
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med., 09 May 2024
Sec. Coronary Artery Disease
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1362893
Background: Elevated lipoprotein (a) level was recognized as an independent risk factor for significant adverse cardiovascular events in acute coronary syndrome (ACS) patients. Despite this recognition, the consensus in the literature regarding the prognostic significance of elevated lipoprotein (a) in ACS was also limited. Consequently, we conducted a thorough systematic review and meta-analysis to evaluate the prognostic relevance of elevated lipoprotein (a) level in individuals diagnosed with ACS.
Methods and results: A thorough literature review was conducted by systematically searching PubMed, Embase, and Cochrane databases until September 2023. This review specifically examined cohort studies exploring the prognostic implications of elevated lipoprotein (a) level in relation to major adverse cardiovascular events (MACE), including death, stroke, non-fatal myocardial infarction (MI), and coronary revascularization, in patients with ACS. The meta-analysis utilized aggregated multivariable hazard ratios (HR) and their respective 95% confidence intervals (CI) to evaluate prognostic implications between high and low lipoprotein (a) levels [the cut-off of high lipoprotein (a) level varies from 12.5 to 60 mg/dl]. Among 18,168 patients in the identified studies, elevated lipoprotein (a) was independently associated with increased MACE risk (HR 1.26; 95% CI: 1.17–1.35, P < 0.00001) and all-cause mortality (HR 1.36; 95% CI: 1.05–1.76, P = 0.02) in ACS patients. In summary, elevated lipoprotein (a) levels independently forecast MACE and all-cause mortality in ACS patients. Assessing lipoprotein (a) levels appears promising for risk stratification in ACS, offering valuable insights for tailoring secondary prevention strategies.
Systematic Review Registration: PROSPERO (CRD42023476543).
Acute coronary syndromes (ACS) encompasses ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina (UA) (1–4). They are common in older adults (5), and yet the incident has increased in younger people (6, 7). Annually, over 7 million individuals worldwide receive ACS diagnoses (8, 9). Roughly 5% succumb before hospital discharge (2, 8–10). Subsequent ACS survivors frequently face major adverse cardiovascular events (MACE), which encompass recurrent ischemic incidents and mortality (11–13). Despite advancements in medical therapy, the one-year incidence of MACE post-ACS has risen to 9.2% (14). Identifying a predictive indicator for MACE after ACS is imperative for improved prognostic outcomes. Recently, lipoprotein(a) [Lp (a)] has emerged as an independent risk factor linked to MACE following ACS (15–19). Nevertheless, conflicting evidence surrounds the prognostic significance of elevated blood Lp (a) levels in ACS patients. For instance, the FORTIAM investigation (20), a multicenter cohort study in Spain involving 1,371 acute myocardial infarction (AMI) patients across 15 hospitals, revealed a poorer prognosis in those with elevated Lp (a) levels at admission. Consistent findings were reported by Andrea Kallmeyer et al. and Si-qi Yang et al. (21, 22). Conversely, a Vietnam-based observational cohort study (23) yielded disparate results, suggesting no correlation between Lp (a) levels ≥ 50 mg/dl during AMI and MACE or all-cause mortality. No comprehensive systematic review or meta-analysis has yet assessed the prognostic significance of elevated Lp (a) levels in ACS patients regarding MACE and all-cause mortality. This meta-analysis aims to investigate the prognostic relevance of baseline blood lipoprotein(a) levels in predicting MACE and all-cause mortality among ACS patients.
The present evidence-based analysis adheres to the guidelines stipulated in the 2020 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement (24). The study protocol was prospectively registered in the PROSPERO database under the registration number CRD42023476543. The comprehensive PRISMA 2020 checklist is provided in Supplementary Table S1. Our systematic literature review employed a comprehensive search strategy, utilizing PubMed, Embase, and Cochrane databases, with the search scope extending until September 2023. We specifically targeted English-language studies exploring the incidence of MACE and/or all-cause mortality in ACS patients. MACE were defined as all-cause mortality, stroke, non-fatal MI, and coronary revascularization. The comparison focused on individuals with high levels of Lp (a) vs. those with low Lp (a) levels. We searched the databases using the following terms: “Lipoprotein(a)”, “Lipoprotein Lp (a) “, “Lipoprotein a”, “Acute Coronary Syndrome”, “Coronary Syndrome, Acute”, “Syndrome, Acute Coronary”, “Syndromes, Acute Coronary”, “Myocardial Infarction”, “Infarction, Myocardial”, “Cardiovascular Stroke”, “Stroke, Cardiovascular”, “Myocardial Infarct”, “Infarcts, Myocardial”, “Heart Attack”, “Angina, Unstable”, “Unstable Anginas”, “Angina Pectoris, Unstable”, “Unstable Angina Pectori”, “Unstable Angina”, “Angina at Rest”, “Angina, Preinfarction”, “Preinfarction Angina”, “Myocardial Preinfarction Syndrome”, “Preinfarction Syndrome, Myocardial”, and “Syndromes, Myocardial Preinfarction” et al. We searched PubMed with the following detailed search strategy: ((((“Angina, Unstable"[Mesh]) OR (((((((((((((((((((Angina, Unstable) OR (Anginas, Unstable)) OR (Unstable Anginas)) OR (Angina Pectoris, Unstable)) OR (Angina Pectori, Unstable)) OR (Unstable Angina Pectori)) OR (Unstable Angina Pectoris)) OR (Unstable Angina)) OR (Angina at Rest)) OR (Angina, Preinfarction)) OR (Anginas, Preinfarction)) OR (Preinfarction Angina)) OR (Preinfarction Anginas)) OR (Myocardial Preinfarction Syndrome)) OR (Myocardial Preinfarction Syndromes)) OR (Preinfarction Syndrome, Myocardial)) OR (Preinfarction Syndromes, Myocardial)) OR (Syndrome, Myocardial Preinfarction)) OR (Syndromes, Myocardial Preinfarction))) OR ((“Myocardial Infarction"[Mesh]) OR ((((((((((((((Myocardial Infarction) OR (Infarction, Myocardial)) OR (Infarctions, Myocardial)) OR (Myocardial Infarctions)) OR (Cardiovascular Stroke)) OR (Cardiovascular Strokes)) OR (Stroke, Cardiovascular)) OR (Strokes, Cardiovascular)) OR (Myocardial Infarct)) OR (Infarct, Myocardial)) OR (Infarcts, Myocardial)) OR (Myocardial Infarcts)) OR (Heart Attack)) OR (Heart Attacks)))) OR ((“Acute Coronary Syndrome"[Mesh]) OR ((((((Acute Coronary Syndrome) OR (Acute Coronary Syndromes)) OR (Coronary Syndrome, Acute)) OR (Coronary Syndromes, Acute)) OR (Syndrome, Acute Coronary)) OR (Syndromes, Acute Coronary)))) AND (((((“Lipoprotein(a) “) OR (“ Lipoprotein Lp (a) “)) OR (“Lipoprotein (a) “)) OR (“Lipoprotein a “)) OR (“Lipoprotein (a-)”)). The exhaustive search methodology is outlined in Supplementary Table S2. Additionally, a thorough manual review of reference lists from all eligible studies was undertaken. Two investigators independently performed the search and assessment of included studies. Any discrepancies in the literature search were resolved through consensus after careful deliberation.
Eligible studies met the following criteria: (1) utilization of randomized controlled, cohort, or case–control designs; (2) examination of adults with diagnosed ACS; (3) primary focus on comparing MACE and/or all-cause mortality between ACS patients with high and low Lp (a) levels; (4) assessment of at least one MACE (e.g., death, stroke, non-fatal MI, or coronary revascularization) with concurrent evaluation of all-cause mortality; and (5) availability of sufficient data to compute the Hazard Ratio (HR). Exclusions comprised reviews, letters, editorial comments, case reports, conference abstracts, pediatric articles, unpublished works, and non-English publications.
According to ACS criteria, the syndrome comprises UA, NSTEMI, and STEMI. Therefore, studies that focused on stable angina, chronic coronary artery disease (CAD) were also excluded.
Two independent investigators (G.C.W and M.Y.X) performed data extraction. Discrepancies were resolved through intervention by a third investigator (S.T.L or D.X.J) to establish consensus. Extracted data from the studies included primary author, publication year, study duration, location, design, ACS types, sample size, follow-up duration, participant age, body mass index (BMI), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c) levels, history of prior medical therapy, diabetes prevalence, Lp (a) threshold, MACE, and mortality-related outcomes. For studies reporting continuous variables as median with range or interquartile range, a validated mathematical approach was employed to derive mean and standard deviation (21–28).
Study quality was assessed using the Newcastle–Ottawa Scale (NOS) (29), categorizing studies scoring seven to nine points as high quality (30). Two independent reviewers evaluated evidence quality in eligible studies, resolving discrepancies through consensus discussions.
Evidence synthesis was performed using Review Manager version 5.4.1 (Cochrane Collaboration, Oxford, UK). Survival variables were assessed using Hazard Ratios (HR), and metrics were presented with corresponding 95% confidence intervals (CIs). Heterogeneity assessment utilized the inconsistency index (I2) (31), where I2 values exceeding 50% indicate significant heterogeneity. In instances of significant heterogeneity (I2 > 50%), a random-effects model estimated combined HR; otherwise, a fixed-effect model was applied (31). Furthermore, we conducted one-way sensitivity analyses to assess the influence of included studies on combined outcomes showing substantial heterogeneity. Publication bias was evaluated visually using funnel plots generated with Review Manager 5.4.1 (Cochrane Collaboration, Oxford, UK) and statistically through Egger's regression tests (32) implemented with Stata version 15.0 (Stata Corp, College Station, TX, USA) for outcomes with 10 or more included studies. A P-value < 0.05 indicated statistically significant publication bias.
The schematic representation of the methodical search and selection procedure is depicted in Figure 1. A comprehensive literature search across PubMed (n = 1,165), Embase (n = 1,713), and Cochrane (n = 172) databases identified a total of 3,050 pertinent articles. Following the elimination of duplicate publications, a meticulous review of titles and abstracts resulted in the inclusion of 2024 articles for further consideration. Ultimately, 18 full-text articles, encompassing a total of 18,168 patients (13,843 male, 4,325 female), were selected for the comprehensive pooled analysis (20–23, 25–28, 33–42). Of these articles, 5 were prospective cohort studies (21, 23, 34, 35, 40), 10 were retrospective cohort studies (20, 22, 25, 26, 28, 33, 36–39), and 3 were observational cohorts (4, 27, 41). The encompassed investigations in this analysis spanned the period from 2009 to 2023. The sample sizes exhibited considerable variability, ranging from 88 to 2007 participants, resulting in a cumulative cohort of 18,168 patients. The duration of follow-up varied notably, extending from 2.93 to 66 months. It is noteworthy that the defined cutoff value for Lp (a) demonstrated heterogeneity across the studies incorporated in this review. Table 1 comprehensively outlines the distinctive characteristics, level of evidence, and quality assessments of each included study. The median (range) quality score, indicative of methodological rigor, was determined to be 7 (5–9). Notably, 14 studies were acknowledged for their high quality, indicating the strength of their methodologies and findings within the scope of this comprehensive analysis (21, 23, 26–28, 33–40, 42). The quality assessment details of all eligible are presented in Supplementary Table S3.
Fourteen studies (20–23, 25, 26, 28, 33, 36–41) investigated the prognostic significance of elevated Lp (a) levels in relation to MACE (Figure 2A). The meta-analysis utilizing a random-effects model revealed a pooled HR of 1.26 (95% CI: 1.17–1.35) for high compared to low category of Lp (a) levels, demonstrating a statistically significant association. However, notable heterogeneity was observed (I2 = 88%; P < 0.00001). A visual inspection of the funnel plot did not reveal any significant publication bias (Figure 2B). Nevertheless, Egger's test yielded a statistically significant result (P < 0.0001). Sensitivity analyses were performed by excluding two studies, both conducted by the same author (26, 28). The removal of these studies resulted in a discernible impact on the original pooled effect sizes of MACE, suggesting that the observed heterogeneity may be attributed to these specific studies.
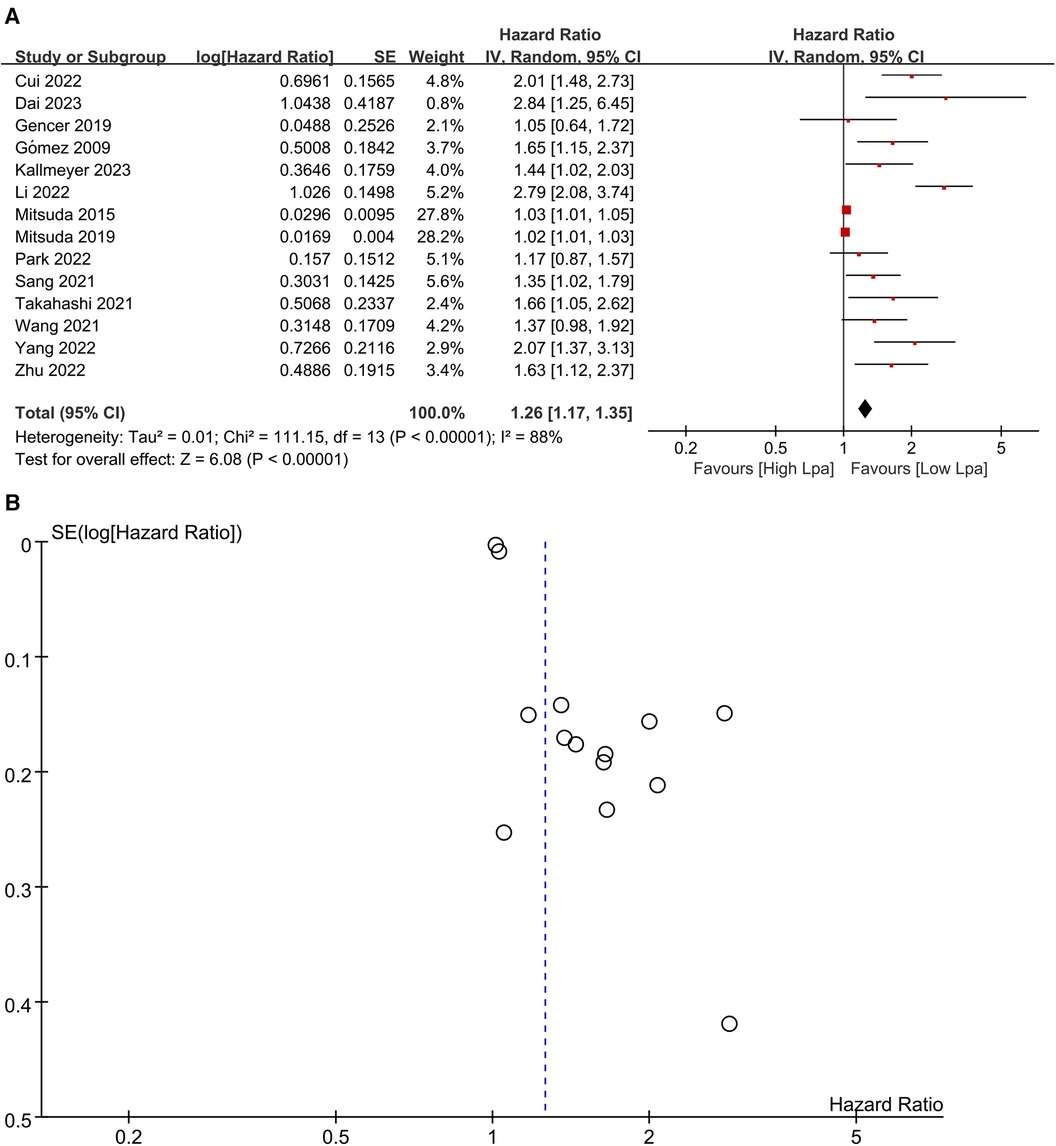
Figure 2. (A) Forest plots of major adverse cardiovascular events outcomes. (B) Funnel plots of major adverse cardiovascular events outcomes.
Seven studies (22, 23, 25, 27, 33, 34, 40) were included in the analysis to investigate the relationship between elevated Lp (a) levels and the risk of all-cause mortality (Figure 3A). Utilizing a random-effects model, the pooled Hazard Ratio (HR) for all-cause mortality was 1.36 (95% CI: 1.05–1.76) when comparing high to low category of Lp (a) levels. There was no significant heterogeneity observed (I2 = 49%; P = 0.02). Examination of the funnel plot revealed no significant publication bias (Figure 3B), and Egger's test did not show statistical significance (P = 0.518). Sensitivity analyses, involving the removal of each study one at a time, demonstrated minimal alterations to the original pooled effect sizes for all-cause mortality. This supported the stability of the results. In summary, the comprehensive analysis of these seven studies indicates a statistically significant association between elevated Lp (a) levels and an increased risk of all-cause mortality. The findings are robust, as evidenced by the lack of publication bias, low heterogeneity, and consistent results in sensitivity analyses.
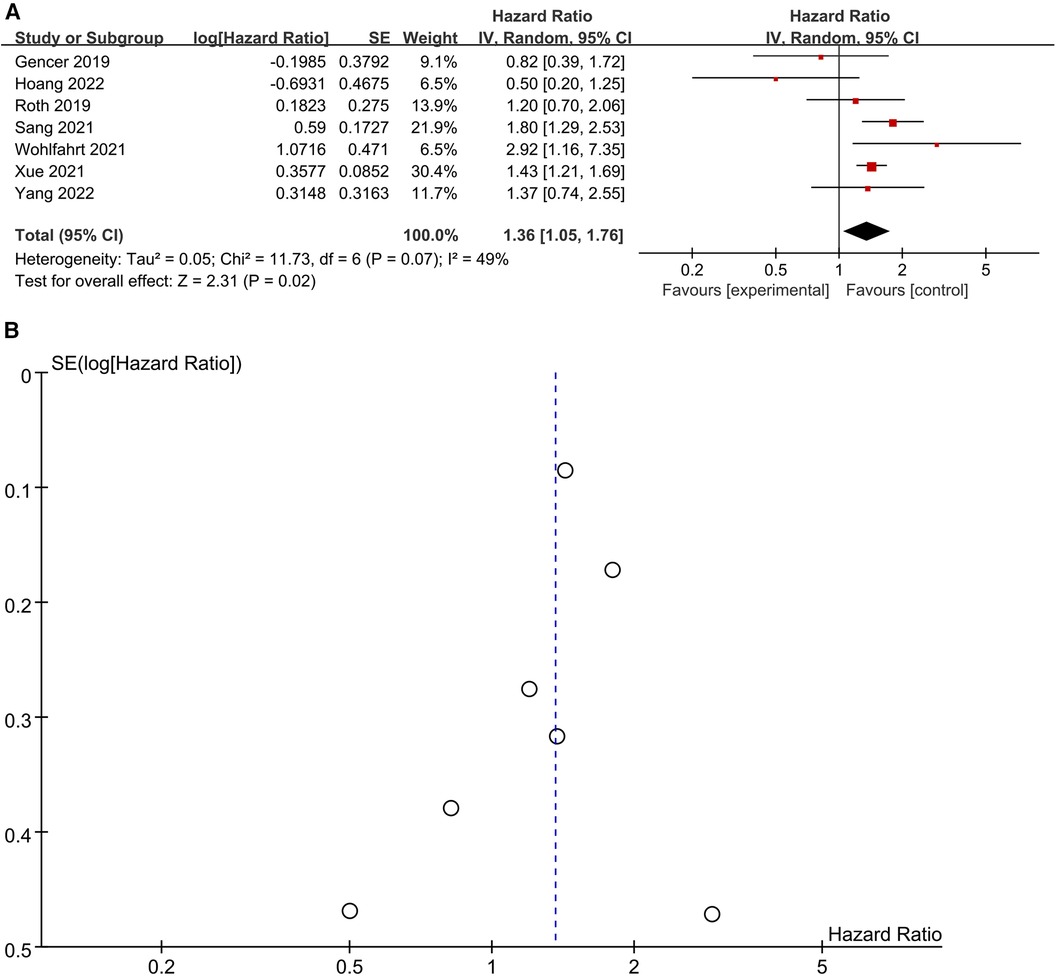
Figure 3. (A) Forest plots of all-cause mortality outcomes. (B) Funnel plots of all-cause mortality outcomes.
In addition, we conducted a subgroup analysis to assess the correlation between elevated Lp (a) levels and the occurrence of MACE, as well as all-cause mortality specifically within the subgroup of patients diagnosed with ACS. We divided the patients into two subgroups according to the study design (prospective cohort vs. retrospective cohort), follow-up duration (≥3 years vs. < 3 years), Lp (a) level (Lp (a) > 30 mg/dl vs. Lp (a) threshold ≤30 mg/dl), area (Asia vs. Europe), and divided into 3 subgroups according to the types of ACS (ACS vs. AMI vs. STEMI). The findings from the subgroup analysis reveal a strong correlation between elevated Lp (a) levels and an increased risk of MACE across all subgroups, with the exception of the STEMI subgroup (HR 1.02, 95% CI: 1.00–1.04, P = 0.02), but the difference of all-cause mortality was not statistically significant in subgroup with prospective cohort (HR 0.94 95%CI: 0.50–1.78, P = 0.86), follow-up duration < 3 years (HR 1.23 95%CI: 0.82–1.86, P = 0.32), Europe area (HR 1.34 95%CI: 0.72–2.49, P = 0.36), and Lp (a) threshold > 30 mg/dl (HR 0.84 95%CI: 0.36–1.95, P = 0.69).
ACS stands as a predominant contributor to global morbidity and mortality, acting as a primary catalyst for coronary heart disease-associated hospitalizations and fatalities, thereby imposing a substantial disease burden (43). Lp (a), comprising apolipoprotein (a), apolipoprotein B-100, and lipid components like cholesterol, phospholipids, and triglycerides (44), has been identified as a promoter of atherosclerosis, inflammation, and thrombosis, emerging as an independent risk factor for ACS (45, 46). The 2019 ESC/EAS guidelines for dyslipidemia management advocate for at least one Lp (a) level assessment for every adult during their lifetime (47). The determination of Lp (a) levels may aid in identifying patients necessitating more intensive therapeutic interventions in ACS. Future investigations are imperative to discern whether reducing Lp (a) levels can confer cardiovascular benefits to ACS patients. The precise mechanisms driving Lp (a)'s predictive value in ACS patients remain enigmatic. Plausibly, Lp (a) exhibits dual pathogenicity, manifesting proinflammatory and antifibrinolytic effects (48). An alternative rationale suggests elevated Lp (a) may compromise endothelial and anticoagulant functions by promoting endothelial dysfunction and increasing phospholipid oxidation (49, 50). Ongoing research delves into the potential role of Lp (a) in risk stratification and residual risk modification (51), prompting the present meta-analysis.The primary findings of this meta-analysis underscore that an elevated Lp (a) level serves as an independent predictor of MACE and all-cause mortality in ACS patients. Individuals with high Lp (a) levels exhibited approximately 26% and 36% higher risks of MACE (HR 1.26, 95% CI: 1.17–1.35, P < 0.00001) and all-cause mortality (HR 1.36, 95% CI: 1.05–1.76, P = 0.02), respectively, compared to those with low Lp (a) levels. Notably, the findings in subgroup with STEMI for MACE were not significantly different in this paper, which may be due to the shorter follow-up periods in studies with STEMI. In addition, most of the studies about STEMI were large-scale and retrospective. Furthermore, the inclusion criteria varied slightly according to different studies (e.g., a Japan-based study in 2019 (28) included patients with index STEMI, while others did not make the declaration). On the other hand, the different results in subgroup analysis for all-cause mortality suggested that the prognostic value of Lp (a) level for all-cause mortality is associated with the study design, follow-up duration, and patients’ races (from different areas and with various Lp (a) levels). It is presumed that the results in subgroup analysis did not show statistical significance, maybe due to the smaller samples with longer follow-up durations (n = 4,885 for <3 years vs. n = 1,475 for ≥3 years), which suggests that the prognostic value of high Lp (a) levels for all-cause mortality may not be present in the short term. The differences in Lp (a) thresholds among various areas may result from genetic racial differences (52). All of which may lead to the differences among studies. The 2019 ESC/EAS guidelines and a statement from the American Heart Association suggest that adults should assess Lp (a) concentration once in lifetime, preferably in initial lipid panel test (47, 53). It is necessary to explain Lp (a) test results in the context of other risks and absolute global cardiovascular risk for ACS patients, and emphasize the association with cardiovascular events risk. Multiple testing may be required depending on the patient's condition and risk factors. It is important to educate individuals with high Lp (a) levels [defined as ≥70 mg/dl(150 nmol/L), maybe lower for Chinese (52)]. to maintain a healthy lifestyle, intensive management of other risk factors (e.g body mass, blood pressure, blood glucose etc.), and add proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors when necessary, as PCSK9 inhibitors have been confirmed to lower Lp (a) levels (52). However, specific Lp (a)-lowering drugs are not available. Therefore, further studies are needed to investigate these interventions and evaluate the clinical benefits, to guide clinical practice for high Lp (a) levels in ACS patients. By decreasing high Lp (a) levels, it is possible to further reduce the risk of MACE and mortality, thus alleviating the disease burden worldwide. This meta-analysis represents a groundbreaking investigation, confirming Lp (a)'s prognostic significance in predicting MACE and all-cause mortality among ACS patients. However, some evidence demonstrates that cathepsin s, soluble LOX-1, and LDL-electronegativity, which have been implicated in the pathogenesis of atherosclerotic cardiovascular disease, have been related with prognosis in patients with ACS (54–56). All of these biomarkers, elevated during ACS, not measured and analysed in the included studies, could be associated with worse outcomes in ACS patients, may limit the interpretation of results. Additionally, several considerations merit attention. Firstly, the absence of individual-level data may introduce variability in pooled outcomes based on patient characteristics. Secondly, diverse cutoff values for elevated blood Lp (a) levels across studies hinder the establishment of a standardized threshold for Lp (a) elevation. Thirdly, the heterogeneity in pooled outcomes may stem from ACS subgroup, study design, follow-up duration, Lp (a) measurement methods, and cutoff values.The prognostic role of Lp (a) using continuous data couldn't be assessed due to insufficient information. Additionally, the exclusion of randomized controlled trials was necessary due to data limitations. Moreover, besides cathepsin s, soluble LOX-1, and LDL-electronegativity, the ACS, as acute inflammation, has potential to increase the level of Lp (a) and thus affect its measurement. Considering the potential confounders and above limitations, results of the present pooled analysis should be interpreted with caution. In spite of several limitations of our study, we confirmed that an elevated Lp (a) level is an independent predictor of MACE and all-cause mortality in ACS patients. The funnel plots and Egger's test examination revealed no significant publication bias, which makes our results more robust. Our evidence-based analysis validated previous studies reporting the different MACE outcomes in high level Lp (a) vs. low level patients with ACS (21, 25, 28, 36–38). More well-designed, large-scale prospective randomized studies with long-term follow-up are needed to further compare the MACE, all-cause mortality in high level vs. low level of Lp (a) patients with ACS.
This meta-analysis underscores that elevated Lp (a) levels independently predict MACE and all-cause mortality in ACS patients. Because of minimal influence from lifestyle, diet, and medical interventions, Lp (a) measurement has the potential to enhance risk stratification in ACS. Future well-designed studies are crucial to investigate variations in Lp (a) prognostic significance among ACS subgroups. Furthermore, these investigations should conclusively establish Lp (a)'s role in refining risk stratification and modifying residual risk in ACS-diagnosed individuals.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
GW: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Validation, Writing – original draft, Writing – review & editing. SL: Conceptualization, Formal Analysis, Methodology, Project administration, Software, Supervision, Validation, Writing – review & editing. MX: Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. CL: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. FP: Investigation, Software, Writing – original draft. DJ: Investigation, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1362893/full#supplementary-material
1. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. (2021)144(22):e368–454. doi: 10.1161/CIR.0000000000001030
2. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42(14):1289–367. doi: 10.1093/eurheartj/ehaa575
3. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation:145(3):e18–e114. doi: 10.1161/CIR.0000000000001038
4. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction. Circulation. (2018) 138(20):e618–51. doi: 10.1161/CIR.0000000000000617
5. Dai X, Busby-Whitehead J, Alexander KP. Acute coronary syndrome in the older adults. J Geriatr Cardiol. (2016) 13(2):101–8. doi: 10.11909/j.issn.1671-5411.2016.02.012
6. Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. (2019) 139(8):1047–56. doi: 10.1161/CIRCULATIONAHA.118.037137
7. Bhatt DL. Percutaneous coronary intervention in 2018. JAMA. (2018) 319(20):2127–8. doi: 10.1001/jama.2018.5281
8. Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. (2017) 389(10065):197–210. doi: 10.1016/S0140-6736(16)30677-8
9. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. (2020) 141(9):e139–596. doi: 10.1161/CIR.0000000000000757
10. Steg PG, Goldberg RJ, Gore JM, Fox KA, Eagle KA, Flather MD, et al. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the global registry of acute coronary events (GRACE). Am J Cardiol. (2002) 90(4):358–63. doi: 10.1016/s0002-9149(02)02489-x
11. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. (2006) 295(2):180–9. doi: 10.1001/jama.295.2.180
12. Steg PG, Bhatt DL, Wilson PW, D'Agostino R Sr, Ohman EM, Röther J, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. (2007) 297(11):1197–206. doi: 10.1001/jama.297.11.1197
13. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. (2010) 304(12):1350–7. doi: 10.1001/jama.2010.1322
14. Hess CN, Clare RM, Neely ML, Tricoci P, Mahaffey KW, James SK, et al. Differential occurrence, profile, and impact of first recurrent cardiovascular events after an acute coronary syndrome. Am Heart J. (2017) 187:194–203. doi: 10.1016/j.ahj.2017.01.016
15. Welsh P, Welsh C, Celis-Morales CA, Brown R, Ho FK, Ferguson LD, et al. Lipoprotein(a) and cardiovascular disease: prediction, attributable risk fraction, and estimating benefits from novel interventions. Eur J Prev Cardiol. (2022) 28(18):1991–2000. doi: 10.1093/eurjpc/zwaa063
16. Guo C, Cao H, Shan G, Zhao W, Zhang H, Niu K, et al. Elevated lipoprotein(a) and risk of coronary heart disease according to different lipid profiles in the general Chinese community population: the CHCN-BTH study. Ann Transl Med. (2021) 9:26. doi: 10.21037/ATM-20-3899
17. Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen city heart study. Circulation. (2008) 117:176–84. doi: 10.1161/CIRCULATIONAHA.107.715698
18. Jin JL, Cao YX, Zhang HW, Sun D, Hua Q, Li YF, et al. Lipoprotein(a) and cardiovascular outcomes in patients with coronary artery disease and prediabetes or diabetes. Diabetes Care. (2019) 42:1312–8. doi: 10.2337/dc19-0274
19. Nestel PJ, Barnes EH, Tonkin AM, Simes J, Fournier M, White HD, et al. Plasma lipoprotein(a) concentration predicts future coronary and cardiovascular events in patients with stable coronary heart disease. Arterioscler Thromb Vasc Biol. (2013) 33:2902–8. doi: 10.1161/ATVBAHA.113.302479
20. Gómez M, Valle V, Arós F, Sanz G, Sala J, Fiol M, et al. Oxidized LDL, lipoprotein (a) and other emergent risk factors in acute myocardial infarction (FORTIAM study). Rev Esp Cardiol. (2009) 62(4):373–82. doi: 10.1016/s1885-5857(09)71664-0
21. Kallmeyer A, Pello Lázaro AM, Blanco-Colio LM, Aceña Á, González-Lorenzo Ó, Tarín N, et al. Absence of high lipoprotein(a) levels is an independent predictor of acute myocardial infarction without coronary lesions. J Clin Med. (2023) 12:960. doi: 10.3390/jcm12030960
22. Yang SQ, Liu HX, Yu XQ, Tong L, Chen X, Qi LY, et al. Elevated lipoprotein(a) levels as an independent predictor of long-term recurrent events in patients with acute coronary syndrome: an observational, retrospective cohort study. Coron Artery Dis. (2022) 33(5):385–93. doi: 10.1097/MCA.0000000000001134
23. Hoang SV, Pham QDD, Nguyen KM, Huynh KLA, Ngo TT, Le HNM, et al. Association between lipoprotein(a) concentration and adverse cardiovascular events in Vietnamese pa-tients with acute myocardial infarction: an observational cohort study. Biomed Res Ther. (2022) 9(1):4873–83. doi: 10.15419/bmrat.v9i1.724
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). (2021) 372:n71. doi: 10.1136/bmj.n71
25. Sang T, Cheng N, Dang A, Lv N, Zhang W, Li Y, et al. Lipoprotein (a) is associated with poor long-term prognosis in patients aged 80 years and older with acute coronary syndrome. J Clin Lipidol. (2021) 15(3):466–76. doi: 10.1016/j.jacl.2021.04.003
26. Mitsuda T, Uemura Y, Ishii H, Takemoto K, Uchikawa T, Koyasu M, et al. Lipoprotein(a) levels predict adverse vascular events after acute myocardial infarction. Heart Vessels. (2016) 31(12):1923–9. doi: 10.1007/s00380-016-0823-0
27. Roth C, Krychtiuk KA, Gangl C, Schrutka L, Distelmaier K, Wojta J, et al. Lipoprotein (a) plasma levels are not associated with survival after acute coronary syndromes: an observational cohort study. PLoS One. (2020) 15(1):e0227054. doi: 10.1371/journal.pone.0227054
28. Mitsuda T, Uemura Y, Ishii H, Tanaka A, Takemoto K, Koyasu M, et al. Prognostic impact of lipoprotein(a) levels during lipid management with statins after ST-elevation acute myocardial infarction. Coron Artery Dis. (2019) 30(8):600–7. doi: 10.1097/MCA.0000000000000798
29. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2011). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
30. Kim SR, Kim K, Lee SA, Kwon SO, Lee JK, Keum N, et al. Effect of red, processed, and white meat consumption on the risk of gastric cancer: an overall and dose–response meta-analysis. Nutrients. (2019) 11(4):826. doi: 10.3390/nu11040826
31. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. doi: 10.1002/sim.1186
32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed). (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
33. Wang Y, Zhao X, Zhou P, Liu C, Chen R, Sheng Z, et al. Impact of postprocedural high-sensitivity C-reactive protein on lipoprotein(a)-associated cardiovascular risk with ST-segment elevation myocardial infarction with percutaneous coronary intervention. Am J Cardiol. (2021) 150:8–14. doi: 10.1016/j.amjcard.2021.03.038
34. Xue Y, Jian S, Zhou W, Zhou Q, Xiang J, Zhu Y, et al. Associations of lipoprotein(a) with coronary atherosclerotic burden and all-cause mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Front Cardiovasc Med. (2021) 8:638679. doi: 10.3389/fcvm.2021.638679
35. Park JS, Cho KH, Hong YJ, Kim MC, Sim DS, Kim JH, et al. Baseline lipoprotein(a) levels and long-term cardiovascular outcomes after acute myocardial infarction. J Korean Med Sci. (2023) 38(13):e102. doi: 10.3346/jkms.2023.38.e102
36. Zhu L, Zheng J, Gao B, Jin X, He Y, Zhou L, et al. The correlation between lipoprotein(a) elevations and the risk of recurrent cardiovascular events in CAD patients with different LDL-C levels. BMC Cardiovasc Disord. (2022) 22(1):171. doi: 10.1186/s12872-022-02618-5
37. Dai K, Shiode N, Yoshii K, Kimura Y, Matsuo K, Jyuri Y, et al. Impact of lipoprotein (a) on long-term outcomes in patients with acute myocardial infarction. Circ J. (2023) 87(10):1356–61. doi: 10.1253/circj.CJ-23-0221
38. Takahashi D, Wada H, Ogita M, Yasuda K, Nishio R, Takeuchi M, et al. Impact of lipoprotein(a) as a residual risk factor in long-term cardiovascular outcomes in patients with acute coronary syndrome treated with statins. Am J Cardiol. (2022) 168:11–6. doi: 10.1016/j.amjcard.2021.12.014
39. Cui C-Y, Ye T, Cheng L-C, Tong L, Tong L, Zhang Z, et al. Lipoprotein a combined with fibrinogen as an independent predictor of long-term prognosis in patients with acute coronary syndrome: a multi-center retrospective study. J Cardiovasc Dev Dis. (2022) 9:322. doi: 10.3390/jcdd9100322
40. Gencer B, Rigamonti F, Nanchen D, Vuilleumier N, Kern I, Aghlmandi S, et al. Prognostic value of elevated lipoprotein(a) in patients with acute coronary syndromes. Eur J Clin Investig. (2019) 49(7):e13117. doi: 10.1111/eci.13117
41. Li Q, Chen Y, Yu L, Zhu L, Wang Z, Jiao S, et al. The relationship between lipoprotein(a) and cardiovascular events in acute coronary syndrome patients with and without chronic kidney disease. Atherosclerosis. (2022) 349:204–10. doi: 10.1016/j.atherosclerosis.2022.04.007
42. Wohlfahrt P, Jenča D, Melenovský V, Franeková J, Jabor A, Šramko M, et al. Very low lipoprotein(a) and increased mortality risk after myocardial infarction. Eur J Intern Med. (2021) 91:33–9. doi: 10.1016/j.ejim.2021.04.012
43. Xu J, Murphy SL, Kockanek KD, Arias E. Mortality in the United States, 2018. NCHS Data Brief. (2020) 355:1–8. PMID: 32487294.
44. Cegla J, France M, Marcovina SM, Neely R, Dermot G. Lp (a) level: when and how to measure it. Ann Clin Biochem. (2021) 58:16–21. doi: 10.1177/0004563220968473
45. Tada H, Takamura M, Kawashiri M. Lipoprotein(a) as an old and new causal risk factor of atherosclerotic cardiovascular disease. J Atheroscler Thromb. (2019) 26:583–91. doi: 10.5551/jat.RV17034
46. Emerging Risk Factors Collaboration, Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. (2009) 302(4):412–23. doi: 10.1001/jama.2009.1063
47. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. (2020) 41:111–88. doi: 10.1093/eurheartj/ehz455
48. Miles LA, Fless GM, Levin EG, Scanu AM, Plow EF. A potential basis for the thrombotic risks associated with lipoprotein(a). Nature. (1989) 339(6222):301–3. doi: 10.1038/339301a0
49. Schachinger V, Halle M, Minners J, Berg A, Zeiher AM. Lipoprotein(a) selectively impairs receptor-mediated endothelial vasodilator function of the human coronary circulation. J Am Coll Cardiol. (1997) 30(4):927–34. doi: 10.1016/S0735-1097(97)00237-4
50. Leibundgut G, Arai K, Orsoni A, Yin H, Scipione C, Miller ER, et al. Oxidized phospholipids are present on plasminogen, affect fibrinolysis, and increase following acute myocardial infarction. J Am Coll Cardiol. (2012) 59(16):1426–37. doi: 10.1016/j.jacc.2011.12.033
51. Lau F D, Giugliano RP. Lipoprotein(a) and its significance in cardiovascular disease: a review. JAMA Cardiol. (2022) 7(7):760–9. doi: 10.1001/jamacardio.2022.0987
52. Patel AP, Wang M, Pirruccello JP, Ellinor PT, Ng K, Kathiresan S, et al. Lp (a) (lipoprotein[a]) concentrations and incident atherosclerotic cardiovascular disease: new insights from a large national biobank. Arterioscler Thromb Vasc Biol. (2021) 41(1):465–74. doi: 10.1161/ATVBAHA.120.315291
53. Reyes-Soffer G, Ginsberg HN, Berglund L, Duell PB, Heffron SP, Kamstrup PR, et al. Lipoprotein(a): a genetically determined, causal, and prevalent risk factor for atherosclerotic cardiovascular disease: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. (2022) 42(1):e48–60. doi: 10.1161/ATV.0000000000000147
54. Stamatelopoulos K, Mueller-Hennessen M, Georgiopoulos G, Lopez-Ayala P, Sachse M, Vlachogiannis NI, et al. Cathepsin S levels and survival among patients with non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. (2022) 80(10):998–1010. doi: 10.1016/j.jacc.2022.05.055
55. Kraler S, Wenzl FA, Georgiopoulos G, Obeid S, Liberale L, von Eckardstein A, et al. Soluble lectin-like oxidized low-density lipoprotein receptor-1 predicts premature death in acute coronary syndromes. Eur Heart J. (2022 May 14) 43(19):1849–60. doi: 10.1093/eurheartj/ehac143
Keywords: acute coronary syndromes, lipoprotein (a), prognosis, outcome, mortality
Citation: Wang G, Xia M, Liang C, Pu F, Liu S and Jia D (2024) Prognostic value of elevated lipoprotein (a) in patients with acute coronary syndromes: a systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1362893. doi: 10.3389/fcvm.2024.1362893
Received: 29 December 2023; Accepted: 24 April 2024;
Published: 9 May 2024.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Yifan Wang, University of Zurich, Switzerland© 2024 Wang, Xia, Liang, Pu, Liu and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sitai Liu ODQ2NzQ2MjQ4QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.