
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 26 June 2024
Sec. Atherosclerosis and Vascular Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1373097
This article is part of the Research Topic Intravascular and Non-invasive Imaging of Inflammatory Conditions in Atherosclerosis View all 7 articles
 Qing Yang1,†
Qing Yang1,† Qi Liu2,3,†
Qi Liu2,3,† Changqing Yin1
Changqing Yin1 Xiaoyu Zhang2,3
Xiaoyu Zhang2,3 Xi Chen4
Xi Chen4 Dmytro Pylypenko5
Dmytro Pylypenko5 Hao Chen6
Hao Chen6 Qiang Shu2,3*‡
Qiang Shu2,3*‡ Dexin Yu1*‡
Dexin Yu1*‡
Objective: To identify the correlation between thrombosis and atherosclerosis in systemic lupus erythematosus (SLE) patients with antiphospholipid antibodies (aPLs) (SLE/aPLs) through high-resolution magnetic resonance imaging (HR-MRI) of the carotid artery.
Methods: A single-center, cross-sectional study was conducted. We collected consecutive patients with SLE/aPLs and healthy controls who underwent carotid HR-MRI examinations. The morphometric characteristics of the common carotid artery (CCA), internal carotid artery (ICA), external carotid artery (ECA), and carotid bulb (Sinus) were measured, and the differences in morphometric parameters between different groups were analyzed.
Results: A total of 144 carotid arteries were analyzed. Compared with the control group, the wall area, wall thickness (WT and WTmax), and normalized wall index of CCA, ICA, ECA, and Sinus were increased in patients with SLE/aPLs, and the total vascular area (TVA) of CCA, ICA, and Sinus, and the bifurcation angle (BIFA) of ICA-ECA were also increased. A negative lupus anticoagulant (LAC) (with or without positive anticardiolipin antibody (aCL) or anti-β2glycoprotein antibody (aβ2GPI)) contributed to illustrating lower increased TVA and thickened vessel walls of CCA and ICA in SLE/aPLs patients without thrombotic events. Logistic regression analysis showed that WTmaxSinus and WTmaxGlobal were independent risk factors for thrombotic events in SLE/aPLs patients. The receiver operator characteristic curve showed that the cut-off value of WTmaxSinus was 2.855 mm, and WTmaxGlobal was 3.370 mm.
Conclusion: HR-MRI ensures the complete and accurate measurement of carotid morphometric parameters. Compared with the control group, the carotid artery in patients with SLE/aPLs is mainly characterized by diffusely thickened vessel walls, and the patients with thrombotic events showed additional higher vascular area of CCA and ICA, and BIFA of ICA-ECA without significant change in lumen area. The carotid arteries of SLE/aPLs patients with thrombotic events exhibited significant vessel wall thickening in all segments except ECA compared to those without thrombotic events. LAC-negative and non-thrombotic events distinguish relatively early atherosclerosis in the carotid arteries in patients with SLE/aPLs. Patients with SLE/aPLs that possess circumscribed thickened carotid vessel walls (>3.370 mm), particularly thickened at the Sinus (>2.855 mm), may require management strategies for the risk of thrombotic events.
Antiphospholipid syndrome (APS) is a rare acquired autoimmune disease characterized by recurrent thrombosis and/or pregnancy complications, with the persistent existence of one or more antiphospholipid antibodies (aPLs), including lupus anticoagulant (LAC), anticardiolipin antibody (aCL), and anti-β2glycoprotein antibody (aβ2GPI) (1, 2). Acquired thrombosis is one of the hallmarks of the onset and progression of APS symptoms (3). Secondary APS is often associated with autoimmune rheumatic diseases, particularly in individuals with systemic lupus erythematosus (SLE) (4, 5). SLE patients with positive aPLs (SLE/aPLs) are associated with a more severe disease course, acquired organ damage, and higher cardiovascular disease risk (6–8). However, currently, there is a lack of relevant research on the susceptibility characteristics of thrombosis in patients with SLE/aPLs.
Recent studies suggest that the pathophysiology of thrombosis may accelerate atherosclerosis in patients with APS (9–11) and that traditional risk factors for atherosclerosis are closely associated with the prevalence and mortality of APS and thrombosis (6, 12, 13). Some studies have observed increased intima-media thickness (IMT) of the carotid artery and atherosclerotic plaques in patients with APS or aPLs carriers (12–15). In contrast, others have not observed a difference between IMT of the carotid artery in patients with APS and healthy individuals (16–18). It is well-recognized that the method of measuring IMT using Doppler ultrasound is relatively primary and limited, which may be the reason for the contradictory conclusions obtained by different studies. Therefore, it is essential to comprehensively and systematically evaluate the morphometric characteristics of critical regions of the carotid arteries in patients with SLE/aPLs.
High-resolution magnetic resonance imaging (HR-MRI) is an emerging auxiliary examination technique that can clearly identify carotid and cerebral arterial lesions that are difficult to discern using standard imaging techniques. It can better characterize the structure of the vessel wall and, combined with image reconstruction, provide accurate morphometric measurements of the entire scanned region of interest for researchers (19–21). Based on this, we believe that HR-MRI can contribute to a comprehensive and systematic morphometric evaluation of the carotid arteries in patients with SLE/aPLs, aiming to overcome the limitations of measurement parameters and sites in carotid Doppler ultrasound. However, there are currently no relevant applications or reports available.
The purpose of this study is to investigate the characteristic morphometric changes of the carotid arteries by measuring the wall and lumen structure of the internal carotid artery (ICA), external carotid artery (ECA), common carotid artery (CCA), and carotid bulb (Sinus) in patients with SLE/aPLs using HR-MRI, thereby elucidating the intrinsic connection between the morphometric characteristics of the carotid arteries and thrombosis from the perspective of radiology and providing a theoretical basis and data to support the early screening and clinical prevention and treatment of thrombosis in patients with SLE/aPLs.
This study was approved by the Institutional Review Committee of Qilu Hospital of Shandong University (#KYLL-202208-048), and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee. All participants fully satisfied the patient's right to know and signed the informed consent form before enrolling in the study.
Inpatients who underwent a carotid HR-MRI examination from June 2021 to October 2023 at Qilu Hospital of Shandong University were prospectively and continuously selected to participate in this cross-sectional study. Patients enrolled in the SLE/aPLs group were over 18 years old and fulfilled the SLE/aPLs criteria, which referred to meeting the 2019 EULAR/ACR SLE classification criteria (22) and having positive aPLs. The patients' clinical information was recorded in detail to determine whether it met the revised Sapporo classification criteria for APS (1). The “clinical non-criteria manifestation” described by Gilberto et al. in 2020 (23) was also recorded, considering that our understanding of APS is constantly improving. Patients in the SLE/aPLs group were further classified into the SLE/aPLs with thrombosis group (thrombosis group) and the SLE/aPLs without thrombosis group (non-thrombosis group) based on whether they had objectively documented or ongoing symptomatic or asymptomatic thrombotic events. Patients suffering malignancy or hematologic disorders, or having a history of non-atherosclerotic brain and carotid artery diseases such as Moya-Moya disease, dissection, and fibromuscular dysplasia, or previous carotid intervention such as arterial stenting, end-arterial decortication surgery, and neck radiation therapy, or with any contraindications to MR examination were excluded from this study. The control group included healthy participants during the same period without any history of autoimmune disease or thrombosis. These participants received extra HR-MRI scans after satisfying the right to know fully and obtaining consent from the patients and their accompanying relatives.
Demographic, clinical, and laboratory data were collected and recorded, and the interval between the record and the HR-MRI scan was less than two weeks. In addition to documented thrombotic events and course of disease, traditional cardiovascular risk factors such as arterial hypertension, hyperlipidemia, diabetes mellitus, obesity (body mass index (BMI) ≥ 30 kg/m2), smoking habits, and previous and/or current treatment were collected.
Criteria for positive aPLs: aPLs must be positive in two or more tests with intervals of more than 12 weeks. Anticardiolipin antibodies (IgG/IgM) were determined by enzyme-linked immunosorbent assay (ELISA) and were considered positive with the presence of medium/high titer (>40/>80 IgG phospholipid (GPL) unites or >40/>80 IgM phospholipid (MPL) unites) (13, 24–26). Anti-β2glycoprotein antibodies (IgG/IgM) were detected by chemiluminescence assay (CIA) and were considered positive with >7.4 chemiluminescence units (CU) for IgG or >3.6 CU for IgM. LAC was identified using the diluted Russell Viper Venom Time and was considered positive with a normalized ratio (NR) ≥1.2. The number of positive aPLs was classified as single-positive aPLs, double-positive aPLs, and triple-positive aPLs (13, 24–29). The Department of Clinical Laboratory of Qilu Hospital of Shandong University tested and reported all the samples and set the reference range of the positive criteria according to the internal quality control.
All MRI scans were performed using a Discovery 750 3.0T magnetic resonance scanner (General Electric Medical System, WI, USA), equipped with a dedicated 40-channel cerebral vascular and carotid integrated coil (Medcoil Healthcare Co Ltd, Suzhou, China).
A three-dimensional (3D) T1- and T2-weighted HR-MRI system was used to analyze the geometry and vessel walls of carotid arteries. The scanning range of the 3D-CUBE sequence completely covered the arterial vascular structure at the bifurcation of the carotid arteries on both sides. The 3D-CUBE-T1 weighted imaging parameters were as follows: repetition time: 1,100 ms; echo time: 15 ms; field of view: 22 cm; matrix: 320 × 320; layer thickness: 0.6 mm; number of excitations: 1; scanning direction: coronal position; excitation mode: selective; voxel size: 0.6 mm × 0.6 mm × 0.6 mm; locs per slab: 90; scanning time: 5 min and 35s. During the scan, a gadolinium contrast agent (Magnevist) was used as an enhanced contrast medium.
The 3D-CUBE-T1 enhanced sequence data were imported into the ADW 4.7 and utilized workstation (General Electric Medical System, WI, USA) to reconstruct images with a slice thickness of 1.0 mm and a spacing between images of 1.0 mm. The cross-sectional images of carotid arteries were obtained by extending the bifurcation sites of ECA and ICA to the proximal and distal ends by 2.0 cm, respectively, perpendicular to the lumen axis of CCA, ECA, ICA, and Sinus. In addition, curved projection reformation along the lumen above the axis was performed to obtain a longitudinal view of the carotid artery.
Two trained radiologists (with more than five years of clinical experience in MR imaging of carotid arteries) independently evaluated all collected HR-MRI images without accessing clinical information. We assessed the bilateral carotid arteries independently, and a complete carotid artery image should include a segment of CCA, ICA, ECA, and an intact Sinus for accurate measurement of the vessel wall and lumen. The quality of HR-MRI images was graded as excellent [high signal-to-noise ratio (SNR), no artifacts, complete suppression of intravascular blood flow signal, precise arterial wall contour, and a distinct boundary with the lumen], good (high SNR, with minimal motion artifacts, incomplete suppression of intravascular blood flow signal, precise arterial wall contour, and a distinct boundary with the lumen), marginal (low SNR, with motion artifacts, having arterial wall contour but indistinguishable wall structure), and poor (low SNR, with significant motion artifacts, unclear display of arterial blood vessels). Images graded as marginal and poor were excluded from this study.
Image analysis was performed using VesselMASS V2019-EXP software (Leiden University Medical Center, the Netherlands). After the eligible reconstructed images were imported into the software, the contours of the outer and inner vessel walls of the carotid artery in the image were manually delineated, followed by correction using the software. The morphometric parameters of CCA, ICA, ECA, and Sinus were measured separately in each layer. The total vessel area (TVA), lumen area (LA), wall thickness (WT), wall area (WA, WA = TVA − LA), normalized wall index (NWI, NWI = WA/TVA × 100%), and lumen area ratio of ICA/CCA (LAICA/LACCA-ratio) were measured and calculated. The highest value of WT measured in all slices (WTmax) was selected. The bifurcation angle (BIFA) was defined as the angle between the inner wall of the ECA and ICA measured on a longitudinal view image obtained by curved projection reformation. In addition, we described concentric and eccentric enhancement of the vessel walls to assess vasculitis and atherosclerotic plaques (30, 31). Enhancement was recorded as concentric if it was uniform and involved the entire circumference of the vessel wall and as eccentric if it was nonuniform, mainly on one side of the vessel wall and not involving the entire circumference (32).
Our null hypotheses considered that patients in the thrombosis group had carotid morphometric parameters similar to those in the non-thrombosis group. A power calculation based on an article (18) and our pre-experiment results assessing the thickness of IMT (Doppler ultrasound) or vessel wall (MRI) of the Sinus in patients in the thrombosis group and non-thrombosis group revealed that a minimum of 29 to 32 arteries were required in each group for an α at 0.05 and a power of 80%.
Statistical analyses were conducted using SPSS 19.0 software (IBM, USA). Data were presented as mean ± standard deviation for continuous variables and percentages for categorical variables. Independent Student's t-test was used to compare continuous data between two groups, and one-way analysis of variance was employed for comparisons involving more than two groups. Bonferroni correction was applied to adjust for multiple comparisons. The χ2 and Fisher's exact tests were used for univariable comparisons between categorical variables. Binary logistic regression analysis was performed to identify independent risk factors for thrombotic events, and the odds ratio (OR) and corresponding 95% confidence interval (CI) of each factor were calculated. The factors with significant differences in descriptive statistics between the thrombosis and non-thrombosis groups were analyzed using univariable binary logistic regression analysis, and the factors verified by univariable regression analysis were entered into the multivariable regression analysis. Significant independent risk factors were selected and plotted on a receiver operator characteristic (ROC) curve using Prism 9 software (GraphPad Software, USA), and the area under the curve (AUC), along with the cut-off value, was calculated. All results were presented as 2-tailed values, and a P-value of <0.05 was considered statistically significant.
After applying the inclusion and exclusion criteria, 52 patients with SLE/aPLs were enrolled in this study, among whom 29 met the Revised Sapporo criteria of APS and 23 had positive aPL only. Of the 29 patients with APS, 24 met the thrombotic criteria only, 1 met the obstetric criteria only, and 4 met both the thrombotic and obstetric criteria. Among the 52 patients with SLE/aPLs, 28 patients with at least one well-documented thrombotic event or ongoing thrombosis were defined as the thrombosis group, as well as the other 24 patients without a history of thrombosis and thrombosis excluded by ancillary examinations were defined as the non-thrombosis group. Additionally, 20 healthy participants without any history of autoimmune disease and thrombosis were classified into the control group. The main demographic and clinical characteristics of the study population are shown in Table 1. There was no statistically significant difference in baseline characteristics between the thrombosis, non-thrombosis, and control groups (P > 0.05). The thrombosis group had a higher rate of antithrombotic therapy than the non-thrombosis group (Total: 75.0% vs. 45.8%, P = 0.031; Warfarin only: 39.3% vs. 0.0%, P = 0.001). Additionally, the thrombosis group had a higher number of positive aPLs (single positive: double positive: triple positive, 28.6%; 42.9%; 28.6% vs. 62.5%; 20.8%; 16.7%, P = 0.048) and LAC positive rate (64.3% vs. 25.0%, P = 0.005) compared with the non-thrombosis group. The APS “non-criteria” manifestations of the thrombosis and non-thrombosis groups are shown in Supplementary Table S1, and no significant difference was shown between these two groups (P > 0.05).
A total of 144 carotid arteries were measured. Within the SLE/aPLs group, more patients in the thrombosis group had eccentric enhancement than those in the non-thrombosis group (16/56 (28.6%) vs. 3/48 (6.3%), P = 0.003), and the presence of concentric enhancement in the two groups was low (2/56 (3.6%) vs. 0/48 (0%), P = 0.498). Quantitative measurements and calculations of TVA, LA, WA, WT, WTmax, and NWI of the CCA, ICA, ECA, and Sinus, including global WTmax, LAICA/LACCA-ratio and BIFA were shown in Table 2. The WA, WT, and WTmax of CCA, ICA, and Sinus, together with TVACCA, NWISinus, and WTmaxGlobal, all showed significant differences between the three groups (P < 0.05). WT, WTmax, and NWI of ECA, as well as NWICCA and NWIICA, showed statistically significant differences between the thrombosis group and the control group and between the non-thrombosis group and the control group (P < 0.05) but not significant between the thrombosis and non-thrombosis groups (P > 0.05). The TVAICA, TVASinus, and BIFA only demonstrated significant differences between the thrombosis and control groups (P < 0.05).
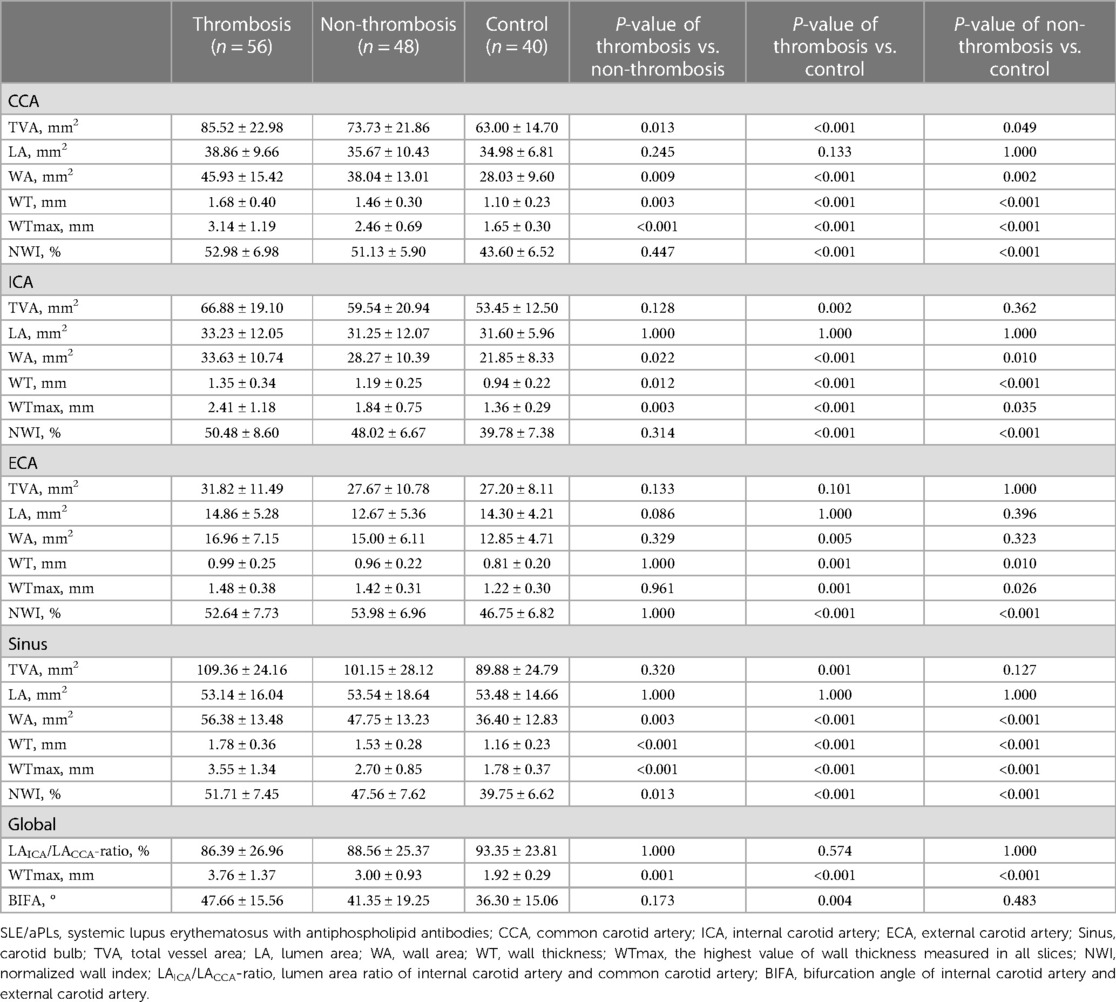
Table 2 Comparison of morphometric characteristics between carotid arteries of patients with SLE/aPLs and healthy control.
We further stratified patients according to the status (positive or negative) of LAC that differed between the thrombosis and non-thrombosis groups (Table 1) and performed statistical analysis (Table 3). The results showed that LAC-positive patients had higher WTmaxCCA than LAC-negative patients in the thrombosis group (3.35 ± 1.32 mm vs. 2.76 ± 0.79 mm, P = 0.041). LAC-positive patients showed higher TVACCA, WACCA, WTCCA, and WTmaxCCA than LAC-negative patients in the non-thrombosis group (P < 0.05). In contrast, in the LAC-positive group, patients with thrombotic events presented higher WT, WTmax, and NWI in the Sinus than those without thrombotic events (P < 0.05). Among LAC-negative patients, the TVA, WA, WT, and WTmax of CCA and ICA, as well as WTSinus, were higher in patients combined with thrombotic events than patients without thrombotic events (P < 0.05). It can be concluded that a negative LAC (with or without positive aCL or aβ2GPI) contributed to illustrating lower increased TVA and thickened vessel walls of CCA and ICA in SLE/aPLs patients without thrombotic events.
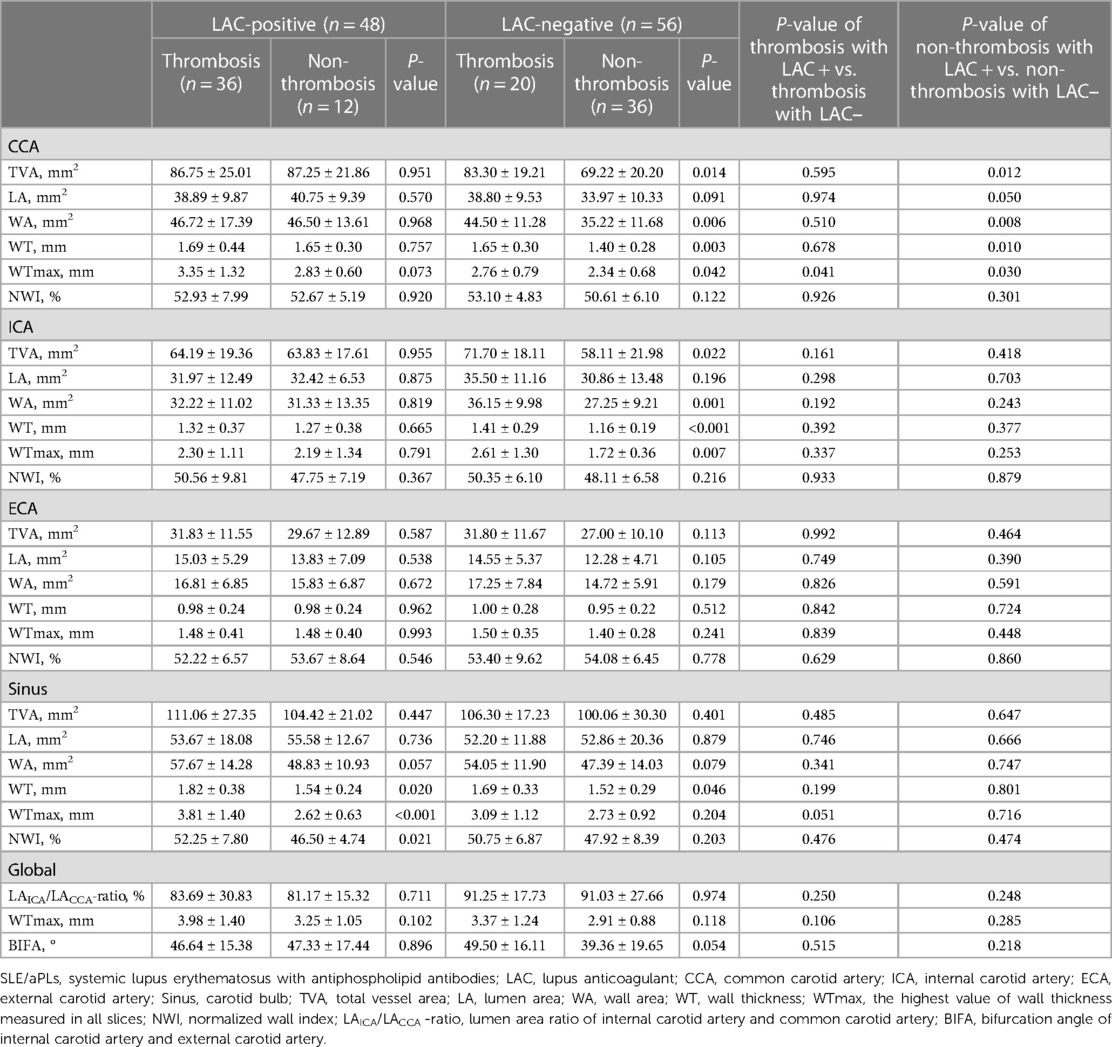
Table 3 Comparison of morphometric characteristics between carotid arteries of patients with SLE/aPLs accompanying positive or negative LAC.
Since the number of positive aPLs varied between the thrombosis group and the non-thrombosis group (Table 1), we categorized all patients with SLE/aPLs into different aPLs-positive groups (single-positive, double-positive, and triple-positive) and performed statistical analysis (Supplementary Table S2). The results demonstrated statistical differences in specific indicators among the groups with varying numbers of positive aPLs. Compared to LAC, the discriminatory ability of the number of positive aPLs was limited. Additionally, we compared the carotid morphometric parameters of patients with high titer aCL with those with medium and low titer. The results showed that only the LAECA (16.69 ± 6.16 mm2 vs. 13.33 ± 5.12 mm2, P = 0.021), WTmaxSinus (3.71 ± 1.13 mm vs. 3.06 ± 1.21 mm, P = 0.021), and BIFA (58.63° ± 19.31° vs. 42.23° ± 16.10°, P < 0.001) were with significant differences between patients with high aCL titer and with medium/low aCL titer (Supplementary Table S3).
We performed univariable and multivariable binary logistic regression analysis with thrombotic events as the outcome variable. The carotid morphometric parameters (Table 2) that were statistically different (P < 0.05) between the thrombosis group and the non-thrombosis group by univariable regression analysis (Table 4) were used as covariates to identify independent risk factors for thrombotic events in patients with SLE/aPLs. We adjusted age, gender, and BMI as confounding factors (model 1). The results showed that WTmaxICA (OR = 5.207, 95% CI, 1.207−26.393, P = 0.046), WTmaxSinus (OR = 5.654, 95% CI, 1.236−25.870, P = 0.026), and WTmaxGlobal (OR = 0.102, 95% CI, 0.018−0.589, P = 0.011) were independently associated with the concurrence of thrombotic events and incorporated into the final model (Table 4). Furthermore, we adjusted age, gender, BMI, course of disease, antithrombotic treatment, LAC, and number of positive aPLs (model 2). The results showed that WTmaxSinus (OR = 10.054, 95% CI, 1.540–65.653, P = 0.016) and WTmaxGlobal (OR = 0.067, 95% CI, 0.009–0.520, P = 0.010) were independently associated with the concurrence of thrombotic events and incorporated into the final model (Table 4).
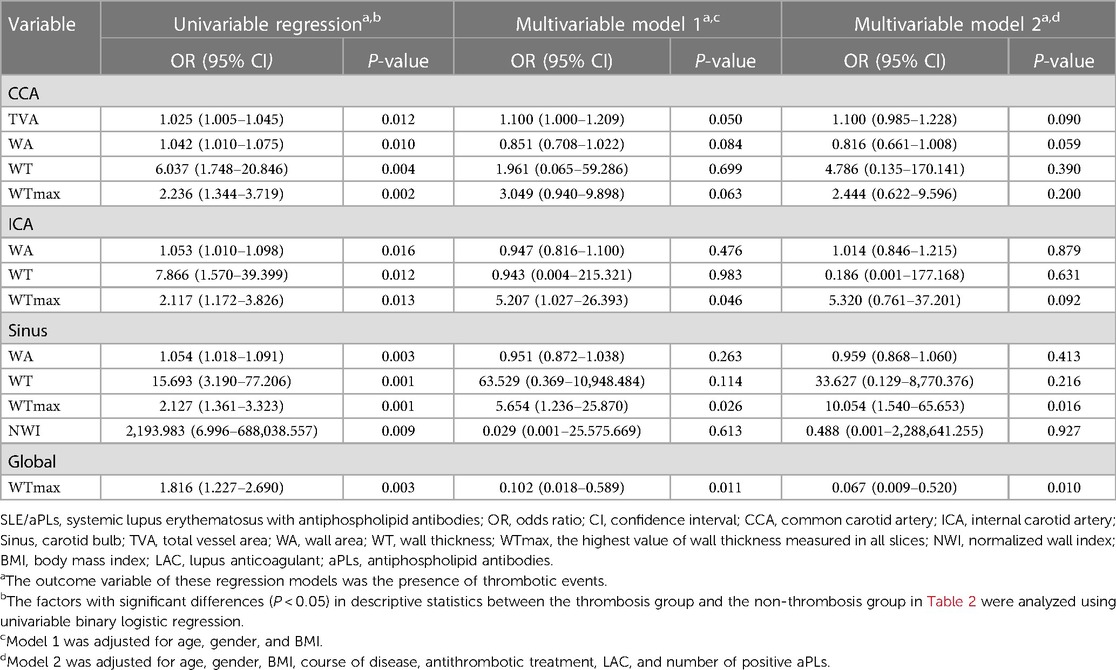
Table 4 Independent risk factors for SLE/aPLs combined with thrombotic events based on binary logistic regression analysis.
Based on this, we further plotted ROC curves for WTmaxSinus and WTmaxGlobal. The AUC and cut-off values for each curve are shown in Figure 1. These data suggested that patients with SLE/aPLs who possessed a circumscribed thickened carotid vessel wall (>3.370 mm), particularly thickened at the Sinuses (>2.855 mm), should be careful with the occurrence of thrombotic events.
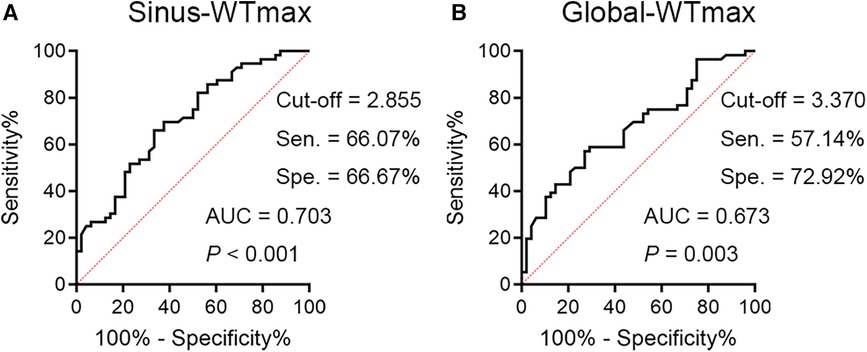
Figure 1 The ROC curves for WTmaxSinus and WTmaxGlobal are shown. (A) The ROC curve for WTmaxSinus has an AUC of 0.703, 95% CI 0.603–0.802, with a cut-off value of 2.855 mm (sensitivity = 66.07%, specificity = 66.67%). (B) The ROC curve for WTmaxGlobal has an AUC of 0.673, 95% CI 0.570–0.775, with a cut-off value of 3.370 mm (sensitivity = 57.14%, specificity = 72.92%). Sen, sensitivity; Spe, specificity.
In this study, we explored the intricate relationship between morphometric parameters of carotid arteries and APS clinical phenotypes. Mechanistically, aPLs promote atherosclerosis and thrombosis in the body (33). The elevated occurrence rates of thrombotic events and atherosclerosis in SLE and APS patients have been extensively reported (6, 10, 12, 34, 35). However, these studies have been limited in reporting the incidence rates of thrombosis and atherosclerosis without exploring the correlation between the two. Research on the correlation between thrombosis and atherosclerosis is limited, and the conclusions are not yet clear (36–38). Therefore, we aim to establish a connection between thrombosis and atherosclerosis through imaging studies, providing a basis for vascular management and thrombosis prevention in the clinical diagnosis and treatment of SLE/aPLs patients.
Previous imaging studies of the carotid artery in APS patients primarily utilized Doppler ultrasound to observe and measure the vessel wall, with IMT as a key or sole data collection indicator (12, 13, 15, 18, 35). However, the experience and technique of the Doppler ultrasound operator significantly affect the measurement accuracy and standard consistency in different studies (39, 40). This study reconstructed HR-MRI images to present a clear, precise, and complete carotid artery morphology, ensuring accurate measurement of morphometric parameters. The clarity of the carotid vessel wall and lumen acquired by HR-MRI facilitates an overall understanding of characteristic morphometric changes, providing a solid foundation for accurately measuring morphometric parameters (20, 21, 41, 42). Consistent with previous reports based on carotid Doppler ultrasound and IMT measurement (12–15), HR-MRI observed accelerated atherosclerosis in SLE/aPLs patients compared to healthy participants.
The carotid artery is a susceptible site for atherosclerosis and serves as a crucial window for predicting and assessing the trends of atherosclerosis in the coronary and cerebral arteries (21, 43–46). In this study, we systematically measured the morphometric parameters of the carotid arteries in SLE/aPLs patients using HR-MRI. Our data suggested that the carotid arteries in patients with SLE/aPLs were primarily characterized by vessel wall thickening at CCA, ICA, ECA, and Sinus, and the patients with thrombotic events showed additional higher TVA of CCA and ICA, and BIFA of ICA-ECA without significant change in LA (Figure 2). The carotid arteries of those with thrombotic events exhibited significant vessel wall thickening in all segments except ECA compared to those without thrombotic events. Consistent with this, Yeo et al. also described diffuse proliferative vasculopathy and vessel wall thickening in the carotid arteries in APS patients (47). Overall, a specific correlation exists between thrombosis and atherosclerosis progression in patients with SLE/aPLs.
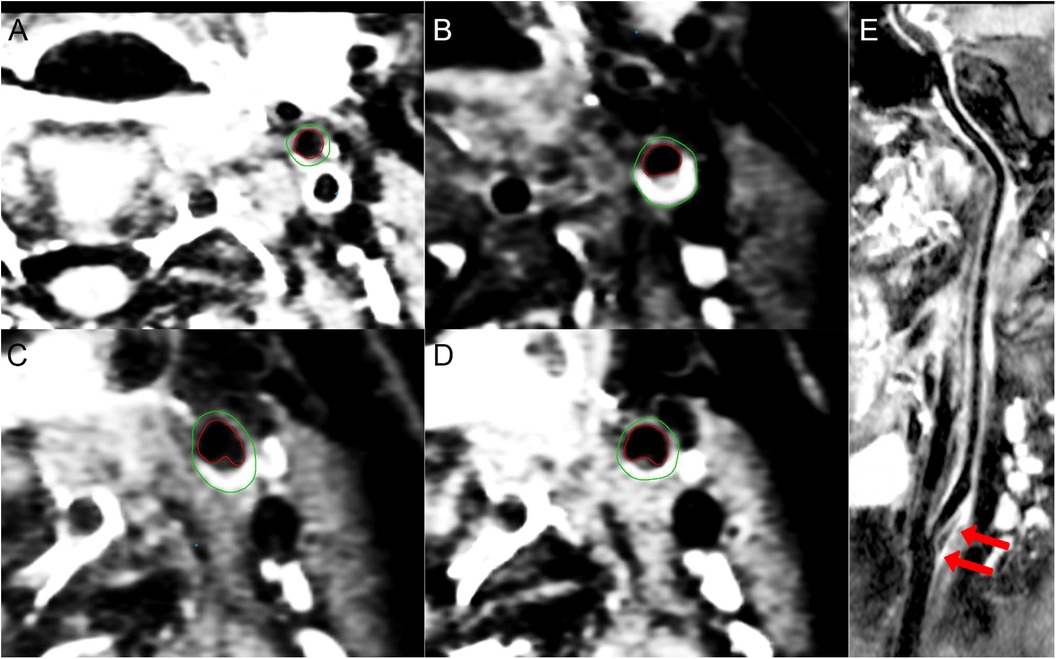
Figure 2 3D-CUBE-T1 weighted images of a 33-year-old woman presents with SLE/aPLs with positive aCL, aβ2GPI, and LAC combined with ischemic cerebral infarction in the left anterior circulation. (A–D) The contours of the outer (green) and inner (red) vessel walls of the left ECA (A), ICA (B), sinus (C), and CCA (D), respectively. Eccentric thickening and enhancement of the vessel wall can be seen at the carotid bifurcation (C) and the origin of the ICA (B). (E) longitudinal view image of curved projection reformation of the left carotid artery showing diffuse vessel wall thickening and atherosclerotic plaque formation at the origin of the left ICA (arrows). The BIFA of ICA-ECA is 38°.
Studies by Ajeganova et al. (6), Tektonidou et al. (10), and Kravvariti et al. (12) have reported a higher prevalence of carotid atherosclerosis in SLE/aPLs patients. Our results also demonstrate thickening of the vessel walls in CCA, ICA, ECA, and Sinus in patients with SLE/aPLs compared to the healthy participants. Moreover, patients in the thrombosis group exhibited further thickening of the CCA, ICA, and Sinus vessel walls, elevated TVACCA, and higher incidence of eccentricity enhancement (which generally means atherosclerotic plaque) than the non-thrombosis group. Consistent with this, Di Minno et al.'s study also observed a higher incidence of atherosclerotic plaques in patients with APS and thrombosis (13). Bettiol et al. reported that the thickness of CCA-IMT, Bulb-IMT, and the incidence of arterial atherosclerosis plaques in the APS thrombus group was significantly higher than in the APS obstetrics and control groups (18). Logistic regression analysis showed that WTmaxSinus and WTmaxGlobal were independent risk factors for thrombotic events in patients with SLE/aPLs. Based on these results, we hypothesize that SLE/aPLs promotes thrombosis and atherosclerosis more substantially than traditional risk factors. The maximum thickness of the vessel wall of the artery is a crucial and intuitive indicator for evaluating atherosclerosis, reflecting the infiltration and destruction process of autoimmune inflammation on the vasculature. The thrombosis, which is also related to the inflammation process of SLE/aPLs, is thus linked to atherosclerosis. Particularly, we want to point out that patients with SLE commonly have small-vessel vasculitis (48, 49). In contrast, reports of large-vessel vasculitis are rare (50). So, we believe this may be why we only collected two cases of patients with carotid artery vasculitis (concentric enhancement). At present, researchers believe that SLE and APS promote carotid atherosclerosis, as mentioned above, rather than large-vessel vasculitis.
Furthermore, the results showed that both LAC-negative and non-thrombotic events distinguished relatively early atherosclerosis in the carotid arteries in patients with SLE/aPLs. The close association between LAC and thrombosis has been widely reported and accepted (51–53). However, limited studies are exploring the association between LAC and atherosclerosis. In a large multicentre population-wide study, LAC-positive women were at higher risk of stroke and myocardial infarction than LAC-negative women. Still, the authors did not specify whether atherosclerosis is the cause of stroke and myocardial infarction (54). The present study's results supplement and support a positive correlation between LAC and arterial lesions.
A positive number of aPLs (aCLs, aβ2GPI, and LAC) has been associated with APS symptoms, with a higher number of positive aPLs linked to increased IMT and atherosclerotic plaque incidence (12, 13, 18). The study by Yoo et al. reported that in patients with ischemic stroke, more than two positive aPLs predict persistent positive aPLs, which indicated the causes of ischemic stroke arising by APS (55), whereas, the present study observed limited intra- and inter-group morphometric parameter changes in the positive number of aPLs in the thrombosis and non-thrombosis groups. In addition, previous studies reported the correlation between aPL titer and atherosclerosis. A higher titer means a higher incidence of atherosclerosis (12, 13, 56). Reciprocally, our results showed little difference in carotid artery morphology between patients with high aCL titer and medium/low aCL titer. The possible reason may be that we only distinguished the titers of aCL [lacking convincing reference for the high titer threshold for detecting aβ2GPI using chemiluminescence (26)], and the sample size of patients with high aCL titer was relatively small in this study. Another point that needs to be explained is that this study focuses on whether there are differences in carotid morphometric parameters between SLE/aPLs patients with and without thrombotic events. However, there was no difference in aCL titer between the thrombosis and non-thrombosis groups, which may suggest a weak correlation between higher aCL titer and thrombotic events in SLE/aPLs patients. In other words, the difference in carotid morphometric parameters between the thrombosis and non-thrombosis groups may not be due to higher aCL titer. Alternatively, the reasons mentioned above are only for our data and analysis. Considering the limited sample size of this study, we believe that a multicentre study or meta-analysis may help identify additional indicators of carotid morphometric parameters associated with the number of positive aPLs and higher aCL/aPLs titers.
Apart from the limitations described in the previous section, the limitations of this study may also include sample size restrictions, as our data did not demonstrate any differences in LA between the various groups. Furthermore, as a cross-sectional study, this research was limited to the measurement and statistical observation of morphometric parameters of the carotid arteries. Whether the morphometric changes reflected by these parameters are correlated with the clinical symptoms and prognosis, and whether timely medical intervention is necessary still requires further support from prospective cohort studies.
In summary, HR-MRI ensures the complete and accurate measurement of carotid morphometric parameters. The carotid arteries in patients with SLE/aPLs are mainly characterized by diffusely thickened vessel walls, and the patients with thrombotic events showed additional higher TVA of CCA and ICA, and BIFA of ICA-ECA without significant change in LA. The carotid arteries of SLE/aPLs patients with thrombotic events exhibited significant vessel wall thickening in all segments except ECA compared to those without thrombotic events. LAC-negative and non-thrombotic events distinguish relatively early atherosclerosis in the carotid arteries in patients with SLE/aPLs. Patients with SLE/aPLs that possess circumscribed thickened carotid vessel walls (>3.370 mm), particularly thickened at the Sinus (>2.855 mm), may require management strategies for the risk of thrombotic events.
The raw data supporting the conclusions of this article will be made available by the authors, upon reasonable request.
The studies involving humans were approved by The Ethics Committee of Qilu Hospital of Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
QY: Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Data curation, Conceptualization. QL: Writing – original draft, Validation, Methodology, Investigation, Data curation, Conceptualization. CY: Writing – original draft, Validation, Software, Data curation. XZ: Writing – original draft, Validation, Investigation, Data curation. XC: Writing – original draft, Visualization, Validation, Software, Methodology, Formal Analysis, Data curation. DP: Writing – review & editing, Validation. HC: Writing – review & editing, Validation, Formal Analysis, Data curation. QS: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Funding acquisition, Conceptualization. DY: Funding acquisition, Writing – review & editing, Supervision, Software, Resources, Project administration, Methodology, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The study was supported by the Shandong Innovation and Entrepreneurship Community of Antibody Drug Project [grant number SDUZHJ(2023)0716] and the ECCM Program of Clinical Research Center of Shandong University [grant number 2021SDUCRCB010].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1373097/full#supplementary-material
1. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemostasis. (2006) 4(2):295–306. doi: 10.1111/j.1538-7836.2006.01753.x
2. Gómez-Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. J Autoimmun. (2014) 48–49:20–5. doi: 10.1016/j.jaut.2014.01.006
3. Sanna G, Bertolaccini ML, Cuadrado MJ, Khamashta MA, Hughes GRV. Central nervous system involvement in the antiphospholipid (hughes) syndrome. Rheumatology. (2003) 42(2):200–13. doi: 10.1093/rheumatology/keg080
4. Belizna CC, Richard V, Primard E, Kerleau JM, Cailleux N, Louvel JP, et al. Early atheroma in primary and secondary antiphospholipid syndrome: an intrinsic finding. Semin Arthritis Rheum. (2008) 37(6):373–80. doi: 10.1016/j.semarthrit.2007.08.002
5. Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, Ramón ED, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. (2015) 74(6):1011–8. doi: 10.1136/annrheumdis-2013-204838
6. Ajeganova S, Hafström I, Frostegård J. Patients with SLE have higher risk of cardiovascular events and mortality in comparison with controls with the same levels of traditional risk factors and intima-media measures, which is related to accumulated disease damage and antiphospholipid syndrome: a case–control study over 10 years. Lupus Sci Med. (2021) 8(1):e000454. doi: 10.1136/lupus-2020-000454
7. Frodlund M, Vikerfors A, Grosso G, Skogh T, Wetterö J, Elvin K, et al. Immunoglobulin A anti-phospholipid antibodies in Swedish cases of systemic lupus erythematosus: associations with disease phenotypes, vascular events and damage accrual. Clin Exp Immunol. (2018) 194(1):27–38. doi: 10.1111/cei.13180
8. Unlu O, Zuily S, Erkan D. The clinical significance of antiphospholipid antibodies in systemic lupus erythematosus. Eur J Rheumatol. (2016) 3(2):75–84. doi: 10.5152/eurjrheum.2015.0085
9. Benagiano M, Gerosa M, Romagnoli J, Mahler M, Borghi MO, Grassi A, et al. Β2 glycoprotein I recognition drives Th1 inflammation in atherosclerotic plaques of patients with primary antiphospholipid syndrome. J Immunol. (2017) 198(7):2640–8. doi: 10.4049/jimmunol.1600305
10. Tektonidou MG, Papassotiriou I, Sfikakis PP. Growth differentiation factor 15 (GDF-15) as potential cardiovascular risk biomarker in antiphospholipid syndrome. Rheumatology. (2022) 61(1):394–9. doi: 10.1093/rheumatology/keab277
11. Corban MT, Duarte-Garcia A, McBane RD, Matteson EL, Lerman LO, Lerman A. Antiphospholipid syndrome: role of vascular endothelial cells and implications for risk stratification and targeted therapeutics. J Am Coll Cardiol. (2017) 69(18):2317–30. doi: 10.1016/j.jacc.2017.02.058
12. Kravvariti E, Konstantonis G, Tentolouris N, Sfikakis PP, Tektonidou MG. Carotid and femoral atherosclerosis in antiphospholipid syndrome: equivalent risk with diabetes mellitus in a case-control study. Semin Arthritis Rheum. (2018) 47(6):883–9. doi: 10.1016/j.semarthrit.2017.10.015
13. Di Minno MND, Emmi G, Ambrosino P, Scalera A, Tufano A, Cafaro G, et al. Subclinical atherosclerosis in asymptomatic carriers of persistent antiphospholipid antibodies positivity: a cross-sectional study. Int J Cardiol. (2019) 274:1–6. doi: 10.1016/j.ijcard.2018.06.010
14. Svensson C, Eriksson P, Zachrisson H, Sjowall C. High-frequency ultrasound of multiple arterial areas reveals increased intima media thickness, vessel wall appearance, and atherosclerotic plaques in systemic lupus erythematosus. Front Med (Lausanne). (2020) 7:581336. doi: 10.3389/fmed.2020.581336
15. Selmi C, De Santis M, Battezzati PM, Generali E, Lari SA, Ceribelli A, et al. Anti-phospholipid antibody prevalence and association with subclinical atherosclerosis and atherothrombosis in the general population. Int J Cardiol. (2020) 300:209–13. doi: 10.1016/j.ijcard.2019.10.042
16. Engelen L, Ferreira I, Stehouwer CD, Boutouyrie P, Laurent S, Collaboration obotRVfAM. Reference intervals for common carotid intima-media thickness measured with echotracking: relation with risk factors. Eur Heart J. (2012) 34(30):2368–80. doi: 10.1093/eurheartj/ehs380
17. Andrade D, Bortolotto L, Bonfá E, Borba E. Primary antiphospholipid syndrome: absence of premature atherosclerosis in patients without traditional coronary artery disease risk factors. Lupus. (2016) 25(5):472–8. doi: 10.1177/0961203315617841
18. Bettiol A, Emmi G, Finocchi M, Silvestri E, Urban ML, Mattioli I, et al. Obstetric antiphospholipid syndrome is not associated with an increased risk of subclinical atherosclerosis. Rheumatology. (2020) 59(12):3709–16. doi: 10.1093/rheumatology/keaa116
19. Wang J, Wang L, Shen Y, Gong X, Ju Y. Relationship between carotid artery angle and plaque morphology in acute cerebral infarction patients. Neurologist. (2022) 27(5):240–4. doi: 10.1097/NRL.0000000000000410
20. Lu M, Zhang L, Yuan F, Peng P, Zhang H, Liu S, et al. Comparison of carotid atherosclerotic plaque characteristics between symptomatic patients with transient ischemic attack and stroke using high-resolution magnetic resonance imaging. BMC Cardiovasc Disord. (2022) 22(1):190. doi: 10.1186/s12872-022-02624-7
21. Liu YT, Zhang ZM, Li ML, Gao S, Feng F, Xu WH. Association of carotid artery geometries with middle cerebral artery atherosclerosis. Atherosclerosis. (2022) 352:27–34. doi: 10.1016/j.atherosclerosis.2022.05.016
22. Assan F, Seror R, Mariette X, Nocturne G. New 2019 SLE EULAR/ACR classification criteria are valuable for distinguishing patients with SLE from patients with pSS. Ann Rheum Dis. (2021) 80(8):e122. doi: 10.1136/annrheumdis-2019-216222
23. Pires da Rosa G, Bettencourt P, Rodriguez-Pinto I, Cervera R, Espinosa G. “Non-criteria” antiphospholipid syndrome: a nomenclature proposal. Autoimmun Rev. (2020) 19(12):102689. doi: 10.1016/j.autrev.2020.102689
24. Barbhaiya M, Zuily S, Naden R, Hendry A, Manneville F, Amigo M-C, et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann Rheum Dis. (2023) 82(10):1258–70. doi: 10.1136/ard-2023-224609
25. Truglia S, Capozzi A, Mancuso S, Manganelli V, Rapino L, Riitano G, et al. Relationship between gender differences and clinical outcome in patients with the antiphospholipid syndrome. Front Immunol. (2022) 13:932181. doi: 10.3389/fimmu.2022.932181
26. Vandevelde A, Chayoua W, de Laat B, Gris JC, Moore GW, Musiał J, et al. Semiquantitative interpretation of anticardiolipin and antiβ2glycoprotein I antibodies measured with various analytical platforms: communication from the ISTH SSC subcommittee on lupus anticoagulant/antiphospholipid antibodies. J Thromb Haemostasis. (2022) 20(2):508–24. doi: 10.1111/jth.15585
27. De Moerloose P, Reber G, Musial J, Arnout J. Analytical and clinical performance of a new, automated assay panel for the diagnosis of antiphospholipid syndrome. J Thromb Haemostasis. (2010) 8(7):1540–6. doi: 10.1111/j.1538-7836.2010.03857.x
28. Wan L-Y, Gu J-Y, Liu T-T, Hu Q-Y, Jia J-C, Teng J-L, et al. Clinical performance of automated chemiluminescent methods for anticardiolipin and anti-β2-glycoprotein I antibodies detection in a large cohort of Chinese patients with antiphospholipid syndrome. Int J Lab Hematol. (2020) 42(2):206–13. doi: 10.1111/ijlh.13156
29. Yin D, Chayoua W, Kelchtermans H, de Groot PG, Moore GW, Gris JC, et al. Detection of anti-domain I antibodies by chemiluminescence enables the identification of high-risk antiphospholipid syndrome patients: a multicenter multiplatform study. J Thromb Haemostasis. (2020) 18(2):463–78. doi: 10.1111/jth.14682
30. Alexander MD, Yuan C, Rutman A, Tirschwell DL, Palagallo G, Gandhi D, et al. High-resolution intracranial vessel wall imaging: imaging beyond the lumen. J Neurol Neurosurg Psychiatry. (2016) 87(6):589–97. doi: 10.1136/jnnp-2015-312020
31. Mandell DM, Mossa-Basha M, Qiao Y, Hess CP, Hui F, Matouk C, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. Am J Neuroradiol. (2017) 38(2):218–29. doi: 10.3174/ajnr.A4893
32. Patzig M, Forbrig R, Küpper C, Eren O, Saam T, Kellert L, et al. Diagnosis and follow-up evaluation of central nervous system vasculitis: an evaluation of vessel-wall MRI findings. J Neurol. (2022) 269(2):982–96. doi: 10.1007/s00415-021-10683-7
33. Tektonidou MG. Cardiovascular disease risk in antiphospholipid syndrome: thrombo-inflammation and atherothrombosis. J Autoimmun. (2022) 128:102813. doi: 10.1016/j.jaut.2022.102813
34. Wirestam L, Jönsson F, Enocsson H, Svensson C, Weiner M, Wetterö J, et al. Limited association between antibodies to oxidized low-density lipoprotein and vascular affection in patients with established systemic lupus erythematosus. Int J Mol Sci. (2023) 24(10):8987. doi: 10.3390/ijms24108987
35. Bolla E, Tentolouris N, Sfikakis PP, Tektonidou MG. Metabolic syndrome in antiphospholipid syndrome versus rheumatoid arthritis and diabetes mellitus: association with arterial thrombosis, cardiovascular risk biomarkers, physical activity, and coronary atherosclerotic plaques. Front Immunol. (2023) 13:1077166. doi: 10.3389/fimmu.2022.1077166
36. Evensen LH, Folsom AR, Pankow JS, Hansen JB, Allison MA, Cushman M, et al. Hemostatic factors, inflammatory markers, and risk of incident venous thromboembolism: the multi-ethnic study of atherosclerosis. J Thromb Haemostasis. (2021) 19(7):1718–28. doi: 10.1111/jth.15315
37. Folsom AR, de Vries PS, Cushman M. No prospective association of a polygenic risk score for coronary artery disease with venous thromboembolism incidence. J Thromb Haemostasis. (2021) 19(11):2841–4. doi: 10.1111/jth.15501
38. Prandoni P, Ghirarduzzi A, Prins MH, Pengo V, Davidson BL, Sørensen H, et al. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemostasis. (2006) 4(9):1891–6. doi: 10.1111/j.1538-7836.2006.02058.x
39. Zhang L, Lyu Q, Zhou W, Li X, Ni Q, Jiang S, et al. High systemic immune-inflammation index is associated with carotid plaque vulnerability: new findings based on carotid ultrasound imaging in patients with acute ischemic stroke. Front Neurol. (2022) 13:959531. doi: 10.3389/fneur.2022.959531
40. Li M, Le W-J, Tao X-F, Li M-H, Li Y-H, Qu N. Advantage in bright-blood and black-blood magnetic resonance imaging with high-resolution for analysis of carotid atherosclerotic plaques. Chin Med J (Engl). (2015) 128(18):2478–84. doi: 10.4103/0366-6999.164933
41. Strecker C, Krafft AJ, Kaufhold L, Hullebrandt M, Treppner M, Ludwig U, et al. Carotid geometry and wall shear stress independently predict increased wall thickness—a longitudinal 3D MRI study in high-risk patients. Front Cardiovasc Med. (2021) 8:723860. doi: 10.3389/fcvm.2021.723860
42. Cao Y, Sun Y, Zhou B, Zhao H, Zhu Y, Xu J, et al. Atherosclerotic plaque burden of middle cerebral artery and extracranial carotid artery characterized by MRI in patients with acute ischemic stroke in China: association and clinical relevance. Neurol Res. (2017) 39(4):344–50. doi: 10.1080/01616412.2017.1281196
43. Tang Y, Zhang J, Liu W, Jin W, Li S, Qian Z, et al. Analysis of carotid vulnerable plaque MRI high-risk features and clinical risk factors associated with concomitant acute cerebral infarction. BMC Cardiovasc Disord. (2023) 23(1):173. doi: 10.1186/s12872-023-03199-7
44. Eun Jun J, Hwang YC, Jeong Ahn K, Yeon Chung H, Jahng GH, Park S, et al. Association between carotid atherosclerosis and presence of intracranial atherosclerosis using three-dimensional high-resolution vessel wall magnetic resonance imaging in asymptomatic patients with type 2 diabetes. Diabetes Res Clin Pract. (2022) 191:110067. doi: 10.1016/j.diabres.2022.110067
45. Bos D, Arshi B, van den Bouwhuijsen QJA, Ikram MK, Selwaness M, Vernooij MW, et al. Atherosclerotic carotid plaque composition and incident stroke and coronary events. J Am Coll Cardiol. (2021) 77(11):1426–35. doi: 10.1016/j.jacc.2021.01.038
46. Suzuki M, Okawa M, Okuno Y, Yang T, Takenobu Y, Shiomi H, et al. Prevalence of carotid artery stenosis with coronary artery disease in Japanese patients: a single-center study. J Neurol Sci. (2022) 443:120492. doi: 10.1016/j.jns.2022.120492
47. Yeo J, Hwang I, Sohn C-H, Lee EE, Lee S-T, Lee EB, et al. Proliferative vasculopathy associated with antiphospholipid antibodies in patients with neurological symptoms. Front Med (Lausanne). (2022) 9:913203. doi: 10.3389/fmed.2022.913203
48. Su L, Qi Z, Guan S, Wei L, Zhao Y. Exploring the risk factors for ischemic cerebrovascular disease in systemic lupus erythematosus: a single-center case-control study. Front Immunol. (2022) 13:978910. doi: 10.3389/fimmu.2022.978910
49. Ide S, Kakeda S, Miyata M, Iwata S, Ohkubo N, Nakayamada S, et al. Intracranial vessel wall lesions in patients with systematic lupus erythematosus. J Magn Reson Imaging. (2018) 48(5):1237–46. doi: 10.1002/jmri.25966
50. Li J, Wang X, Zhang H, Wang W, Pei Y, Xie T, et al. Aorta coarctation and systemic lupus erythematosus: a case report. Medicine (Baltimore). (2019) 98(28):e16397. doi: 10.1097/MD.0000000000016397
51. Chaturvedi S, McCrae KR. Diagnosis and management of the antiphospholipid syndrome. Blood Rev. (2017) 31(6):406–17. doi: 10.1016/j.blre.2017.07.006
52. Arachchillage DRJ, Laffan M. Pathogenesis and management of antiphospholipid syndrome. Br J Haematol. (2017) 178(2):181–95. doi: 10.1111/bjh.14632
53. Arreola-Diaz R, Majluf-Cruz A, Sanchez-Torres L, Hernandez-Juarez J. The pathophysiology of the antiphospholipid syndrome: a perspective from the blood coagulation system. Clin Appl Thromb Hemost. (2022) 28:10760296221088576. doi: 10.1177/10760296221088576
54. Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol. (2009) 8(11):998–1005. doi: 10.1016/S1474-4422(09)70239-X
55. Yoo JS, Kim YS, Kim HY, Kwon HS, Koh S-H, Heo SH, et al. Comparison of patients with transient and sustained increments of antiphospholipid antibodies after acute ischemic stroke. J Neurol. (2021) 268(7):2541–9. doi: 10.1007/s00415-021-10432-w
Keywords: carotid artery, high-resolution magnetic resonance imaging, systemic lupus erythematosus, antiphospholipid antibodies, thrombosis, morphometry
Citation: Yang Q, Liu Q, Yin C, Zhang X, Chen X, Pylypenko D, Chen H, Shu Q and Yu D (2024) Risk factors for thrombotic events in systemic lupus erythematosus patients with antiphospholipid antibodies: insights from morphometric measurements of carotid arteries. Front. Cardiovasc. Med. 11:1373097. doi: 10.3389/fcvm.2024.1373097
Received: 19 January 2024; Accepted: 21 May 2024;
Published: 26 June 2024.
Edited by:
Hiroyoshi Mori, Showa University Fujigaoka Hospital, JapanReviewed by:
Tommaso Bucci, University of Liverpool, United Kingdom© 2024 Yang, Liu, Yin, Zhang, Chen, Pylypenko, Chen, Shu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dexin Yu, eXVkZXhpbjAzMzBAc2luYS5jb20=; Qiang Shu, c2h1cWlhbmdAc2R1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.