on SUCRA values is presented in Table 3.
As shown in Table 2, dapagliflozin [RR = 0.49, CI (0.24–0.97)] significantly reduced urinary and reproductive system infections compared to placebo. However, canagliflozin, sotagliflozin, and empagliflozin did not exhibit a significant difference in reducing these infections. There was no difference observed among the different SGLT2i treatments. These findings are visually represented in the network plot shown in Figure 3E. The ranking based on SUCRA value is as follows: canagliflozin (92%), sotagliflozin (60%), placebo (54%), empagliflozin (34%), and dapagliflozin (10%).
Table 2 shows that dapagliflozin [RR = 50.55, CI (35.27–65.84)] and empagliflozin [RR = 40.35, CI (0.19–80.52)] significantly improved walking distance compared to placebo, whereas canagliflozin [RR = 71.62, CI (−8.36–151.60)] showed no difference. There was no difference observed among the different SGLT2i treatments. These comparisons are graphically illustrated in the network plot shown in Figure 3F. The ranking based on SUCRA values is as follows: canagliflozin (80%), dapagliflozin (65%), empagliflozin (51%), and placebo (2%).
As presented in Table 2, empagliflozin [MD = −537.81, CI (−1,021.40 to −53.22)] and dapagliflozin [MD = −365.33, CI (−504.83 to −225.83)] showed significant differences in improving NT-proBNP in patients with HF compared to placebo. However, canagliflozin [MD = −159.66, CI (−543.13–223.81)] showed no significant impact on proBNP levels. There was no difference observed among the different SGLT2i treatments. These results are illustrated in the network plot shown in Figure 3G. The ranking based on SUCRA values is as follows: empagliflozin (87%), dapagliflozin (70%), canagliflozin (34%), and placebo (8%).
As detailed in Table 2, compared to placebo, there was no significant difference in the improvement of KCCQ scores in patients with HF between dapagliflozin [MD = 4.72, CI (2.52–6.91)] and empagliflozin [MD = 3. 6, CI (0.61–6.62)]. No significant differences were observed among the different SGLT2i treatments. These findings are visually represented in the network plot shown in Figure 3H. The ranking based on SUCRA values is as follows: dapagliflozin 86%), empagliflozin (63%), and placebo (1%).
As shown in Table 2, compared with placebo, dapagliflozin [MD = −2.67, CI (−3.44 to −1.91)] showed significant statistical differences in improving LAVi, while empagliflozin [MD = 0.06, CI (−3.05–3.63)] showed no significant change. These findings are depicted in the network plot shown in Figure 3I. The ranking based on SUCRA values is as follows: dapagliflozin (96%), empagliflozin (27%), and placebo (25%).
As shown in Table 2, compared with placebo, dapagliflozin [MD = −0.73, CI (−3.52–2.07)], ipragliflozin [MD = −0.40, CI (−6.96–6.16)], and canagliflozin [MD = −3.07, CI (−6.96–0.81)] showed no difference in improving E/e', and there were no significant variations observed among the different SGLT2i treatments. The network plot is presented in Figure 3J. The ranking based on SUCRA values is as follows: dapagliflozin (75%), placebo (60%), ipragliflozin (52%), and canagliflozin (11%).
The results presented in Table 2 indicated that empagliflozin [MD = −6.94, CI (−12.52 to −1.36)] and dapagliflozin [MD = −4.52, CI (−6.96 to −2.08)] significantly reduced LVMi compared to placebo. There was no significant difference observed among the different SGLT2i treatments. The network plot is shown in Figure 3K. The ranking based on SUCRA values is as follows: ipragliflozin (85%), empagliflozin (66%), dapagliflozin (45%), and placebo (3%).
Table 2 demonstrates that dapagliflozin [MD = −12.60, CI (−21.83 to −3.37)] reduced LVEDV in patients with HF compared to placebo, while ipragliflozin, empagliflozin, and canagliflozin did not show a significant impact in this aspect. No differences were observed among the different SGLT2i treatments. The network plot illustrating these findings is presented in Figure 3L. The ranking based on SUCRA values is as follows: canagliflozin (76%), empagliflozin (67%), ipragliflozin (49%), dapagliflozin (44%), and placebo (12%).
Table 2 demonstrates that dapagliflozin [MD = −8.98, CI (−15.64 to −2.33)] reduced LVESV in patients with HF compared to placebo. In contrast, ipragliflozin, canagliflozin, and empagliflozin did not exhibit statistically significant differences in this aspect, indicating no significant variance among the different SGLT2i treatments. The network plot illustrating these relationships is presented in Figure 3M. The ranking based on SUCRA values is as follows: canagliflozin (80%), dapagliflozin (65%), ipragliflozin (43%), empagliflozin (48%), and placebo (11%).
As indicated in Table 2, dapagliflozin [MD = 5.05, CI (3.81–6.29)] significantly increased LVEF in patients with HF compared to placebo, whereas canagliflozin, empagliflozin, and ipragliflozin showed no significant differences. No differences were observed among the different SGLT2i treatments. These findings are visually represented in the network plot shown in Figure 3N. The ranking based on SUCRA values is as follows: dapagliflozin (78%), empagliflozin (66%), canagliflozin (53%), ipragliflozin (41%), and placebo (10%).
Table 2 illustrates that empagliflozin [MD = −0.03, CI (−0.04 to −0.02)] significantly increased hematocrit (HCT) in patients with HF compared to placebo. Dapagliflozin did not show any significant difference, and there was no disparity observed among the different SGLT2i treatments. The network plot illustrating these findings is presented in Figure 3O. The ranking based on SUCRA values is as follows: placebo (95%), dapagliflozin (53%), and empagliflozin (1%).
The network plot in Figure 4 illustrates that dapagliflozin [RR = 0.44, CI (0.15–1.23)], empagliflozin [RR = 0.75, CI (0.11–5.15)], and sotagliflozin [RR = 0.42, CI (0.05–3.66)] did not significantly reduce the composite of hospitalization for HF and CV death compared to placebo, and there was no difference between the different SGLT2i, as shown in Table 4. The ranking based on SUCRA values is presented in Table 5. Compared with placebo, dapagliflozin significantly improved hospitalization for HF [RR = 0.51, CI (0.33–0.80)] and CV death [RR = 0.73, CI (0.54–0.97)], with no significant differences noted between the different SGLT2i treatments. These findings are outlined in Table 4, and the associated SUCRA rankings are presented in Table 5. Compared to placebo, dapagliflozin reduced all-cause death [RR = 0.69, CI (0.48–0.99)], and dapagliflozin [RR = 0.40, CI (0.18–0.93)] increased a composite of urinary and reproductive infections, with no differences observed between the different SGLT2i treatments. The SUCRA values for these comparisons are also presented in Table 5.
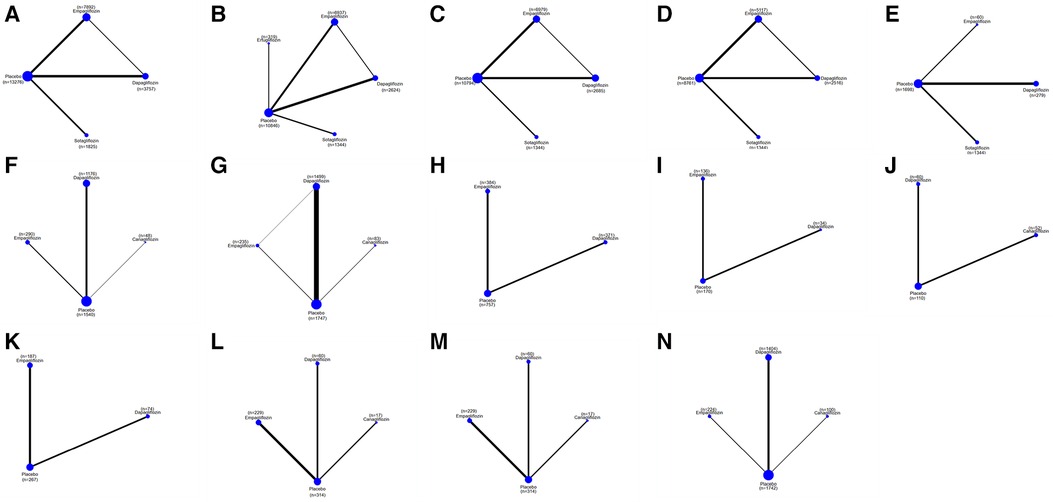
Figure 4. A network plot of each comparison in all eligible trials in HFrEF. (A) The network plot of each comparison in terms of a composite of hospitalization for HF and CV death. (B) The network plot of each comparison in terms of hospitalization for HF. (C) The network plot of each comparison in terms of CV death. (D) The network plot of each comparison in terms of all-cause death. (E) The network plot of each comparison in terms of a composite of urinary and reproductive infections. (F) The network plot of each comparison in terms of 6 min walk distance. (G) The network plot of each comparison in terms of NT-proBNP. (H) The network plot of each comparison in terms of KCCQ. (I) The network plot of each comparison in terms of LAVi. (J) The network plot of each comparison in terms of E/e’. (K) The network plot of each comparison in terms of LVMi. (L) The network plot of each comparison in terms of LVEDV. (M) The network plot of each comparison in terms of LVESV. (N) The network plot of each comparison in terms of LVEF.
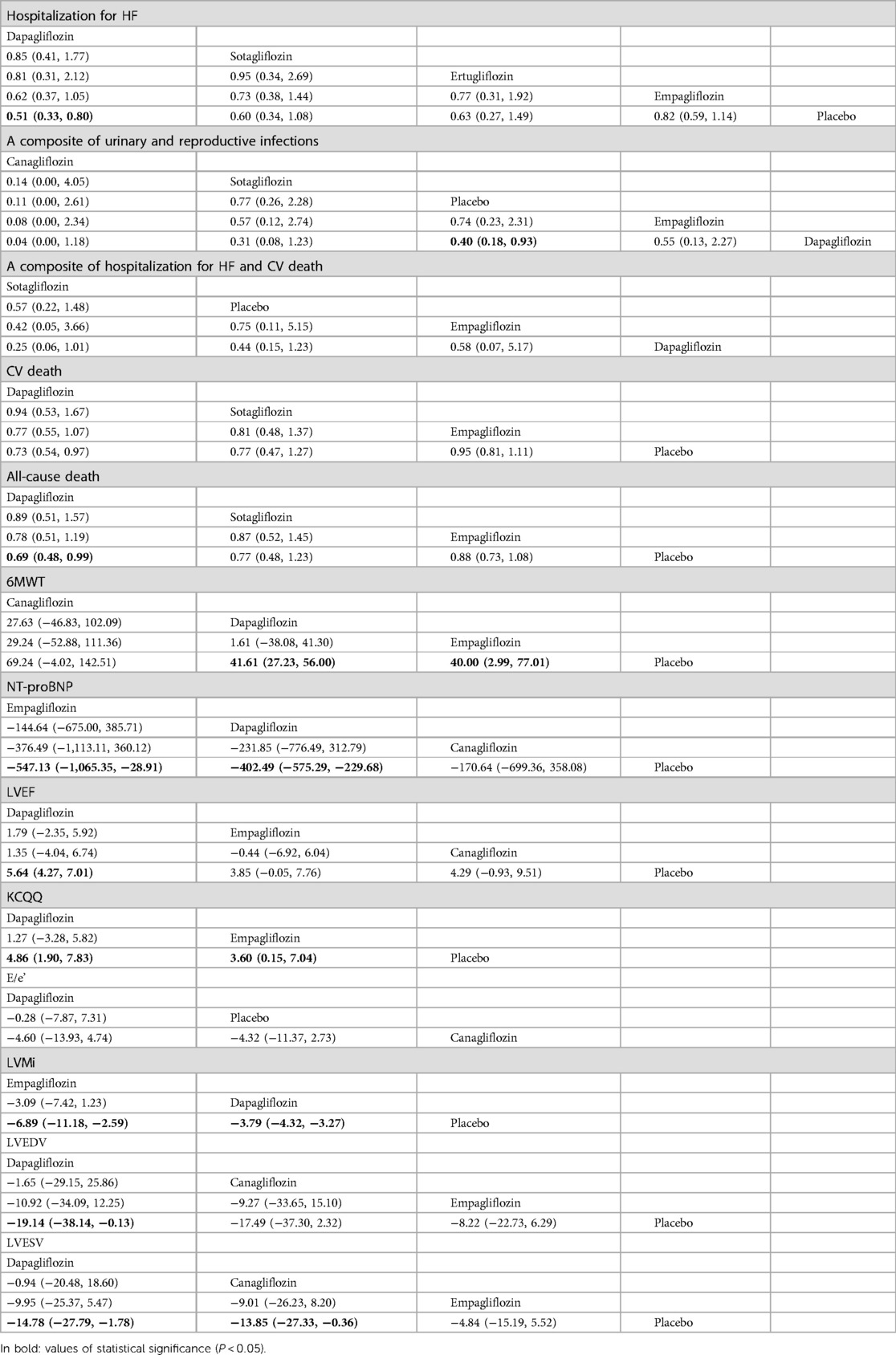
Table 4. Comparison of the efficacy and safety of different SGLT2i in treating HFrEF [RR and mean difference (95% CI)].
Compared to placebo, dapagliflozin improved the 6MWT of HFrEF patients [MD = 41.61, CI (27.23–56.00)] and proBNP levels [MD = −402.49, CI (−575.29 to −299.68)], and empagliflozin improved the 6MWT of HF patients [MD = 40.00, CI (2.99–77.01)] and proBNP levels [MD = −547.13, CI (−1,065.35 to −28.91)]. No significant differences were observed between different SGLT2i. The ranking based on SUCRA values is shown in Table 5. Compared to placebo, SGLT2i did not improve E/e' in cardiac structure. Compared to placebo, dapagliflozin significantly reduced LVMi [MD = −3.79, CI (−4.33 to −3.27)] and increased LVEF [MD = 5.64, CI (4.27 to 7.01)] LVEDV [MD = −19.14, CI (−38.14 to −0.13)], LVESV [MD = −14.78, CI (−27.79 to −1.78)], and KCCQ score [MD = 4.86, CI (1.90–7.83)]. Compared to placebo, canagliflozin reduced LVESV [MD = −13.85, CI (−27.33 to −0.36] in patients with HFrEF. Compared to placebo, empagliflozin reduced LVMi [MD = −6.89, CI (−11.18 to −2.59)] and KCCQ score [MD = 3.60, CI (0.15–7.04)]. The ranking based on SUCRA values is shown in Table 5.
The network plot presented in Figure 5 indicates that compared to placebo, sotagliflozin, dapagliflozin, and empagliflozin did not significantly reduce a composite of hospitalization for HF and CV death, individual hospitalizations for HF and CV death, or all-cause mortality, as detailed in Table 6. No significant differences were observed between the different SGLT2i treatments in these outcomes. The ranking based on SUCRA values is shown in Table 7.
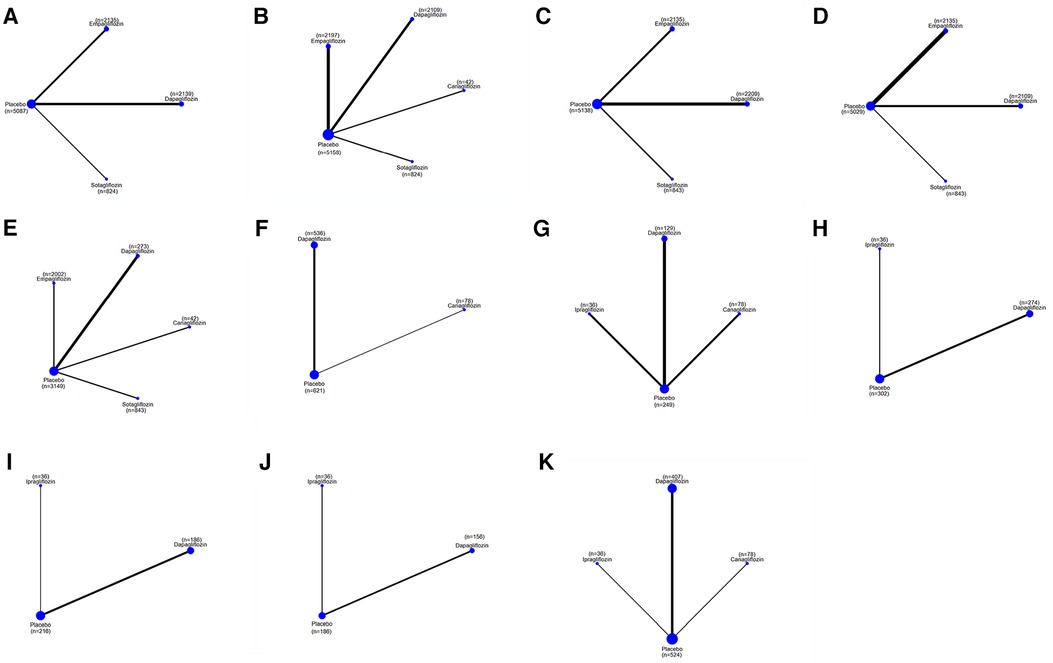
Figure 5. A network plot of each comparison in all eligible trials in HFpEF. (A) The network plot of each comparison in terms of a composite of hospitalization for HF and CV death. (B) The network plot of each comparison in terms of hospitalization for HF. (C) The network plot of each comparison in terms of CV death. (D) The network plot of each comparison in terms of all-cause death. (E) The network plot of each comparison in terms of a composite of urinary and reproductive infections. (F) The network plot of each comparison in terms of NT-proBNP. (G) The network plot of each comparison in terms of E/e’ (H) The network plot of each comparison in terms of LVMi. (I) The network plot of each comparison in terms of LVEDV. (J) The network plot of each comparison in terms of LVESV. (K) The network plot of each comparison in terms of LVEF.
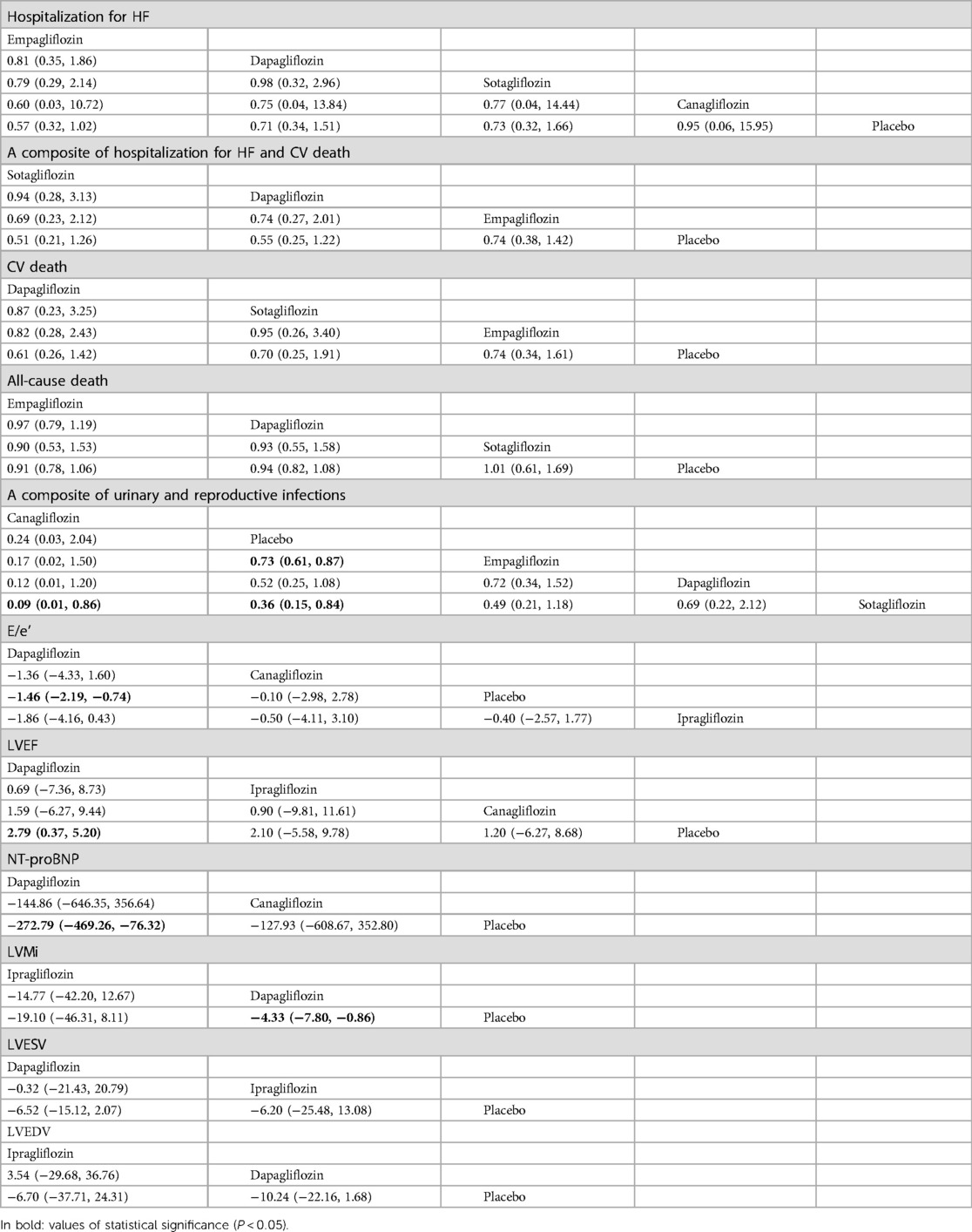
Table 6. Comparison of the efficacy and safety of different SGLT2i in treating HFpEF [RR and mean difference (95% CI)].
In terms of safety for patients with HFpEF, empagliflozin [RR = 0.73, CI (0.61–0.87)] and sotagliflozin [RR = 0.36, CI (0.15–0.84)] increased the risk of urinary and reproductive tract infections compared to placebo, while canagliflozin [RR = 0.09, CI (0.01–0.86)] presented a lower risk than sotagliflozin. The ranking based on SUCRA values for safety is as follows: canagliflozin (96%), placebo (75%), empagliflozin (44%), dapagliflozin (26%), and sotagliflozin (8%).
Compared to placebo, dapagliflozin [MD = −272.79, CI (−469.26 to −76.32)] showed significant differences in reducing NT-proBNP levels, while canagliflozin showed no difference. There were no significant differences among the different SGLT2i treatments. The ranking based on SUCRA values is as follows: dapagliflozin (87%), canagliflozin (48%), and placebo (14%). In addition, compared to placebo, dapagliflozin improved E/e' [MD = −1.46, CI (−2.19 to −0.74)], LVMi [MD = −4.33, CI (−7.80 to −0.86)], and LVEF [MD = 2.79, CI (0.37–5.20)] in terms of adverse cardiac remodeling. No differences were noted for canagliflozin and ipragliflozin or among different SGLT2i. There was no statistical difference in improving LVESV and LVEDV compared to placebo and among the different SGLT2i treatments. The ranking based on SUCRA values is detailed in Table 7.
Comparison-corrected funnel plots were utilized to assess publication bias in the study, focusing on a range of outcome indicators such as a composite of hospitalization for HF and CV death, hospitalization for HF, CV death, all-cause death, urinary and reproductive infections, 6MWT, NT-ProBNP, KCCQ, LAVi, E/e', LVMi, LVEDV, LVESV, LVEF, and HCT. The network funnel plot revealed the presence of small sample effects in the comparison between dapagliflozin and placebo for a composite of hospitalization for HF and CV death (Figure 6A), a composite of urinary and reproductive infections (Figure 6E), CV death (Figure 6C), 6MWT and NT-ProBNP (Figures 6F,G), LVMi (Figure 6K), LVESV (Figure 6M), and LVEDV (Figure 6L). The comparison between canagliflozin and placebo showed a small sample effect in hospitalization for HF (Figure 6B), while the comparison between empagliflozin and placebo indicated small sample effects in CV death (Figure 6C), LVESV (Figure 6M), LVEF (Figure 6N), and all-cause death (Figure 6D).
Figure 6. Funnel plot of pairwise comparison among each SGLT2i treatment. (A) The funnel plot of a composite of hospitalization for HF and CV death. (B) The funnel plot of hospitalization for HF. (C) The funnel plot of CV death. (D) The funnel plot of all-cause death. (E) The funnel plot of a composite of urinary and reproductive infections. (F) The funnel plot of 6 min walk distance. (G) The funnel plot of NT-proBNP. (H) The funnel plot of KCCQ. (I) The funnel plot of LAVi. (J) The funnel plot of E/e’. (K) The funnel plot of LVMi. (L) The funnel plot of LVEDV. (M) The funnel plot of LVESV. (N) The funnel plot of LVEF. (O) The funnel plot of HCT.
This review analyzed 77 RCT involving 43,561 patients using Bayesian network meta-analysis for a comprehensive evaluation. The study encompassed more than 10 outcome indicators, including a composite of hospitalization for HF and CV death, hospitalization for HF and CV death, a composite of urinary and reproductive effects, and an assessment of the cardiac structure. Subgroup analysis was performed based on the ejection fraction of HF. Although the efficacy of SGLT2i varies slightly with different LVEF baselines of patients, it may be beneficial in patients with HF regardless of LVEF baseline. Compared with the placebo, SGLT2i demonstrated a significant advantage in reducing a composite of hospitalization for HF and CV death, hospitalization for HF and CV death, and KCQQ scores while showing no significant impact on reducing all-cause mortality. Indirect comparisons between different SGLT2i suggest improvements in a composite of hospitalization for HF and CV death, hospitalization for HF, and CV death. Sotagliflozin outperformed empagliflozin and dapagliflozin in reducing hospitalization for HF and CV death. However, there is no difference between empagliflozin and dapagliflozin. Nevertheless, given the limited research on sotagliflozin, further investigation is warranted.
Regarding the safety profile in total HF patients, SGLT2i are associated with an increased risk of urinary and reproductive system infections, with dapagliflozin showing the highest risk among them. However, there is no distinction between various types of SGLT2i. A previous meta-analysis (83) showed that, except for dapagliflozin, SGLT2i did not increase incidences of urinary and reproductive system infections, which is consistent with our findings. Moreover, the US Food and Drug Administration has included this potential side effect in its list of adverse reactions. HCT was utilized as a reference indicator to assess low blood volume. The meta-analysis demonstrated that SGLT2i resulted in a rise in HCT relative to placebo, implying an elevated hypotension hazard for SGLT2i. The primary mechanism of action of SGLT2i involves the inhibition of the SGLT2 transporter, predominantly located in the S1 segment of the proximal tubules (84), increasing the excretion of glucose in the urine. Nevertheless, inhibiting SGLT2i also diminishes sodium reabsorption in the proximal tubules, potentially increasing sodium excretion. Previous studies (85, 86) reported a correlation between the administration of SGLT2i and a reduction of body weight and blood pressure.
The network meta-analysis results indicated that dapagliflozin and empagliflozin significantly improved NT-proBNP and 6MWT. However, no statistically significant difference was observed among different SGLT2i. While SGLT2i have shown promise in treating HF, it is crucial to determine whether they directly influence the heart's structural function. Therefore, we collected relevant indicators, such as LAVi, E/e', LVMi, and LVEDV, to systematically evaluate the changes in cardiac structure in HF patients treated with SGLT2i. The results showed that SGLT2i significantly reduced LVMi, LVEDV, LAVi, and LVESV and increased LVEF, reflecting significant benefits in improving cardiac systolic and diastolic function. Cardiac anatomy and functional parameters are vital in predicting the prognosis and quality of life in HF patients. Animal studies conducted on T2DM models revealed the positive effects of SGLT2i on left ventricular hypertrophy and dilation (87, 88), as well as cardiac systolic and diastolic function. LAVi and E/e' are predictive factors (89) used to evaluate cardiac diastolic function. Our research revealed that SGLT2i did not reduce E/e' in HFrEF patients. Nonetheless, subgroup analysis indicated SGLT2i could enhance the E/e' and LAVi of patients with HFpEF, potentially due to differing mechanisms between HFpEF and HFrEF.
According to the grading of HFpEF by the European and American Heart Association, this study conducted subgroup analysis based on ejection fraction. The HFpEF (EF ≥ 50%) group did not show significant differences in reducing a composite of hospitalization for HF and CV death, hospitalization for HF, CV death, and all-cause death. No significant differences were observed between different SGLT2i. However, some meta-analyses have shown that (90–92) SGLT2i can reduce a composite of HF and CV death hospitalizations. However, the previous study defined HFpEF as EF greater than 40%, which differs from our study. Therefore, our study should be more convincing. Regarding the safety of HFpEF patients, SGLT2i also present risks of urinary and reproductive infections, with empagliflozin and sotagliflozin being notable culprits. Canagliflozin has demonstrated higher safety compared to sotagliflozin in this aspect. In terms of improving ventricular remodeling, compared to placebo, SGLT2i have shown improvement in LAVi, E/e', LVEF, and LVMi. However, no significant differences were observed in LVESV and LVEDV, and there was no difference between different SGLT2i. The mechanism of HFpEF remains unclear, and left ventricular diastolic dysfunction is considered the main pathophysiological mechanism underlying the occurrence of HFpEF (93). Our research also confirmed that SGLT2i can improve the diastolic function of HFpEF patients. Typically, remodeling the structure of the patient's heart can significantly enhance their prognosis and quality of life. Unfortunately, there has been limited research on the quality of life scores of HFpEF patients; thus, this outcome measure was not included in our analysis.
In the subgroup analysis of HFrEF (EF <50%), the network meta-analysis results revealed significant effects of SGLT2i in reducing hospitalization for HF, CV death, all-cause death, NT-ProBNP, and 6MWT. Interestingly, this finding contrasts with the statistical results obtained before conducting the subgroup analysis, indicating the importance of evaluating the contribution of SGLT2i to HF based on ejection fraction. Furthermore, SGLT2i showed significant advantages in improving all-cause death. The indirect comparison revealed no statistical difference between different SGLT2i. Regarding the safety of HFrEF, dapagliflozin significantly increased the risk of a composite of urinary and reproductive infections compared to placebo. Additionally, our analysis revealed that SGLT2i could enhance KCCQ scores in HFrEF patients. Regarding ventricular remodeling, our study revealed that SGLT2i reduced LVMi, LVESV, and LVEDV and increased LVEF. These findings suggest that SGLT2i can enhance diastolic and systolic function in patients with HFrEF, thereby potentially augmenting the prognostic outcomes for these patients. The therapeutic effect of SGLT2i on cardiac structural remodeling was found to be significantly better in HFrEF patients compared to HFpEF patients, with SGLT2i demonstrating superiority in improving cardiac diastolic function in HFpEF patients. Consistent with our findings, a previous meta-analysis (94) showed that empagliflozin had a more significant effect in improving cardiac structure.
This study presents several limitations. ① This study mainly focuses on empagliflozin and dapagliflozin, with relatively little research available on canagliflozin, sotagliflozin, ipragliflozin, and ertugliflozin. Future research should explore these alternative SGLT2i to provide a more comprehensive understanding of their efficacy and safety profiles. ② Currently, there is only one direct comparison between dapagliflozin and empagliflozin available in current literature, leading to an indirect evaluation of the efficacy and safety of canagliflozin, sotagliflozin, ipragliflozin, and ertugliflozin in treating HF patients. Consequently, a potential bias exists between the reported results and the actual drug performances, underscoring the need for further direct-controlled trials to validate their efficacy and safety profiles. ③ There are variations in baseline characteristics such as gender, age, race, and chronic medical conditions among the included studies, potentially resulting in clinical heterogeneity. ④ Variations in follow-up durations between the six SGLT2i drug studies and within individual studies for each drug could introduce bias into the study results. ⑤ The limited number of studies on HF with HFpEF (EF ≥ 50%) underscores the necessity for more research to substantiate the relevant findings.
In summary, SGLT2i can significantly improve the prognosis of all patients with HF despite the associated increased risk of urinary and reproductive infections. Overall, HF patients benefit from enhanced cardiac remodeling, with those with HFrEF experiencing the most substantial benefits. Indirect comparisons between different SGLT2i revealed no significant differences in HFrEF. Among the six types of SGLT2i, sotagliflozin demonstrated superiority over empagliflozin and dapagliflozin in reducing hospitalization for HF and cardiovascular death in total HF. Canagliflozin exhibited higher safety than sotagliflozin regarding urinary and reproductive infections in patients with HFpEF. Overall, SGLT2i showed better efficacy in patients with HFrEF than those with HFpEF.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
XL: Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing. HZ: Methodology, Project administration, Writing – review & editing. YC: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Writing – review & editing. YH: Investigation, Resources, Software, Writing – original draft. XF: Data curation, Investigation, Methodology, Writing – original draft. KZ: Investigation, Methodology, Writing – review & editing. MW: Resources, Validation, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Funded by Military Logistical Special Project for Health Care (23KYBJ06).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1379765/full#supplementary-material
1. Yan T, Zhu S, Yin X, Xie C, Xue J, Zhu M, et al. Burden, trends, and inequalities of heart failure globally, 1990–2019: a secondary analysis based on the global burden of disease 2019 study. J Am Heart Assoc. (2023) 12(6):e027852. doi: 10.1161/JAHA.122.027852
2. Bellanca L, Linden S, Farmer R. Incidence and prevalence of heart failure in England: a descriptive analysis of linked primary and secondary care data—the PULSE study. BMC Cardiovasc Disord. (2023) 23(1):374. doi: 10.1186/s12872-023-03337-1
3. Roger VL, Banaag A, Korona-Bailey J, Wiley TMP, Turner CE, Haigney MC, et al. Prevalence of heart failure stages in a universal health care system: the military health system experience. Am J Med. (2023) 136(11):1079–86.e1. doi: 10.1016/j.amjmed.2023.07.007
4. Hanon O, Belmin J, Benetos A, Chassagne P, De Decker L, Jeandel C, et al. Consensus of experts from the French Society of Geriatrics and Gerontology on the management of heart failure in very old subjects. Arch Cardiovasc Dis. (2021) 114(3):246–59. doi: 10.1016/j.acvd.2020.12.001
5. Peng X, Wang J, Li J, Li Y, Wang X, Liu X, et al. Gender-specific prevalence and trend of heart failure in China from 1990 to 2019. ESC Heart Fail. (2023) 10(3):1883–95. doi: 10.1002/ehf2.14361
6. Savarese G, Uijl A, Lund LH, Anker SD, Asselbergs F, Fitchett D, et al. Empagliflozin in heart failure with predicted preserved versus reduced ejection fraction: data from the EMPA-REG OUTCOME trial. J Card Fail. (2021) 27(8):888–95. doi: 10.1016/j.cardfail.2021.05.012
7. Anker SD, Butler J, Usman MS, Filippatos G, Ferreira JP, Bocchi E, et al. Efficacy of empagliflozin in heart failure with preserved versus mid-range ejection fraction: a pre-specified analysis of EMPEROR-preserved. Nat Med. (2022) 28(12):2512–20. doi: 10.1038/s41591-022-02041-5
8. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. (2021) 384(2):129–39. doi: 10.1056/NEJMoa2030186
9. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. (2021) 384(2):117–28. doi: 10.1056/NEJMoa2030183
10. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. (2019) 140(18):1463–76. doi: 10.1161/CIRCULATIONAHA.119.042929
11. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381(21):1995–2008. doi: 10.1056/NEJMoa1911303
12. Jensen J, Omar M, Kistorp C, Poulsen MK, Tuxen C, Gustafsson I, et al. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: a double-blinded, randomized, and placebo-controlled trial. Am Heart J. (2020) 228:47–56. doi: 10.1016/j.ahj.2020.07.011
13. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. (2020) 383(15):1413–24. doi: 10.1056/NEJMoa2022190
14. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF). Circulation. (2021) 143(6):516–25. doi: 10.1161/CIRCULATIONAHA.120.052186
15. Santos-Gallego CG, Vargas-Delgado AP, Requena-Ibanez JA, Garcia-Ropero A, Mancini D, Pinney S, et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. (2021) 77(3):243–55. doi: 10.1016/j.jacc.2020.11.008
16. Omar M, Jensen J, Ali M, Frederiksen PH, Kistorp C, Videbaek L, et al. Associations of empagliflozin with left ventricular volumes, mass, and function in patients with heart failure and reduced ejection fraction: a substudy of the empire HF randomized clinical trial. JAMA Cardiol. (2021) 6(7):836–40. doi: 10.1001/jamacardio.2020.6827
17. Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes Mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. (2020) 142(23):2205–15. doi: 10.1161/CIRCULATIONAHA.120.050255
18. Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. (2021) 42(6):700–10. doi: 10.1093/eurheartj/ehaa943
19. Cao H, Zhang Z, Qiu X. Efficacy analysis of dapagliflozin in the treatment of patients with chronic heart failure and reduced ejection fraction. Mod Diagn Treat. (2022) 33(21):3263–5. doi: 10.1007/s11739-024-03532-8
20. Cai G, Lin X. Effectiveness of sacubitril valsartan combined with dagliflozin in the treatment of heart failure with reduced ejection fraction and type 2 diabetes mellitus. Diabetes N World. (2021) 24(20):79–82.6. doi: 10.16658/j.cnki.1672-4062.2021.20.079
21. Dai R, Liu L, Yang X, Su Q, Qian Z. Study of dagliflozin in patients with intermediate ejection fraction heart failure combined with type 2 diabetes mellitus. J Pract Med. (2020) 36(18):2505–9. doi: 10.3969/j.issn.1006-5725.2020.18.010
22. Deng Y. Clinical efficacy analysis of dagliflozin in the treatment of elderly patients with HFrEF combined with T2DM. Mod Diagn Treat. (2022) 33(16):2407–9.
23. Fan H, Wang Y, Wang X, Dong X, Shao X, Zhang Y. Effect of recombinant human brain natriuretic peptide in combination with dagliflozin on the prognosis of heart failure patients with reduced ejection fraction. Chinese J N Drugs Clin. (2023) 42(2):141–4. doi: 10.14109/j.cnki.xyylc.2023.02.15
24. Ni R, Feng J. The efficacy and safety of dagliflozin in the treatment of elderly patients with reduced ejection fraction heart failure. Efficacy and safety of dagliflozin in the treatment of elderly patients with heart failure with reduced ejection fraction. Lingnan J Cardiovasc Dis. (2023) 29(01):68–73. doi: 10.26921/d.cnki.ganyu.2023.001117
25. He G. Effect of dagliflozin on cardiac function in patients with heart failure with reduced ejection fraction. Smart Health. (2022) 8(35):87–90. doi: 10.19335/j.cnki.2096-1219.2022.35.021
26. Su Y, Cheng J, Liu Y, Yang J, He H. Effect of dagliflozin combined with sacubitril valsartan sodium tablets on patients with type 2 diabetes mellitus combined with heart failure with reduced ejection fraction. Chin Foreign Med Res. (2022) 20(19):150–3. doi: 10.14033/j.cnki.cfmr.2022.19.039
27. Jia P. Clinical study of dagliflozin in elderly patients with heart failure combined with type 2 diabetes mellitus with reduced ejection fraction. [M.S.]. Hebei Medical University (2021). doi: 10.27111/d.cnki.ghyku.2021.000272
28. Xu L, Wang W, Zhang Y, Shi B. Clinical efficacy of dagliflozin in the treatment of type 2 diabetes mellitus combined with heart failure with mildly reduced ejection fraction. J Hainan Med Coll. (2023) 29(09):681–7. doi: 10.13210/j.cnki.jhmu.20230322.002
29. Li X, Lin Z. Clinical value of dagliflozin in elderly patients with heart failure with reduced ejection fraction combined with type 2 diabetes mellitus. China Med Guide. (2020) 18(35):1–3. doi: 10.15912/j.cnki.gocm.2020.35.002
30. Liu Y, Tang Z, Yang J, Liu J. Effect of dagliflozin on serum chloride and short-term prognosis in patients with heart failure with reduced ejection fraction. Chin Contemp Med. (2022) 29(31):64–71. doi: 10.3969/j.issn.1674-4721.2022.31.017
31. Zhang L, Wang J, Pan H. Effect of early application of dagliflozin in patients with heart failure with reduced ejection fraction. China Health Stand Admin. (2023) 14(2):167–71. doi: 10.3969/j.issn.1674-9316.2023.02.036
32. Wang F, Ma T, Zhao Q, Yang D. Effectiveness of dagliflozin in non-diabetic heart failure patients with mildly reduced ejection fraction. Chin Contemp Med. (2023) 30(04):82–6. doi: 10.3969/j.issn.1674-4721.2023.04.021
33. Yang P, Zhang Q, Wang X. Efficacy of dagliflozin in the treatment of heart failure patients with reduced ejection fraction. J Guangxi Med Univ. (2021) 38(7):1436–41. doi: 10.16190/j.cnki.45-1211/r.2021.07.031
34. Wu W, Zhang SY, Liu C, Shen J, Wang N, Wang Q, et al. A randomised controlled trial of the effect of empagliflozin on peak oxygen uptake in patients with HFmrEF. Chin J Cardiovasc Dis. (2022) 50(7):676–83. doi: 10.3760/cma.j.cn112148-20220120-00059
35. Zhang Z. Study of dagliflozin in patients with heart failure combined with type 2 diabetes mellitus with mildly reduced ejection fraction. [M.S.]. Hebei Medical University (2022).
36. Zheng H, Zhou B. Efficacy and prognostic analysis of sodium-glucose cotransporter protein-2 inhibitors in the treatment of heart failure with reduced ejection fraction. Med Inform. (2021) 34(10):92–6. doi: 10.3969/j.issn.1006-1959.2021.10.025
37. Ferreira JP, Anker SD, Butler J, Filippatos G, Iwata T, Salsali A, et al. Impact of anaemia and the effect of empagliflozin in heart failure with reduced ejection fraction: findings from EMPEROR-reduced. Eur J Heart Fail. (2022) 24(4):708–15. doi: 10.1002/ejhf.2409
38. Jensen J, Omar M, Kistorp C, Tuxen C, Gustafsson I, Kober L, et al. Effects of empagliflozin on estimated extracellular volume, estimated plasma volume, and measured glomerular filtration rate in patients with heart failure (empire HF renal): a prespecified substudy of a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. (2021) 9(2):106–16. doi: 10.1016/S2213-8587(20)30382-X
39. Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, et al. Empagliflozin in patients with heart failure, reduced ejection fraction, and volume overload: EMPEROR-reduced trial. J Am Coll Cardiol. (2021) 77(11):1381–92. doi: 10.1016/j.jacc.2021.01.033
40. Palau P, Amiguet M, Dominguez E, Sastre C, Mollar A, Seller J, et al. Short-term effects of dapagliflozin on maximal functional capacity in heart failure with reduced ejection fraction (DAPA-VO2): a randomized clinical trial. Eur J Heart Fail. (2022) 24(10):1816–26. doi: 10.1002/ejhf.2560
41. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387(12):1089–98. doi: 10.1056/NEJMoa2206286
42. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385(16):1451–61. doi: 10.1056/NEJMoa2107038
43. Tanaka A, Hisauchi I, Taguchi I, Sezai A, Toyoda S, Tomiyama H, et al. Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: a randomized trial (CANDLE). ESC Heart Fail. (2020) 7(4):1585–94. doi: 10.1002/ehf2.12707
44. Carbone S, Billingsley HE, Canada JM, Bressi E, Rotelli B, Kadariya D, et al. The effects of canagliflozin compared to sitagliptin on cardiorespiratory fitness in type 2 diabetes mellitus and heart failure with reduced ejection fraction: the CANA-HF study. Diabetes Metab Res Rev. (2020) 36(8):e3335. doi: 10.1002/dmrr.3335
45. Pitt B, Bhatt DL, Szarek M, Cannon CP, Leiter LA, McGuire DK, et al. Effect of sotagliflozin on early mortality and heart failure-related events: a post hoc analysis of SOLOIST-WHF. JACC Heart Fail. (2023) 11(8 Pt 1):879–89. doi: 10.1016/j.jchf.2023.05.026
46. McMurray JJV, Docherty KF, de Boer RA, Hammarstedt A, Kitzman DW, Kosiborod MN, et al. Effect of dapagliflozin versus placebo on symptoms and 6-minute walk distance in patients with heart failure: the DETERMINE randomized clinical trials. Circulation. (2024) 149(11):825–38. doi: 10.1161/CIRCULATIONAHA.123.065061
47. Fu Q, Zhou L, Fan Y, Liu F, Fan Y, Zhang X, et al. Effect of SGLT-2 inhibitor, dapagliflozin, on left ventricular remodeling in patients with type 2 diabetes and HFrEF. BMC Cardiovasc Disord. (2023) 23(1):544. doi: 10.1186/s12872-023-03591-3
48. Gao M, Wang B. Effect of dagliflozin on short-term prognosis of type 2 diabetes mellitus patients with HFpEF. Shenzhen J Integr Chin West Med. (2022) 32(10):99–102. doi: 10.16458/j.cnki.1007-0893.2022.10.030
49. Wei Y, Wang J, Cheng F, Xu H, Wang J. Clinical efficacy and mechanism of action of cagliflozin in the treatment of female heart failure patients with diabetes mellitus and reduced ejection fraction. J Pract Cardiovasc Pulmon Vasc Dis. (2020) 28(09):26–9 +39. doi: 10.3969/j.issn.1008-5971.2020.09.006
50. Chen A, Liang G, Pan M, Zhai Y, Bai J, Yi Y, et al. Comparison of the efficacy and safety of dagliflozin and empagliflozin in patients with type 2 diabetes mellitus combined with heart failure with reduced ejection fraction. Straits Pharmacol. (2023) 35(08):72–6. doi: 10.3969/j.issn.1006-3765.2023.08.019
51. Du B, Yang Q, Liu Y, Ma C. Clinical effect analysis of sacubitril valsartan combined with dagliflozin in the treatment of patients with reduced ejection fraction heart failure without diabetes mellitus. Syst Med. (2023) 8(19):101–4. doi: 10.19368/j.cnki.2096-1782.2023.19.101
52. Gong Z, Zhang Y, Fang L, Shen Z, Yuan Y. Clinical study of sodium-glucose cotransporter protein-2 inhibitor combined with sacubitril valsartan sodium in the treatment of heart failure patients with reduced ejection fraction. Adv Mod Biomed. (2023) 23(07):1299–303 +357. doi: 10.13241/j.cnki.pmb.2023.07.019
53. He P. Efficacy of dagliflozin in elderly chronic heart failure combined with type 2 diabetes mellitus and hyperuricaemia. Front Med. (2023) 13(35):53–5.
54. Lv G, Zhang H, Li H, Zheng X, Geng L, An H, et al. Effect of dapagliflozin on left ventricular remodeling and renal function in patients with T2DM complicated with HFrEF. J Cardiovasc Cerebrovasc Dis Integr Trad Chin West Med. (2023) 21(19):3594–7. doi: 10.12102/j.issn.1672-1349.2023.19.022
55. Pan L, Sun G, Huang Z, Haung F. Effects of engeleukin combined with rhBNP therapy on ventricular remodelling and serum NT-proBNP, hs-CRP, and IL-6 levels in patients with heart failure with reduced ejection fraction. J Clin Exp Med. (2023) 22(18):1934–8. doi: 10.3969/j.issn.1671-4695.2023.18.009
56. Peng X, Guo D, Hou Y, Wu J, Jiang S, Zheng X, et al. Clinical efficacy of dagliflozin in the treatment of patients with intermediate ejection fraction heart failure combined with type 2 diabetes mellitus. World Abstr Curr Med Inform. (2023) 24(99):99–102, 7. doi: 10.3969/j.issn.1671-3141.2023.099.020
57. Wu F, Chen G. Clinical efficacy of sacubitril valsartan combined with dagliflozin in the treatment of heart failure with reduced ejection fraction. Clin Ration Use Drugs. (2023) 16(36):1–4. doi: 10.15887/j.cnki.13-1389/r.2023.36.001
58. Wu J. Clinical study of dagliflozin in the treatment of chronic heart failure patients with reduced ejection fraction. Prim Med Forum. (2023) 27(25):56–58, 79. 10.19435/j.1672-1721.2023.25.018
59. Wu N. Application effect of dagliflozin on heart failure patients with reduced ejection fraction. Henan Med Res. (2023) 32(21):3995–9. doi: 10.3969/j.issn.1004-437X.2023.21.037
60. Yang L, Wang Y, Sun X, Xue X, Bai R, He J, et al. Clinical effect of dagliflozin combined with sacubitril valsartan sodium in the treatment of heart failure with reduced ejection fraction. Clin Med Res Pract. (2023) 8(27):37–40. doi: 10.19347/j.cnki.2096-1413.202327010
61. Zhao C, Gu C, Jiang S, Lv K. Effect of dagliflozin on heart failure patients with reduced ejection fraction. West Med. (2023) 35(07):1052–6. doi: 10.3969/j.issn.1672-3511.2023.07.022
62. Chen W, Zhao Z, Cui L, Ma X, Shen L. Efficacy and safety of dagliflozin combined with sacubitril valsartan in the treatment of heart failure patients with reduced ejection fraction. Int J Med Health. (2024) 30(2):301–5. doi: 10.3760/cma.j.issn.1007-1245.2024.02.025
63. Gong Q, You Q, Wang H. Effect of dagliflozin on cardiac function and serum NT-proBNP and cTnⅠ levels in heart failure patients with reduced ejection fraction. Clinical Med Eng. (2024) 31(02):197–8. doi: 10.3969/j.issn.1674-4659.2024.02.0197
64. Lu Q, Deng Y, Yang Z, Huang H, Qin P, Luo W, et al. Effects of dagliflozin on myocardial work and energy metabolism in patients with ejection fraction-decreased heart failure without type 2 diabetes. Chin Pharm. (2024) 27(01):100–8. doi: 10.12173/j.issn.1008-049X.202312138
65. Zhang L, Zhang Z, Li S, Chen Y. Evaluation of the therapeutic effect of nosintor combined with dagliflozin in heart failure with reduced ejection fraction. China Modn Drug Appl. (2024) 18(04):21–4. doi: 10.14164/j.cnki.cn11-5581/r.2024.04.005
66. Ueda T, Kasama S, Yamamoto M, Nakano T, Ueshima K, Morikawa Y, et al. Effect of the sodium-glucose cotransporter 2 inhibitor canagliflozin for heart failure with preserved ejection fraction in patients with type 2 diabetes. Circ Rep. (2021) 3(8):440–8. doi: 10.1253/circrep.CR-21-0030
67. Akasaka H, Sugimoto K, Shintani A, Taniuchi S, Yamamoto K, Iwakura K, et al. Effects of ipragliflozin on left ventricular diastolic function in patients with type 2 diabetes and heart failure with preserved ejection fraction: the EXCEED randomized controlled multicenter study. Geriatr Gerontol Int. (2022) 22(4):298–304. doi: 10.1111/ggi.14363
68. Oldgren J, Laurila S, Akerblom A, Latva-Rasku A, Rebelos E, Isackson H, et al. Effects of 6 weeks of treatment with dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on myocardial function and metabolism in patients with type 2 diabetes: a randomized, placebo-controlled, exploratory study. Diabetes Obes Metab. (2021) 23(7):1505–17. doi: 10.1111/dom.14363
69. Bu X, Li C, Li L, Liu Y. Efficacy of dagliflozin in heart failure with preserved ejection fraction in the elderly. Chin J Geriatr Cardiovasc Dis. (2022) 24(7):689–92. doi: 10.3969/j.issn.1009-0126.2022.07.005
70. Li L. Observation on the efficacy of dagliflozin in patients with ejection fraction preserved heart failure with type 2 diabetes mellitus. [Master]. Henan University (2021).
71. Liu S, Luo L, Zhao C. Therapeutic efficacy of dagliflozin in elderly patients with type 2 diabetes mellitus combined with ejection fraction preserved heart failure and its effect on cardiac function. Chin Med. (2022) 17(4):539–43. doi: 10.12114/j.issn.1008-5971.2022.00.061
72. Luo P. Effectiveness of dagliflozin in the treatment of patients with type 2 diabetes mellitus with heart failure with normal ejection fraction. Zhongguancang Me Sci Technol Co. (2022) 34(12):29–31 +5. doi: 10.3969/j.issn.1672-0369.2022.12.009
73. Sun H. Study of dagliflozin in patients with ejection fraction preserved heart failure combined with type 2 diabetes mellitus. [MSc]. Nanchang University (2021).
74. Xu X, Xu S. Effect of dagliflozin on cardiac function and ventricular structure in patients with type 2 diabetes mellitus combined with heart failure with preserved ejection fraction. World Abstr Curr Med Inform. (2021) 21(84):38–41. doi: 10.3969/j.issn.1671-3141.2021.84.011
75. Yang F. Study on the effect of dagliflozin on cardiac function in patients with type 2 diabetes mellitus combined with heart failure with preserved ejection fraction. [Master]. Bengbu Medical College (2022).
76. Zeng F, Liu J, Zhang L. Efficacy analysis of dagliflozin in the treatment of heart failure with preserved ejection fraction. J Anhui Med Coll. (2023) 22(06):52–4. doi: 10.20072/j.cnki.issn2097-0196.2023.06.018
77. Duan H, Zhao X. Cardioprotective effect of dagliflozin on type 2 diabetes mellitus patients with combined ejection fraction preserved heart failure. Chin Health Care. (2023) 41(21):184–7.
78. Liang M, Lin W. Study on the mechanism of clinical benefit of adding dagliflozin to the standard treatment of nosintocin in the treatment of heart failure with preserved ejection fraction. Chin For Med Res. (2023) 2(36):15–7.
79. Liu S, Shang S, Su H, Liu C. Effects of dagliflozin on insulin resistance, ventricular remodelling and MHR and NT-proBNP levels in patients with T2DM with HFPEF. J Mol Diagn Ther. (2023) 15(2):285–9. doi: 10.3969/j.issn.1674-6929.2023.02.027
80. Lv X. Effect of dagliflozin combined with conventional heart failure treatment regimen for type 2 diabetes mellitus combined with ejection fraction preserved heart failure on patients’ cardiac function and serological indices. Pharmacoecon China. (2023) 18(9):80–2, 6. doi: 10.12010/j.issn.1673-5846.2023.09.018
81. Wang J, Qian M, Wang Y. Effect of SGLT-2 inhibitors on extracellular volume fraction in patients with diabetes mellitus combined with heart failure with preserved ejection fraction. Chin J Health Care Med. (2023) 25(2):142–6. doi: 10.3969/j.issn.1674-3245.2023.02.006
82. Zhang Y, Yang W. Observation on the efficacy of dagliflozin in elderly type 2 diabetes mellitus combined with ejection fraction preserved heart failure. J Aerosp Med. (2024) 35(01):11–5. doi: 10.3969/j.issn.2095-1434.2024.01.005
83. Alkabbani W, Zongo A, Minhas-Sandhu JK, Eurich DT, Shah BR, Alsabbagh MW, et al. Sodium-Glucose cotransporter-2 inhibitors and urinary tract infections: a propensity score-matched population-based cohort study. Can J Diabetes. (2022) 46(4):392–403.e13. doi: 10.1016/j.jcjd.2021.12.005
84. Kelly MS, Lewis J, Huntsberry AM, Dea L, Portillo I. Efficacy and renal outcomes of SGLT2 inhibitors in patients with type 2 diabetes and chronic kidney disease. Postgrad Med. (2019) 131(1):31–42. doi: 10.1080/00325481.2019.1549459
85. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. (2010) 375(9733):2223–33. doi: 10.1016/S0140-6736(10)60407-2
86. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. (2013) 15(9):853–62. doi: 10.1111/dom.12127
87. Li C, Zhang J, Xue M, Li X, Han F, Liu X, et al. SGLT2 Inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. (2019) 18(1):15. doi: 10.1186/s12933-019-0816-2
88. Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol. (2017) 16(1):9. doi: 10.1186/s12933-016-0489-z
89. Prasad SB, Guppy-Coles K, Stanton T, Armstrong J, Krishnaswamy R, Whalley G, et al. Relation of left atrial volumes in patients with myocardial infarction to left ventricular filling pressures and outcomes. Am J Cardiol. (2019) 124(3):325–33. doi: 10.1016/j.amjcard.2019.05.007
90. Tsampasian V, Elghazaly H, Chattopadhyay R, Ali O, Corballis N, Chousou PA, et al. Sodium glucose co-transporter 2 inhibitors in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Prev Cardiol. (2022) 29(6):e227–9. doi: 10.1093/eurjpc/zwab189
91. Pandey AK, Dhingra NK, Hibino M, Gupta V, Verma S. Sodium-glucose cotransporter 2 inhibitors in heart failure with reduced or preserved ejection fraction: a meta-analysis. ESC Heart Fail. (2022) 9(2):942–6. doi: 10.1002/ehf2.13805
92. Zhao L, Guo W, Huang W, Wang L, Huang S. Benefit of sodium-glucose cotransporter-2 inhibitors on survival outcome is related to the type of heart failure: a meta-analysis. Diabetes Res Clin Pract. (2022) 187:109871. doi: 10.1016/j.diabres.2022.109871
93. Ross L, Patel S, Stevens W, Burns A, Prior D, La Gerche A, et al. The clinical implications of left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol. (2022) 40(10):1986–92. doi: 10.55563/clinexprheumatol/irc0ih
Keywords: sodium–glucose cotransporter 2 inhibitors, heart failure, cardiac structural remodeling, heart failure with reduced ejection fraction, heart failure with preserved ejection fraction
Citation: Lan X, Zhu H, Cao Y, Hu Y, Fan X, Zhang K and Wu M (2024) Effects of different sodium–glucose cotransporter 2 inhibitors in heart failure with reduced or preserved ejection fraction: a network meta-analysis. Front. Cardiovasc. Med. 11:1379765. doi: 10.3389/fcvm.2024.1379765
Received: 31 January 2024; Accepted: 22 April 2024;
Published: 23 May 2024.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Hector A. Cabrera-Fuentes, University of Giessen, Germany© 2024 Lan, Zhu, Cao, Hu, Fan, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjie Cao, MTg2MTAwODk3MjdAMTYzLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.