
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 01 May 2024
Sec. Coronary Artery Disease
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1367919
Background: Neutrophil-to-high-density lipoprotein cholesterol ratio (NHR), monocyte-to-high-density lipoprotein cholesterol ratio (MHR), lymphocyte-to-high-density lipoprotein cholesterol ratio (LHR), platelet-to-high-density lipoprotein cholesterol ratio (PHR), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), and aggregate index of systemic inflammation (AISI) have been identified as immune-inflammatory biomarkers associated with the prognosis of cardiovascular diseases. However, the relationship of these biomarkers with the prognosis of myocardial infarction with non-obstructive coronary arteries (MINOCA) remains unclear.
Method: Patients with MINOCA who underwent coronary angiography at the 920th Hospital of Joint Logistics Support Force were included in our study. Clinical baseline characteristics and laboratory testing data were collected from the hospital record system. The patients were divided into two groups on the basis of major adverse cardiovascular events (MACE) occurrence. Multiple logistic regression analysis was conducted to assess the relationship between NHR, MHR, LHR, PHR, SII, SIRI, AISI, and MACE. Receiver operating characteristic (ROC) curves were generated to evaluate the predictive value of NHR, MHR, LHR, PHR, SII, SIRI, and AISI for MACE in patients with MINOCA. The accuracy of the prediction was indicated by the area under the curve (AUC) value.
Results: The study included 335 patients with MINOCA. (81 in the MACE group and 254 in the No-MACE group). The MACE group had higher levels of NHR, MHR, LHR, PHR, SII, SIRI, and AISI than the No-MACE group. Multiple logistic regression analysis adjusted for confounding factors indicated that the higher levels of NHR, MHR, PHR, SII, SIRI, and AISI were associated with the occurrence of MACE in patients with MINOCA (P < 0.001). The AUC values for NHR, MHR, PHR, SII, SIRI, and AISI were 0.695, 0.747, 0.674, 0.673, 0.688, and 0.676, respectively. The combination of NHR, MHR, PHR, SII, SIRI, and AISI improved the accuracy of predicting MACE in patients with MINOCA (AUC = 0.804).
Conclusion: Higher levels of NHR, MHR, PHR, SII, SIRI, and AISI were associated with the occurrence of MACE, and the combination of NHR, MHR, PHR, SII, SIRI, and AISI improved the accuracy for predicting the incidence of MACE events in patients with MINOCA.
Cardiovascular disease (CVD) is a leading cause of death worldwide, and a major obstacle to sustainable human health (1, 2). Coronary heart disease (CHD) is the most prevalent CVD globally and causes a severe threat to human health (3, 4). Coronary angiography is considered the gold standard for diagnosing CHD and for assessing anatomical lesions in the coronary artery (5). In patients with acute myocardial infarction (AMI) undergoing coronary angiography, obstructive myocardial infarction (MI-CAD) is a common form of AMI (6, 7). However, approximately 5%–10% patients with AMI do not show significant coronary artery obstruction (normal or less than 50% narrowing of coronary arteries); this condition is termed myocardial infarction with non-obstructive coronary arteries (MINOCA) (6, 7).
MINOCA is a heterogeneous disease caused by various factors, including coronary plaque rupture, coronary artery spasm, spontaneous coronary artery dissection, and coronary artery embolism or thrombosis (7, 8). Among these factors, atherosclerotic plaque rupture is the most common one (6, 7). Although immune inflammation is not the direct cause of MINOCA, previous studies have indicated that inflammation is a crucial factor in the development of atherosclerosis, and inflammation causes endothelial injury and plaque rupture, resulting in thrombus formation (9–11). Immune inflammation is also closely associated with the prognosis of AMI (12–14). Therefore, early intervention of the immune inflammatory response may effectively reduce the incidence of adverse events in patients with MINOCA.
As immune-inflammatory biomarkers, neutrophil-to-high-density lipoprotein cholesterol ratio (NHR), monocyte-to-high-density lipoprotein cholesterol ratio (MHR), lymphocyte-to-high-density lipoprotein cholesterol ratio (LHR), platelet-to-high-density lipoprotein cholesterol ratio (PHR), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), and aggregate index of systemic inflammation (AISI) have been widely used in the prognostic assessment of diseases such as cancer and CVDs (15, 16). Currently, there is limited research on the effect of immune-inflammatory biomarkers on the prognosis of MINOCA; Patients with MINOCA had better prognosis in the risk of major long-term adverse cardiovascular events (MACE) than those with MI-CAD (17, 18). However, recent studies have shown that patients with MINOCA exhibit the same risk of MACE as those with MI-CAD (19–21). The study of Armillotta et al. showed that the rate of hospitalization for heart failure at long-term follow-up was similar between patients with MI-CAD and those with MINOCA (22). This finding suggested that patients with MINOCA deserve attention in clinical practice. The recognition of simple and practical prognostic indicators to identify high-risk patients and implement more proactive interventions not only enhances the focus on patients with MINOCA but also contributes to refining preventive strategies for MINOCA.
The main objectives of the present study were to (1) investigate the relationship between NHR, MHR, LHR, PHR, SII, SIRI, and AISI and MACE in patients with MINOCA and (2) to determine the predictive abilities of NHR, MHR, LHR, PHR, SII, SIRI, and AISI for adverse events in patients with MINOCA. The concept of our study is of noteworthy interest, since the negative prognostic role of inflammation in MINOCA patients has been observed in previous study (23).
This study conducted a retrospective analysis of data of patients with MINOCA from the hospital record system of the 920th Hospital of Joint Logistics Support Force, People's Liberation Army of China (PLA) from January 1, 2019 to March 31, 2023 (24). Clinical baseline characteristics and laboratory testing data at baseline were collected from the hospital record system. Because the study outcome was the occurrence of MACE, information regarding MACE was collected by telephone from August 2023. The inclusion standards were as follows: (1) patients who underwent coronary angiography and (2) patients who met the diagnostic criteria for MINOCA. The exclusion criteria were as follows: (1) patients with a history of coronary revascularization treatment, including thrombolysis, percutaneous coronary intervention (PCI), or coronary artery bypass grafting; (2) patients whose coronary angiography suggested stenosis ≥50%; (3) patients with elevated levels of cardiac enzymes due to nonischemic causes such as acute myocarditis, acute heart failure, or pulmonary embolism; and (4) patients with incomplete clinical data or who were lost to follow-up.
The study followed the principles of the Declaration of Helsinki and received approval from the Ethics Committee of the 920th Hospital of Joint Logistics Support Force, PLA (approval number: 2015067). Written informed consent was obtained from the study patients.
The diagnostic criteria for MINOCA were based on the “Fourth Universal Definition of Myocardial Infarction” (6) as follows: (1) a definitive diagnosis of AMI, defined as troponin level above the 99th percentile of the upper reference limit, and with conclusive clinical evidence of myocardial ischemia; (2) coronary angiography confirming the absence of significant coronary stenosis or stenosis <50%; and (3) exclusion of other nonischemic conditions that could cause an elevation in the level of cardiac enzymes (25).
MACE was defined as including all-cause death, nonfatal myocardial infarction, nonfatal stroke, revascularization, and rehospitalization due to unstable angina or heart failure (26).
The following patient data were extracted from the hospital's electronic medical record system for analysis: patient age, gender, medical history, admission heart rate, admission blood pressure, in-hospital medication, discharge diagnosis, and laboratory results. Medical history included hypertension, diabetes, hyperlipidemia, smoking history, and stroke. In-hospital medications included dual antiplatelet therapy, beta-blockers, statins, and angiotensin-converting enzyme inhibitors/angiotensin receptor blockers. Laboratory test indicators included the levels of troponin, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, lymphocytes, platelets, and monocytes. NHR, MHR, LHR, PHR, SII, SIRI, and AISI were calculated using the following formulas:
NHR = ratio of neutrophil count to HDL level;
MHR = ratio of monocyte count to HDL level;
LHR = ratio of lymphocyte count to HDL level;
PHR = ratio of platelet count to HDL level;
SII = platelet count multiplied by neutrophil-to-lymphocyte ratio;
SIRI = monocyte count multiplied by neutrophil-to-lymphocyte ratio;
AISI = neutrophil count multiplied by platelet count multiplied by monocyte-to-lymphocyte ratio.
Statistical analysis was conducted using IBM SPSS Version 26.0 (IBM Corp., Armonk, NY, USA). GraphPad Prism 9 was used for graphical representation of the results. Continuous variables (metric data) that followed a normal distribution were expressed as mean ± standard deviation. The data with non-normal distribution were expressed as quartiles and interquartile range (P25–P75). Student's t-test or Mann–Whitney U-test was used to analyze baseline characteristics and laboratory examination data. Logistic regression analysis was used to investigate the relationship between clinical data, laboratory examination results, and the occurrence of MACE. Independent predictors of MACE in MINOCA patients were determined by including variables with P < 0.01 from univariate analysis into multivariate regression models. Receiver operating characteristic (ROC) analysis was used to predict the effect of NHR, MHR, LHR, PHR, SII, SIRI, and AISI on the occurrence of MACE in patients with MINOCA. The accuracy of the prediction was indicated by the area under the curve (AUC) value. A P-value of <0.05 was considered statistically significant.
As showed in Figure 1, we selected 335 patients with MINOCA from 5,930 AMI patients who underwent coronary angiography at the 920th Hospital from January 1, 2019, to March 31, 2023. The median follow-up duration was 28 (18, 40) months. Among the 335 patients, 81 patients (24.2%) experienced MACE, and 254 patients (75.8%) did not experience MACE.
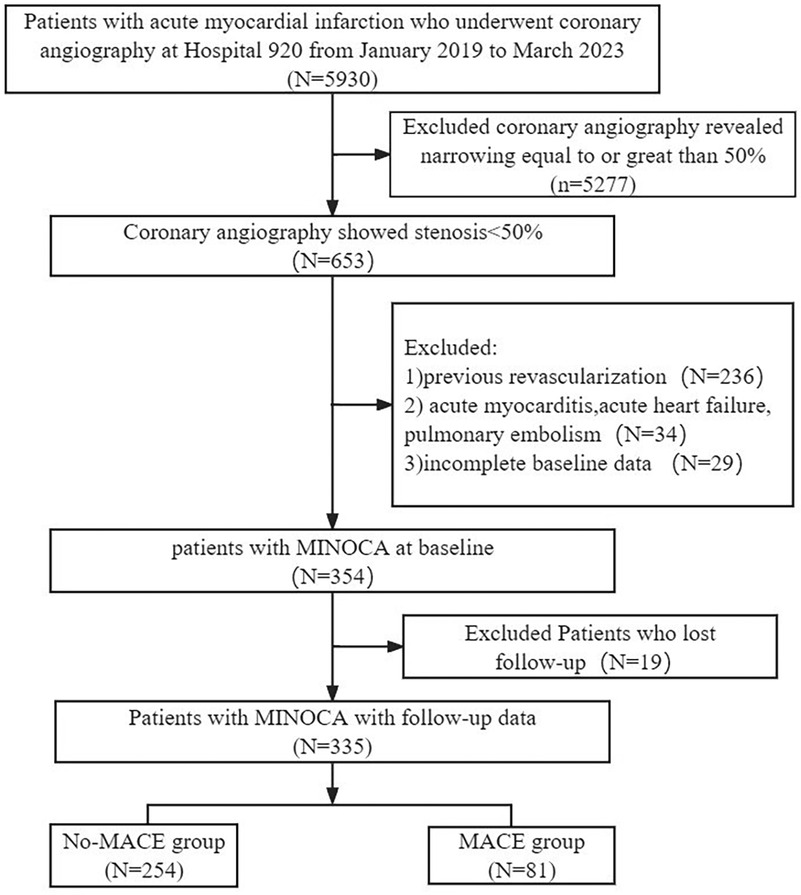
Figure 1. Study flowchart. Flowchart of inclusion and exclusion of the study participants. MACE, major adverse cardiovascular events; MINOCA, myocardial infarction with non-obstructive coronary arteries.
Table 1 has shown the baseline characteristics of the MACE and No-MACE groups. The average age of all study patients was 61.24 ± 12.18 years, and 56.1% patients were males. Compared to the No-MACE group, the MACE group patients were older (60.05 ± 11.81 years vs. 64.96 ± 12.63 years, P < 0.001), had a higher proportion of males (52.8% vs. 66.7%, P = 0.028), and had a higher proportion of stroke (4.3% vs. 12.3%, P = 0.010). No significant differences were observed between the No-MACE group and the MACE group in the proportion of hypertension, diabetes, hyperlipidemia, smoking, and in-hospital use of secondary prevention medications for CHD (all P > 0.05).
Table 2 showed differences in the laboratory examination data for all MINOCA patients and the MACE and No-MACE groups. Compared to the No-MACE group, the MACE group had lower levels of HDL cholesterol, LDL cholesterol, and lymphocytes (P < 0.05). Additionally, the MACE group showed significantly higher levels of creatinine, monocytes, NHR, MHR, PHR, SII, SIRI, and AISI (P < 0.05); however, no significant differences were observed in total cholesterol, neutrophil count, platelets, and LHR between the No-MACE and MACE groups (P > 0.05).
As shown in the boxplot in Figure 2, compared to the No-MACE group, the MACE group had higher levels of NHR, MHR, PHR, SII, SIRI, and AISI.
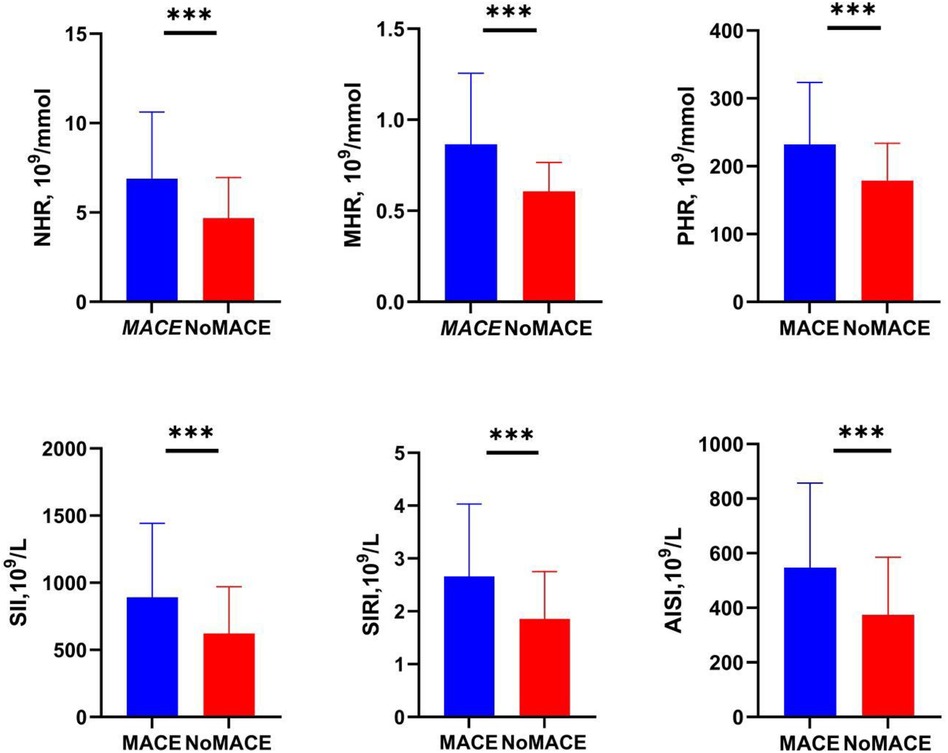
Figure 2. Levels of inflammatory markers in the MACE and No-MACE groups. MACE, major adverse cardiovascular events; NHR, neutrophil-to-high-density lipoprotein cholesterol ratio; MHR, monocyte-to-high-density lipoprotein cholesterol ratio; LHR, lymphocyte-to-high-density lipoprotein cholesterol ratio; PHR, platelet-to-high-density lipoprotein cholesterol ratio; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; AISI, systemic inflammation composite index. ***P < 0.0001.
Table 3 showed the results of the association between laboratory examination results and the occurrence of MACE. Univariate logistic regression analysis indicated that gender, age, NHR, MHR, PHR, SII, SIRI, and AISI were associated with the occurrence of MACE. After adjusting for DAPT, statin, ACEI or ARB, beta-blocker, creatinine levels, total cholesterol and LHR, variables with statistical significance (P < 0.05) were included in the multivariate logistic regression analysis model. Gender, age, NHR, PHR, SIRI, and AISI were still significantly associated with the occurrence of MACE (P < 0.05).
ROC curve analysis was used to assess the ability of NHR, MHR, PHR, SII, SIRI and AISI to predict MACE occurrence in patients with MINOCA. The results showed that these inflammatory factors had a high predictive value for MACE occurrence in MINOCA patients as follows NHR: AUC = 0.695, 95% CI = 0.625–0.766, P = 0.000; MHR: AUC = 0.747, 95% CI = 0.686–0.809, P = 0.000; PHR: AUC = 0.674, 95% CI = 0.602–0.746, P = 0.000; SII: AUC = 0.673, 95% CI = 0.604–0.742, P = 0.000; SIRI: AUC = 0.688, 95% CI = 0.622–0.754, P = 0.000; AISI: AUC = 0.676, 95% CI = 0.610–0.742, P = 0.000. The AUC value for predicting MACE in patients with MINOCA by using the combination of NHR + MHR + PHR + SII + SIRI + AISI was 0.804 (95% CI = 0.749–0.859). Therefore, compared to a single index, the combined indicator significantly improved the diagnostic efficiency of predicting the occurrence of MACE (Figure 3).
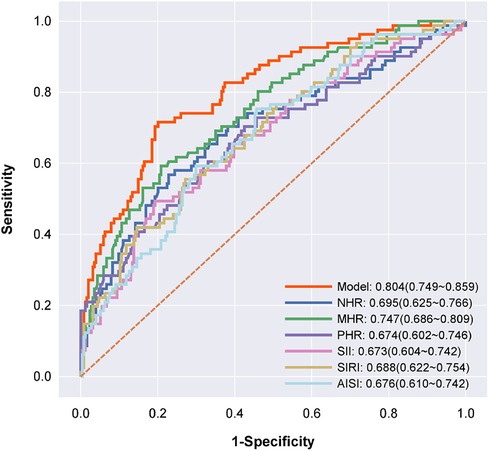
Figure 3. Receiver operating characteristic curve analyses for predicting major adverse cardiovascular events. Receiver operating characteristic curve analyses for predicting major adverse cardiovascular events. The model (red) includes NHR + MHR + PHR + SII + SIRI + AISI; NHR (dark blue); MHR (green); PHR (violet); SII (pink); SIRI (yellow); and AISI (baby blue), P < 0.001.
This study found that higher levels of NHR, MHR, PHR, SII, SIRI, and AISI were associated with the occurrence of MACE, and the combination of NHR, MHR, PHR, SII, SIRI, and AISI improved the accuracy for predicting the incidence of MACE events in patients with MINOCA.
MINOCA is a special type of AMI wherein myocardial infarction is present without significant obstruction of the coronary artery (6). Although patients with MINOCA do not have coronary artery blockages, they are still at a relatively high risk of onset of adverse cardiovascular events (19, 21). Therefore, early risk stratification for MINOCA patients is critical to identify high-risk individuals and implement more proactive and decisive intervention measures (27). We found that patients with MINOCA constituted 5.6% of patients with AMI. A previous study showed that MINOCA accounts for 1%–13% of AMI patients (7). A meta-analysis indicated that the incidence of MINOCA in patients with MI was 6% (28), which was similar to the findings of the present study. This meta-analysis also found that 40% of patients with MINOCA were females. Our study had a similar conclusion, with males comprising 56.1% of all patients with MINOCA. Thus, there were more males than females among patients with MINOCA in our study. MINOCA is categorized as a CVD, and inflammation plays a crucial role in the development and progression of CVDs. Inflammatory reactions can lead to the formation and rupture of atherosclerotic plaques, thereby triggering severe cardiovascular events such as myocardial infarction and stroke (29). Atherosclerosis was considered a cholesterol storage disease characterized by the accumulation of cholesterol and thrombus fragments in the arterial wall (30). However, recent studies have shown the proliferation of smooth muscle cells (SMCs) in atherosclerotic plaque lesions (30, 31). In recent years, the significance of inflammation in the development of atherosclerosis has gained increasing recognition. Inflammation has been proposed as a possible trigger also in patients with spontaneous coronary artery dissection, which occurs more frequently in women and can cause myocardial infarction and sudden cardiac death, and differential diagnosis with atherosclerotic MINOCA is crucial, since therapy is totally different, but the role of anti-inflammatory drugs remains uncertain (32, 33).
Atherosclerosis is currently regarded as a chronic inflammatory disease of the arterial wall (34), characterized by immune-inflammatory dysfunction involving interactions between immune cells and vascular cells (11, 35). Monocytes initiate intracellular lipid accumulation by releasing proinflammatory cytokines, reactive oxygen species, and proteolytic enzymes, thereby promoting fibrous cap fragility and the formation of lipid core thrombi (10, 36). Neutrophils exacerbate tissue damage and inflammation in the late stages of atherosclerosis by triggering the lysis and death of SMCs (37). Conversely, lymphocytes may impede the progression of atherosclerosis, thereby playing a protective role (38). Platelets have a dual role in atherosclerosis: platelet adhesion to the endothelial surface may signal the recruitment and extravasation of monocytes, thereby playing a critical role in the initiation of atherosclerotic plaque formation (39), and platelet activation can initiate thrombus formation, which promotes blood clotting (40). Elevated LDL and reduced HDL levels are the crucial factors in the occurrence and progression of atherosclerosis (41, 42). Therefore, understanding the relationship between inflammation and CVDs is very critical to prevent and treat CVDs. Recent studies have suggested that NHR (15), MHR (43, 44), LHR (45), PHR (46), SII (47), SIRI (48), and AISI (49) are novel inflammatory markers with significant clinical relevance in the occurrence, development, and prognosis of many diseases because of their ease of acquisition. NHR (15) can serve as a predictive indicator for the long-term clinical outcomes of elderly patients with AMI, exceeding MHR and LDL-C/HDL-C. Higher MHR and SIRI levels were associated with an increased risk of metabolic diseases and CVD. Low LHR (49) can serve as a mortality predictor in septic patients. PHR has a high predictive ability in diagnosing and assessing metabolic syndrome and could serve as a biomarker for diagnosing the risk of atherosclerotic thrombosis (46). SII and SIRI are significantly correlated with MACE occurrence in patients with AMI and thus could serve as useful indicators to predict MACE (16). In adults with hypertension, an elevated AISI value was significantly associated with an increased risk of CVD-related death, thus functioning as an early warning parameter for adverse outcomes (50).
Our study further extends the role of inflammatory biomarkers to patients with MINOCA. We found that the MACE group had higher levels of inflammatory markers than the No-MACE group, and high levels of inflammatory markers were independent risk factors for MACE in patients with MINOCA. We further assessed the diagnostic value of inflammatory markers in predicting MACE in patients with MINOCA. We found that NHR, MHR, PHR, SII, SIRI, and AISI could predict the occurrence of MACE in patients with MINOCA, with AUC values of 0.695, 0.747, 0.674, 0.673, 0.688, and 0.676, respectively. We further integrated these six inflammatory markers into a model and found that the integrated model could enhance the diagnostic value of predicting MACE in patients with MINOCA, with an AUC value of 0.804. This finding indicates that the use of the combined model in clinical practice can better predict the occurrence of MACE in patients with MINOCA, thus providing convenient diagnostic assistance for clinicians. Moreover this study confirms that the prognosis of MINOCA patients is not benign, especially in high-risk subgroups such as patients with positive inflammation markers of with unmet secondary prevention targets (51).
According to the findings of the present study, future research can further explore the relationship between NHR, MHR, PHR, SII, SIRI, and AISI with specific MACE outcomes, such as causes of death and mortality rates. Additionally, it would be worthwhile to consider integrating these inflammatory biomarkers with other predictive models or scoring systems to enhance the accuracy of prognostic assessment for patients with MINOCA.
There are some limitations in this study. First, this study analyzed only the overall MACE events in patients with MINOCA and did not specifically examine the factors leading to MACE events, such as reasons for rehospitalization, specific causes of death, and time of death. Second, the retrospective study design may have introduced information bias. Third, the study sample was drawn from a single center, and although it possesses a degree of representativeness, it may not be fully representative of the overall population. Additionally, cardiac magnetic resonance (CMR) is crucial to identify whether the underlying mechanism of acute myocardial injury is ischemic or non-ischemic and is related to cardiomyopathy, myocarditis, or Takotsubo syndrome) (52, 53); however, this study was based only on the clinician's experience and many patients did not undergo CMR examination for identifying the underlying mechanism of acute myocardial injury, and it is possible that some myocarditis have been included in the population, thus affecting results (54). Left ventricular ejection fraction (LVEF) and renal failure are critical indicators of cardiovascular outcomes. C-reactive protein (CRP) is also a common biomarker of inflammation. Because of the small database, the data regarding LVEF, renal failure, and CRP were unavailable; consequently, these indicators were not included in the present study and require further research in future investigations. Finally, the sample size of this study was relatively small, and it was designed as a cross-sectional study. Thus, a cause-and-effect relationship cannot be established, and further long-term follow-up studies are required to confirm the stability and reliability of the conclusions.
The present study revealed correlation between NHR, MHR, PHR, SII, SIRI, and AISI with MACE occurrence in patients with MINOCA. Higher levels of inflammatory biomarkers were associated with a higher risk of MACE. The combination model was more valuable in predicting MACE events in patients with MINOCA. This study has reported a new predictive method for patients with MINOCA, which could enable physicians to more accurately assess disease risk in patients. In practical terms, our research has provided clinicians a simple, cost-effective, and noninvasive approach for screening and predicting the risk of MACE occurrence in patients with MINOCA. This can aid in early intervention and treatment, eventually improving the prognosis of patients with MINOCA.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the 920 Hospital of Kunming Medical University (2015067). The patients/participants provided their written informed consent to participate in this study.
HZ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. XL: Conceptualization, Formal Analysis, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. WW: Data curation, Investigation, Writing – original draft. YZ: Data curation, Investigation, Writing – original draft. GG: Data curation, Formal Analysis, Writing – original draft. SL: Data curation, Software, Writing – original draft. BL: Data curation, Software, Writing – original draft. RG: Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the Science and Technology Department of Yunnan Province (202101AY070001-030) and the 920th Hospital of Joint Logistics Support Force, PLA (2020YGD11).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
MACE, major adverse cardiovascular events; MINOCA, myocardial infarction with non-obstructive coronary arteries; NHR, neutrophil-to-high-density lipoprotein cholesterol ratio; MHR, monocyte-to-high-density lipoprotein cholesterol ratio; LHR, lymphocyte-to-high-density lipoprotein cholesterol ratio; PHR, platelet-to-high-density lipoprotein cholesterol ratio; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; AISI, aggregate index of systemic inflammation; CVD, cardiovascular disease; CHD, coronary heart disease; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; SMCs, smooth muscle cells; HDL, high-density lipoprotein; LDL, low-density lipoprotein; ROC, receiver operating characteristic; AUC, area under the curve.
1. Clark H. NCDs: a challenge to sustainable human development. Lancet. (2013) 381(9866):510–1. doi: 10.1016/s0140-6736(13)60058-6
2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
3. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. (2017) 70(1):1–25. doi: 10.1016/j.jacc.2017.04.052
4. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. (2010) 362(23):2155–65. doi: 10.1056/NEJMoa0908610
5. Li N, Liu J, Ren Y, Cheng J. Diagnostic value of the cardiopulmonary exercise test in coronary artery disease. J Thorac Dis. (2022) 14(3):607–13. doi: 10.21037/jtd-22-24
6. Kristian T. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72(18):2231–64. doi: 10.1016/S0735-1097(18)32772-4
7. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. (2017) 38(3):143–53. doi: 10.1093/eurheartj/ehw149
8. Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J. (2015) 36(8):475–81. doi: 10.1093/eurheartj/ehu469
9. Libby P, Pasterkamp G, Crea F, Jang IK. Reassessing the mechanisms of acute coronary syndromes. Circ Res. (2019) 124(1):150–60. doi: 10.1161/circresaha.118.311098
10. Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. (2017) 136(12):1155–66. doi: 10.1161/circulationaha.117.029870
11. Wang H, Liu Z, Shao J, Lin L, Jiang M, Wang L, et al. Immune and inflammation in acute coronary syndrome: molecular mechanisms and therapeutic implications. J Immunol Res. (2020) 2020:4904217. doi: 10.1155/2020/4904217
12. Pietilä KO, Harmoinen AP, Jokiniitty J, Pasternack AI. Serum C-reactive protein concentration in acute myocardial infarction and its relationship to mortality during 24 months of follow-up in patients under thrombolytic treatment. Eur Heart J. (1996) 17(9):1345–9. doi: 10.1093/oxfordjournals.eurheartj.a015068
13. Kumar V, Prabhu SD, Bansal SS. CD4(+) T-lymphocytes exhibit biphasic kinetics post-myocardial infarction. Front Cardiovasc Med. (2022) 9:992653. doi: 10.3389/fcvm.2022.992653
14. Kumar V, Rosenzweig R, Asalla S, Nehra S, Prabhu SD, Bansal SS. TNFR1 contributes to activation-induced cell death of pathological CD4(+) T lymphocytes during ischemic heart failure. JACC Basic Transl Sci. (2022) 7(10):1038–49. doi: 10.1016/j.jacbts.2022.05.005
15. Huang JB, Chen YS, Ji HY, Xie WM, Jiang J, Ran LS, et al. Neutrophil to high-density lipoprotein ratio has a superior prognostic value in elderly patients with acute myocardial infarction: a comparison study. Lipids Health Dis. (2020) 19(1):59. doi: 10.1186/s12944-020-01238-2
16. Wei X, Zhang Z, Wei J, Luo C. Association of systemic immune inflammation index and system inflammation response index with clinical risk of acute myocardial infarction. Front Cardiovasc Med. (2023) 10:1248655. doi: 10.3389/fcvm.2023.1248655
17. Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjöld A, Gard A, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. (2017) 135(16):1481–9. doi: 10.1161/circulationaha.116.026336
18. Bainey KR, Welsh RC, Alemayehu W, Westerhout CM, Traboulsi D, Anderson T, et al. Population-level incidence and outcomes of myocardial infarction with non-obstructive coronary arteries (MINOCA): insights from the Alberta contemporary acute coronary syndrome patients invasive treatment strategies (COAPT) study. Int J Cardiol. (2018) 264:12–7. doi: 10.1016/j.ijcard.2018.04.004
19. Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, et al. Presentation, clinical profile, and prognosis of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA): results from the VIRGO study. J Am Heart Assoc. (2018) 7(13):e009174. doi: 10.1161/jaha.118.009174
20. Planer D, Mehran R, Ohman EM, White HD, Newman JD, Xu K, et al. Prognosis of patients with non-ST-segment-elevation myocardial infarction and nonobstructive coronary artery disease: propensity-matched analysis from the acute catheterization and urgent intervention triage strategy trial. Circ Cardiovasc Interv. (2014) 7(3):285–93. doi: 10.1161/circinterventions.113.000606
21. Andersson HB, Pedersen F, Engstrøm T, Helqvist S, Jensen MK, Jørgensen E, et al. Long-term survival and causes of death in patients with ST-elevation acute coronary syndrome without obstructive coronary artery disease. Eur Heart J. (2018) 39(2):102–10. doi: 10.1093/eurheartj/ehx491
22. Armillotta M, Amicone S, Bergamaschi L, Angeli F, Rinaldi A, Paolisso P, et al. Predictive value of killip classification in MINOCA patients. Eur J Intern Med. (2023) 117:57–65. doi: 10.1016/j.ejim.2023.08.011
23. Eggers KM, Baron T, Hjort M, Nordenskjöld AM, Tornvall P, Lindahl B. Clinical and prognostic implications of C-reactive protein levels in myocardial infarction with nonobstructive coronary arteries. Clin Cardiol. (2021) 44(7):1019–27. doi: 10.1002/clc.23651
24. Li X, Lu L, Yuan Q, Yang L, Du L, Guo R. Validity of regional network systems on reperfusion therapy in diabetes mellitus and non-diabetes mellitus patients with ST-segment elevation myocardial infarction. Front Cardiovasc Med. (2022) 9:991479. doi: 10.3389/fcvm.2022.991479
25. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American heart association. Circulation. (2019) 139(18):e891–908. doi: 10.1161/cir.0000000000000670
26. Gao S, Ma W, Huang S, Lin X, Yu M. Sex-specific clinical characteristics and long-term outcomes in patients with myocardial infarction with non-obstructive coronary arteries. Front Cardiovasc Med. (2021) 8:670401. doi: 10.3389/fcvm.2021.670401
27. Reynolds HR, Smilowitz NR. Myocardial infarction with nonobstructive coronary arteries. Annu Rev Med. (2023) 74:171–88. doi: 10.1146/annurev-med-042921-111727
28. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. (2015) 131(10):861–70. doi: 10.1161/circulationaha.114.011201
29. Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. (2021) 117(13):2525–36. doi: 10.1093/cvr/cvab303
30. Ross R, Glomset JA. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. (1976) 295(7):369–77. doi: 10.1056/nejm197608122950707
31. Ross R, Glomset JA. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. (1976) 295(8):420–5. doi: 10.1056/nejm197608192950805
32. Ciliberti G, Westaby J, Papadakis M, Behr ER, Sharma S, Finocchiaro G, et al. Coronary artery dissection and myocardial infarction with nonobstructed coronary arteries: insights from a UK nationwide autopsy-based registry-brief report. Arterioscler Thromb Vasc Biol. (2023) 43(5):787–92. doi: 10.1161/atvbaha.122.318401
33. Foà A, Canton L, Bodega F, Bergamaschi L, Paolisso P, De Vita A, et al. Myocardial infarction with nonobstructive coronary arteries: from pathophysiology to therapeutic strategies. J Cardiovasc Med (Hagerstown). (2023) 24(Suppl 2):e134–e46. doi: 10.2459/jcm.0000000000001439
34. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. (1999) 340(2):115–26. doi: 10.1056/nejm199901143400207
35. Spirig R, Tsui J, Shaw S. The emerging role of TLR and innate immunity in cardiovascular disease. Cardiol Res Pract. (2012) 2012:181394. doi: 10.1155/2012/181394
36. Gratchev A, Sobenin I, Orekhov A, Kzhyshkowska J. Monocytes as a diagnostic marker of cardiovascular diseases. Immunobiology. (2012) 217(5):476–82. doi: 10.1016/j.imbio.2012.01.008
37. Fernández-Ruiz I. Neutrophil-driven SMC death destabilizes atherosclerotic plaques. Nat Rev Cardiol. (2019) 16(8):455. doi: 10.1038/s41569-019-0214-1
38. Sharma M, Schlegel MP, Afonso MS, Brown EJ, Rahman K, Weinstock A, et al. Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression. Circ Res. (2020) 127(3):335–53. doi: 10.1161/circresaha.119.316461
39. Massberg S, Brand K, Grüner S, Page S, Müller E, Müller I, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. (2002) 196(7):887–96. doi: 10.1084/jem.20012044
40. Nording HM, Seizer P, Langer HF. Platelets in inflammation and atherogenesis. Front Immunol. (2015) 6:98. doi: 10.3389/fimmu.2015.00098
41. Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. (2013) 2013:152786. doi: 10.1155/2013/152786
42. Rosenson RS, Brewer HB Jr, Ansell BJ, Barter P, Chapman MJ, Heinecke JW, et al. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat Rev Cardiol. (2016) 13(1):48–60. doi: 10.1038/nrcardio.2015.124
43. Liu Z, Fan Q, Wu S, Wan Y, Lei Y. Compared with the monocyte to high-density lipoprotein ratio (MHR) and the neutrophil to lymphocyte ratio (NLR), the neutrophil to high-density lipoprotein ratio (NHR) is more valuable for assessing the inflammatory process in Parkinson’s disease. Lipids Health Dis. (2021) 20(1):35. doi: 10.1186/s12944-021-01462-4
44. Wang P, Guo X, Zhou Y, Li Z, Yu S, Sun Y, et al. Monocyte-to-high-density lipoprotein ratio and systemic inflammation response index are associated with the risk of metabolic disorders and cardiovascular diseases in general rural population. Front Endocrinol (Lausanne). (2022) 13:944991. doi: 10.3389/fendo.2022.944991
45. Yu S, Guo X, Li G, Yang H, Zheng L, Sun Y. Lymphocyte to high-density lipoprotein ratio but not platelet to lymphocyte ratio effectively predicts metabolic syndrome among subjects from rural China. Front Cardiovasc Med. (2021) 8:583320. doi: 10.3389/fcvm.2021.583320
46. Jialal I, Jialal G, Adams-Huet B. The platelet to high density lipoprotein -cholesterol ratio is a valid biomarker of nascent metabolic syndrome. Diabetes Metab Res Rev. (2021) 37(6):e3403. doi: 10.1002/dmrr.3403
47. Liu Y, Liu J, Liu L, Cao S, Jin T, Chen L, et al. Association of systemic inflammatory response index and pan-immune-inflammation-value with long-term adverse cardiovascular events in ST-segment elevation myocardial infarction patients after primary percutaneous coronary intervention. J Inflamm Res. (2023) 16:3437–54. doi: 10.2147/jir.S421491
48. Hamad DA, Aly MM, Abdelhameid MA, Ahmed SA, Shaltout AS, Abdel-Moniem AE, et al. Combined blood indexes of systemic inflammation as a mirror to admission to intensive care unit in COVID-19 patients: a multicentric study. J Epidemiol Glob Health. (2022) 12(1):64–73. doi: 10.1007/s44197-021-00021-5
49. Liu W, Tao Q, Xiao J, Du Y, Pan T, Wang Y, et al. Low lymphocyte to high-density lipoprotein ratio predicts mortality in sepsis patients. Front Immunol. (2023) 14:1279291. doi: 10.3389/fimmu.2023.1279291
50. Xiu J, Lin X, Chen Q, Yu P, Lu J, Yang Y, et al. The aggregate index of systemic inflammation (AISI): a novel predictor for hypertension. Front Cardiovasc Med. (2023) 10:1163900. doi: 10.3389/fcvm.2023.1163900
51. Ciliberti G, Guerra F, Pizzi C, Merlo M, Zilio F, Bianco F, et al. Characteristics of patients with recurrent acute myocardial infarction after MINOCA. Prog Cardiovasc Dis. (2023) 81:42–7. doi: 10.1016/j.pcad.2023.10.006
52. Mileva N, Paolisso P, Gallinoro E, Fabbricatore D, Munhoz D, Bergamaschi L, et al. Diagnostic and prognostic role of cardiac magnetic resonance in MINOCA: systematic review and meta-analysis. JACC Cardiovasc Imaging. (2023) 16(3):376–89. doi: 10.1016/j.jcmg.2022.12.029
53. Bergamaschi L, Foà A, Paolisso P, Renzulli M, Angeli F, Fabrizio M, et al. Prognostic role of early cardiac magnetic resonance in myocardial infarction with nonobstructive coronary arteries. JACC Cardiovasc Imaging. (2024) 17(2):149–61. doi: 10.1016/j.jcmg.2023.05.016
54. Bucciarelli V, Bianco F, Francesco AD, Vitulli P, Biasi A, Primavera M, et al. Characteristics and prognosis of a contemporary cohort with myocardial infarction with non-obstructed coronary arteries (MINOCA) presenting different patterns of late gadolinium enhancements in cardiac magnetic resonance imaging. J Clin Med. (2023) 12(6):2266. doi: 10.3390/jcm12062266
Keywords: myocardial infarction with non-obstructive coronary arteries, inflammation, coronary artery disease, biomarker, immune inflammation
Citation: Zhou H, Li X, Wang W, Zha Y, Gao G, Li S, Liu B and Guo R (2024) Immune-inflammatory biomarkers for the occurrence of MACE in patients with myocardial infarction with non-obstructive coronary arteries. Front. Cardiovasc. Med. 11:1367919. doi: 10.3389/fcvm.2024.1367919
Received: 12 January 2024; Accepted: 18 April 2024;
Published: 1 May 2024.
Edited by:
Tommaso Gori, University Medical Centre, Johannes Gutenberg University Mainz, GermanyReviewed by:
Matteo Armillotta, University of Bologna, Italy© 2024 Zhou, Li, Wang, Zha, Gao, Li, Liu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruiwei Guo Z3J3NzcxMjEwQDE2My5jb20=
†These authors have contributed equally to this work
‡ORCID Ruiwei Guo orcid.org/0000-0002-3617-6169
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.