- 1Department of Cardiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Key Laboratory of Biological Targeted Therapy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Hubei Provincial Engineering Research Center of Immunological Diagnosis and Therapy for Cardiovascular Diseases, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Department of Cardiology, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, China
Hereditary transthyretin amyloid (ATTRv) cardiomyopathy (CM) is caused by mutations in the TTR gene. TTR mutations contribute to TTR tetramer destabilization and dissociation, leading to excessive deposition of insoluble amyloid fibrils in the myocardium and finally resulting in cardiac dysfunction. In this article, we report a case of a Chinese patient with transthyretin mutation p.D58Y and provide detailed information on cardiac amyloidosis, including transthoracic echocardiography, cardiac magnetic resonance, and SPECT imaging for the first time. Our report aims to provide a better understanding of ATTR genotypes and phenotypes.
Introduction
Amyloidosis is a heterogeneous group of clinical diseases characterized by the misfolding and deposition of amyloid proteins in different human tissues and organs, resulting in cell damage, tissue destruction, and organ dysfunction. In the case of cardiac amyloidosis (CA), nine amyloid fibril precursors have been reported to cause CA, including transthyretin (TTR) and immunoglobulin light chain, which account for more than 95% amyloid cardiomyopathy (1–3). The pathobiology of transthyretin amyloid includes TTR misfolding and aggregation, resulting in restrictive cardiomyopathy, heart failure, and other clinical symptoms (4–6). TTR is a 56 kDa homo-tetrameric protein composed of four 127-amino acid monomers. It is encoded by genes located on human chromosome 18q12.1. TTR is mainly transcribed in liver cells and can also be found in the brain, retina, and choroid plexus. Its primary role is to transport thyroxine- and retinol-binding proteins, with the stability of its structure closely associated with its function.
Transthyretin amyloidosis (ATTR) comprises wild-type (genetically normal, ATTRwt) and hereditary (substitution or deletion mutations, ATTRv) amyloidosis. In ATTR amyloidosis, the TTR protein instability is caused by genetic variations in ATTRv and age-related instability in ATTRwt. This instability leads to the disassociation of tetramers into monomers, which finally polymerize into insoluble fibrils. Based on a TTR mutation database (http://amyloidosismutations.com/mut-attr.php), more than TTR gene 140 variants were reported (7). Within these, the V30M (p.Val50Met, valine-to-methionine at amino acid position 50) mutation was by far the most studied one and believed to be the most common one worldwide (8). On the other hand, Val122Ile (p.Val142Ile) is the most common mutation responsible for familial amyloid cardiomyopathy. The clinical spectrums of ATTRv varied from exclusive neurological involvement, mix phenotype, to predominant cardiac involvement (9). In p.V50M, the extracardiac manifestations especially neurologic phenotypes were observed. Other mutations, such as p.V142I, p.T80A, and p.I88l, on the contrary, were reported to be predominantly cardiac phenotypes.
In clinical practice, ATTR-CA diagnosis is challenging. The red flags and caveats in CA should attract enough attention. In recent years, ATTR-CA has been increasingly diagnosed owing to improved clinical awareness and advances in diagnosis methods. In addition, the prevalence of ATTR-CA, especially ATTRwt, is believed to be much higher than previously thought (10, 11). Despite the advances in ATTR mechanisms, research, and therapeutic approaches, there is still a long way to go for ATTR treatment. For untreated ATTRwt patients, the median survival from diagnosis is 3.6–4.8 years; for ATTRv patients, 5.8 years; and for Val122Ile patients, only 2.6 years (2). Fortunately, there is now a TTR tetramer stabilizer, tafamidis, available for ATTR treatment. In addition, clinical trials involving TTR-specific antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs) are currently underway and are expected to be effective.
Here, we report a rare transthyretin mutation p.D58Y in a Chinese patient and provide detailed CA information for the first time. Our report aims to enhance our understanding of the genotypic and phenotypic spectra of ATTR hereditary.
Case presentation
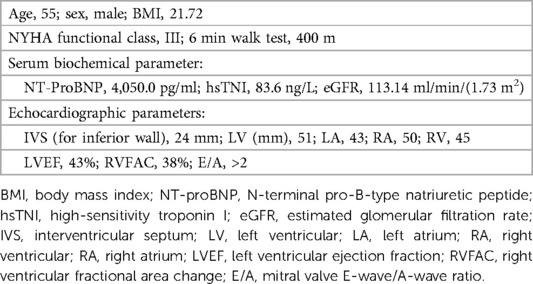
A previously healthy 55-year-old man underwent a physical examination where a reduction in ejection fraction (EF) was found accidentally. Subsequently, he was prescribed anticardiac remodeling drugs. Clinical characteristics are presented in Table 1. The patient suffered from syncope during climbing 10 days before admission. He had a history of hypertension for 8 years with the highest systolic blood pressure of 158 mmHg. Additionally, he underwent cholecystectomy 1 year prior and reported chronic diarrhea for several years without undergoing examination.
ECG showed old inferior and interior wall myocardial infarction (Figure 1A). For laboratory testing, NT-proBNP was found significantly increased at 4,050.0 pg/ml (threshold value, 125 pg/ml). Complete blood count, myocardial enzymes, and biochemical examination were normal.
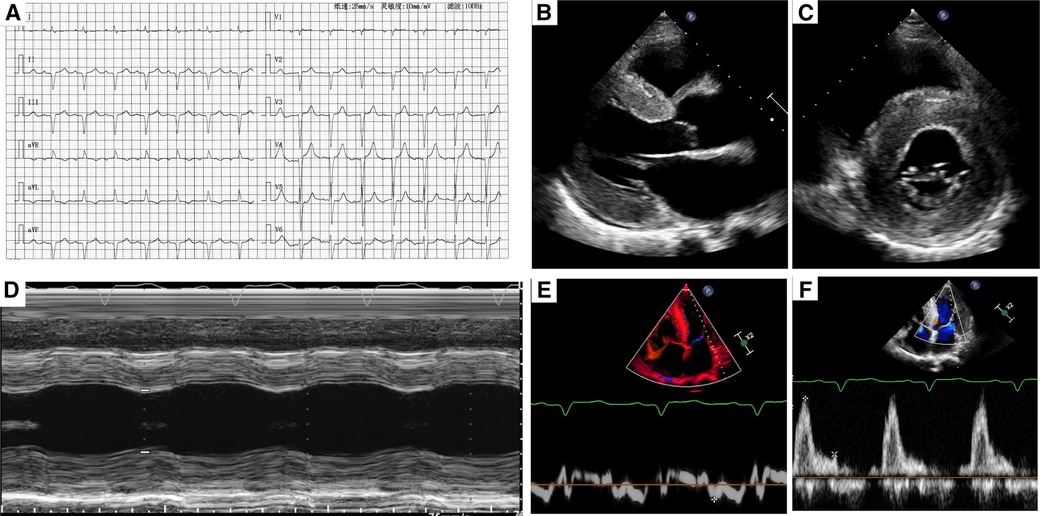
Figure 1. (A) A typical electrocardiogram. (B,C) Two-dimensional transthoracic echocardiography showed left ventricular hypertrophy and myocardium speckled pattern. (D) The contractility function was reduced. (E,F) Diastolic dysfunction (stage III) was found by pulse-waved Dopper echocardiogram.
Transthoracic echocardiography demonstrated diffuse hypertrophy of the left ventricular wall and “ground-glass” enhancement of myocardial echogenicity. The thickness for basal, middle, and apical segments of the interventricular septum was 24, 22, and 21 mm, respectively. The corresponding thickness of the segments for the inferior wall was 24, 18, and 22 mm, respectively. In addition, the contractility function was reduced with an estimated LVEF of 43%. The right ventricular wall motion was normal with a right ventricular fractional area change of 38%. Furthermore, bi-atrial enlargement and diastolic dysfunction (stage III) were observed. What's more, mild to moderate insufficiency was found in the aortic, mitral, and tricuspid valves (Figures 1B–E).
Coronary arteriography showed coronary artery was normal. In addition, a dynamic electrocardiogram showed that the patient had no fast or chronic arrhythmia but only 625 ventricular premature beats in 24 h. The average heart rate was 62 bpm (min, 62; max, 93 bpm). What's more, craniocerebral MRI and MRA did not show significant abnormality.
Cardiac magnetic resonance was then used to evaluate cardiomyopathy due to his diffuse hypertrophy and reduced EF value. The first-pass perfusion imaging showed decreased subendocardial perfusion in the left ventricular walls. Delayed-enhancement magnetic resonance imaging revealed the left ventricle with diffuse granular enhancement, mainly under the endocardium (Figures 2A,B). The patient had no family history of amyloidosis. Serum kappa, lambda light chains, and kappa/lambda free light chains ratio were normal, and no monoclonal protein was detected. Subsequently, a SPECT imaging using 99mTc-PYP scintigraphy was done. The result showed a significantly higher cardiac uptake than bone (SQA grade 3), indicating the possibility of transthyretin amyloid deposition (Figure 2C–E). A genetic analysis of the TTR was then performed, and the result revealed a rare mutation NM_000371.4 c.172G>T resulting in p.D58Y for the Chinese population (chr18:31592998-31592998, human genome 38).
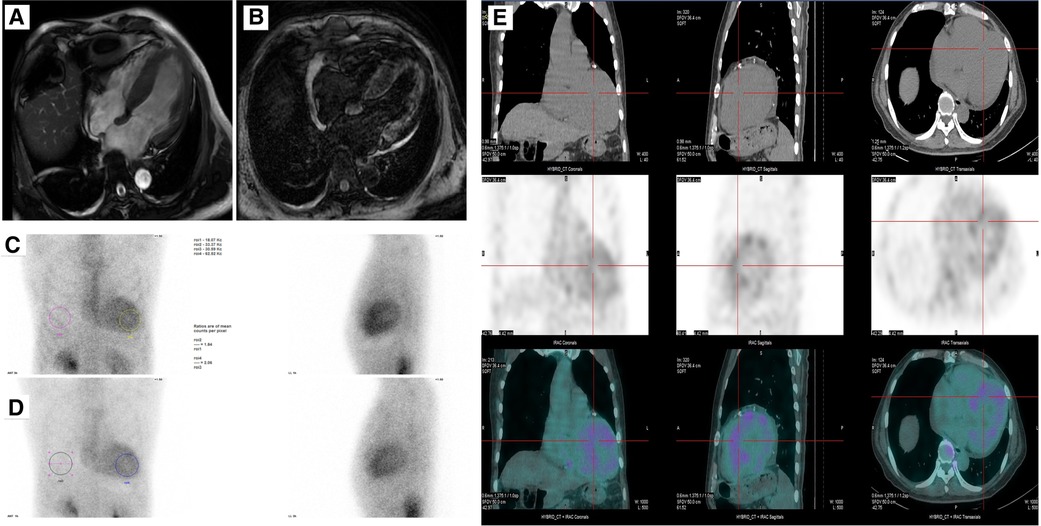
Figure 2. (A,B) Cine and late gadolinium-enhanced MRI. (C–E) A SPECT imaging using 99mTc-PYP scintigraphy showing uptake of 99mTc-PYP by cardiac at 3 h and 1 h.
According to the new British ATTRm prognostic staging scores, this patient was in stage II (NT-proBNP, >3,000 pg/ml), indicating a median survival time of approximately 46.7 months (12). The patient is currently undergoing treatment with tafamidis (20 mg p.o. qd) and is under regular follow-up.
Discussion
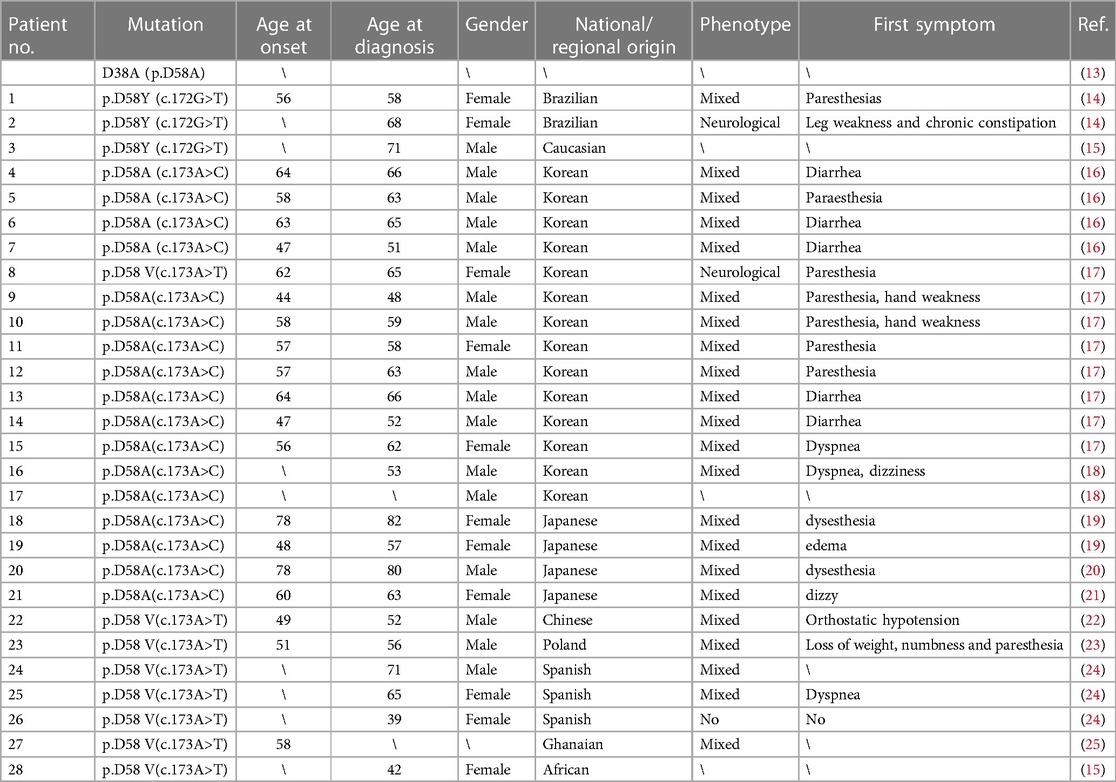
Here, we reported a rare transthyretin mutation p.D58Y(c.172G > T) in a Chinese patient. Due to historical reasons, there was inconsistency among different groups in the numbering of amino acids. Some labs number them according to the beginning of the mature protein, while others include the 20-amino acid signal sequences in numbering the unprocessed gene product. There have been some reports of the same amino acid mutation of the TTR gene, including p.D58A, p.D58V, and p.D58Y, in which the amino acid aspartic acid (D) was replaced by alanine (A), valine (V), and tyrosine (Y), respectively (Table 2).
According to the 14-year update Transthyretin Amyloidosis Outcomes Survey (THAOS), which included 5,894 ATTR patients, there were 36 p.D58A patients (0.6% of all ATTR patients) involved (13). Unfortunately, no details about these 36 patients were given. Lavigne et al. (14) reported two Brazilian sisters with p.D58Y mutation, who suffered from late-onset neuropathy associated with CA. The first patient suffered from hand paresthesias at age 56. After 2 years of disease progression, she developed postural hypotension and syncope episodes and was then found to have CA. However, no evidence of cardiac involvement was provided. The second patient suffered from a rapidly progressive leg weakness and chronic constipation. A severe sensory and motor axonal neuropathy was revealed by electromyography. It is worth noting that her cardiac function was normal. What's more, another two other groups reported p.D58Y mutants but again without details (15, 26, 27). Hence, in contrast to previous studies, we provided here detailed clinical phenotypes for ATTR p.D58Y mutation, especially the CA information for the first time.
Interestingly, after carefully searching, the other similar mutations for this amino acid aspartic acid were most reported in East Asia. Jang et al. (16) reported 4 p.D58A patients from seven mutations and indicated that p.D58A was the predominant TTR mutation in Korean patients. They found that Asp58Ala had older disease onset ages and higher gastrointestinal involvement incidence. Similarly, Choi et al. (17) reported the characteristics of 18 South Korean ATTR patients and found that p.D58A was the most common mutation pattern (8/18). These eight patients shared similar clinical symptoms, such as polyneuropathy and cardiac disease. Three of them complained of severe diarrhea with weight loss, which was consistent with our case. This patient had suffered from diarrhea for several years, and it cannot be ruled out whether diarrhea was associated with amyloidosis. In addition, Cho et al. (18) reported a p.D58A variant Korean male with clinical polyneuropathy presentation, whose amyloid was distributed in the heart and colonic mucosa. It seems that amyloid deposition distributed in the heart and colonic mucosa with polyneuropathy was p.D58A ATTR's clinical feature. Moreover, future researches are needed to clarify whether digestive tracts are involved in these ATTR D38 (aspartic acid at position 58) mutations. After fully communicating with the patient and his family in this case, there were no other somatic neuropathy or autonomic dysfunctions. However, it could be better if assessments such as electromyography and histopathological examination of the nerve were performed in future follow-ups with the patient's consent.
In addition, Yazak et al. (19) and Yazaki et al. (28). reported two Japanese female patients with p.D58A mutation. They found that these mutants suffered earlier cardiac dysfunction with cardiomegaly and heart failure than other mutants. They also found pulmonary parenchyma was diffusely amyloid deposition by autopsy in p.D58A patients. Moreover, another two labs, Tachibana and Kishikawa, also reported one Asp38Ala case each (20, 21). For Chinese data, He et al. (22) summarized the clinical characteristics and prognosis of 23 Chinese ATTR-CM patients, including p.D58V. They reported that the clinical presentations of this p.D58V patient were heart and nerve mix phenotype with orthostatic hypotension as the initial manifestation. In addition, they found that Asp38Val belonged to the early-onset group (<50 years old).
In addition, tmutations were also reported outside East Asia. Gillmore et al. (15) mentioned one African female with p.D58V mutation, but no details were given. Lipowska et al. (23) reported the genetic and clinical presentation of transthyretin-related familial amyloid polyneuropathy (ATTR-FAP) in Poland. They reported one 51-year-old Asp38Val (p.Asp58Val) patient with first manifestation: loss of weight, numbness, and paresthesia in feet. Augustin et al. (24) reported a large pedigree from a 71-year-old Spanish man with p.D58V patient who had predominant late-onset heart involvement with variable polyneuropathy. What's more, Lachmann et al. (25) reported one Ghanaian patient with p.D58V mutation, who presented with CA but the predominant clinical feature was neuropathy.
The worldwide presence of Asp mutation showed the importance of this amino acid in ATTR pathogenicity. As we know, the ATTR structure stability was closely associated with its function. The kinetic and thermodynamic stability studies would be very important for better understanding ATTR mutants. Recently, Ma et al. (29) characterized the kinetic and thermodynamic stabilities of a novel Chinese c.175G>T p.D59Y mutant, which was very close to the c.172G>T p.D58Y mutant in our study. They found that p.D59Y was less stable than WT TTR and tafamidis showed potential benefits to p.D59Y ATTRv. It was interesting that not all TTR variants were associated with amyloidosis. Using ligand binding and structural studies, Almeida et al. (30) found that the Met 119 variant would induce residue Leu-110 to move to a position closer to the hormone, resulting in increased T4 binding affinity. Rosen et al. (31) and Curtis et al. (32) also reported increased T4 affinity in Met 119 variant. What's more, in opposition to other TTR variants, Coelho et al. (33) found that Met 119 substitution may induce tetrameric structure stabilization and result in lower amyloidogenic potential. The effects of these D38 (aspartic acid at position 58) mutations on ATTR structure stability and pathophysiology is an important question and needs to be addressed in the future. Furthermore, the main limitations of this study include the lack of biopsy and pedigree and the short follow-up period.
Conclusion
In this article, we presented a rare case of a Chinese patient with transthyretin mutation p.D58Y and provided detailed CA information for the first time. Presenting this p.D58Y ATTR case aims to provide a better understanding of ATTR genotypes and phenotypes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Wuhan Union Hospital Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL: Funding acquisition, Writing – original draft, Writing – review & editing. JP: Methodology, Writing – original draft, Writing – review & editing. BL: Funding acquisition, Visualization, Writing – original draft, Writing – review & editing. ZC: Data curation, Validation, Writing – review & editing. ZC: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the National Natural Science Foundation of China (Nos. 82070317, 22274105 to JL, 82100298 to BL and 81900519 to JP).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology working group on myocardial and pericardial diseases. Eur J Heart Fail. (2021) 23(4):512–26. doi: 10.1002/ejhf.2140
2. Kittleson MM, Ruberg FL, Ambardekar AV, Brannagan TH, Cheng RK, Clarke JO, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. (2023) 81(11):1076–126. doi: 10.1016/j.jacc.2022.11.022
3. Koike H, Okumura T, Murohara T, Katsuno M. Multidisciplinary approaches for transthyretin amyloidosis. Cardiol Ther. (2021) 10(2):289–311. doi: 10.1007/s40119-021-00222-w
4. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. (2012) 126(10):1286–300. doi: 10.1161/CIRCULATIONAHA.111.078915
5. Gonzalez-Duarte A, Ulloa-Aguirre A. A brief journey through protein misfolding in transthyretin amyloidosis (ATTR amyloidosis). Int J Mol Sci. (2021) 22(23):13158. doi: 10.3390/ijms222313158
6. Sekijima Y. Transthyretin (ATTR) amyloidosis: clinical spectrum, molecular pathogenesis and disease-modifying treatments. J Neurol Neurosur PS. (2015) 86(9):1036–43. doi: 10.1136/jnnp-2014-308724
7. Rowczenio DM, Noor I, Gillmore JD, Lachmann HJ, Whelan C, Hawkins PN, et al. Online registry for mutations in hereditary amyloidosis including nomenclature recommendations. Hum Mutat. (2014) 35(9):E2403–12. doi: 10.1002/humu.22619
8. Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (transthyretin amyloid outcome survey). J Am Coll Cardiol. (2016) 68(2):161–72. doi: 10.1016/j.jacc.2016.03.596
9. Rapezzi C, Quarta CC, Obici L, Perfetto F, Longhi S, Salvi F, et al. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. (2013) 34(7):520–8. doi: 10.1093/eurheartj/ehs123
10. Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. (2008) 40(3):232–9. doi: 10.1080/07853890701842988
11. Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, et al. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. Jama Cardiol. (2016) 1(8):880–9. doi: 10.1001/jamacardio.2016.2839
12. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. (2018) 39(30):2799–806. doi: 10.1093/eurheartj/ehx589
13. Dispenzieri A, Coelho T, Conceicao I, Waddington-Cruz M, Wixner J, Kristen AV, et al. Clinical and genetic profile of patients enrolled in the transthyretin amyloidosis outcomes survey (THAOS): 14-year update. Orphanet J Rare Dis. (2022) 17(1):236. doi: 10.1186/s13023-022-02359-w
14. Lavigne MC, Marques VD, Lourenco CM, Cabrini DI, Nunez JC, Herrera RF, et al. Transthyretin Asp38Tyr: a new mutation associated to a late onset neuropathy. J Peripher Nerv Syst. (2015) 20(1):60–2. doi: 10.1111/jns.12112
15. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. (2016) 133(24):2404–12. doi: 10.1161/CIRCULATIONAHA.116.021612
16. Jang MA, Lee GY, Kim K, Kim SJ, Kim JS, Lee SY, et al. Asp58Ala is the predominant mutation of the TTR gene in Korean patients with hereditary transthyretin-related amyloidosis. Ann Hum Genet. (2015) 79(2):99–107. doi: 10.1111/ahg.12101
17. Choi K, Seok JM, Kim BJ, Choi YC, Shin HY, Sunwoo IN, et al. Characteristics of South Korean patients with hereditary transthyretin amyloidosis. J Clin Neurol. (2018) 14(4):537–41. doi: 10.3988/jcn.2018.14.4.537
18. Cho HJ, Yoon JY, Bae MH, Lee JH, Yang DH, Park HS, et al. Familial transthyretin amyloidosis with variant Asp38Ala presenting with orthostatic hypotension and chronic diarrhea. J Cardiovasc Ultrasound. (2012) 20(4):209–12. doi: 10.4250/jcu.2012.20.4.209
19. Yazak M, Take YI, Katoh M, Ikeda SI. Postmortem findings in two familial amyloidosis patients with transthyretin variant Asp38Ala. Amyloid. (2000) 7(4):270–7. doi: 10.3109/13506120009146441
20. Tachibana N, Tokuda T, Yoshida K, Taketomi T, Nakazato M, Li YF, et al. Usefulness of MALDI/TOF mass spectrometry of immunoprecipitated serum variant transthyretin in the diagnosis of familial amyloid polyneuropathy. Amyloid. (1999) 6(4):282–8. doi: 10.3109/13506129909007341
21. Kishikawa M, Nakanishi T, Miyazaki A, Shimizu A, Kusaka H, Fukui M, et al. A new amyloidogenic transthyretin variant, [D38A], detected by electrospray ionization/mass spectrometry. Amyloid. (1999) 6(4):278–81. doi: 10.3109/13506129909007340
22. He S, Tian Z, Guan H, Li J, Fang Q, Zhang S. Clinical characteristics and prognosis of Chinese patients with hereditary transthyretin amyloid cardiomyopathy. Orphanet J Rare Dis. (2019) 14(1):251. doi: 10.1186/s13023-019-1235-x
23. Lipowska M, Drac H, Rowczenio D, Gilbertson J, Hawkins PN, Lasek-Bal A, et al. Transthyretin-related familial amyloid polyneuropathy (ATTR-FAP) in Poland—genetic and clinical presentation. Neurol Neurochir Pol. (2020) 54(6):552–60. doi: 10.5603/PJNNS.a2020.0100
24. Augustin S, Llige D, Andreu A, Gonzalez A, Genesca J. Familial amyloidosis in a large Spanish kindred resulting from a D38V mutation in the transthyretin gene. Eur J Clin Invest. (2007) 37(8):673–8. doi: 10.1111/j.1365-2362.2007.01836.x
25. Lachmann HJ, Booth DR, Booth SE, Bybee A, Gilbertson JA, Gillmore JD, et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. New Engl J Med. (2002) 346(23):1786–91. doi: 10.1056/NEJMoa013354
26. Martinez-Naharro A, Kotecha T, Norrington K, Boldrini M, Rezk T, Quarta C, et al. Native T1 and extracellular volume in transthyretin amyloidosis. JACC Cardiovasc Imaging. (2019) 12(5):810–9. doi: 10.1016/j.jcmg.2018.02.006
27. Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, et al. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. (2015) 132(16):1570–9. doi: 10.1161/CIRCULATIONAHA.115.016567
28. Yazaki M, Tokuda T, Nakamura A, Higashikata T, Koyama J, Higuchi K, et al. Cardiac amyloid in patients with familial amyloid polyneuropathy consists of abundant wild-type transthyretin. Biochem Bioph Res Co. (2000) 274(3):702–6. doi: 10.1006/bbrc.2000.3203
29. Ma Q, Wang M, Huang Y, Nie Y, Zhang X, Yang DD, et al. Identification of a novel transthyretin mutation D39Y in a cardiac amyloidosis patient and its biochemical characterizations. Front Cardiovasc Med. (2023) 10:1091183. doi: 10.3389/fcvm.2023.1091183
30. Almeida MR, Damas AM, Lans MC, Brouwer A, Saraiva MJ. Thyroxine binding to transthyretin met 119. Comparative studies of different heterozygotic carriers and structural analysis. Endocrine. (1997) 6(3):309–15. doi: 10.1007/BF02820508
31. Rosen HN, Murrell JR, Liepnieks JJ, Benson MD, Cody V, Moses AC. Threonine for alanine substitution at position 109 of transthyretin differentially alters human transthyretin’s affinity for iodothyronines. Endocrinology. (1994) 134(1):27–34. doi: 10.1210/endo.134.1.8275943
32. Curtis AJ, Scrimshaw BJ, Topliss DJ, Stockigt JR, George PM, Barlow JW. Thyroxine binding by human transthyretin variants: mutations at position 119, but not position 54, increase thyroxine binding affinity. J Clin Endocr Metab. (1994) 78(2):459–62. doi: 10.1210/jcem.78.2.7906282
Keywords: transthyretin amyloid cardiomyopathy, amyloid, genetics, mutation, case report
Citation: Lin J, Peng J, Lv B, Cao Z and Chen Z (2024) Case Report: A rare transthyretin mutation p.D58Y in a Chinese case of transthyretin amyloid cardiomyopathy. Front. Cardiovasc. Med. 11:1374241. doi: 10.3389/fcvm.2024.1374241
Received: 21 January 2024; Accepted: 10 May 2024;
Published: 22 May 2024.
Edited by:
Nobuhiro Tahara, Kurume University, Japan© 2024 Lin, Peng, Lv, Cao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhijian Chen, ZHJjaGVuemhpamlhbkBodXN0LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Jibin Lin
Jibin Lin Jiangtong Peng1,2,3,†
Jiangtong Peng1,2,3,† Bingjie Lv
Bingjie Lv Zhijian Chen
Zhijian Chen