- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Republic of Korea
- 2Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Republic of Korea
- 3Department of Epidemiology and Health Informatics, Korea University, Seoul, Republic of Korea
- 4Department of Internal Medicine, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Republic of Korea
- 5Department of Cardiology, Chungbuk National University College of Medicine, Chungbuk National University College of Medicine, Cheongju, Republic of Korea
- 6Division of Pulmonary Medicine and Allergy, Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Republic of Korea
Background: Although the association between tuberculosis (TB) and cardiovascular disease (CVD) has been reported in several studies and is explained by mechanisms related to chronic inflammation, few studies have comprehensively evaluated the association between TB and CVD in Korea.
Methods: Using the Korea National Health and Nutrition Survey, we classified individuals according to the presence or absence of previous pulmonary TB was defined as the formal reading of a chest radiograph or a previous diagnosis of pulmonary TB by a physician. Using multivariable logistic regression analyses, we evaluated the association between the 10-year atherosclerotic cardiovascular disorder (ASCVD) risk and TB exposure, as well as the 10-year ASCVD risk according to epidemiological characteristics.
Results: Among the 69,331 participants, 4% (n = 3,101) had post-TB survivor group. Comparing the 10-year ASCVD risk between the post-TB survivor and control groups, the post-TB survivor group had an increased 10-year ASCVD risk in the high-risk group (40.46% vs. 24.00%, P < 0.001). Compared to the control group, the intermediate- and high-risk groups had also significantly increased 10-year ASCVD risks (odds ratio [OR] 1.14, 95% confidence interval [CI] 1.04–1.23 and OR 1.69, 95% CI 1.59–1.78, respectively) in the post-TB survivor group. In the association of CVD among post-TB survivors according to epidemiologic characteristics, age [adjusted OR (aOR) 1.10, 95% CI 1.07–1.12], current smoking (aOR 2.63, 95% CI 1.34–5.14), a high family income (aOR 2.48, 95% CI 1.33–4.62), diabetes mellitus (aOR 1.97, 95% CI 1.23–3.14), and depression (aOR 2.06, 95% CI 1.03–4.10) were associated with CVD in the post-TB survivor group.
Conclusions: Our study findings suggest a higher 10-year ASCVD risk among TB survivors than healthy participants. This warrants long-term cardiovascular monitoring and management of the post-TB population.
Background
Tuberculosis (TB) is the leading cause of mortality due to infectious diseases and is among the top 10 leading causes of death worldwide (1). With global efforts, such as the implementation of the End TB Strategy, the survival rate after TB treatment is increasing (2), and the long-term management of TB survivors is expected to become more important.
TB causes chronic inflammation and immune activation, leading to the development of atherosclerosis and other cardiovascular conditions, even after TB treatment. Cardiovascular diseases (CVDs) are caused by TB-induced direct cardiac involvement, such as pericardial TB. However, increases in CVD incidence in patients with TB have been reported (3–7). For example, in Taiwan, a large population-based retrospective study found that patients with a history of TB had an approximately 3-fold increased risk of developing CVD compared to those without TB (8). Likewise, another study conducted in Taiwan found that TB is associated with an increased risk of ischemic stroke (9). In addition to the mechanistic effect of chronic inflammation on CVD, the increased CVD risk in patients with TB may be related to factors such as socioeconomic status, lifestyle factors, and comorbidities including diabetes mellitus (DM) and hypertension, which are associated with both TB and CVD (10, 11).
Despite the importance of this increased cardiovascular risk, no study has focused on the association between TB and atherosclerotic cardiovascular disorder (ASCVD) in Korea. We did not find any study that reported a correlation between TB and the 10-year ASCVD risk other than the actual CVD rate. Therefore, using a nationwide surveillance system, we analyzed the 10-year ASCVD risk of post-TB survivors compared to that of participants not affected by TB.
Methods
Study population
The Korea National Health and Nutrition Survey (KNHANES) is a population-based nationwide surveillance system maintained by the Korea Disease Control and Prevention Agency since 1998 to assess the health and nutritional status of Koreans. We used data from KNHANES IV (2007–2009), V (2010–2012), VI (2013–2015), VII (2016–2018), and VIII (2019). The study population was selected using a stratified multistage sampling method. During the 13 years of the study period, 105,732 participants without age limitations were enrolled. Among these, participants with missing weight variables or 10-year ASCVD (n = 36,401) were excluded; thus, 69,331 participants were included in this study. The eligible participants were classified into two groups according to their previous TB diagnosis. Previous pulmonary TB was defined as the formal reading of a chest radiograph or a previous diagnosis of pulmonary TB by a physician (Figure 1). The study protocol was approved by the Institutional Review Board of Chungbuk National University Hospital (application no.2023-05-001). The KNHANES were approved by the relevant institutional review boards and all participants provided informed consent.
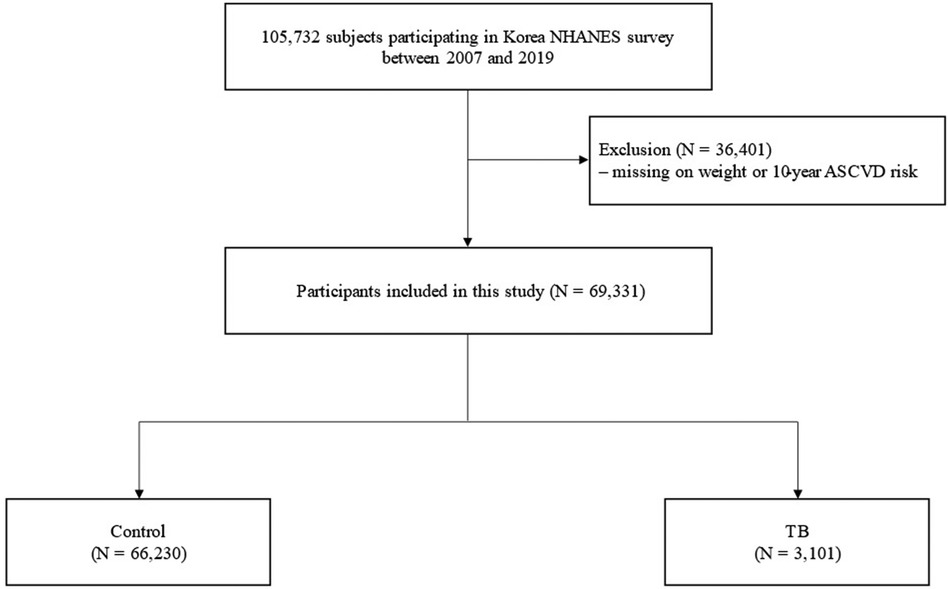
Figure 1. Flow chart. ASCVD, atherosclerotic cardiovascular disorder; NHANES, National health and nutrition examination survey; TB, pulmonary tuberculosis.
Measurements
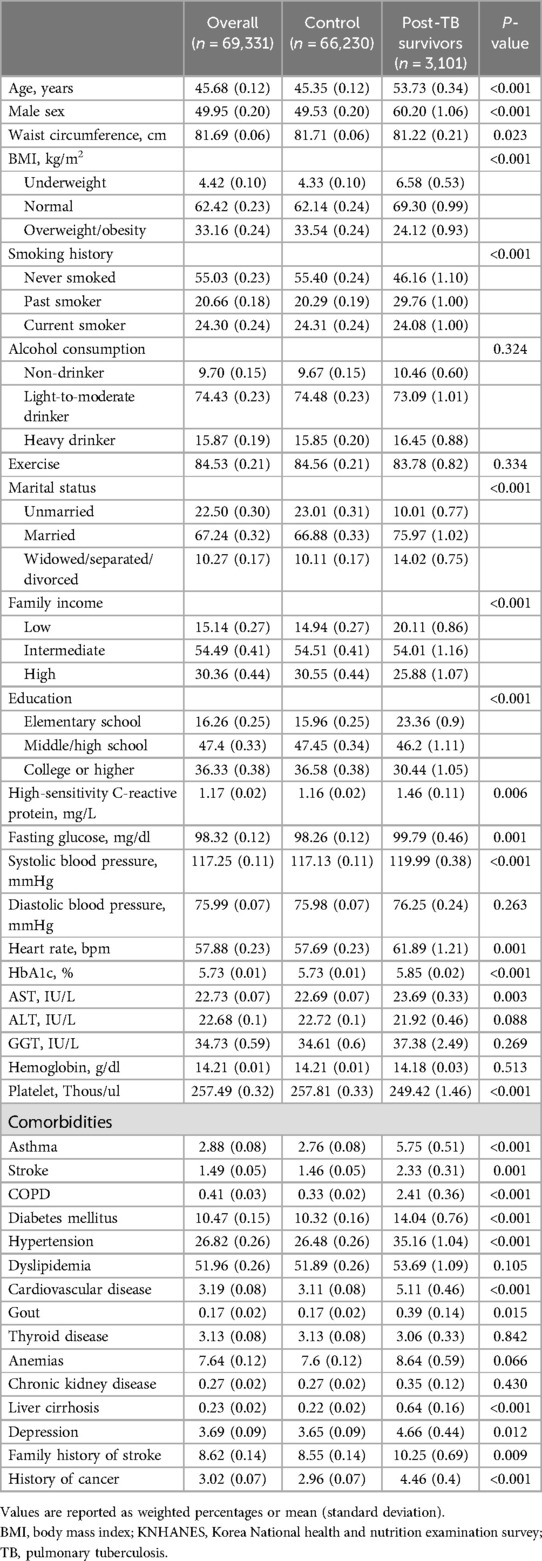
Available data on age, sex, weight circumference, body mass index (BMI), smoking history, alcohol history, marital status, income status, and educational status were collected from the Korea NHANES database. BMI was calculated as weight in kilograms divided by height in meters squared, and categorized according to Asian-specific criteria: underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), and obese (≥25.0–29.9 kg/m2) (12). Participants who consumed more than 30 g/day of alcohol were classified as heavy drinkers. Comorbidities [asthma, stroke, chronic obstructive pulmonary disease (COPD), DM, hypertension, dyslipidemia, gout, thyroid disease, anemias, chronic kidney disease, depression, family history of stroke and history of cancer] were defined based on self-reported diagnoses by a physician. DM was defined as a fasting glucose level ≥126 mg/dl, the current use of antidiabetic medications, or a self-reported diagnosis of DM by a physician (13). Hypertension was defined as a self-reported diagnosis by a physician, the use of antihypertensive medication, a systolic blood pressure ≥140 mmHg, or a diastolic blood pressure ≥90 mmHg (14). Dyslipidemia was defined as a self-reported diagnosis by a physician, the use of lipid-lowering medication, a total cholesterol concentration ≥240 mg/dl, or a fasting triglyceride concentration ≥200 mg/dl (15).
Assessment of the 10-year ASCVD risk
CVD is a major cause of disease and death worldwide (16). In 1976, the Framingham Heart Study revealed multiple risk factors and developed for the first time coronary heart disease risk equations (17). Since then, various equations for CVD risk calculation have been published by public health studies and used in clinical practice. Among these, the 10-year ASCVD risk published by the American Heart Association is the most frequently used (18) and allows the calculation of the 10-year risk of cardiovascular problems, such as heart attack or stroke. This risk estimate considers age, sex, race, cholesterol levels, blood pressure, medication use, DM, and smoking status. The ASCVD risk score is expressed as a percentage. A 0%–4.9% risk is considered low, a 5.0%–7.4% risk is considered borderline, a 7.5%–20% risk is considered intermediate, and a risk greater than 20% is considered high (18).
Outcomes
The main objective of our study was to investigate the correlation between CVD risk and prior TB diagnosis. We also evaluated epidemiological factors associated with CVD in patients with TB.
Statistical analysis
All analyses were performed using the survey commands in STATA 15.1 version (StataCorp LP, College Station, TX, USA) to account for the complex sampling design and survey weights. All data are presented as weighted percentages with standard errors. Data were compared using Student's t-test for continuous variables and Pearson's χ2 test for categorical variables. Multivariate logistic regression analyses were performed to evaluate the association between TB and CVD. Adjusted odds ratios (aORs) and 95% confidence intervals (CIs) were estimated after adjusting for potential confounding factors (age, BMI, alcohol consumption, marital status, family income, education, and comorbidities). Variables used to calculate the 10-year ASCVD risk (age, sex, race, blood pressure, medication use, DM status, and smoking status) were not adjusted for in the multivariable analysis to minimize collinearity. All tests were two-sided, and P-values < 0.05 were considered to indicate significant differences.
Results
Baseline characteristics
The baseline characteristics of the participants are presented in Table 1. Of the 69,331 participants, approximately 4% (n = 3,101) had TB (post-TB survivor group), and 96% (n = 66,230) had never had TB (control group). Compared with the control group, the post-TB survivor group had an older mean age (53.73 years vs. 45.35 years), a higher proportion of male participants (60.20% vs. 49.53%), a higher proportion of underweight participants (6.58% vs. 4.33%), a higher proportion of smokers (53.84% vs. 44.60%), a lower proportion of unmarried participants (10.01% vs. 23.01%), a lower proportion of high familial income (25.88% vs. 30.55%), and a lower level of education (P < 0.001 for all variables). Regarding comorbidities, the post-TB survivor group had a higher proportion of participants with asthma (5.75% vs. 2.76%), stroke (2.33% vs. 1.46%), COPD (2.41% vs. 0.33%), DM (14.04% vs. 10.32%), hypertension (35.16% vs. 26.48%), CVD (5.11% vs. 3.11%), liver cirrhosis (0.64% vs. 0.22%) and history of cancer (4.46% vs. 2.96%, P < 0.001 for all variables). The proportion of participants with depression was also higher in the post-TB survivor group (4.66% vs. 3.65%, P = 0.012). Moreover, the proportion of participants with dyslipidemia was higher in the post-TB survivor group without reaching statistical significance (53.69% vs. 51.89%, P = 0.105).
Comparison of 10-year ASCVD risk between post-TB and control groups
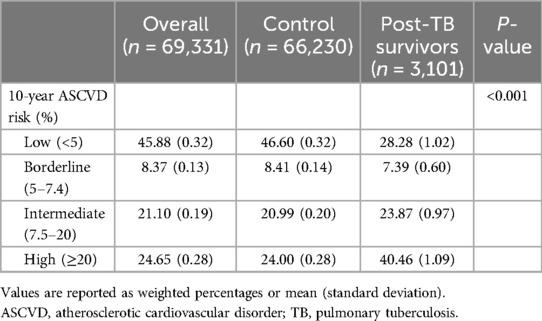
A comparison of the 10-year ASCVD risk between the post-TB survivor and control groups is shown in Table 2. Compared to the control group, the post-TB survivor group had a higher 10-year ASCVD risk than the control group in the high-risk stratum (40.46% vs. 24.00%, P < 0.001).
Association of 10-year ASCVD risk with prior TB diagnosis
Figure 2 shows the association between the 10-year ASCVD risk and TB exposure. Compared to the control group, the intermediate and high-risk strata of the post-TB survivor group had significantly higher 10-year ASCVD risks (OR 1.14, 95% CI 1.04–1.23 in the intermediate risk group; OR 1.69, 95% CI 1.59–1.78 in the high-risk group).
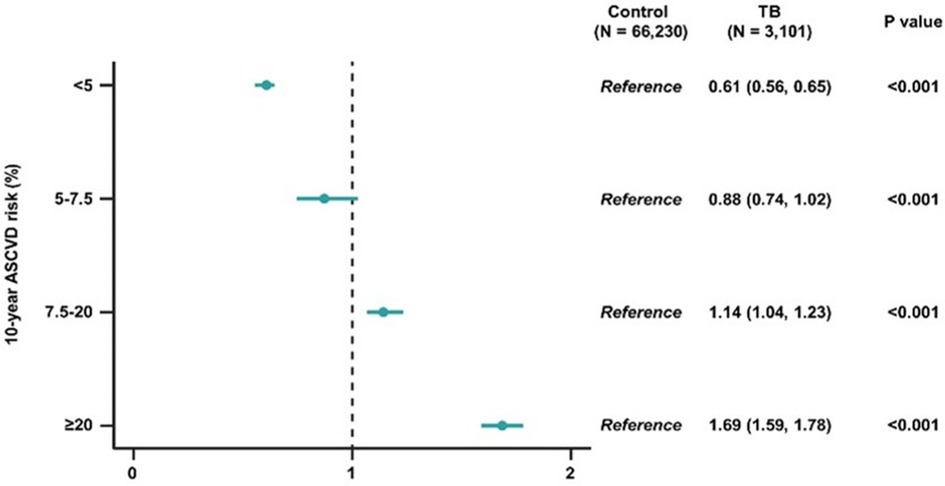
Figure 2. Odds ratios and 95% confidence intervals of 10-year ASCVD risk in post-TB survivors, compared to participants without TB. ASCVD, atherosclerotic cardiovascular disorder; TB, pulmonary tuberculosis.
Factors associated with CVD among patients with TB
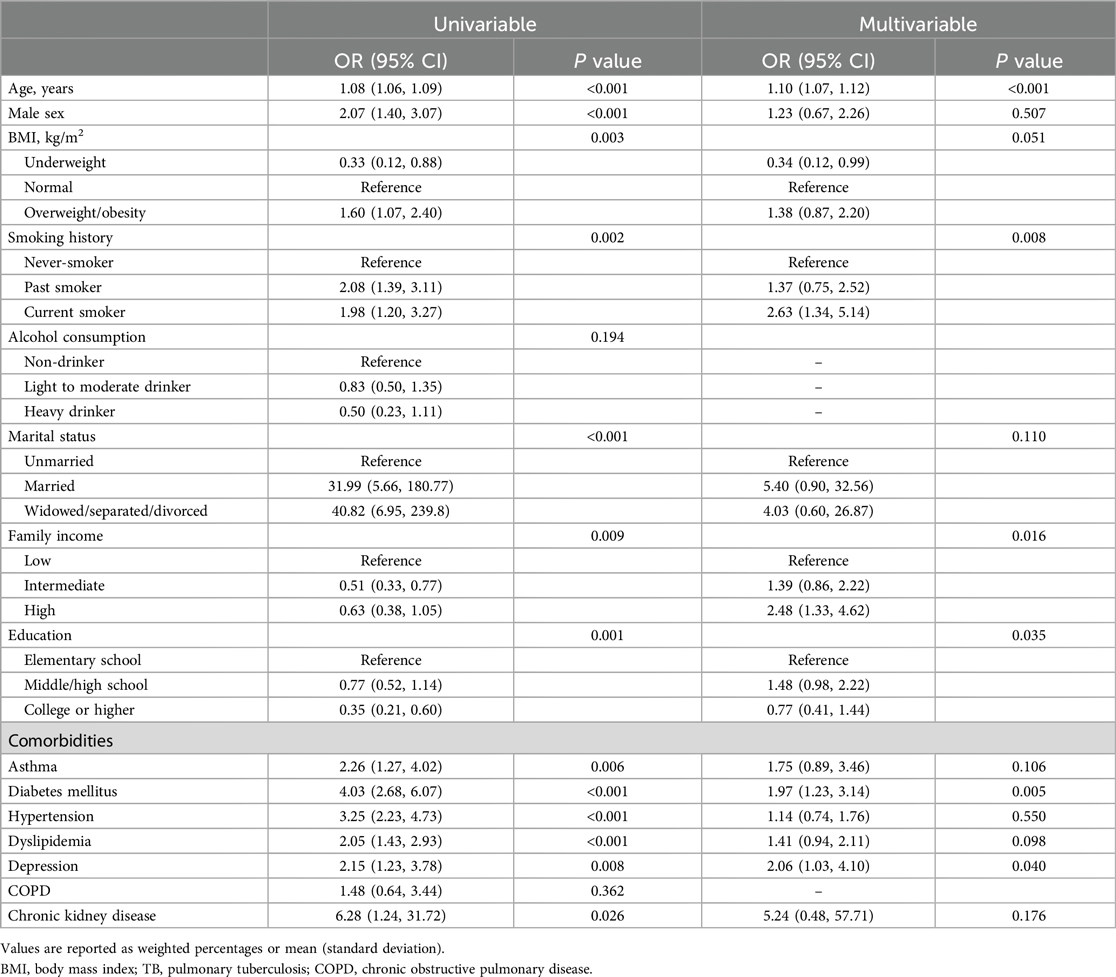
The multivariable analysis results of CVD association among patients with TB are shown in Table 3. In multivariable analyses, age [adjusted OR (aOR) 1.10, 95% CI 1.07–1.12], current smoker (aOR 2.63, 95% CI 1.34–5.14), high family income (aOR 2.48, 95% CI 1.33–4.62), DM (aOR 1.97, 95% CI 1.23–3.14) and depression (aOR 2.06, 95% CI 1.03–4.10) were factors significantly associated with CVD among patients with TB.
Discussion
We investigated the association between TB survival and CVD risk (defined as the 10-year ASCVD risk) using nationally representative data from Korea. Our study showed that TB survivors had a higher risk of ASCVD than participants without prior TB diagnosis indicating long-term cardiovascular consequences of a TB infection. Additionally, among patients with TB, age, current smoking, high family income, DM and depression were factors significantly associated with CVD.
Previous studies have consistently shown a high risk of CVD development in TB survivors (4, 9, 19, 20). As an example, a meta-analysis conducted in Taiwan showed that patients with TB had a 1.76-fold increased risk of developing coronary heart disease compared to controls (19). In another study, the incidence of ischemic stroke was 1.52 times higher in tuberculosis patients (21). Our study also showed that the post-TB survivor group had a significantly higher 10-year ASCVD risks (OR 1.69, 95% CI 1.59–1.78 in the high-risk group) compared to the control group, aligning with similar results from previous studies. The mechanisms underlying this association are not fully understood; however, one possible mechanism has been proposed (16). TB is known to cause chronic inflammation and immune activation, leading to the development of atherosclerosis and other cardiovascular conditions, even after TB treatment. Possible mechanisms include increased expression of proinflammatory cytokines, monocyte/macrophage immune activation, CD4+ Th1 and Th17 cell immune activation, and autoimmunity mediated by antibodies against mycobacterial hit shock protein 65 (16). Via one or several of these mechanisms, patients surviving TB have a higher incidence of chronic diseases, such as ischemic stroke, CVD, chronic kidney disease, and pulmonary complications, than healthy individuals (7, 22–24).
Our study showed that epidemiologic factors, age, and current smoking were associated with CVD in post-TB survivors. Age (25, 26) and smoking (27–29) are well known as common risk factors for tuberculosis and CVD. Along the same line, it can be interpreted that they are also factors related to the occurrence of CVD in post-TB survivors. Additionally, we showed that a high family income was associated with CVD in post TB-survivors. This finding is contrary to the previous notion that a lower income level is associated with myocardial infarction, sudden cardiac death and the risk of TB (30, 31). Unfortunately, the reason for this phenomenon cannot be fully explained given the observational nature of our study, so further research on this is considered necessary.
Regarding comorbidities, our findings showed that DM and depression were factors significantly associated with CVD in post TB survivors. A systematic review of 13 observational studies reports that DM increases the risk of developing tuberculosis threefold (32). Although the mechanism is not fully understood, it is believed that DM may contribute to TB development due to dysfunctional immune responses (33). Additionally, because DM is also known to be a risk factor for CVD (34), DM is considered a factor associated with CVD in TB survivors. Similarly, the prevalence of CVD in patients with depression is over two-to-three times that in the general population (35). Some mechanisms are biologically plausible for depression to increase the incidence of CVD. These include changes in the autonomic nervous system (36), coagulation factors, such as fibrinogen, and proinflammatory cytokines (37). Patients with depression also tend to have poor adherence to medical treatment. These factors may lead to association between CVD and in patients with depression (38).
The association between TB and the risk of ASCVD has important implications for the long-term management of TB survivors. Considering the increased risk of ASCVD in TB survivors, our findings highlight the need to closely monitor cardiovascular risk factors and provide appropriate interventions, such as control of hypertension and hyperglycemia or smoking cessation to mitigate the risk of TB survivors, especially in those with comorbidities. Further exploration of the underlying mechanisms associated with TB infection and CVD, such as the role of specific inflammatory markers or immune responses, will enhance our understanding of its pathophysiology and guide targeted interventions.
Our study has three major limitations. First, this was a cross-sectional study; thus, the actual CVD risk might not be accurate for TB survivors. Second, this study was performed in a single country; therefore, our results may not be generalizable. Similar studies should be conducted in other countries and ethnicities. Third, in KHANES, it is difficult to identify the various types of ASCVD or CVD that the participants have, such as MI, CAD without MI, PVD, etc (39, 40). However, this study lacks sufficient analysis of the various types of ASCVD or CVD in post-TB survivor. Further research is needed on post-TB survivor.
Future directions
Future researches should include prospective studies to trace trajectory of the long-term cardiovascular health of TB survivors and investigate specific inflammatory markers linked to increased ASCVD risk in TB survivors for targeted interventions. Finally similar studies should be conducted in diverse populations to generalize findings and understand regional variations in TB related ASCVD risk.
Conclusion
In conclusion, this study found a higher 10-year ASCVD risk among TB survivors than among control participants, and this finding warrants long-term cardiovascular monitoring and management in this population. Our study suggests that a comprehensive approach that includes early identification and treatment of comorbidities, lifestyle modifications, and cardiovascular risk reduction strategies can help improve the long-term cardiovascular outcomes of TB infection.
Key messages
1. TB survivors are a higher 10-year ASCVD risk than among control participants.
2. Among TB patients, age, current smoker, high family income, DM and depression were factors significantly associated with CVD.
3. Comprehensive approaches that include the early identification and treatment of comorbidities, lifestyle modifications, and cardiovascular risk reduction strategies can help improve the long-term cardiovascular consequences of TB infection.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Korea National Health and Nutrition Survey and Institutional Review Board of Chungbuk National University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JY: Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. S-HK: Funding acquisition, Project administration, Writing – original draft, Writing – review & editing, Validation. JaS: Project administration, Supervision, Writing – review & editing. SG: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft. JeS: Project administration, Supervision, Writing – review & editing. D-HB: Project administration, Supervision, Writing – review & editing, Methodology. JC: Project administration, Supervision, Validation, Writing – review & editing. KL: Project administration, Supervision, Validation, Writing – review & editing. KC: Project administration, Supervision, Validation, Writing – review & editing. HL: Conceptualization, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing, Methodology. BY: Writing – review & editing, Investigation, Methodology, Supervision, Conceptualization, Project administration, Validation, Visualization, Writing – original draft. KM: Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the research grant of the Chungbuk National University Hospital in 2023.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1364337/full#supplementary-material
References
1. World Health Organization. Global Tuberculosis Report 2021. Geneva: World Health Organization (2021). Available online at: https://www.who.int/publications/i/item/9789240037021 (Accessed August 5, 2024).
2. World Health Organization. The End TB Strategy. Geneva: World Health Organization (2015). Available online at: https://www.who.int/publications/i/item/WHO-HTM-TB-2015.19 (Accessed August 5, 2024).
3. Adefuye MA, Manjunatha N, Ganduri V, Rajasekaran K, Duraiyarasan S, Adefuye BO. Tuberculosis and cardiovascular complications: an overview. Cureus. (2022) 14(8):e28268. doi: 10.7759/cureus.28268
4. Chidambaram V, Ruelas Castillo J, Kumar A, Wei J, Wang S, Majella MG, et al. The association of atherosclerotic cardiovascular disease and statin use with inflammation and treatment outcomes in tuberculosis. Sci Rep. (2021) 11(1):15283. doi: 10.1038/s41598-021-94590-x
5. Kumar NP, Fukutani KF, Shruthi BS, Alves T, Silveira-Mattos PS, Rocha MS, et al. Persistent inflammation during anti-tuberculosis treatment with diabetes comorbidity. Elife. (2019) 8:e46477. doi: 10.7554/eLife.46477
6. Lee HR, Yoo JE, Choi H, Han K, Jung JH, Park J, et al. Tuberculosis and risk of ischemic stroke: a nationwide cohort study. Stroke. (2022) 53(11):3401–9. doi: 10.1161/STROKEAHA.122.039484
7. Lee HR, Yoo JE, Choi H, Han K, Lim YH, Lee H, et al. Tuberculosis and the risk of ischemic heart disease: a nationwide cohort study. Clin Infect Dis. (2023) 76(9):1576–84. doi: 10.1093/cid/ciac946
8. Salindri AD, Wang JY, Lin HH, Magee MJ. Post-tuberculosis incidence of diabetes, myocardial infarction, and stroke: retrospective cohort analysis of patients formerly treated for tuberculosis in Taiwan, 2002–2013. Int J Infect Dis. (2019) 84:127–30. doi: 10.1016/j.ijid.2019.05.015
9. Sheu JJ, Chiou HY, Kang JH, Chen YH, Lin HC. Tuberculosis and the risk of ischemic stroke: a 3-year follow-up study. Stroke. (2010) 41(2):244–9. doi: 10.1161/STROKEAHA.109.567735
10. Cantwell MF, McKenna MT, McCray E, Onorato IM. Tuberculosis and race/ethnicity in the United States: impact of socioeconomic status. Am J Respir Crit Care Med. (1998) 157(4 Pt 1):1016–20. doi: 10.1164/ajrccm.157.4.9704036
11. Jakovljević D, Sarti C, Sivenius J, Torppa J, Mähönen M, Immonen-Räihä P, et al. Socioeconomic status and ischemic stroke: the FINMONICA stroke register. Stroke. (2001) 32(7):1492–8. doi: 10.1161/01.STR.32.7.1492
12. Kim MK, Lee WY, Kang JH, Kang JH, Kim BT, Kim SM, et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul). (2014) 29(4):405–9. doi: 10.3803/EnM.2014.29.4.405
13. Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem. (2011) 57(6):e1–47. doi: 10.1373/clinchem.2010.161596
14. Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). G Ital Cardiol (Rome). (2018) 19(11 Suppl 1):3S–73S. doi: 10.1714/3026.30245
15. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. (2019) 290:140–205. doi: 10.1016/j.atherosclerosis.2019.08.014
16. Huaman MA, Henson D, Ticona E, Sterling TR, Garvy BA. Tuberculosis and cardiovascular disease: linking the epidemics. Trop Dis Travel Med Vaccines. (2015) 1(1):10. doi: 10.1186/s40794-015-0014-5
17. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham study. Am J Cardiol. (1976) 38(1):46–51. doi: 10.1016/0002-9149(76)90061-8
18. Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB Sr., Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. (2014) 63(25 Pt B):2935–59. doi: 10.1016/j.jacc.2013.11.005
19. Wongtrakul W, Charoenngam N, Ungprasert P. Tuberculosis and risk of coronary heart disease: a systematic review and meta-analysis. Indian J Tuberc. (2020) 67(2):182–8. doi: 10.1016/j.ijtb.2020.01.008
20. Hossain MB, Johnston JC, Cook VJ, Sadatsafavi M, Wong H, Romanowski K, et al. Role of latent tuberculosis infection on elevated risk of cardiovascular disease: a population-based cohort study of immigrants in British Columbia, Canada, 1985–2019. Epidemiol Infect. (2023) 151:e68. doi: 10.1017/S0950268823000559
21. Basham CA, Smith SJ, Romanowski K, Johnston JC. Cardiovascular morbidity and mortality among persons diagnosed with tuberculosis: a systematic review and meta-analysis. PLoS One. (2020) 15(7):e0235821. doi: 10.1371/journal.pone.0235821
22. Ruzangi J, Iwagami M, Smeeth L, Mangtani P, Nitsch D. The association between chronic kidney disease and tuberculosis; a comparative cohort study in England. BMC Nephrol. (2020) 21(1):420. doi: 10.1186/s12882-020-02065-4
23. Wei Y, Tang S, Xie Z, He Y, Zhang Y, Xie Y, et al. Pulmonary tuberculosis-related ischemic stroke: a retrospective case control study. J Inflamm Res. (2022) 15:4239–49. doi: 10.2147/JIR.S368183
24. Fan H, Wu F, Liu J, Zeng W, Zheng S, Tian H, et al. Pulmonary tuberculosis as a risk factor for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ann Transl Med. (2021) 9(5):390. doi: 10.21037/atm-20-4576
25. Kim H, Kim S, Han S, Rane PP, Fox KM, Qian Y, et al. Prevalence and incidence of atherosclerotic cardiovascular disease and its risk factors in Korea: a nationwide population-based study. BMC Public Health. (2019) 19:1–11. doi: 10.1186/s12889-018-6343-3
26. Byng-Maddick R, Noursadeghi M. Does tuberculosis threaten our ageing populations? BMC Infect Dis. (2016) 16(1):1–5. doi: 10.1186/s12879-016-1451-0
27. Pan A, Wang Y, Talaei M, Hu FB. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. (2015) 132(19):1795–804. doi: 10.1161/CIRCULATIONAHA.115.017926
28. van Zyl Smit RN, Pai M, Yew W-W, Leung C, Zumla A, Bateman E, et al. Global lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD. Eur Respir J. (2010) 35(1):27–33. doi: 10.1183/09031936.00072909
29. Silva DR, Muñoz-Torrico M, Duarte R, Galvão T, Bonini EH, Arbex FF, et al. Risk factors for tuberculosis: diabetes, smoking, alcohol use, and the use of other drugs. J Bras Pneumol. (2018) 44:145–52. doi: 10.1590/s1806-37562017000000443
30. Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. (2018) 137(20):2166–78. doi: 10.1161/CIRCULATIONAHA.117.029652
31. He J, Zhu Z, Bundy JD, Dorans KS, Chen J, Hamm LL. Trends in cardiovascular risk factors in US adults by race and ethnicity and socioeconomic status, 1999–2018. JAMA. (2021) 326(13):1286–98. doi: 10.1001/jama.2021.15187
32. Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. (2008) 5(7):e152. doi: 10.1371/journal.pmed.0050152
33. Restrepo BI, Schlesinger LS. Impact of diabetes on the natural history of tuberculosis. Diabetes Res Clin Pract. (2014) 106(2):191–9. doi: 10.1016/j.diabres.2014.06.011
34. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. (2019) 140:e596–646. doi: 10.1161/CIR.0000000000000678
35. Hare DL, Toukhsati SR, Johansson P, Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur Heart J. (2014) 35(21):1365–72. doi: 10.1093/eurheartj/eht462
36. de Jonge P, Mangano D, Whooley MA. Differential association of cognitive and somatic depressive symptoms with heart rate variability in patients with stable coronary heart disease: findings from the heart and soul study. Psychosom Med. (2007) 69(8):735–9. doi: 10.1097/PSY.0b013e31815743ca
37. Brouwers C, Mommersteeg PM, Nyklíček I, Pelle AJ, Westerhuis BL, Szabó BM, et al. Positive affect dimensions and their association with inflammatory biomarkers in patients with chronic heart failure. Biol Psychol. (2013) 92(2):220–6. doi: 10.1016/j.biopsycho.2012.10.002
38. Liu Q, Wang H, Liu A, Jiang C, Li W, Ma H, et al. Adherence to prescribed antihypertensive medication among patients with depression in the United States. BMC Psychiatry. (2022) 22(1):764. doi: 10.1186/s12888-022-04424-x
39. Tonelli M, Muntner P, Lloyd A, Manns BJ, Klarenbach S, Pannu N, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. (2012) 380(9844):807–14. doi: 10.1016/S0140-6736(12)60572-8
Keywords: tuberculosis, cardiovascular disease, 10-year atherosclerotic cardiovascular disorder risk, TB survivors, nationwide database
Citation: Yang J, Kim SH, Sim JK, Gu S, Seok JW, Bae DH, Cho JY, Lee KM, Choe KH, Lee H, Yang B and Min KH (2024) Tuberculosis survivors and the risk of cardiovascular disease: analysis using a nationwide survey in Korea. Front. Cardiovasc. Med. 11:1364337. doi: 10.3389/fcvm.2024.1364337
Received: 16 January 2024; Accepted: 29 July 2024;
Published: 9 August 2024.
Edited by:
Leonardo Roever, Brazilian Evidence-Based Health Network, BrazilReviewed by:
Bidita Khandelwal, Sikkim Manipal University, IndiaTsegahun Manyazewal, Addis Ababa University, Ethiopia
Weihong Chen, Huazhong University of Science and Technology, China
© 2024 Yang, Kim, Sim, Gu, Seok, Bae, Cho, Lee, Choe, Lee, Yang and Min. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bumhee Yang, eWJod29ybGQwNDE1QGdtYWlsLmNvbQ==; Kyung Hoon Min, bWlua3l1bmdob29uQGtvcmVhLmFjLmty
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Jiyoul Yang
Jiyoul Yang Sun-Hyung Kim
Sun-Hyung Kim Jae Kyeom Sim2,†
Jae Kyeom Sim2,† Dae-Hwan Bae
Dae-Hwan Bae Ki Man Lee
Ki Man Lee Hyun Lee
Hyun Lee Bumhee Yang
Bumhee Yang Kyung Hoon Min
Kyung Hoon Min