
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 09 May 2024
Sec. Cardiac Rhythmology
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1372505
This article is part of the Research TopicAtrial Fibrillation: Selection of Management Strategy and Evaluation of OutcomesView all 39 articles
 JungMin Choi1,†
JungMin Choi1,† So-Ryoung Lee1,2,†
So-Ryoung Lee1,2,† Eue-Keun Choi1,2*
Eue-Keun Choi1,2* Kyung-Yeon Lee1
Kyung-Yeon Lee1 Hyo-Jeong Ahn1
Hyo-Jeong Ahn1 Soonil Kwon1
Soonil Kwon1 Bongseong Kim3
Bongseong Kim3 Kyung-Do Han4
Kyung-Do Han4 Seil Oh1,2
Seil Oh1,2 Gregory Y. H. Lip2,5,6
Gregory Y. H. Lip2,5,6
Background: Patients with hypertension are at a high risk of atrial fibrillation (AF). Recent research has indicated the varying effects of antihypertensive medications on developing AF.
Objectives: We investigated the relationship between different types of antihypertensive medications and the risk of AF occurrence.
Methods: We analyzed data from 113,582 subjects with national health screening examinations between 2009 and 2014. The study population was categorized according to antihypertensive medication type. The primary outcome was the incidence of AF.
Results: Among 113,582 subjects (mean age 59.4 ± 12.0 years, 46.7% men), 93,557 received monotherapy [angiotensin receptor blockers (ARB), angiotensin-converting enzyme inhibitors (ACEi), beta-blockers, calcium channel blockers (CCB), or diuretics], while 34,590 received combination therapy (ARB/beta-blockers, ARB/CCB, ARB/diuretics, or ARB/CCB/diuretics). During a mean follow-up duration of 7.6 ± 2.1 years, 3.9% of patients were newly diagnosed with AF. In monotherapy, ACEi and CCB had similar AF risks as ARB, while beta-blockers and diuretics showed higher AF risks than ARB. In combination therapy, ARBs/CCBs and ARBs/diuretics had the lowest AF risk, whereas ARBs/beta-blockers had the highest compared to ARB/CCB. Among the specific ARBs, the AF risk varied insignificantly, except for telmisartan and candesartan.
Conclusions: In hypertensive patients receiving monotherapy, ACEi and CCB showed a similar AF risk as ARBs, while beta-blockers and diuretics were associated with a higher risk. Among those receiving combination therapy, ARBs/CCBs and ARBs/diuretics had the lowest AF risk, whereas ARBs/beta-blockers showed the highest risk. Various types of ARBs have different associations with AF risk.
Hypertension is one of the most common comorbidities, with a prevalence of 30% among adults in 2019 (1–3). Elevated blood pressure leads to an increased risk of cardiovascular complications, such as coronary heart disease, stroke, and an increased risk of mortality (4–8). Hypertension is also a widely recognized risk factor for atrial fibrillation (AF) (9, 10). Diagnosis of hypertension, whether it is being treated or not, has been shown to increase the likelihood of AF by 70% in a previous systematic review and lowers the spontaneous restoration rate once AF is developed (11, 12). The co-prevalence of hypertension in AF patients increases the risk of major cardiovascular events and mortality compared to those without hypertension (13, 14). Based on these findings, researchers have attempted to identify the antihypertensive medication with the highest efficacy in preventing AF (15–17). The use of renin-angiotensin system inhibitors (RASi) has been associated with a 33% reduction in the risk of AF occurrence (15). The increased attention to the antiarrhythmic effects of various antihypertensive medications has led to studies comparing their differing prophylactic effects on developing AF (16, 18–21). However, the potential impact of antihypertensive medications on the risk of new-onset AF, particularly within different combinations of antihypertensive medication and specific types of angiotensin II receptor blockers (ARB), has not been addressed before.
This nationwide cohort study evaluated the effect of different antihypertensive medications using a large nationwide population.
This study utilized the comprehensive claims database from the Korean National Health Insurance Service (NHIS), which functions as the exclusive insurer for approximately 52 million individuals and corresponds to the entire population of South Korea in 2019 (22, 23). The Korean National Health Information Database (NHID) contains sociodemographic, healthcare usage, health screening, and healthcare provider data (22, 23). The National Health Screening Database provides detailed information on laboratory findings and lifestyle questionnaires (23). The healthcare utilization database comprises records of prescriptions linked with diagnoses based on the International Classification of Disease, Tenth Revision of Clinical Modification (ICD-10-CM) (22, 23).
The study was conducted following the principles of the Declaration of Helsinki. The data were anonymized; thus, the study was exempt from the Institutional Review Board (IRB) review of Seoul National University Hospital (IRB no. E-2109-118-1255). In addition, because data from the NHIS were de-identified, obtaining informed consent was not feasible. The use of the NHIS database from 2009 to 2014 will be authorized in 2023.
Figure 1 summarizes the patient flow. Subjects from the Korean National Health Insurance Service claims database who underwent screening between 1 January 2009, and 31 December 2014 (n = 556,888) were screened. Patients who were prescribed antihypertensive medications under hypertension diagnosis were selected (n = 120,481). Patients younger than 20 years (n = 1,500), those with preexisting AF before enrollment (n = 5,177), and those who developed AF before the 1-year follow-up period (n = 1,567) were excluded from the analysis.
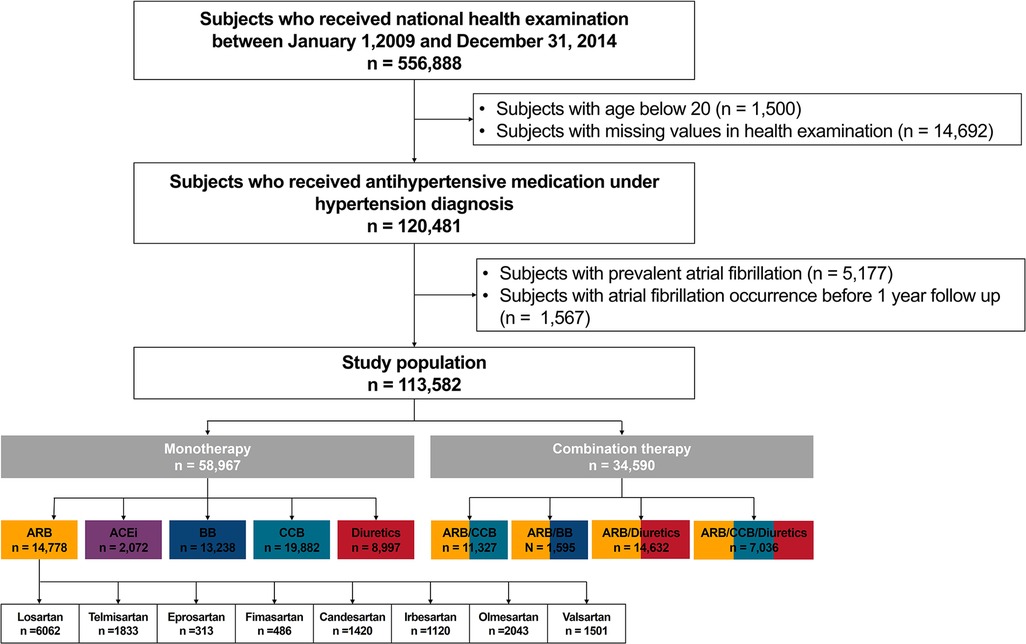
Figure 1. Study design. ARB, angiotensin II receptor blocker; ACEi, angiotensin-converting enzyme inhibitor; BB, beta-blocker; CCB, calcium channel blocker; NHIC, national health insurance corporation.
The study population was by antihypertensive prescription: monotherapy [one of ARB, angiotensin-converting enzyme inhibitors (ACEi), beta-blocker, CCB, or diuretics], and combination therapy (combinations of two or three agents: ARB/CCB, ARB/beta-blockers, ARB/diuretics, and ARB/CCB/diuretics). The ARB monotherapy group was further subdivided by specific types: losartan, telmisartan, eprosartan, fimasartan, candesartan, irbesartan, olmesartan, and valsartan.
The covariates included patient demographics, comorbidities, medications, lifestyle, and health screening results (more details in Supplementary Table S1). The bottom 20% of NHIS participant income was deemed low. General health screening examinations collected metrics including systolic and diastolic blood pressure (SBP and DBP, respectively), weight, height, body mass index, and waist circumference measurements. Laboratory findings included fasting glucose level, estimated glomerular filtration rate (eGFR), total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein, and triglycerides (23, 24). The self-reported questionnaire included information on smoking patterns, alcohol consumption, and regular exercise (23, 25, 26).
The primary outcome was AF incidence during follow-up. AF was defined by ICD-10-CM codes (I48; AF and atrial flutter) (Supplementary Table S1) (23). The index year was the first NHIS health exam, excluding those developing AF within 1 year. Patients were monitored until AF incidence, NHIS exclusion, or study end on 31 December 2018, whichever event occurred first.
Baseline characteristics are displayed as either mean ± standard deviation (SD) or median (interquartile range, IQR) for continuous variables and as numbers (percentages) for categorical variables, as deemed suitable. Student's t-tests and χ2 tests were used for continuous and categorical variables, respectively. The incidence rate (IR) was calculated as the number of events per 1,000 person-years (PY) during follow-up.
Survival analysis was performed using the Kaplan–Meier method to determine AF cumulative incidence with different antihypertensives. Cox proportional hazard regression models assessed the hazard ratio (HR) and 95% confidence intervals (CI). Four Cox models were progressively adjusted for covariates: (1) unadjusted model (model 1); (2) model adjusted for age and sex (model 2); (3) model adjusted for age, sex, comorbidities (diabetes mellitus, dyslipidemia, previous myocardial infarction, heart failure, peripheral artery disease, chronic kidney disease, stroke/transient ischemic attack, chronic obstructive pulmonary disease, thyroid disease, and sleep apnea), and social habits (smoking, alcohol consumption, regular exercise, and low income) (model 3); and (4) model 3 with addition of health screening examination measurements [SBP, fasting glucose level, total cholesterol, and body mass index (BMI)] and hypertension duration (model 4).
Subgroup analyses were conducted based on age categories (<65, 65–74, and ≥75 years), sex, obesity (BMI < 25 kg/m2 and ≥25 kg/m2), presence of abdominal obesity (men ≥ 90 cm or women ≥ 85 cm of waist circumference), smoking patterns (never, former, and current), alcohol consumption (none, mild to moderate, and heavy), income levels (low and others), and duration of hypertension (<2 years and ≥2 years). To assess the impact of antihypertensive medication on AF risk among patients without additional AF risks such as comorbidities, further sensitivity analyses were conducted on a healthy population that excluded individuals with comorbidities (diabetes mellitus, dyslipidemia, previous myocardial infarction, heart failure, prior ischemic stroke/transient ischemic attack, peripheral artery disease, chronic kidney disease, chronic obstructive pulmonary disease, and thyroid diseases).
Statistical significance was set at p < 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina, USA).
A total of 113,582 participants were included in the final study population. Details of the baseline characteristics for the distinct monotherapy groups are presented in Table 1, whereas those for the combination therapy group are shown in Supplementary Table S2. Specific details regarding the ARB medications are provided in Supplementary Table S3. The mean age of the total population was 59.4 ± 12.0 years old, with 46.7% men and a mean hypertension duration of 4.5 ± 3.4 years. The most prevalent comorbidity was dyslipidemia (38.4%), followed by diabetes mellitus (23.0%). Among the study population, 47.3% were obese, and 35.7% satisfied the criteria for abdominal obesity.
During a mean follow-up duration of 7.6 ± 2.1 years, AF was newly diagnosed in 3,741 (3.9%) patients. The adjusted HR (aHR) with 95% CI and IR according to the type of antihypertensive agent used in patients receiving antihypertensive monotherapy is shown in Figure 2A and Supplementary Table S4. In those on monotherapy, subjects administered beta-blocker exhibited the highest increase in AF risk (aHR, 1.51; 95% CI, 1.33–1.71, p < 0.001), followed by diuretics (aHR, 1.37; 95% CI, 1.19–01.58, p < 0.001), when compared to those receiving ARBs. Subjects taking ACEi or CCBs showed comparable risk of AF with ARBs (aHR, 1.19; 95% CI, 0.96–1.47 for ACEi, and aHR, 1.00; 95% CI, 0.89–1.12 for CCB, respectively, p < 0.001).

Figure 2. Hazard ratio and incidence rate comparison in each antihypertensive medication group; (A) monotherapy group (B) combination therapy group (C) specific ARB type in ARB only prescribed group (D) specific ARB type in combination with other medication group. Model 4: model adjusted for age, sex, comorbidities (diabetes mellitus, dyslipidemia, previous myocardial infarction, heart failure, peripheral artery disease, chronic kidney disease, stroke/transient ischemic attack, chronic obstructive pulmonary disease, thyroid disease, and sleep apnea), and social habits (smoking, alcohol consumption, regular exercise, and low income), health screening examination measurements (SBP, fasting glucose level, total cholesterol, and BMI) and hypertension duration. ARB, angiotensin II receptor blocker; ACEi, angiotensin-converting enzyme inhibitor; BB, beta-blockers; CCB, calcium channel blocker; CI, confidence interval; HTN, hypertension.
Figure 2B shows the aHR (95% CI) and IR based on a particular type of antihypertensive medication in the combination therapy group. Comprehensive details are provided in Supplementary Table S4.
Within the population receiving combination antihypertensive therapy, individuals prescribed ARB/beta-blockers exhibited the highest risk (aHR, 1.54; 95% CI, 1.25–1.92, p < 0.001), followed by ARB/CCB/Diuretics with an aHR of 1.18, 95% CI, 1.03–1.36 (p < 0.001) compared to those taking ARB/CCB. Patients with ARB/diuretics did not show a significant difference in AF risk compared to those taking ARB/CCB (adjusted HR, 0.99; 95% CI, 0.87–1.12, p < 0.001).
Figure 2C illustrates the aHR (95% CI) and IR for specific ARB types in patients who were exclusively prescribed ARB monotherapy. Figure 2D presents the same data for patients receiving ARBs in combination therapy. Additional information is provided in Supplementary Table S5.
In the subset of individuals receiving ARB monotherapy, most ARB medications exhibited no notable variation in AF risk compared with losartan. In the population receiving ARB as part of antihypertensive combination therapy, when compared to losartan, candesartan and telmisartan were associated with a higher risk of AF (aHR, 1.33; 95% CI, 1.15–1.54, and 1.20; 95% CI, 1.05–1.36) (p = 0.004). Other ARBs did not show significant differences compared to losartan.
Figure 3 illustrates the cumulative incidence of AF with distinct antihypertensive medications.
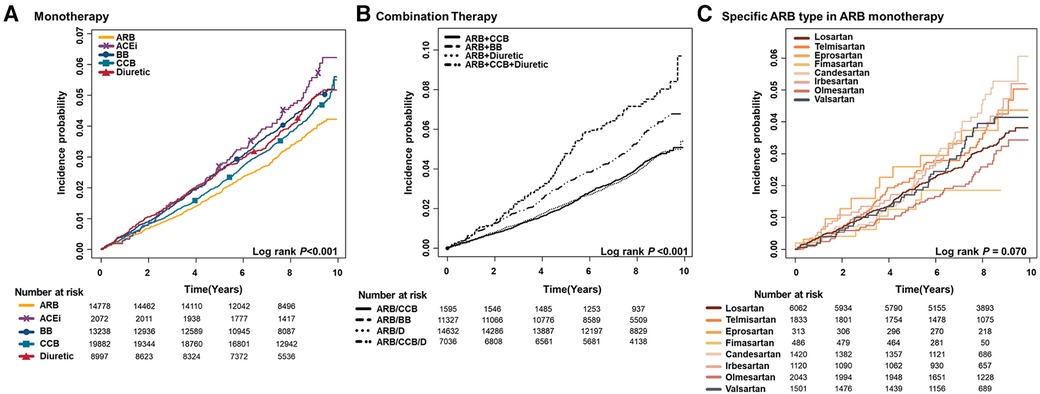
Figure 3. Cumulative incidence curves of AF stratified by antihypertensive medication; (A) monotherapy (B) combination (C) specific type of ARB in ARB monotherapy patients. ARB, angiotensin II receptor blocker; ACEi, angiotensin-converting enzyme inhibitor; BB, beta-blocker; CCB, calcium channel blocker; D, diuretic.
Subgroup analyses conducted for ARB monotherapy, combination therapy, and specific ARB types are provided in Supplementary Tables S6–S8, respectively.
In the monotherapy group, the different effects of antihypertensive medication types on AF risk were notably attenuated among individuals aged >75 years (p for interaction = 0.034). No significant interactions were observed in the other subgroups, including sex, body mass index, abdominal obesity, smoking habits, alcohol consumption, income level, SBP, and duration of hypertension. Within the combination therapy group, in obese patients, compared to ARB/CCB, the relative AF risk of ARB/CCB/diuretics was accentuated (aHR, 1.30; 95% CI, 1.09–1.56, p = 0.007). A similar pattern was observed for those with abdominal obesity (aHR, 1.34; 95% CI, 1.10–1.63, p = 0.014) and SBP over 130 mmHg (aHR, 1.34; 95% CI, 1.13–1.60, p = 0.012). Subgroup analyses of specific ARB types within the ARB monotherapy group did not indicate significant interactions across subgroups.
In the sensitivity analyses of monotherapy group, subjects taking ACEi, beta-blocker, and diuretics were associated with a higher risk of AF compared to ARB (aHR, 2.40; 95% CI, 1.41–4.08 for ACEi, aHR, 1.85; 95% CI, 1.34–2.55 for beta-blocker, and aHR, 1.59; 95% CI, 1.12–2.27 for diuretics, p < 0.001) (Supplementary Table S8). In the combination therapy group, the result was consistent with the main results, showing ARB/beta-blocker to exhibit the highest AF risk compared to ARB/CCB (aHR, 2.75; 95% CI, 1.44–5.24, p = 0.023). Sensitivity analysis of specific ARB types is shown in Supplementary Table S9. ARB, in combination with other antihypertensive medications, did not show a significant difference in the risk of AF occurrence (p = 0.596).
The main findings of this study are as follows: (1) In the hypertensive patient group receiving antihypertensive monotherapy, those treated with ACE inhibitors and CCBs showed a similar AF risk to those treated with ARBs, while those treated with beta-blockers and diuretics showed a higher AF risk; (2) In the group receiving combination therapy, those treated with ARB/diuretics and ARB/CCB/diuretics showed a similar AF risk to those treated with ARB/CCBs, while those treated with ARB/beta-blockers showed a higher AF risk; and (3) Among the specific types of ARBs, the AF risk did not differ significantly except for telmisartan and candesartan in both the monotherapy group and the combination therapy group.
The protective effect of RASi on the risk of AF occurrence for both primary and secondary prevention has been shown in previous studies with other ethnicities (15, 17–19, 27). The Losartan Intervention For End Point Reduction in Hypertension (LIFE) trial showed that losartan intervention was associated with a lower incidence of new-onset AF and associated stroke than atenolol (20).
Comparisons between RASi and diuretics or beta-blockers have been conflicting in previous studies, with randomized clinical trial data only available for ACEis and not ARBs (16, 28, 29). Previous randomized clinical trials have failed to show significant benefits of ACEi compared with diuretics and beta-blockers in AF risk (28, 29). However, a recent nationwide population study on both ACEi and ARB showed a lower incidence of AF in patients prescribed RASi compared with beta-blockers or diuretics (16). This difference may be attributed to the specific type of RASi used in the clinical trial, as we observed slight variations in AF risk among different subtypes of ARBs in our study.
The antiarrhythmic effect on developing AF was most prominent among individuals with left ventricular hypertrophy or heart failure (30). The beneficial effect of RASi on developing AF is thought to be attributable to atrial electrical remodeling of RASi, as seen in animal models (31, 32). Furthermore, recent studies have compared the effect of angiotensin receptor-neprilysin inhibitor (ARNI) with ARB and found a better reduction of atrial electrical instability in the ARNI group in a retrospective study and animal experiment (33). However, there is a limitation in the generalizability of the result, as ARNI is only recommended for heart failure management and not hypertension in current guidelines, and further research is needed (34).
As a result of previous studies on antihypertensive medication, RASi has been stated in the European Society of Cardiology AF management guidelines as an upstream therapy among patients with left ventricular dysfunction, left ventricular hypertrophy, or hypertension (35). Also, as stated in management guidelines, based on a holistic or integrated care management approach, hypertension control is very important for patients who are already diagnosed with AF (35, 36). The coexistence of hypertension in patients with AF increases the risk of stroke, and guidelines emphasize attention to good BP control in AF patients with hypertension to reduce AF and the risk of stroke and bleeding (35). Indeed, adherence to the Atrial fibrillation Better Care (ABC) pathway is associated with improved clinical outcomes (37, 38).
In this study, the use of beta-blockers was associated with a higher risk of AF than ARB monotherapy or ARB/CCB combination therapy. Also, a recent study found the beta-blocker usage to be associated with impaired left atrial function in patients with hypertension and without heart failure or AF (39). In the course of hypertension, uncontrolled blood pressure was associated with reduced early diastolic filling, atrial remodeling, and increased AF inducibility (40, 41). The difference in the preventative effects of atrial remodeling between RASi and beta-blockers may have led to unfavorable outcomes in terms of AF and stroke risk among beta-blocker users compared to RASi users as seen in previous studies (16, 20). Additionally, the prescription of beta blockers to patients with hypertension may be due to suspicion of undiagnosed AF.
In line with the findings of a previous Danish study, our study also observed a higher risk of AF among individuals prescribed diuretics (16). This increased risk may be attributable to the fact that the prescription of diuretics in patients with hypertension could be due to a symptomatic prescription of heart failure that has not yet been diagnosed. While our study found no benefits of diuretics in general hypertensive patients, meta-analyses of heart failure patients suggest that mineralocorticoid receptor blockade may have the potential to reduce AF risk (42). A recent study on a novel, selective, nonsteroidal mineralocorticoid receptor antagonist, finerenone, also found potential for reducing AF risk in patients with chronic kidney disease and type 2 diabetes (43).
To our knowledge, this study is the first to compare the AF risk among different types of ARBs. Previous studies have focused on the comparison between ARBs and placebo or other types of antihypertensive medications, such as diuretics or beta-blockers, for new-onset AF (18–20). The head-to-head comparison of the different types of ARBs has not yet been performed. Although this study had a retrospective design, it is novel in exploring the differences between ARBs in incident AF risk. In this study, most ARBs showed similar AF risks to losartan, except for telmisartan and candesartan, which showed a higher AF risk. These drugs have both been shown to decrease AF risk compared to placebo in previous studies (18, 19). However, caution is needed when interpreting the results, as our study used losartan as a reference and not a placebo. The variation among different types of ARBs may be due to their differing binding affinities, lipophilicities, and metabolism (44). For example, candesartan and telmisartan are known to have the highest binding affinity to AT1 receptor antagonist (45). Telmisartan has distinct characteristics such as partial agonistic effect on the peroxisome proliferator-activated receptor and stronger inhibitory effects on arachidonic acid (46, 47). Candesartan is the only drug among ARBs to be a prodrug and is converted during gastrointestinal absorption (48). These unique characteristics of candesartan and telmisartan might have led to a different effect on AF risk. Furthermore, differences in the study population should also be considered. For example, the TRANSCEND trial (the Telmisartan Randomized AssessmeNt Study in ACE iN-tolerant subjects with cardiovascular disease) and CHARM (the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity) focused on high-risk patients with cardiovascular disease or diabetes with end-organ damage or symptomatic congestive heart failure, respectively (18, 19).
However, further prospective research will be needed to reach a definitive conclusion.
This study has several limitations. First, AF may have been underestimated in this study because the definition of AF was limited to the ICD-10-CM code diagnosis without a review of electrocardiograms. However, using ICD-10-CM codes for AF diagnosis has shown a predictive value of 94.1% in a previous study (49). Second, only blood pressure at baseline was included, and information on blood pressure control was not considered. The degree of high blood pressure showed a linear increase in systolic blood pressure and AF risk (50). Thus, controlling hypertension with antihypertensive medications may have affected the incidence of AF. Third, the specific dose of antihypertensive medication or the echocardiographic data of the patients could not be evaluated. This limitation was due to the nature of the NHID used in this study. The current NHID dataset does not provide the specific doses of antihypertensive medications or echocardiographic data. Further research is required to compare the effects of different doses of antihypertensive medications. Fourth, changes in antihypertensive medication or diagnosis of new comorbidities could not be accounted for, as only the baseline values were retrieved. These changes may have affected the incidence of AF. Fifth, beta-blockers and diuretics are not only used for hypertension alone but also for other purposes, such as heart failure. Thus, the influence of comorbidities not reported in the NHID (such as impending heart failure) on the incidence cannot be ignored. However, because the sensitivity analyses showed a consistent trend, such confounders' effect was presumed negligible.
In monotherapy, ACEi and CCB exhibited a similar AF risk to that of ARB, while beta-blockers and diuretics were associated with a higher risk. Among those receiving combination therapy, the lowest AF risk was observed with ARBs/CCBs and ARBs/diuretics, whereas ARBs/beta-blockers had the highest risk. Various types of ARBs exhibit varying degrees of association with the risk of AF, suggesting that there may be a nuanced relationship between different types of ARBs and the likelihood of developing AF.
The datasets presented in this article are not readily available due to legislation by the Korean government. Requests to access the datasets should be directed to Korean National Health Insurance Service.
The studies involving humans were approved by Institutional Review Board (IRB) review of Seoul National University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JC: Visualization, Writing – original draft, Writing – review & editing. S-RL: Writing – original draft, Writing – review & editing. E-KC: Writing – review & editing. K-YL: Writing – review & editing. H-JA: Writing – review & editing. SK: Writing – review & editing. BK: Writing – original draft. K-DH: Writing – review & editing. SO: Writing – review & editing. GL: Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
The authors declare that this study received funding from Boryung Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
We thank the National Health Insurance Service for the approval of data usage.
S-RL: Speaking fees from Bayer, BMS/Pfizer, Biosense Webster, Daiichi-Sankyo, Sanofi-Aventis, Daewoong Pharmaceutical Co., Samjinpharm, Seers Technology, Biotronik, Boston Scientific, and Medtronic. Consultant for Biosense Webster. E-KC: Research grants or speaking fees from Abbott, Bayer, BMS/Pfizer, Biosense Webster, Chong Kun Dang, Daewoong Pharmaceutical Co., Daiichi-Sankyo, DeepQure, Dreamtech Co., Ltd., Jeil Pharmaceutical Co., Ltd., Medtronic, Samjinpharm, Seers Technology, and Skylabs. GL: Consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, Anthos and Daiichi-Sankyo. GL is a National Institute for Health and Care Research Senior Investigator and co-principal investigator of the AFFIRMO project on multimorbidity in AF, which received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No. 899871. No personal fees were received.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1372505/full#supplementary-material
ARB, angiotensin II receptor blockers; IRB, institutional review board; NHID, national health information database; NHIS, national health insurance service; RASi, renin-angiotensin system inhibitors.
1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398(10304):957–80. doi: 10.1016/s0140-6736(21)01330-1
2. Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. (2021) 18(11):785–802. doi: 10.1038/s41569-021-00559-8
3. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16(4):223–37. doi: 10.1038/s41581-019-0244-2
4. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. (2016) 387(10022):957–67. doi: 10.1016/s0140-6736(15)01225-8
5. Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J 3rd. Factors of risk in the development of coronary heart disease–six year follow-up experience. The framingham study. Ann Intern Med. (1961) 55:33–50. doi: 10.7326/0003-4819-55-1-33
6. GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392(10159):1923–94. doi: 10.1016/s0140-6736(18)32225-6
7. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. (2002) 360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8
8. Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PLoS One. (2013) 8(7):e65174. doi: 10.1371/journal.pone.0065174
9. Lip GYH, Coca A, Kahan T, Boriani G, Manolis AS, Olsen MH, et al. Hypertension and cardiac arrhythmias: a consensus document from the European heart rhythm association (EHRA) and ESC council on hypertension, endorsed by the heart rhythm society (HRS), Asia-Pacific heart rhythm society (APHRS) and sociedad latinoamericana de estimulación cardíaca y electrofisiología (SOLEACE). Europace. (2017) 19(6):891–911. doi: 10.1093/europace/eux091
10. Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the atherosclerosis risk in communities (ARIC) study. Circulation. (2011) 123(14):1501–8. doi: 10.1161/circulationaha.110.009035
11. Ball J, Carrington MJ, McMurray JJ, Stewart S. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. (2013) 167(5):1807–24. doi: 10.1016/j.ijcard.2012.12.093
12. Mariani MV, Pierucci N, Piro A, Trivigno S, Chimenti C, Galardo G, et al. Incidence and determinants of spontaneous cardioversion of early onset symptomatic atrial fibrillation. Medicina. (2022) 58(11):1513. doi: 10.3390/medicina58111513
13. Kim D, Yang PS, Kim TH, Jang E, Shin H, Kim HY, et al. Ideal blood pressure in patients with atrial fibrillation. J Am Coll Cardiol. (2018) 72(11):1233–45. doi: 10.1016/j.jacc.2018.05.076
14. Antikainen RL, Peters R, Beckett NS, Rajkumar C, Bulpitt CJ. Atrial fibrillation and the risk of cardiovascular disease and mortality in the hypertension in the very elderly trial. J Hypertens. (2020) 38(5):839–44. doi: 10.1097/hjh.0000000000002346
15. Schneider MP, Hua TA, Böhm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin-angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. (2010) 55(21):2299–307. doi: 10.1016/j.jacc.2010.01.043
16. Marott SCW, Nielsen SF, Benn M, Nordestgaard BG. Antihypertensive treatment and risk of atrial fibrillation: a nationwide study. Eur Heart J. (2013) 35(18):1205–14. doi: 10.1093/eurheartj/eht507
17. Schaer BA, Schneider C, Jick SS, Conen D, Osswald S, Meier CR. Risk for incident atrial fibrillation in patients who receive antihypertensive drugs: a nested case-control study. Ann Intern Med. (2010) 152(2):78–84. doi: 10.7326/0003-4819-152-2-201001190-00005
18. Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. (2008) 372(9644):1174–83. doi: 10.1016/s0140-6736(08)61242-8
19. Ducharme A, Swedberg K, Pfeffer MA, Cohen-Solal A, Granger CB, Maggioni AP, et al. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Am Heart J. (2006) 152(1):86–92. doi: 10.1016/j.ahj.2005.06.036
20. Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlöf B, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the losartan intervention for end point reduction in hypertension (LIFE) study. J Am Coll Cardiol. (2005) 45(5):712–9. doi: 10.1016/j.jacc.2004.10.068
21. Vermes E, Tardif JC, Bourassa MG, Racine N, Levesque S, White M, et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the studies of left ventricular dysfunction (SOLVD) trials. Circulation. (2003) 107(23):2926–31. doi: 10.1161/01.Cir.0000072793.81076.D4
22. Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol. (2017) 46(3):799–800. doi: 10.1093/ije/dyw253
23. Choi EK. Cardiovascular research using the Korean national health information database. Korean Circ J. (2020) 50(9):754–72. doi: 10.4070/kcj.2020.0171
24. Lee SR, Choi EK, Han KD, Lee SH, Oh S. Effect of the variability of blood pressure, glucose level, total cholesterol level, and body mass index on the risk of atrial fibrillation in a healthy population. Heart Rhythm. (2020) 17(1):12–9. doi: 10.1016/j.hrthm.2019.07.006
25. Lee SR, Choi EK, Ahn HJ, Han KD, Oh S, Lip GYH. Association between clustering of unhealthy lifestyle factors and risk of new-onset atrial fibrillation: a nationwide population-based study. Sci Rep. (2020) 10(1):19224. doi: 10.1038/s41598-020-75822-y
26. Park CS, Han KD, Choi EK, Kim DH, Lee HJ, Lee SR, et al. Lifestyle is associated with atrial fibrillation development in patients with type 2 diabetes mellitus. Sci Rep. (2021) 11(1):4676. doi: 10.1038/s41598-021-84307-5
27. Maggioni AP, Latini R, Carson PE, Singh SN, Barlera S, Glazer R, et al. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the valsartan heart failure trial (Val-HeFT). Am Heart J. (2005) 149(3):548–57. doi: 10.1016/j.ahj.2004.09.033
28. Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A, et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: the captopril prevention project (CAPPP) randomised trial. Lancet. (1999) 353(9153):611–6. doi: 10.1016/s0140-6736(98)05012-0
29. Hansson L, Lindholm LH, Ekbom T, Dahlöf B, Lanke J, Scherstén B, et al. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish trial in old patients with hypertension-2 study. Lancet. (1999) 354(9192):1751–6. doi: 10.1016/s0140-6736(99)10327-1
30. Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S, et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J Am Coll Cardiol. (2005) 45(11):1832–9. doi: 10.1016/j.jacc.2004.11.070
31. Nakashima H, Kumagai K, Urata H, Gondo N, Ideishi M, Arakawa K. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation. (2000) 101(22):2612–7. doi: 10.1161/01.cir.101.22.2612
32. Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. (2003) 41(12):2197–204. doi: 10.1016/s0735-1097(03)00464-9
33. Zhu T, Zhang W, Yang Q, Wang N, Fu Y, Li Y, et al. Effect of angiotensin receptor-neprilysin inhibitor on atrial electrical instability in atrial fibrillation. Front Cardiovasc Med. (2022) 9:1048077. doi: 10.3389/fcvm.2022.1048077
34. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. (2018) 71(6):e13–115. doi: 10.1161/hyp.0000000000000065
35. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
36. Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YH, et al. 2021 focused update consensus guidelines of the Asia pacific heart rhythm society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemost. (2022) 122(1):20–47. doi: 10.1055/s-0041-1739411
37. Romiti GF, Guo Y, Corica B, Proietti M, Zhang H, Lip GYH. Mobile health-technology-integrated care for atrial fibrillation: a win ratio analysis from the mAFA-II randomized clinical trial. Thromb Haemost. (2023) 123(11):1042–8. doi: 10.1055/s-0043-1769612
38. Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D, et al. Adherence to the “atrial fibrillation better care” pathway in patients with atrial fibrillation: impact on clinical outcomes-A systematic review and meta-analysis of 285,000 patients. Thromb Haemost. (2022) 122(3):406–14. doi: 10.1055/a-1515-9630
39. Sardana M, Syed AA, Hashmath Z, Phan TS, Koppula MR, Kewan U, et al. Beta-blocker use is associated with impaired left atrial function in hypertension. J Am Heart Assoc. (2017) 6(2):e005163. doi: 10.1161/jaha.116.005163
40. Eshoo S, Ross DL, Thomas L. Impact of mild hypertension on left atrial size and function. Circ Cardiovasc Imaging. (2009) 2(2):93–9. doi: 10.1161/circimaging.108.793190
41. Lau DH, Mackenzie L, Kelly DJ, Psaltis PJ, Brooks AG, Worthington M, et al. Hypertension and atrial fibrillation: evidence of progressive atrial remodeling with electrostructural correlate in a conscious chronically instrumented ovine model. Heart Rhythm. (2010) 7(9):1282–90. doi: 10.1016/j.hrthm.2010.05.010
42. Liu T, Korantzopoulos P, Shao Q, Zhang Z, Letsas KP, Li G. Mineralocorticoid receptor antagonists and atrial fibrillation: a meta-analysis. Europace. (2016) 18(5):672–8. doi: 10.1093/europace/euv366
43. Filippatos G, Bakris GL, Pitt B, Agarwal R, Rossing P, Ruilope LM, et al. Finerenone reduces new-onset atrial fibrillation in patients with chronic kidney disease and type 2 diabetes. J Am Coll Cardiol. (2021) 78(2):142–52. doi: 10.1016/j.jacc.2021.04.079
44. Michel MC, Foster C, Brunner HR, Liu L. A systematic comparison of the properties of clinically used angiotensin II type 1 receptor antagonists. Pharmacol Rev. (2013) 65(2):809–48. doi: 10.1124/pr.112.007278
45. Timmermans PB. Pharmacological properties of angiotensin II receptor antagonists. Can J Cardiol. (1999) 15 Suppl F:26f–8f.10579749
46. Kakuta H, Kurosaki E, Niimi T, Gato K, Kawasaki Y, Suwa A, et al. Distinct properties of telmisartan on agonistic activities for peroxisome proliferator-activated receptor γ among clinically used angiotensin II receptor blockers: drug-target interaction analyses. J Pharmacol Exp Ther. (2014) 349(1):10–20. doi: 10.1124/jpet.113.211722
47. Senda A, Mukai Y, Toda T, Hayakawa T, Yamashita M, Eliasson E, et al. Effects of angiotensin II receptor blockers on metabolism of arachidonic acid via CYP2C8. Biol Pharm Bull. (2015) 38(12):1975–9. doi: 10.1248/bpb.b15-00577
48. Oparil S. Newly emerging pharmacologic differences in angiotensin II receptor blockers. Am J Hypertens. (2000) 13(1 Pt 2):18s–24s. doi: 10.1016/s0895-7061(99)00250-2
49. Lee SS, Ae Kong K, Kim D, Lim YM, Yang PS, Yi JE, et al. Clinical implication of an impaired fasting glucose and prehypertension related to new onset atrial fibrillation in a healthy Asian population without underlying disease: a nationwide cohort study in Korea. Eur Heart J. (2017) 38(34):2599–607. doi: 10.1093/eurheartj/ehx316
Keywords: atrial fibrillation, hypertension, antihypertensive medication, angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blocker (ARBs), beta blocker, calcium channel blocker (CCB), diuretics
Citation: Choi J, Lee S-R, Choi E-K, Lee K-Y, Ahn H-J, Kwon S, Kim B, Han K-D, Oh S and Lip GYH (2024) Association between types of antihypertensive medication and the risk of atrial fibrillation: a nationwide population study. Front. Cardiovasc. Med. 11:1372505. doi: 10.3389/fcvm.2024.1372505
Received: 18 January 2024; Accepted: 17 April 2024;
Published: 9 May 2024.
Edited by:
Teresa Strisciuglio, University of Naples Federico II, ItalyReviewed by:
Carlo Lavalle, Sapienza University of Rome, Italy© 2024 Choi, Lee, Choi, Lee, Ahn, Kwon, Kim, Han, Oh and Lip. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eue-Keun Choi Y2hvaWVrMTdAc251LmFjLmty
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.