
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 05 July 2024
Sec. Clinical and Translational Cardiovascular Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1379189
 Walter Masson1*
Walter Masson1* Martín Lobo2
Martín Lobo2 Juan Patricio Nogueira3,4
Juan Patricio Nogueira3,4 Alfredo Matias Rodriguez-Granillo5,6
Alfredo Matias Rodriguez-Granillo5,6 Leandro Ezequiel Barbagelata1
Leandro Ezequiel Barbagelata1 Daniel Siniawski1
Daniel Siniawski1
Background: The anti-inflammatory effect could be one of the mechanisms by which semaglutide reduces cardiovascular risk in patients with type 2 diabetes mellitus (T2DM) and/or obesity. Determining the anti-inflammatory effect of semaglutide was the objective of this systematic review and meta-analysis.
Methods: This meta-analysis was performed according to the PRISMA guidelines. A literature search was performed to detect randomised clinical trials that have quantified the effect of semaglutide on C-reactive protein (CRP) levels compared to placebo or a control group (other glucose-lowering drugs). The primary outcome was CRP index (final CRP/basal CRP). A random-effects model was used.
Results: Thirteen randomised clinical trials were considered eligible (n = 26,131). Overall, semaglutide therapy was associated with lower CRP index values compared to the placebo group (SMD −0.56; 95% CI −0.69 to −0.43, I2 92%) or the control group (SMD −0.45; 95% CI −0.68 to −0.23, I2 82%).Such an association was similarly observed when different treatment regimens (subcutaneous vs. oral) or different populations (patients with or without T2DM) were analysed. The sensitivity analysis showed that the results were robust.
Conclusion: The present meta-analysis demonstrated that the use of semaglutide was associated with a reduction in inflammation irrespective of the population evaluated or the treatment regimen used. These findings would explain one of the mechanisms by which semaglutide reduces cardiovascular events.
Systematic Review Registration: PROSPERO [CRD42024500551].
Glucagon-like peptide-1 (GLP-1RA) receptor agonists possess multiple favorable metabolic, anti-inflammatory effects and their use is associated with marked body weight reduction (1–3). More importantly, this drug class has been shown to reduce cardiovascular events in patients with type 2 diabetes mellitus (T2DM) at high cardiovascular risk or with established cardiovascular disease (4, 5). Accordingly, current guidelines recommend the use of GLP-1RAs as first-line antidiabetic therapies in patients at high cardiovascular risk (6).
Semaglutide is a potent GLP-1RA used in the treatment of T2DM with proven cardiovascular benefits (7). It is currently available in formulations for oral and subcutaneous administration (8). In addition, the use of higher doses of semaglutide was associated with a marked decrease in body weight in patients with overweight or obesity (9). Recently, the cardiovascular benefit with the use of high doses of semaglutide was also seen in patients with overweight or obesity and high cardiovascular risk but without T2DM (10).
Multiple mechanisms have been proposed to explain the cardiovascular benefit of semaglutide. These include direct cardiac effects (protection against myocardial ischaemia, reduction of epicardial adipose tissue and improvement of cardiac contractility), vascular (vasodilator effect and improvement of endothelial dysfunction), renal (reduced glomerular filtration rate and proteinuria drop) and metabolic (blood pressure decreased, lipid profile improved and glucose-lowering effect) (11).
Inflammation plays a crucial role in the development and progression of atherosclerosis and its cardiovascular complications (12). Likewise, elevation of inflammatory markers predicts the development of T2DM and its complications (13–15). Strong evidence has shown the anti-inflammatory effect of GLP-1RA (16, 17). The favorable effects on atherogenic lipoproteins and hepatic steatosis indices support the pleiotropic benefits of semaglutide beyond glycemic control (18). Consequently, anti-inflammatory actions could be an additional relevant mechanism to explain the cardiovascular benefit of this type of drugs. A previously published meta-analysis has reported a significant decrease in several anti-inflammatory markers with the use of GLP-1RA (19). However, it did not include trials with semaglutide.
Considering what has previously been discussed, the primary objective of this study was to perform a systematic review and updated meta-analysis on the anti-inflammatory effect of semaglutide.
This study was conducted in accordance with the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) to report systematic reviews (20) (PRISMA checklist in Supplementary Material). This systematic review was recorded in PROSPERO (CRD42024500551).
A literature search was performed identifying clinical trials of semaglutide published until 01 Dec 2023. As two independent reviewers searched the electronic PubMed/MEDLINE, Scielo, Embase and Lilacs databases using the either the Medical Subject Headings (MeSH) terms or keywords “semaglutide” or “GLP-1RA”, combined with “inflammation” or “C-reactive protein (CRP)” or “cytokines”, or using the term “semaglutide” with the “randomised clinical trials” filter, and the data were extracted. To match each individual descriptor and define the search, we use the Boolean operator “AND.” In addition, the authors also searched for ’snow ball’ to find other articles of interest. Only studies conducted in humans were included No language restrictions were used in the search.
The following inclusion criteria were established: (a) Studies that have analysed the anti-inflammatory effect of semaglutide (expressed as ultra-sensitive blood CRP levels) compared to a placebo group or a group with another anti-diabetic regimen; (b) Studies with a duration of follow-up ≥3 months; (c) Randomized clinical trials.
Potential risks of bias were evaluated for all included trials, using a tool developed for this purpose (Rob 2) (21). This tool assesses bias on five different domains: bias arising from randomisation, bias due to deviations from planned intervention, bias due to lack of outcome data, bias in outcome measurement, and bias in selection of reported outcome. Each domain was rated as “high risk”, “low risk” or “with some concerns”, further obtaining an overall rating of each study. Two authors determined the risk of bias for each article. Any disagreement was resolved with a third reviewer.
The effect of semaglutide therapy on CRP reduction was calculated. Effect size measures were expressed as standardised mean differences (SMDs) between CRP indices with their respective 95% confidence intervals (95% CIs). These indices were obtained by dividing the final value by the baseline CRP value into both groups. The 95% CIs were calculated manually when not reported in the original publications (22). Furthermore, statistical I2 was calculated to quantify heterogeneity and inconsistency between studies. A random-effects model was chosen because the trials differ in the populations included or in the follow-up time and because the calculated heterogeneity (I2) was elevated. To compare the average effect between subgroups, we used a Z-test. The level of statistical significance was set to 0.05 (2-tail analysis). Statistical software R (version 3.5.1) was used for the analysis (23).
The sensitivity analysis consists of replicating the results of the meta-analysis, excluding at each step, each of the studies included in the review. If the results obtained are similar, both in the direction/magnitude of the effect and in the statistical significance, the analysis indicate that the result is robust.
Begg & Mazumdar test and the Egger test with mixed effects model were done. Additionally, a funnel plot using the standard error for standardized mean difference was created.
The search included 331 potentially relevant articles after examining the titles/summary, excluding 290 studies because they were duplicate publications or because they did not evaluate the purpose of this study. After careful reading of the remaining articles, 29 studies were removed, because the exposure/event of interest was not reported. A flowchart of the trial selection process is shown in Figure 1.
Thirteen randomised clinical trials (n = 26,131 patients) were identified and considered eligible for this systematic review (10, 24–35). A total of 13,923 subjects were allocated to the semaglutide group and 12,208 individuals were randomised to a control group. Such a control group was assigned to placebo in 10 trials (10, 24, 27–32, 34, 35), to another anti- diabetic drug in 2 trials (exenatide and empagliflozin) (24, 26) and to both options in the remaining trial (liraglutide and placebo) (33).
In total, 5 studies included patients with T2DM (24–27, 29) while another 6 studies evaluated patients with overweight or obesity without T2DM (10, 28, 30, 31, 33, 34). Within the latter, a study specifically including patients with heart failure with preserved ejection fraction is highlighted (35). Finally, a study simultaneously analysed patients with or without T2DM (32). The population characteristics of the studies included in this review are shown in Table 1.
All studies included in this review showed low risk of bias. The quality of the selected studies is shown in Figure 2.
Overall, this meta-analysis shows that semaglutide therapy was associated with lower CRP index values compared to placebo (SMD −0.56; 95% CI −0.69 to −0.43, I2 92%) or when comparing semaglutide treatment vs. a control group consisting of patients medicated with other glucose-lowering drugs (SMD −0.45; 95% CI −0.68 to −0.23, I 382%) (Figure 3).
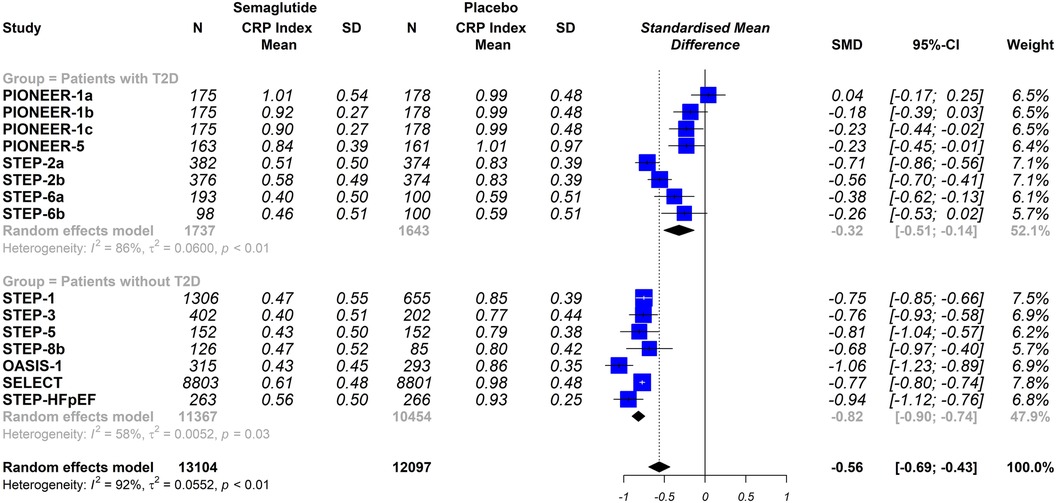
Figure 3 Effect of semaglutide therapy on CRP index. Random-effects model, standardised mean differences (SMD), 95% confidence intervals (CI) and statistical I2. Top: semaglutide vs. placebo trial group. Bottom: group of studies with semaglitide versus other drugs. PIONEER-1 a, b and c: semaglutide 3, 7 and 14 mg orally, respectively. STEP-2 a and b: semaglutide 2.4 mg and 1 mg subcutaneous, respectively. STEP-6 a and b: semaglutide 2.4 mg and 1.7 mg subcutaneously, respectively. STEP-8b: semaglutide 2.4 mg arm vs placebo.
The stratified analysis was performed by analysing only the trials that used a placebo group as comparator, When the trials were analysed according to the different dosing schemes (subcutaneous vs. oral), the results were not statistically significantly different (oral semaglutide group: SMD −0.33; 95% CI −0.75 to 0.08, I2 95%); semaglutide subcutaneous dose group: SMD −0.69; 95% CI −0.78 to −0.60, I2 74%); interaction p = 0.098 (Figure 4). Furthermore, when the trials were analysed according to the included populations (patients with or without T2DM), the results were similar (patients with T2DM: SMD −0.32; 95% CI −0.51 to −0.14, I2 86%); patients without T2DM: SMD −0.82; 95% CI −0.90 to −0.74, I2 58%); interaction p ≤ 0.0001 (Figure 5).
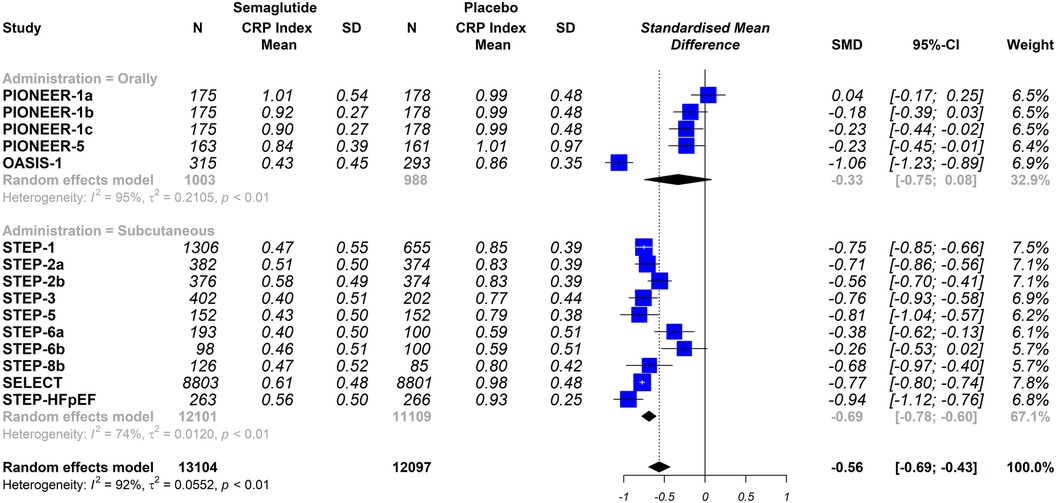
Figure 4 Effect of semaglutide therapy on CRP rate stratified by groups of patients with or without type 2 diabetes mellitus (T2DM). Analysis performed only with trials vs. placebo. Random-effects model, standardised mean differences (SMD), 95% confidence intervals (CI) and statistical I2. PIONEER-1 a, b and c: semaglutide 3, 7 and 14 mg orally, respectively. STEP-2 a and b: semaglutide 2.4 mg and 1 mg subcutaneous, respectively. STEP-6 a and b: semaglutide 2.4 mg and 1.7 mg subcutaneously, respectively. STEP-8b: semaglutide 2.4 mg arm vs placebo.
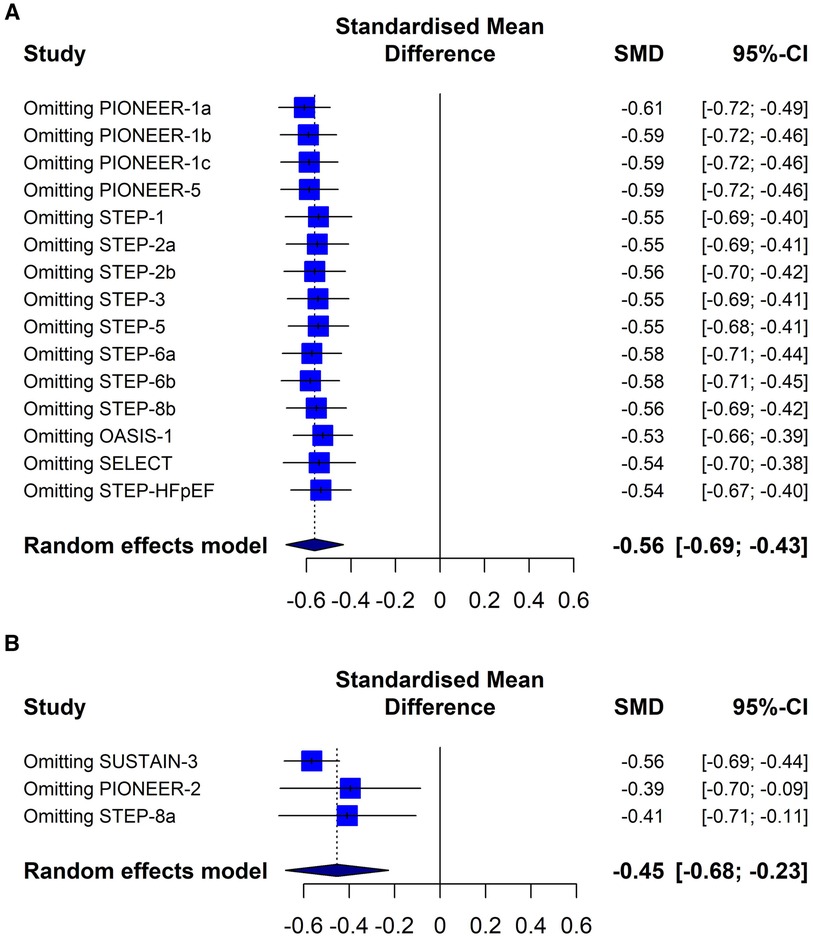
Figure 5 The effect of semaglutide therapy on the stratified CRP rate by the way semaglutide was administered in diabetic (A) and non-diabetic population (B). Analysis performed only with trials vs. placebo. Random-effects model, standardised mean differences (SMD), 95% confidence intervals (CI) and statistical I2. PIONEER-1 a, b and c: semaglutide 3, 7 and 14 mg orally, respectively. STEP-2 a and b: semaglutide 2.4 mg and 1 mg subcutaneous, respectively. STEP-6 a and b: semaglutide 2.4 mg and 1.7 mg subcutaneously, respectively. STEP-8b: semaglutide 2.4 mg arm vs. placebo.
The sensitivity analysis showed the same directionality and magnitude of the overall results when the trials were excluded one by one (Figure 6). The analytical evaluation by the Begg & Mazumdar and the Egger tests do not suggest publication bias (p = 0.472 and p = 0,102, respectively). The graphic representation does not show a clear asymmetry (Supplementary Figure S1).

Figure 6 Sensitivity analysis. (A) Semaglutide versus placebo trial group. (B) Group of studies with semaglitide versus other drugs. PIONEER-1 a, b and c: semaglutide 3, 7 and 14 mg orally, respectively. STEP-2 a and b: semaglutide 2.4 mg and 1 mg subcutaneous, respectively. STEP-6 a and b: semaglutide 2.4 mg and 1.7 mg subcutaneously, respectively. STEP-8b: semaglutide 2.4 mg arm vs. placebo.
In this meta-analysis we observed that treatment with semaglutide compared to placebo or to a control group that included patients medicated with other glucose-lowering drugs, was associated with lower CRP levels, regardless of the regimen used or of the population evaluated. Compared to placebo or the control group receiving other glucose-lowering drugs, the CRP rate in the semaglutide treated group was 44% and 55% lower, respectively. This value is adequately adjusted with the CRP reductions associated with the use of semaglutide observed in some of the studies included in this review (range between 39.1% and 59.6%) (10, 30, 31, 33, 35).
Evidence from epidemiological studies has shown that elevated CRP levels, a surrogate for systemic inflammation, are associated with increased cardiovascular risk (36). Furthermore, in some clinical trials evaluating patients treated with statins, elevated CRP levels were a predictor of the risk of future cardiovascular events and death stronger than cholesterol bound to low-density lipoprotein (LDL-C) itself (37). CRP is predominantly synthesised in the liver, mainly in response to interleukin-6 (IL-6) and to a lesser extent to interleukin 1β (IL-1β) and 17 (IL-17) and tumour necrosis factor alfa (TNF-α) (38). Furthermore, the involvement of inflammasomes is relevant in this process. The latter are complexes of high molecular weight proteins formed in the cytosolic compartment in response to different stimuli. Among the most widely studied in the context of atherosclerosis is the cytosolic multiprotein signaling complex called the NLRP3 inflammasome, which serves as a platform for the activation of caspase-1 and promotes the synthesis of pro-inflammatory cytokines (39).
In clinical practice, CRP values exceeding 3 mg/L are considered as a marker of cardiovascular risk (40, 41). Interestingly, 6 of the 8 studies included in this systematic review reporting baseline CRP values showed elevated levels (between 3 and 4.8 mg/L), reflecting that the populations evaluated, mostly patients with T2DM and obesity, possess a chronic inflammatory state.
Several drugs evaluated in the field of cardiovascular prevention have shown to simultaneously have an anti-inflammatory (CRP lowering) and cardioprotective effect (decrease in cardiovascular atherosclerotic events). These results strongly suggest that in addition to LDL-C, CRP could be a new treatment target. Statins, colchicine, IL1β receptor antagonists and bempedoic acid are some examples (42–45).
Furthermore, many in vitro studies have shown that GLP-1RAs may attenuate or suppress the expression of various inflammatory factors, including TNF-α, IL-6 and endothelial adhesion molecules (46, 47). In addition, GLP-1RAs have been found to inhibit the activation of the NLRP3 inflammasome, thereby reducing the maturation and release of inflammatory cytokines (48). Another systemic anti-inflammatory mechanism induced by liraglutide and semaglutide and involved in the reduction of CRP and cardiovascular risk, is decreased intestinal permeability through activation of Brunner's gland secretion and modulation of intraepithelial lymphocytes function (49). Moreover, GLP-1RAs appear to exert a direct epigenetic effect in patients with T2DM, regulating microRNAs that are involved in maintaining endothelial cell homeostasis (50). Accordingly, the anti-inflammatory mechanisms associated with GLP-1RA treatment could be closely linked to the cardiovascular benefit seen in clinical trials (7, 10). Furthermore, GLP-1RA therapy might play a relevant role in the treatment of other entities that possess a chronic inflammatory component, including steatohepatitis, neurodegenerative disorders, diabetic nephropathy, asthma or psoriasis (51–54).
A previously published meta-analysis showed that the use of GLP-1RA was associated with a significant reduction in CRP levels (18). However, the vast majority of the information comes from studies that have evaluated the use of exenatide and liraglutide. Furthermore, a descriptive and exploratory analysis from the PIONEER and SUSTAIN programs also showed that the use of semaglutide was associated with a reduction in CRP, although this analysis only included 4 trials (55). In this context, our meta-analysis first evaluated all information reported in clinical trials on the association between the use of semaglutide and CRP levels.
Although the initial presentation of semaglutide for clinical use was subcutaneous, administered once weekly, an oral formulationwas recently developed (56). The pharmacokinetic and pharmacodynamic differences between these formulations could be associated with different biological effects (57). In that regard, the results of our study showed that the anti-inflammatory effect was independent of the dosage form used. While the primary outcome in the subgroup of trials using oral semaglutide “rips 0”, the interaction p-value was not statistically significant.
Another interesting finding of this meta-analysis was that the effect on CRP levels was observed in both the population with or without T2DM. The elucidation of various cellular mechanisms linking inflammation with insulin resistance and β-cell dysfunction has revolutionised knowledge about the molecular pathogenesis of T2DM, currently establishing that this entity is a metabolic and inflammatory disorder (58). In this context, and given the link between inflammation and progression of T2DM and its complications, the finding of our study on CRP decline in this population is relevant. On the other hand, it has also been observed that patients with obesity have a chronic inflammatory state of low grade (59). In addition, obesity is considered a pre-diabetic state (60). Consequently, we would be faced with a “continuous” pathophysiology involving adipose tissue, inflammation, insulin resistance, pancreatic dysfunction, the occurrence of T2DM and development of micro- and macrovascular complications. All of this makes the findings from our study on the anti-inflammatory impact of semaglutide in the population without T2DM, mostly with obesity and pre-diabetes, of clinical relevance as well. Looking at the stratified analysis, it would appear that the impact on the CRP rate is greater in the population without T2DM, although such findings could relate to the higher semaglutide doses used in this population.
Finally, our findings showed a clear anti-inflammatory effect of semaglutide when compared to placebo, but also when compared to other anti-diabetic drugs with proven cardiovascular benefit. Interestingly, within the drugs tested were other GLP-1RAs such as exenatide or liraglutide. It would thus appear that the molecular, pharmacokinetic and pharmacodynamic differences in these drugs could influence the anti-inflammatory effect (61).
In summary, alongside its well-established anti-inflammatory properties, the impact on weight loss, glycemic control, and reduction of cardiovascular risk factors—such as blood pressure and lipid profile—explains the cardiovascular benefits of semaglutide (62, 63).
This meta-analysis has some limitations. First, there was clinical heterogeneity due to the different characteristics of the populations and the different follow-up times. Likewise, statistical heterogeneity was high. Although I2 is commonly used for assessment of heterogeneity, it is not a perfect measure and its value depends on the precision and size of the included studies. In this case, high heterogeneity can be attributed more to the magnitude of the effect than to the direction of the effect, being influenced by the low number of patients evaluated in many of the studies. In that regard, the sensitivity analysis showed robust results. Second, we were unable to quantitatively analyze the absolute or percentage reduction in CRP values because these data were not published by the majority of the original studies. Instead, we were able to analyze the index between the final and baseline values for each arm. Interpreting the index clinically proves more challenging than simply describing absolute values. Third, due to the absence of reported data in the original studies, we were unable to analyze additional inflammatory markers. Another frequently used inflammatory markers include acute-phase proteins, essentially serum amyloid A, fibrinogen and procalcitonin, and cytokines, predominantly TNFα, interleukins 1β, 6, 8, 10 and 12 and their receptors and IFNγ (64). However, their use in clinical practice is often limited, due to lacking analytical or clinical validation, or technical challenges. Four, the inability to identify additional therapies and any concomitant pathologies that could affect the inflammatory state represents another limitation of this study. Finally, the analysis of the studies comparing the use of semaglutide with other antidiabetic drugs only included a small number of studies. Therefore, additional information will be required to establish definitive conclusions.
The present updated meta-analysis of randomised clinical trials demonstrated that the use of semaglutide was associated with a marked anti-inflammatory effect. According to the stratified analysis, the effect would occur irrespective of the schemes used or the populations tested. The inflammatory pathway could explain much of the cardiovascular benefit seen in large clinical trials with semaglutide.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
WM: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization. ML: Writing – review & editing, Investigation, Formal Analysis. JN: Writing – original draft, Investigation, Data curation, Conceptualization. AR-G: Investigation, Writing – review & editing, Data curation. LB: Writing – review & editing. DS: Writing – review & editing, Investigation, Data curation, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Journal submission was financially funded by Novo Nordisk Pharma Argentina S.A. The authors take full responsibility for the content and conclusions stated in this manuscript. Novo Nordisk Pharma Argentina S.A. neither influenced the content of this publication nor was it involved in the study design, data collection, analysis, interpretation or review.
WM, ML and DS have served as a speaker from Novo Nordisk.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1379189/full#supplementary-material
Supplementary Figure 1
Funnel plot to assess publication bias.
1. Parab P, Chaudhary P, Mukhtar S, Moradi A, Kodali A, Okoye C, et al. Role of glucagon-like peptide-1 (GLP-1) receptor agonists in cardiovascular risk management in patients with type 2 diabetes mellitus: a systematic review. Cureus. (2023) 15:e45487. doi: 10.7759/cureus.45487
2. Patti AM, Rizvi AA, Giglio RV, Stoian AP, Ligi D, Mannello F. Impact of glucose-lowering medications on cardiovascular and metabolic risk in type 2 diabetes. J Clin Med. (2020) 9(4):912. doi: 10.3390/jcm9040912
3. Patti AM, Nikolic D, Magan-Fernandez A, Giglio RV, Castellino G, Chianetta R, et al. Exenatide once-weekly improves metabolic parameters, endothelial dysfunction and carotid intima-media thickness in patients with type-2 diabetes: an 8-month prospective study. Diabetes Res Clin Pract. (2019) 149:163–9. doi: 10.1016/j.diabres.2019.02.006
4. Guo X, Sang C, Tang R, Jiang C, Li S, Liu N, et al. Effects of glucagon-like peptide-1 receptor agonists on major coronary events in patients with type 2 diabetes. Diabetes Obes Metab. (2023) 25(Suppl 1):53–63. doi: 10.1111/dom.15043
5. Giglio RV, Stoian AP, Al-Rasadi K, Banach M, Patti AM, Ciaccio M. Novel therapeutical approaches to managing atherosclerotic risk. Int J Mol Sci. (2021) 22(9):4633. doi: 10.3390/ijms22094633
6. Marx N, Federici M, Schütt K, Müller-Wieland D, Ajjan RA, Antunes MJ, et al. 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. (2023) 44:4043–140. doi: 10.1093/eurheartj/ehad192
7. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2016) 375:1834–44. doi: 10.1056/NEJMoa1607141
8. Gouveri E, Popovic DS, Papanas N. Potential new therapeutic implications of semaglutide: new colours of the rainbow? Diabetes Ther. (2023) 15:13–8. doi: 10.1007/s13300-023-01506-1
9. Amaro A, Sugimoto D, Wharton S. Efficacy and safety of semaglutide for weight management: evidence from the STEP program. Postgrad Med. (2022) 134(sup1):5–17. doi: 10.1080/00325481.2022.2147326
10. Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. (2023) 389:2221–32. doi: 10.1056/NEJMoa2307563
11. Morales J, Shubrook JH, Skolnik N. Practical guidance for use of oral semaglutide in primary care: a narrative review. Postgrad Med. (2020) 132:687–96. doi: 10.1080/00325481.2020.1788340
12. Boczar KE, Beanlands R. Hearts on fire: the role of inflammation in the pathogenesis of atherosclerotic cardiovascular disease and how we can tend to the flames. Can J Cardiol. (2022) 38:1553–57. doi: 10.1016/j.cjca.2022.05.023
13. Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes—results of the prospective population-based European prospective investigation into cancer and nutrition (EPIC)-Potsdam study. Diabetes. (2003) 52:812–17. doi: 10.2337/diabetes.52.3.812
14. Xu Y, Whitmer K. C-reactive protein and cardiovascular disease in people with diabetes: high-sensitivity CRP testing can help assess risk for future cardiovascular disease events in this population. Am J Nurs. (2006) 106:66–72. doi: 10.1097/00000446-200608000-00027
15. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. (2001) 286:327–34. doi: 10.1001/jama.286.3.327
16. Balogh DB, Wagner LJ, Fekete A. An overview of the cardioprotective effects of novel antidiabetic classes: focus on inflammation, oxidative stress, and fibrosis. Int J Mol Sci. (2023) 24:7789. doi: 10.3390/ijms24097789
17. Read YS, Jun HS. Anti-inflammatory effects of GLP-1-based therapies beyond glucose control. Inflamm Mediat. (2016) 2016:3094642. doi: 10.1155/2016/3094642
18. Patti AM, Giglio RV, Allotta A, Bruno A, Di Bella T, Stoian AP, et al. Effect of semaglutide on subclinical atherosclerosis and cardiometabolic compensation: a real-world study in patients with type 2 diabetes. Biomedicines. (2023) 11(5):1362. doi: 10.3390/biomedicines11051362
19. Bray JJH, Foster-Davies H, Salem A, Hoole AL, Obaid DR, Halcox JPJ, et al. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: a systematic review and meta-analysis of randomised controlled trials. Diabetes Obes Metab. (2021) 23:1806–22. doi: 10.1111/dom.14399
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hofmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n7
21. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 28:l4898. doi: 10.1136/bmj.l4898
22. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
23. Viechtbauer W. Conducting meta-analyses in R with the metaphor package. J Stat Soft. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
24. Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, et al. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. (2018) 41:258–66. doi: 10.2337/dc17-0417
25. Aroda VR, Rosenstock J, Terauchi Y, Altuntas Y, Lalic NM, Morales Villegas EC, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Diabetes Care. (2019) 42:1724–32. doi: 10.2337/dc19-0749
26. Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Lindberg SØ, et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care. (2019) 42:2272–81. doi: 10.2337/dc19-0883
27. Mosenzon O, Blicher TM, Rosenlund S, Eriksson JW, Heller S, Hels OH, et al. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. (2019) 7:515–27. doi: 10.1016/S2213-8587(19)30192-5
28. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. (2021) 384:989–1002. doi: 10.1056/NEJMoa2032183
29. Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2•4mg once weekly in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. (2021) 397:971–84. doi: 10.1016/S0140-6736(21)00213-0
30. Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. (2021) 325:1403–13. doi: 10.1001/jama.2021.1831
31. Wharton S, Batterham RL, Bhatta M, Buscemi S, Christensen LN, Frias JP, et al. Two-year effect of semaglutide 2.4mg on control of eating in adults with overweight/obesity: STEP 5. Nat Med. (2022) 28:2083–91. doi: 10.38/s41591-022-02026-4
32. Kadowaki T, Isendahl J, Khalid U, Lee SY, Nishida T, Ogawa W, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an East Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. (2022) 10:193–206. doi: 10.1016/S2213-8587(22)00008-0
33. Rubino DM, Greenway FL, Khalid U, O'Neil PM, Rosenstock J, Sørrig R, et al. Effect of weekly subcutaneous semaglutide vs. daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. (2022) 327:138–50. doi: 10.1001/jama.2021.23619
34. Knop FK, Aroda VR, do Vale RD, Holst-Hansen T, Laursen PN, Rosenstock J, et al. Oral semaglutide 50mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2023) 402:705–19. doi: 10.1016/S0140-6736(23)01185-6
35. Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. (2023) 389:1069–84. doi: 10.1056/NEJMoa2306963
36. Avan A, Sany SBT, Ghayour-Mobarhan M, Rahimi HR, Tajfard M, Ferns G, et al. Serum C-reactive protein in the prediction of cardiovascular diseases: overview of the latest clinical studies and public health practice. J Cell Physiol. (2018) 233:8508–25. doi: 10.1002/jcp.26791
37. Ridker PM, Bhatt DL, Pradhan AD, Glynn RJ, MacFadyen JG, Nissen SE. Inflammation and cholesterol as predictors of cardiovascular events among patients receiving statin therapy: a collaborative analysis of three randomised trials. Lancet. (2023) 401:1293–301. doi: 10.1016/S0140-6736(23)00215-5
38. Mouliou DS. C-reactive protein: pathophysiology, diagnosis, false test results and a novel diagnostic algorithm for clinicians. Diseases. (2023) 11:132. doi: 10.3390/diseases11040132
39. Wang L, Hauenstein AV. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol Aspects Med. (2020) 76:100889. doi: 10.1016/j.mam.2020.100889
40. Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. (2016) 118:145–56. doi: 10.1161/CIRCRESAHA.115.306656
41. Amezcua-Castillo E, Gonzalez-Pacheco H, Sáenz-San Martin A, Mendez-Ocampo P, Gutierrez-Moctezuma I, Massó F, et al. C-reactive protein: the quintessential marker of systemic inflammation in coronary artery disease-advancing toward precision medicine. Biomedicines. (2023) 11:2444. doi: 10.3390/biomedicines11092444
42. Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. (2005) 352:20–8. doi: 10.1056/NEJMoa042378
43. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. (2018) 391:319–28. doi: 10.1016/S0140-6736(17)32814-3
44. Chen T, Liu G, Yu B. A meta-analysis evaluating efficacy and safety of colchicine for prevention of major cardiovascular events in patients with coronary artery disease. Clin Res Cardiol. (2023) 112:1487–505. doi: 10.1007/s00392-023-02254-9
45. Nissen SE, Lincoff AM, Brennan D, Ray KK, Mason D, Kastelein JJP, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. (2023) 388:1353–64. doi: 10.1056/NEJMoa2215024
46. Hattori Y, Jojima T, Tomizawa A, Satoh H, Hattori S, Kasai K, Kastelein JJP, et al. A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetology. (2010) 53:2256–63. doi: 10.1007/s00125-010-1831-8
47. Krasner NM, Ido Y, Ruderman NB, Cacicedo JM. Glucagon-like peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS One. (2014) 9:e97554. doi: 10.1371/journal.pone.0097554
48. Luo X, Hu Y, He S, Ye Q, Lv Z, Liu J, et al. Dulaglutide inhibits high glucose- induced endothelial dysfunction and NLRP3 inflammasome activation. Arch Biochem Biophys. (2019) 671:203–09. doi: 10.1016/j.abb.2019.07.008
49. Rakipovski G, Rolin B, Nøhr J, Klewe I, Frederiksen KS, Augustin R, et al. The GLP-1 analogs liraglutide and semaglutide reduceatherosclerosis in ApoE/ and LDLr/ mice by a mechanism that includes inflammatory pathways. J Am Coll Cardiol Basic Trans Sci. (2018) 3:844–57. doi: 10.1016/j.jacbts.2018.09.004
50. Giglio RV, Nikolic D, Volti GL, Stoian AP, Banerjee Y, Magan-Fernandez A, et al. Liraglutide increases Serum levels of MicroRNA-27b, −130a and −210 in patients with type 2 diabetes mellitus: a novel epigenetic effect. Metabolites. (2020) 10(10):391. doi: 10.3390/metabo10100391
51. Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, et al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. (2014) 85:579–89. doi: 10.1038/ki.2013.427
52. Ao N, Yang J, Wang X, Du J. Glucagon-like peptide-1 preserves non-alcoholic fatty liver disease through inhibition of the endoplasmic reticulum stress-associated pathway. Hepatol Res. (2016) 46:343–53. doi: 10.1111/hepr.12551
53. Seufert J, Gallwitz B. The extra-pancreatic effects of GLP-1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes Metab. (2014) 16:673–88. doi: 10.1111/dom.12251
54. Malavazos AE, Meregalli C, Sorrentino F, Vignati A, Dubini C, Scravaglieri V, et al. Semaglutide therapy decreases epicardial fat inflammation and improves psoriasis severity in patients affected by abdominal obesity and type-2 diabetes. Endocrinol Diabetes Metab Case Rep. (2023) 2023:23–0017. doi: 10.1530/EDM-23-0017
55. Mosenzon O, Capehorn MS, De Remigis A, Rasmussen S, Weimers P, Rosenstock J. Impact of semaglutide on high-sensitivity C-reactive protein: exploratory patient-level analyses of SUSTAIN and PIONEER randomized clinical trials. Cardiovasc Diabetol. (2022) 21:172. doi: 10.1186/s12933-022-01585-7
56. Cowart K. Oral semaglutide: first-in-class oral GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. Ann Pharmacother. (2020) 54:478–85. doi: 10.1177/1060028019889064
57. Clements JN, Isaacs D, Hartman RE, Gambill K. Pharmacokinetics and clinical implications of oral semaglutide for type 2 diabetes mellitus. Clin Pharmacokinet. (2021) 60:153–63. doi: 10.1007/s40262-020-00951-6
58. Hameed I, Masoodi SR, Mir SA, Nabi M, Ghazanfar K, Ganai BA, et al. Type 2 diabetes mellitus: from a metabolic disorder to an inflammatory condition. World J Diabetes. (2015) 6:598–612. doi: 10.4239/wjd.v6.i4.598
59. White-Flowers G, Almanza-Pérez JC, Lopez-Roa RI, Alarcon-Aguilar FJ, García-Macedo R, Cruz M. Obesity as an inflammatory process. Bol Med Hosp Infant Mex. (2010) 67:88–97.
60. Sangrós FJ, Torrecilla J, Giráldez-García C, Carrillo L, Mancera J, Mur T, et al. Association of general and abdominal obesity with hypertension, dyslipidaemia and prediabetes in the PREDAPS study. Rev Esp Cardiol (Engl Ed). (2018) 71:170–7. doi: 10.1016/j.rec.2017.04.035
61. Jódar E. Characteristics and types of GLP-1 receptor agonists. An opportunity for individualised therapy. Med Clin (Barc). (2014) 143(Suppl 2):12–7. doi: 10.1016/S0025-7753(14)70103-4
62. Ansari HUH, Qazi SU, Sajid F, Altaf Z, Ghazanfar S, Naveed N, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists on body weight and cardiometabolic parameters in individuals with obesity and without diabetes: a systematic review and meta-analysis. Endocr Pract. (2024) 30(2):160–71. doi: 10.1016/j.eprac.2023.11.007
63. Kennedy C, Hayes P, Salama S, Hennessy M, Fogacci F. The effect of semaglutide on blood pressure in patients without diabetes: a systematic review and meta-analysis. J Clin Med. (2023) 12(3):772. doi: 10.3390/jcm12030772
Keywords: semaglutide, inflammation, C-reactive protein, glucagon-like peptide-1 receptor agonists, meta-analysis
Citation: Masson W, Lobo M, Nogueira JP, Rodriguez-Granillo AM, Barbagelata LE and Siniawski D (2024) Anti-inflammatory effect of semaglutide: updated systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1379189. doi: 10.3389/fcvm.2024.1379189
Received: 30 January 2024; Accepted: 21 June 2024;
Published: 5 July 2024.
Edited by:
Sonia Eiras, Health Research Institute of Santiago de Compostela (IDIS), SpainReviewed by:
Abhinav Grover, Medical College of Wisconsin, United States© 2024 Masson, Lobo, Nogueira, Rodriguez-Granillo, Barbagelata and Siniawski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Walter Masson, d2FsdGVyLm1hc3NvbkBob3NwaXRhbGl0YWxpYW5vLm9yZy5hcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.