
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 08 July 2024
Sec. Atherosclerosis and Vascular Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1375040
This article is part of the Research Topic Frontiers in Cardiovascular Medicine: Rising Stars 2023 View all 28 articles
Background: Atherosclerotic cardiovascular disease (ASCVD), a leading cause of global fatalities, has inconsistent findings regarding the impact of muscle symptoms despite promising clinical trials involving PCSK9 inhibitors (PCSK9i) and siRNA as potential therapeutic options.
Methods: The databases EMBASE, PubMed, Web of Science, Cochrane, and ClinicalTrials.gov were thoroughly searched without any restrictions on language. Review Manager 5.3 software was utilized to calculate relative risks with 95% confidence intervals (CIs) for dichotomous data and mean differences or standardized mean differences with 95%CIs for continuous data. To evaluate publication bias, Egger's test was employed using Stata/SE software.
Results: This analysis included 26 studies comprising 28 randomized controlled trials (RCTs) involving a total of 100,193 patients, and 4 different lipid-lowering therapy combinations. For events with creatine kinase >3ULN, evolocumab and alirocumab demonstrated significant advantages compared to inclisiran. Evolocumab showed the best results in terms of both new muscle symptom events and creatine kinase >3ULN.
Conclusions: Based on this network meta-analysis (NMA) results, evolocumab has emerged as a promising treatment option for patients with hyperlipidemia and muscle disorders compared to other PCSK9 inhibitors and inclisiran.
Systematic Review Registration: PROSPERO [CRD42023459558].
ASCVD is one of the leading causes of mortality worldwide, accounting for over one-third of all global deaths (1). Dyslipidemia, characterized by the excessive accumulation of low-density lipoprotein cholesterol (LDL-C) in the vasculature, is recognized as a pivotal risk factor in developing ASCVD (2). Consequently, reducing LDL-C levels is essential for managing ASCVD. Statin therapy had been suggested as the first-line therapy by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines and the European Atherosclerosis Society/European Society of Cardiology (EAS/ESC) guidelines. Despite the widespread use of statin therapy, some patients are unable to tolerate it from the outset (3, 4).A meta-analysis identified the most common reason for statin discontinuation as the development of muscle symptoms, with or without changes in creatine kinase (CK) levels. These symptoms occurred in patients who were on increasing doses of statin therapy or who were using a combination of more than two statins (5). As a result, some new therapies have been developed to enhance LDL-C reduction in high-risk ASCVD patients, including PCSK9i and siRNA therapies (6). Achieving guideline-recommended LDL-C goals in statin-intolerant patients requires the use of personalized lipid-lowering therapies other than statins (7). According to the 2018 AHA/ACC guideline and the 2017 National Lipid Association update, PCSK9 inhibitors were recommended for patients with LDL-C levels ≥70 mg/dl or non-high-density lipoprotein cholesterol (non-HDL-C) ≥100 mg/dl after maximally tolerated LDL-lowering therapies (8, 9). The current incidence of statin intolerance is approximately 9.1% and is associated with an increased statin dosage (5).
PCSK9 inhibitors, such as evolocumab, bococizumab, and alirocumab, have demonstrated the ability to bind with PCSK9, effectively inhibiting its interaction with the low-density lipoprotein receptor (LDLR) (10). Common adverse effects of PCSK9 inhibitors include nasopharyngeal pain, headache, and muscle symptoms. Few reports are available comparing the incidence of muscle-related adverse events induced by different types of PCSK9 inhibitors (11). Inclisiran is a siRNA molecule specifically designed to target the mRNA encoding PCSK9, leading to its degradation and the subsequent suppression of PCSK9 protein production (12). The siRNA-mediated degradation of PCSK9 mRNA effectively blocks the synthesis of PCSK9 protein, offering a new therapeutic approach for treating cardiovascular diseases (13, 14). Inclisiran has shown a substantial effect in lowering LDL-C; however, due to the lack of extensive clinical data, its long-term tolerability and safety remain uncertain compared to PCSK9 inhibitors (15). For inclisiran, adverse events at the injection site have been commonly reported. However, the occurrence of muscle symptoms and the elevation of creatine kinase levels have not been thoroughly investigated (16).
For statin-intolerant patients experiencing rhabdomyolysis and requiring alternative therapies, PCSK9i and inclisiran present viable options (17). However, there is no evidence to suggest that these therapies are superior in terms of muscule-related effects. Therefore, we conducted a systematic review and NMA of RCTs to compare the muscle-related adverse effects of these treatments.
This NMA followed the guidelines set by the Cochrane Collaboration and was reported by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis), as outlined in Figure 1 (18). To ensure the originality, dependability and transparency of the research, the research proposal was registered with the Systematic Review Registry (PROSPERO) under the number CRD42023459558.
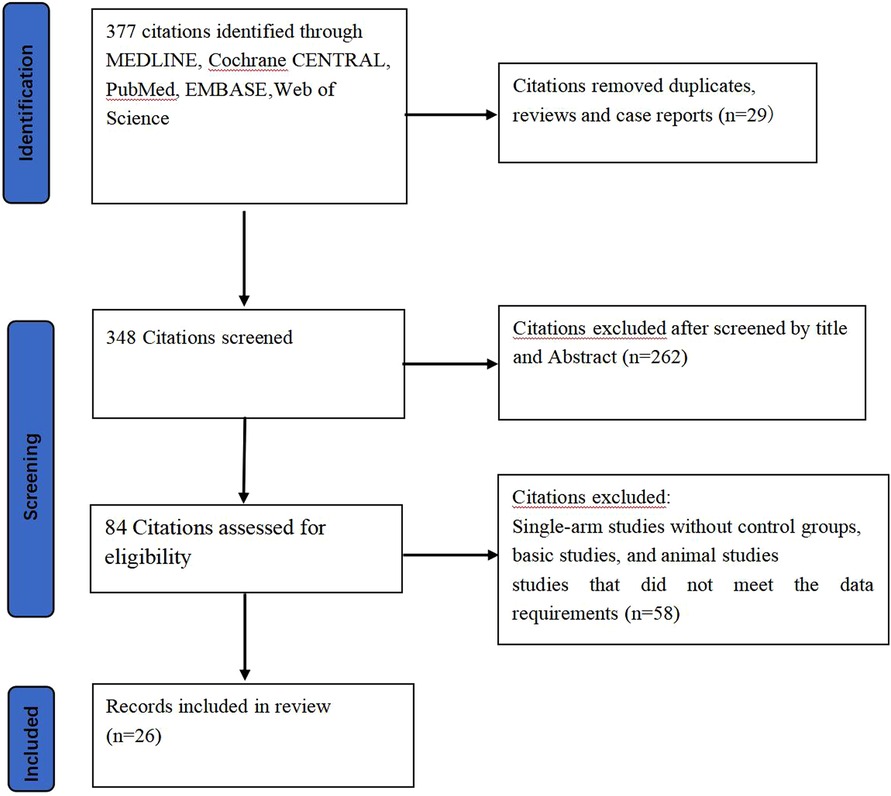
Figure 1 Flowchart. This accompanying flow diagram illustrates the systematic process employed to identify and include pertinent literature in this study.
A detailed literature search was conducted with a language restriction to English using electronic databases including Web of Science, EMBASE, PubMed, Cochrane Library, and Clinical Trials from their inception until December 5, 2023. The search utilized the following keywords: “muscle symptoms,” “creatine kinase,” “inclisiran,” “PSCK9i,” “proprotein convertase subtilisin/kexin type 9 monoclonal antibody,” “PCSK9 inhibitor,” “PCSK9 antibody,” “evolocumab,” “bococizumab,” “alirocumab,” “RG7652,” “AMG145,” “REGN727,” “RN316,” “SAR236553”.
The studies included in this meta-analysis must adhere strictly to the following criteria:
(1) Eligible studies are Phase II or Phase III RCTs.
(2) The RCTs involved treatment with PSCK9i or inclisiran.
(3) The RCTs report outcomes of new muscular symptoms or CK>3ULN.
The following types of studies were excluded:
(1) Multiple publications describing the same cohort.
(2) Specific categories of publications, including editorial articles, conference abstracts, correspondence, literature reviews, and case reports.
(3) Long-term studies on the safety and effectiveness of PCSK9i replicated in patient cohorts.
All selected trials were processed by the PRISMA guidelines for data extraction. To ensure the highest level of data accuracy and comprehensiveness, three researchers independently extracted the relevant data points. In case of any inconsistencies or uncertainties, discussions were promptly held with a fourth author to reach a consensus, ensuring the accuracy and completeness of the collected data. To maintain the originality and uniqueness of the extracted data, we conducted a thorough review and cross-checked the following information: trial name, sample size, publication year, publication source, first author, trial phase, national clinical trial identification number, number of patients, and intervening measure. In addition to the primary clinical outcomes, we specifically collected and analyzed indicators and incidence rates related to adverse muscular reactions. To ensure the high quality of the included studies we used the Cochrane Risk of Bias tool (1.0) to assess the RCTs (19).
To assess the potential impact of PCSK9i therapy on incident muscle symptoms, we conducted meta-analyses using both random- and fixed-effect models to calculate the overall relative risk (RR). Additional details of our data analysis approach were provided in the Supplementary Data. A two-tailed P value less than 0.05 was considered statistically significant for the summary treatment effect estimate. All statistical analyses were performed using Stata 16 and Revman (20).
To conduct a thorough heterogeneity analysis, we used STATA to calculate the I2 values, which provide valuable insights into the degree of heterogeneity in this data. An I2 value less than 25% indicates low heterogeneity, while values between 25% and 50% denotes moderate heterogeneity. An I2 value greater than 75% suggests high heterogeneity. In cases of low heterogeneity, we utilized a fixed-effects model to ensure stability and reliability in the analysis. Conversely, when heterogeneity was moderate or high, a random-effects model was employed to account for the broader range of study variations.
We employed the node-splitting method to further assess the consistency of evidence from both direct and indirect sources, ensuring rigorous examines of the internal validity of the evidence synthesis. Additionally, we utilized funnel plots along with Egger's regression test to detect small-study effects, enhancing the comprehensiveness of our evaluation by including a wide range of studies. This approach blosters the reliability and robustness of our findings (21).
After an extensive search across four databases (Web of Science, PubMed, Cochrane Library, Embase), we identified 377 relevant articles. After removing duplicates and screening the titles and abstracts, we considered 84 full-text articles for eligibility. The detailed selection process was summarized in Figure 1, including 26 articles in this NMA.
This meta-analysis included 100,193 patients across 28 RCTs, evaluating four lipid-lowing therapies: bococizumab (Boc) (22), evolocumab (Evo) (23–38), alirocumab (Ali) (30, 39–46), and inclisiran (Inc) (47). Basic information for each study, including the first author, publication date, lipid-lowering treatment type, patient sex ratio, age, follow-up duration, NCT number, and patient profile is provided in Supplementary Table S1.
In this analysis, these 26 studies compared bococizumab with placebo (1 study), evolocumab with placebo (16 studies), alirocumab with placebo (9 studies), and inclisiran with placebo (1 study). Additionally, one study examined the safety profiles of both evolocumab and alirocumab compared to placebo controls. Figure 2 provides a visual representation of the eligible comparisons in the form of a network plot.
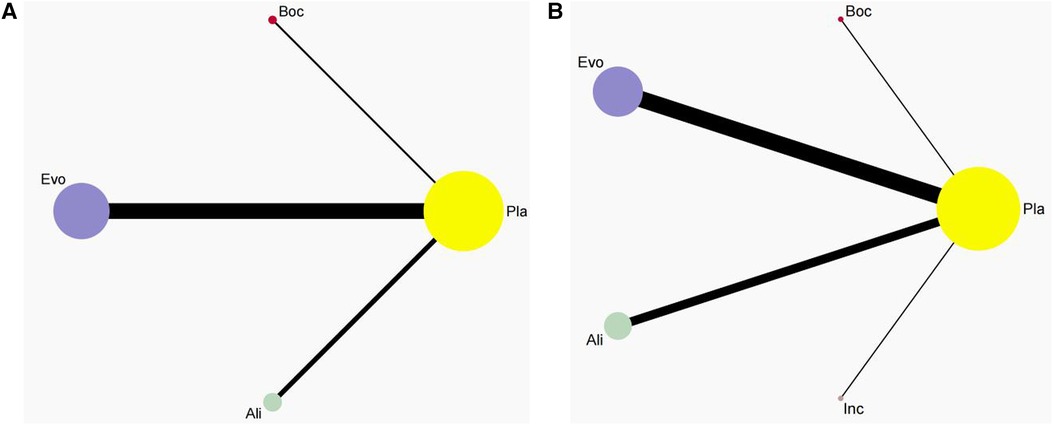
Figure 2 Network plot. This network plot illustrates the safety of three (A) and four(B) different lipid-lowering therapies (PCSK9i and Inclisiran) for patients. In the plot, circles are used to represent each intervention as a node in the network, while lines depict direct comparisons within the framework of RCTs. The thickness of the lines corresponds to the number of RCTs included in each comparison.
Figure 3 presents the outcomes of the risks of bias assessment for the 26 trials included in the study. Overall, the risk of bias was considered low due to the robust design of the RCTs employed. To further ensure methodological rigor, we also reviewed the test protocols for additional details.
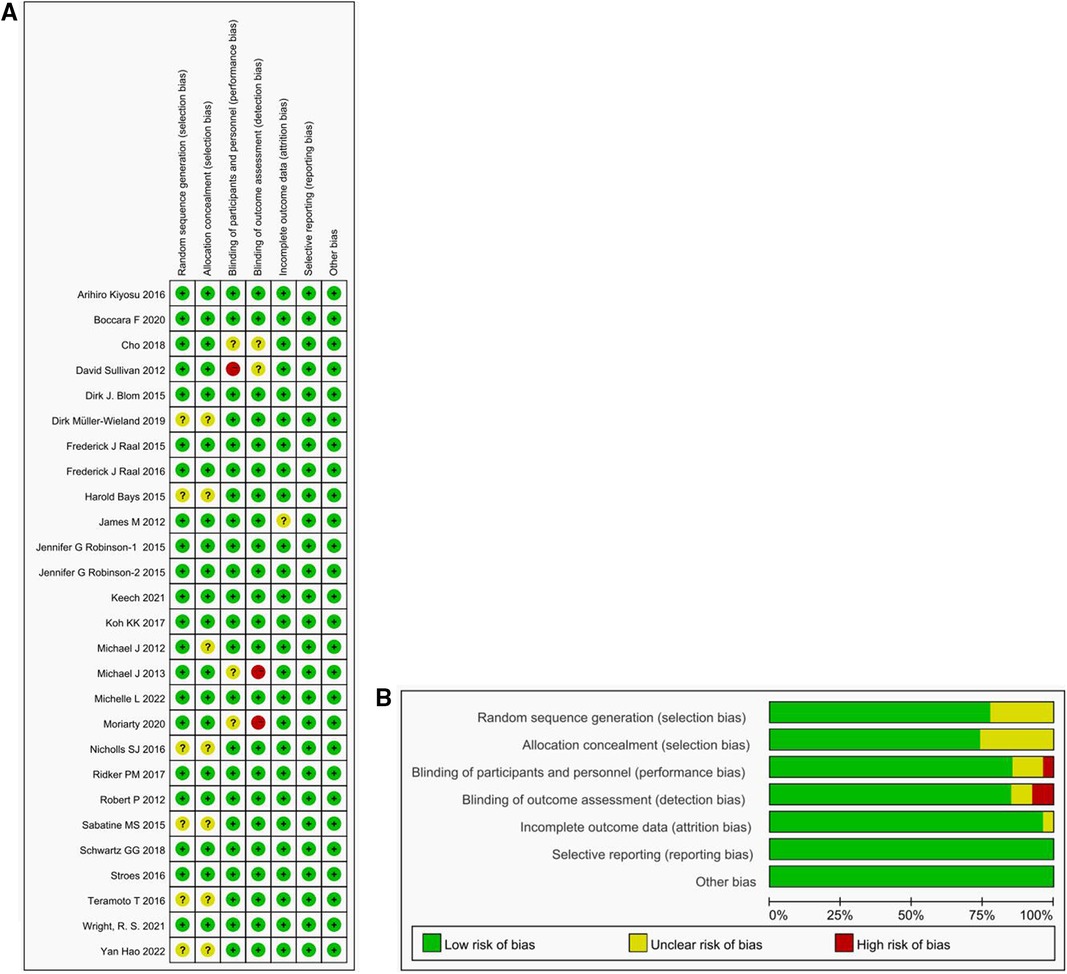
Figure 3 Risk of bias figure. (A) Methodological Quality Summary: This section presents the authors’ assessment of each methodological quality item for each study included in the analysis. The two main sources of bias evaluated are performance bias and detection bias. (B) Methodological Quality Map: We provide their evaluation of the overall quality of each methodology used in the included studies, expressed as a percentage of all included studies. Described as a percentage of all included studies.
Regarding random sequence generation, 20 studies were assessed as having a low risk, while 6 studies had an unclear risk. For allocation concealment, 19 studies had a low risk, and 7 studies had an unclear risk. In terms of performance bias, 22 studies examined a low risk, 3 studies had an unclear risk, and 1 study had a high risk. For detection bias, 22 studies had a low risk, 2 studies had an unclear risk, and 2 studies had a high risk. When evaluating attrition bias, 25 studies were considered to have a low risk, while 1 study had an unclear risk. All trials were rated as having a low risk for the reporting bias, primarily because the data analysis focused on the intention-to-treat population and included an adequate number of relevant endpoints. However, it is worth noting that some studies allowed for crossover, which could introduce potential biases into the results.
Pairwise meta-analyses were conducted for 22 trials reporting new muscle symptoms and for 22 trials reporting events of creatine kinase >3ULN.
Head-to-head comparisons revealed that, compared to placebo, patients treated with bococizumab experienced a higher incidence of muscle symptoms. (RR = 1.09; 95%CI: 0.95–1.25, P = 0.22) and creatine kinase >3ULN (RR = 0.86; 95%CI: 0.68–1.09, P = 0.22). Similarly, evolocumab increased the risk of muscle symptoms (RR = 1.05; 95%CI: 0.97–1.14, P = 0.94) and creatine kinase >3ULN (RR = 0.69; 95%CI: 0.43–0.96, P = 0.26). Additionally, alirocumab elevated the risk of muscle symptoms (RR = 1.16; 95%CI, 0.89–1.51, P = 0.28) and creatine kinase >3ULN (RR = 0.86; 95%CI: 0.66–1.12, P = 0.27). Inclisiran solely heightened the risk of creatine kinase >3ULN (RR = 1.09; 95%CI:0.61–1.93, P = 0.78).
The forest plots in Figures 4, 5 visualize the pairwise comparisons of the incidence of muscle symptom and creatine kinase >3ULN, respectively. As shown in Supplementary Figures S1, S2, the funnel plot shows no significant publication bias in this study.

Figure 4 Forest plot. Forest plot for new muscle symptom events. The safety of PCSK9i in hyperlipidemic patients.
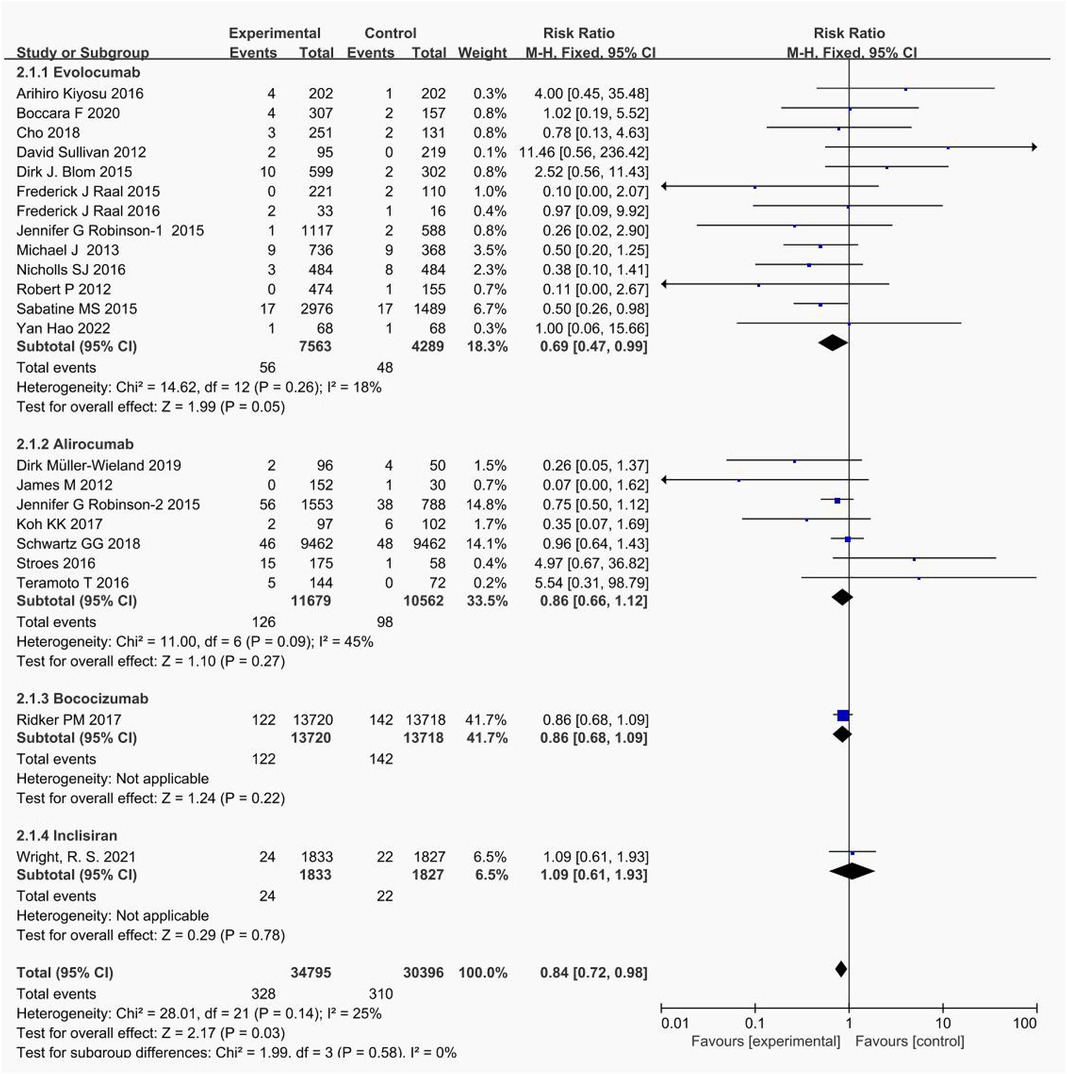
Figure 5 Forest plot. Forest plot for events with creatine kinase >3ULN.The safety of PCSK9i and inclisiran in hyperlipidemic patients.
The non-direct comparative results for new muscle symptom events are displayed in Figure 6. Among PCSK9i, alirocumab posed the highest risk for new onset muscle symptoms, followed by bococizumab, with evolocumab being the least likely to cause such events.
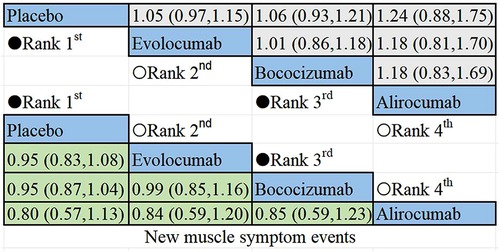
Figure 6 Summary of target outcomes including new muscle symptom events. Safety of PCSK9i in hyperlipidemic patients analyzed by Bayesian network meta-analysis.
The results of the non-direct comparisons for events with creatine kinase >3ULN are presented in Figure 7. Compared to inclisiran, bococizumab (RR = 1.07; 95%CI: 0.57–2.01), evolocumab (RR = 0.52;95% CI: 0.25–1.05), alirocumab (RR = 0.76; 95%CI: 0.4–1.44), and placebo (RR = 0.92; 95% CI: 0.51–1.64) exhibited varying risk patterns. The order of lipid-lowering agents causing new-onset CK>3ULN in descending order of risk: bococizumab > inclisiran > placebo > alirocumab > evolocumab. Evolocumab appeared to carry the lowest risk for elevated creatine kinase levels, while bococizumab posed the highest risk among the lipid-lowering agents.
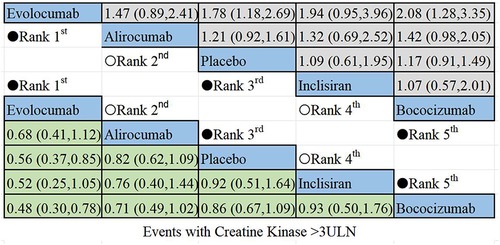
Figure 7 Summary of target outcomes including events with creatine kinase >3ULN. Safety of PCSK9i and inclisiran in hyperlipidemic patients analyzed by Bayesian network meta-analysis.
A subgroup analysis was conducted to evaluate the risk of muscle adverse events and creatine kinase elevation caused by PCSK9i and inclisiran from five perspectives: age (≥60 years or <60 years), gender (female ≥50% or female <50%), different LDL-C level before treatment (≥125 mg/dl or <125 mg/dl), follow-up time (≥52 weeks or <52 weeks), and sample size (≥500 participants or <500 participants) in Supplementary Figures S3–S6. The result showed that gender, age, LDL-C level before treatment, follow-up time, and sample size had no significant impact on the risk of muscle adverse events and creatine kinase elevation caused by PCSK9i and inclisiran in Figure 8.
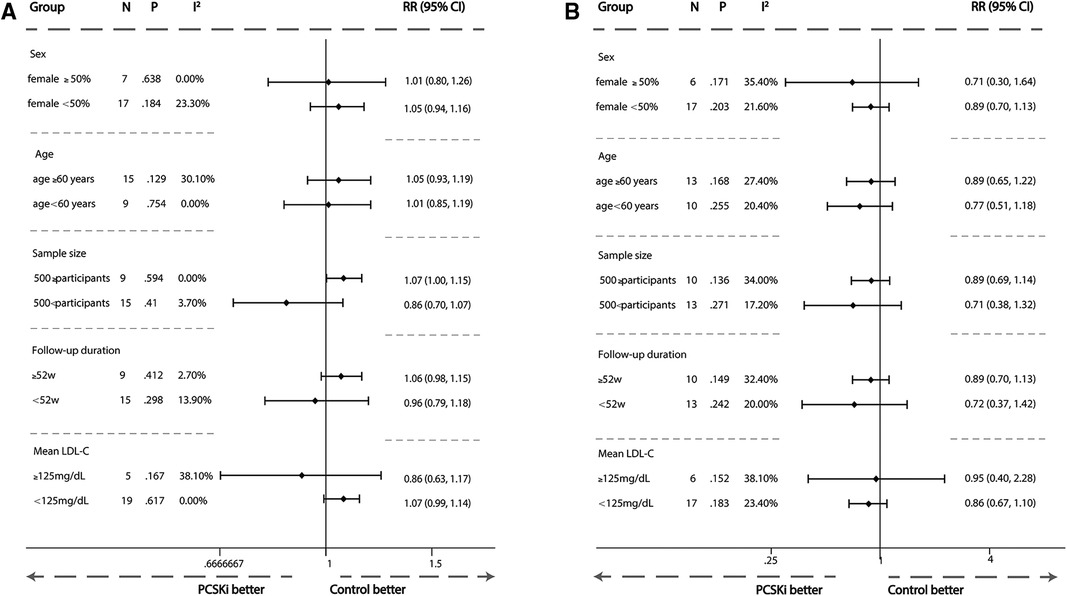
Figure 8 Subgroup meta-analysis of association between PCSK9i therapy and risk of new muscle symptom and creatine kinase >3ULN. (A) Subgroup meta-analysis of association between PCSK9i therapy and risk of incident muscle symptoms. (B) Subgroup meta-analysis of association between PCSK9i and inclisiran therapy with risk of incident Creatine Kinase >3ULN.
In this comprehensive NMA, encompassing a substantial cohort of 100,193 patients who either received high-dose statin treatment or reported intolerance to statins, our results indicated that the use of inclisiran and PCSK9i may lead to various adverse effects throughout therapy. Previous reports had suggested that these lipid-lowering therapies could impact the neurocognitive system of patients or even increase the risk of fractures (48, 49). However, the risk of muscular adverse events associated with PCSK9i and inclisiran had not been comprehensively summarized in a complete meta-analysis until now.
The utilization of NMA represented an advancement compared to traditional meta-analyses, as it allows for indirect comparisons of interventions across RCTs by incorporating a joint comparator group. This approach encompassed a broader range of studies, thereby enhancing the credibility of the findings. When evaluating the relative safety profiles of PCSK9i (such as evolocumab, bococizumab, and alirocumab) and inclisiran in patients with hyperlipidemia, head-to-head clinical trials are invaluable. They provide essential insights that guide clinical decision-making.
The comparison of new muscular symptom events demonstrated that evolocumab exhibited the highest level of safety, followed by bococizumab. In contrast, patients treated with alirocumab showed a relatively higher incidence of new muscular symptoms.
Similarly, when comparing events with CK>3ULN, patients treated with bococizumab had a higher risk of elevated creatine kinase compared to those treated with inclisiran (22, 47). In contrast, other PCSK9 inhibitors, such as evolocumab and alirocumab, demonstrated a better safety profile than inclisiran. with evolocumab having the fewest incidents of creatine kinase elevation. However, it is important to note that inclisiran and bococizumab were each included in only one trial, which may impact the results of the NMA. More RCTs are needed in the future to confirm these findings. Notably, bococizumab has been suspended in recent years due to its higher immunogenicity (50).
Adverse drug reactions were observed to be both more severe and more frequent in female subjects compared to their male counterparts. The pharmacological aspects of these reactions have been comparatively understudied. To develop appropriate individualized dosing regimens, gender differences should receive greater attention (51). This may require additional clinical trials to validate this observation. Moreover, an interesting phenomenon was noted, patients with specific genotypes (e.g., SLCO1B1rs4149056) had more difficulty reaching the LDL-C target value, with a notable gender difference in this effect. Future studies should focus on the safety and efficacy of PCSK9 inhibitors in genetically diserve patients to further explore these differences (52).
Through this comprehensive NMA, we provided valuable insights into the relative safety of PCSK9i and inclisiran in patients with hyperlipidemia. These findings have important implications for clinical decision-making and patient outcomes. It is significant to note that clinicians might face challenges in selecting these therapies due to the high cost of PCSK9 inhibitors. A cost-effectiveness analysis of PCSK9 inhibitors and inclisiran would provide a crucial basis to supporting their use in statin-intolerant patients (53).
Our analysis offered significant insights into the safety of muscle adverse events among patients using different lipid-lowering therapies. However, it is imperative to recognize the necessity for additional studies to validate and expand upon our findings. Considering the intricate and diverse nature of individuals with hyperlipidemia, it is crucial to personalize treatment decisions on an individual basis. Patient stratification based on factors such as ethnicity and age may play a vital role in selecting the most suitable lipid-lowering drug for each patient. By tailoring treatment protocols to align with the distinct clinical profiles of individual patients, we can enhance therapeutic outcomes and reduce the incidence of novel muscule symptoms.
Notably, the limitations of these findings stem from the quality of available evidence, including internal bias and heterogeneity. Incomplete reporting, controversial treatment classifications, and potential misclassification also posed constraints. Furthermore, high-impact interventions might be influenced by other factors associated with higher socioeconomic status in the patient population. Therefore, further exploration of these potential limitations is crucial for enhancing our understanding of the safety lipid-lowering therapy in patients with hyperlipidemia.
Based on the results of this NMA, evolocumab demonstrated the lowest likelihood of causing adverse muscle effects compared to other PCSK9 inhibitors (bococizumab, alirocumab) and inclisiran.This makes evolocumab a promising lipid-lowering option for patients with both hyperlipidemia and muscle disease.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
WL: Writing – original draft. LS: Writing – original draft. SY: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Beijing Municipal Administration of Hospitals Incubating Program, code: PZ2023007.
We gratefully acknowledge the assistance and the instruction of Evidence-Based Medicine of Beijing Shijitan Hospital on statistic methodology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1375040/full#supplementary-material
1. Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MS. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan study. Neurology. (2006) 66:641–6. doi: 10.1212/01.wnl.0000201253.93811.f6
2. Mora S, Wenger NK, Demicco DA, Breazna A, Boekholdt SM, Arsenault BJ, et al. Determinants of residual risk in secondary prevention patients treated with high- versus low-dose statin therapy: the treating to new targets (TNT) study. Circulation. (2012) 125:1979–87. doi: 10.1161/CIRCULATIONAHA.111.088591
3. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41:111–88. doi: 10.1093/eurheartj/ehz455
4. Reiner Z. Resistance and intolerance to statins. Nutr Metab Cardiovasc Dis. (2014) 24:1057–66. doi: 10.1016/j.numecd.2014.05.009
5. Bytyçi I, Penson PE, Mikhailidis DP, Wong ND, Hernandez AV, Sahebkar A, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. (2022) 43:3213–23. doi: 10.1093/eurheartj/ehac015
6. Bergeron N, Phan BA, Ding Y, Fong A, Krauss RM. Proprotein convertase subtilisin/kexin type 9 inhibition: a new therapeutic mechanism for reducing cardiovascular disease risk. Circulation. (2015) 132:1648–66. doi: 10.1161/CIRCULATIONAHA.115.016080
7. Bosco G, Di Giacomo Barbagallo F, Spampinato S, Lanzafame L, Di Pino A, Piro S, et al. Management of statin intolerant patients in the era of novel lipid lowering therapies: a critical approach in clinical practice. J Clin Med. (2023) 12:2444. doi: 10.3390/jcm12062444
8. Ray KK, Molemans B, Schoonen WM, Giovas P, Bray S, Kiru G, et al. EU-wide cross-sectional observational study of lipid-modifying therapy use in secondary and primary care: the DA VINCI study. Eur J Prev Cardiol. (2021) 28:1279–89. doi: 10.1093/eurjpc/zwaa047
9. Wilson PWF, Polonsky TS, Miedema MD, Khera A, Kosinski AS, Kuvin JT. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. (2019) 73:3210–27. doi: 10.1016/j.jacc.2018.11.004
10. Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. (2006) 354:1264–72. doi: 10.1056/NEJMoa054013
11. Grześk G, Dorota B, Wołowiec Ł, Wołowiec A, Osiak J, Kozakiewicz M, et al. Safety of PCSK9 inhibitors. Biomed Pharmacother. (2022) 156:113957. doi: 10.1016/j.biopha.2022.113957
12. Dyrbuś K, Gąsior M, Penson P, Ray KK, Banach M. Inclisiran-new hope in the management of lipid disorders? J Clin Lipidol. (2020) 14:16–27. doi: 10.1016/j.jacl.2019.11.001
13. Ranasinghe P, Addison ML, Dear JW, Webb DJ. Small interfering RNA: discovery, pharmacology and clinical development-an introductory review. Br J Pharmacol. (2023) 180:2697–720. doi: 10.1111/bph.15972
14. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. (2020) 382:1507–19. doi: 10.1056/NEJMoa1912387
15. Merćep I, Friščić N, Strikić D, Reiner Ž. Advantages and disadvantages of inclisiran: a small interfering ribonucleic acid molecule targeting PCSK9-A narrative review. Cardiovasc Ther. (2022) 2022:8129513. doi: 10.1155/2022/8129513
16. Ray KK, Troquay RPT, Visseren FLJ, Leiter LA, Scott Wright R, Vikarunnessa S, et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. (2023) 11:109–19. doi: 10.1016/S2213-8587(22)00353-9
17. Khan SU, Yedlapati SH, Lone AN, Hao Q, Guyatt G, Delvaux N, et al. PCSK9 inhibitors and ezetimibe with or without statin therapy for cardiovascular risk reduction: a systematic review and network meta-analysis. Br Med J. (2022) 377:e069116. doi: 10.1136/bmj-2021-069116
18. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
19. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
20. Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using stata. Epidemiol Health. (2017) 39:e2017047. doi: 10.4178/epih.e2017047
21. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
22. Ridker PM, Tardif JC, Amarenco P, Duggan W, Glynn RJ, Jukema JW, et al. Lipid-reduction variability and antidrug-antibody formation with bococizumab. N Engl J Med. (2017) 376:1517–26. doi: 10.1056/NEJMoa1614062
23. Kiyosue A, Honarpour N, Kurtz C, Xue A, Wasserman SM, Hirayama A. A phase 3 study of evolocumab (AMG 145) in statin-treated Japanese patients at high cardiovascular risk. Am J Cardiol. (2016) 117:40–7. doi: 10.1016/j.amjcard.2015.10.021
24. Boccara F, Kumar PN, Caramelli B, Calmy A, López JAG, Bray S, et al. Evolocumab in HIV-infected patients with dyslipidemia: primary results of the randomized, double-blind BEIJERINCK study. J Am Coll Cardiol. (2020) 75:2570–84. doi: 10.1016/j.jacc.2020.03.025
25. Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. Jama. (2012) 308:2497–506. doi: 10.1001/jama.2012.25790
26. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. (2014) 370:1809–19. doi: 10.1056/NEJMoa1316222
27. Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA part B): a randomised, double-blind, placebo-controlled trial. Lancet. (2015) 385:341–50. doi: 10.1016/S0140-6736(14)61374-X
28. Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. (2015) 385:331–40. doi: 10.1016/S0140-6736(14)61399-4
29. Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. Jama. (2014) 311:1870–82. doi: 10.1001/jama.2014.4030
30. Koh KK, Nam CW, Chao TH, Liu ME, Wu CJ, Kim DS, et al. A randomized trial evaluating the efficacy and safety of alirocumab in South Korea and Taiwan (ODYSSEY KT). J Clin Lipidol. (2018) 12:162–172.e166. doi: 10.1016/j.jacl.2017.09.007
31. Cho L, Dent R, Stroes ESG, Stein EA, Sullivan D, Ruzza A, et al. Persistent safety and efficacy of evolocumab in patients with statin intolerance: a subset analysis of the OSLER open-label extension studies. Cardiovasc Drugs Ther. (2018) 32:365–72. doi: 10.1007/s10557-018-6817-7
32. Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G, et al. Efficacy and safety of longer-term administration of evolocumab (AMG 145) in patients with hypercholesterolemia: 52-week results from the open-label study of long-term evaluation against LDL-C (OSLER) randomized trial. Circulation. (2014) 129:234–43. doi: 10.1161/CIRCULATIONAHA.113.007012
33. Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. (2012) 380:1995–2006. doi: 10.1016/S0140-6736(12)61771-1
34. O'Donoghue ML, Giugliano RP, Wiviott SD, Atar D, Keech A, Kuder JF, et al. Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation. (2022) 146:1109–19. doi: 10.1161/CIRCULATIONAHA.122.061620
35. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. Jama. (2016) 316:2373–84. doi: 10.1001/jama.2016.16951
36. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. (2015) 372:1500–9. doi: 10.1056/NEJMoa1500858
37. Hao Y, Yang YL, Wang YC, Li J. Effect of the early application of evolocumab on blood lipid profile and cardiovascular prognosis in patients with extremely high-risk acute coronary syndrome. Int Heart J. (2022) 63:669–77. doi: 10.1536/ihj.22-052
38. Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. (2012) 380:2007–17. doi: 10.1016/S0140-6736(12)61770-X
39. McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. (2012) 59:2344–53. doi: 10.1016/j.jacc.2012.03.007
40. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. (2015) 372:1489–99. doi: 10.1056/NEJMoa1501031
41. Teramoto T, Kobayashi M, Tasaki H, Yagyu H, Higashikata T, Takagi Y, et al. Efficacy and safety of alirocumab in Japanese patients with heterozygous familial hypercholesterolemia or at high cardiovascular risk with hypercholesterolemia not adequately controlled with statins- ODYSSEY JAPAN randomized controlled trial. Circ J. (2016) 80:1980–7. doi: 10.1253/circj.CJ-16-0387
42. Stroes E, Guyton JR, Lepor N, Civeira F, Gaudet D, Watts GF, et al. Efficacy and safety of alirocumab 150mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc. (2016) 5:e003421. doi: 10.1161/JAHA.116.003421
43. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. (2018) 379:2097–107. doi: 10.1056/NEJMoa1801174
44. Müller-Wieland D, Rader DJ, Moriarty PM, Bergeron J, Langslet G, Ray KK, et al. Efficacy and safety of alirocumab 300mg every 4 weeks in individuals with type 2 diabetes on maximally tolerated statin. J Clin Endocrinol Metab. (2019) 104:5253–62. doi: 10.1210/jc.2018-02703
45. Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni-Berthold I, et al. Alirocumab as add-on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. (2015) 100:3140–8. doi: 10.1210/jc.2015-1520
46. Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, et al. Efficacy and safety of alirocumab in statin-intolerant patients over 3 years: open-label treatment period of the ODYSSEY ALTERNATIVE trial. J Clin Lipidol. (2020) 14:88–97.e82. doi: 10.1016/j.jacl.2020.01.001
47. Wright RS, Ray KK, Raal FJ, Kallend DG, Jaros M, Koenig W, et al. Pooled patient-level analysis of inclisiran trials in patients with familial hypercholesterolemia or atherosclerosis. J Am Coll Cardiol. (2021) 77:1182–93. doi: 10.1016/j.jacc.2020.12.058
48. Lv F, Cai X, Lin C, Yang W, Hu S, Ji L. Proprotein convertase subtilisin/kexin type 9 inhibitors and the risk of fracture: a systematic review and meta-analysis of randomized controlled trials. Calcif Tissue Int. (2023) 113:175–85. doi: 10.1007/s00223-023-01085-0
49. Giugliano RP, Mach F, Zavitz K, Kurtz C, Schneider J, Wang H, et al. Design and rationale of the EBBINGHAUS trial: a phase 3, double-blind, placebo-controlled, multicenter study to assess the effect of evolocumab on cognitive function in patients with clinically evident cardiovascular disease and receiving statin background lipid-lowering therapy-A cognitive study of patients enrolled in the FOURIER trial. Clin Cardiol. (2017) 40:59–65. doi: 10.1002/clc.22678
50. Sindi AAA. Genetics, safety, cost-effectiveness, and accessibility of injectable lipid-lowering agents: a narrative review. J Lipids. (2023) 2023:2025490. doi: 10.1155/2023/2025490
51. Franconi F, Campesi I. Pharmacogenomics, pharmacokinetics and pharmacodynamics: interaction with biological differences between men and women. Br J Pharmacol. (2014) 171:580–94. doi: 10.1111/bph.12362
52. Bosco G, Di Giacomo Barbagallo F, Di Marco M, Miano N, Scilletta S, Spampinato S, et al. The impact of SLCO1B1 rs4149056 on LDL-C target achievement after lipid lowering therapy optimization in men and women with familial hypercholesterolemia. Front Endocrinol (Lausanne). (2024) 15:1346152. doi: 10.3389/fendo.2024.1346152
Keywords: PCSK9 inhibitor, alirocumab, bococizumab, inclisiran, evolocumab, muscle symptom events
Citation: Li W, Sun L and Yan S (2024) PCSK9 inhibitors and inclisiran with or without statin therapy on incident muscle symptoms and creatine kinase: a systematic review and network meta-analysis. Front. Cardiovasc. Med. 11:1375040. doi: 10.3389/fcvm.2024.1375040
Received: 23 January 2024; Accepted: 24 June 2024;
Published: 8 July 2024.
Edited by:
Chieko Mineo, University of Texas Southwestern Medical Center, United StatesReviewed by:
Roberto Scicali, University of Catania, Italy© 2024 Li, Sun and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sichao Yan, eWFuc2ljaGFvQGJqc2p0aC5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.