
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 27 March 2024
Sec. Pediatric Cardiology
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1357747
Here we report a rare morphology of a cardiac fibroma in a child. A 2-year and 8-month-old toddler came for “chronic constipation” and was found to have a heart murmur on cardiac auscultation. Further transthoracic echocardiography suggested “a strong echogenic mass in the left ventricular wall, with some part of “a string of beads” in shape extending into left ventricle outflow tract”, which was atypical for either a tumor, thrombus or vegetation. The child underwent resection of the mass and mitral valvuloplasty. Pathological examination confirmed the mass as a cardiac fibroma.
Primary cardiac fibromas in children are exceedingly rare and predominantly occur in infants and young children under the age of 2 (1, 2). These fibromas are typically solitary and mainly located in the left ventricle, with the right ventricle and ventricular septum being less common sites of occurrence (3). Cases may present with evident clinical symptoms and signs, though some remain asymptomatic (4). Physical examination often sees the presence of a heart murmur in symptomatic children. Echocardiography can detect homogeneous exogenic masses within the cardiac chambers (Figure 1E), while computed tomography or magnetic resonance scans can provide a more precise assessment of the tumor's location, size, number, and hemodynamic alterations (5, 6). Here we report a case of cardiac fibroma with an atypical morphology. Surgical excision and pathology confirmed it as a cardiac fibroma.
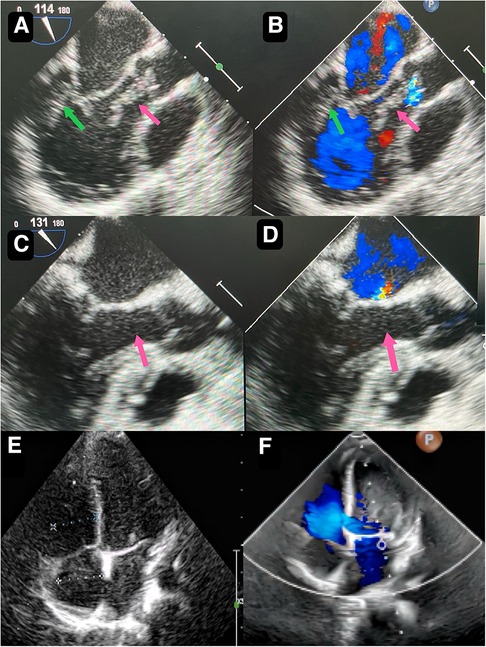
Figure 1. Preoperative transthoracic echocardiography reveals a slightly hyperechoic, approximately 32 mm by 16 mm mass in the posterior wall of the left ventricle (A) echogenic “string of beads” is observed wiggling in the left ventricular outflow tract, with one end connected to the posterior part of the left ventricle and the other end appearing to be connected to the left coronary sinus of the aorta (B) postoperative transesophageal echocardiogram shows complete removal of the mass (C) postoperative transesophageal echocardiogram shows competent mitral valve (D) preoperative transthoracic echocardiogram shows competent mitral valve (E) postoperative transthoracic echocardiogram mitral valve shows trivial regurgitation (F).
A 2-year-and-8-month-old toddler was admitted to the hospital for evaluation of chronic constipation attributed to long-standing low-fiber diet and poor therapeutic effect of prolonged lactulose use on softening stools. A heart murmur was noted on physical examination. The child had no relevant medical history or signs of infection, trauma, cold, or any other predisposing factors. Preoperative transthoracic echocardiography revealed a hyperechoic, approximately 32 mm by 16 mm mass in the posterior wall of the left ventricle (Figure 1A). In addition, an echogenic “string of beads” was observed wiggling in the left ventricular outflow tract, with one end connected to the posterior part of the left ventricle and the other end appearing to be connected to the left coronary sinus of the aorta (Figure 1B). The sizes of the four chambers were considered normal, and the ejection fraction was 63%. Interestingly, this child showed no clinical symptoms and results of coagulation assays, neutrophil, C-reactive protein, complete antinuclear antibody, antineutrophil cytoplasmic antibodies, and antistreptolysin O titer tests were all insignificant. A diagnosis of tumor, thrombus or vegetation was yet to be made.
Though the child was generally doing well and hemodynamics stable, there remained a concerning risk that the string part may break off and result in embolism. After careful consideration and discussion in a multidisciplinary team, surgical excision was planned. During the procedure, an incision was made in posterior leaflet of the mitral valve to expose the mass. It was shown that part of the mass was like a string of beads (Figure 2A), while the other part was embedded in the posterior left ventricular wall, close to the posterolateral papillary muscle (Figure 2B). The mass was predominantly white, with an intact capsule and a tough texture. After successful removal of the mass, water injection test showed significant regurgitation of the mitral valve from anterior leaflet prolapse. Mitral valve repair was performed. The ascending aortotomy was performed to exclude any residual mass in the aorta, though there was no residual mass found. Postoperative transesophageal echocardiogram showed complete removal of the mass (Figure 1C) and competent mitral valve (Figure 1D). Subsequent histopathological analysis confirmed the mass as a cardiac fibroma with myxoid degeneration (Figure 3). The results of the immunohistochemical analysis of the heart tumor specimen was as follows: Ki-67(10%+), DES (+), SMA (+), CR (focal +), CD34 (vascular +), Vim (+), EMA (−), CK (−), CD163 (+), ALK (−). The recovery was uneventful and the patient was discharged on postoperative day 10. Upon Follow-up, investigations including chest radiogram and electrocardiogram revealed no significant abnormalities. Transthoracic echocardiography demonstrated mild hyperechogenicity of the left ventricular papillary muscles, potentially related to postoperative changes. There was trivial regurgitation of the mitral valve (Figure 1F). Left ventricular systolic function was preserved. The patient's family reported no issues with daily activities or exercise tolerance.
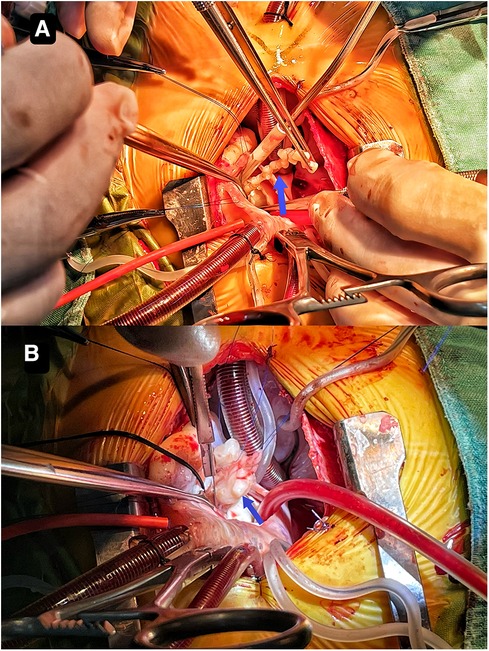
Figure 2. It shows that part of the mass resembles a string of beads (A) the other part is embedded in the posterior left ventricular wall close to the posterolateral papillary muscle (B).
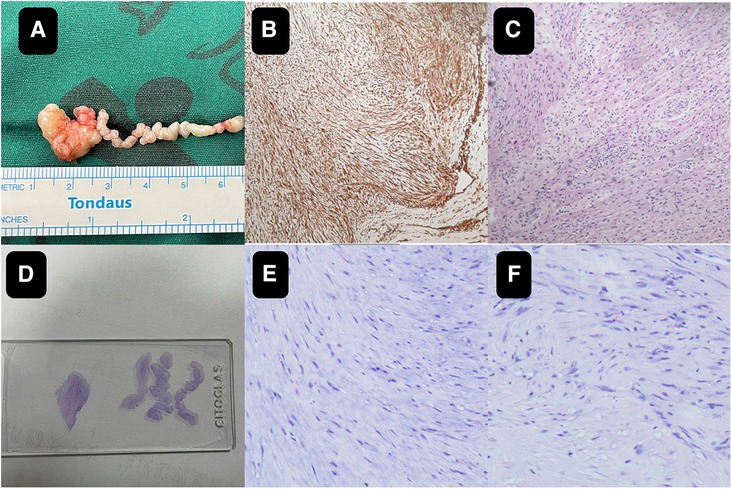
Figure 3. The close-up of the specimen (A) subsequent histopathological analysis confirmed the mass as a cardiac fibroma with myxoid degeneration (B,C). On the pathological slide it shows on the left the mass part and on the right the string part (D) the histology images of the wiggling string part (E) the histology images of the mass part (F).
Cardiac fibromas are extremely rare heart tumors, particularly in children, and typically occur between a few months to a few years of age. The clinical presentation of cardiac fibromas in children can vary greatly. Some patients may not exhibit noticeable symptoms, while others may experience symptoms such as heart murmurs, shortness of breath, and arrhythmia (7). In this particular case, the patient did not have any noticeable symptoms in her daily life, but a heart murmur was detected during physical examination when they sought medical attention for chronic constipation. The diagnosis of cardiac fibromas generally entails various imaging and laboratory tests, including echocardiography, computed tomography, and sometimes cardiac catheterization (8). Echocardiography is the most frequently employed diagnostic tool as it provides for the visualization of the location, size, and characteristics of a cardiac tumor (9, 10).
In the present case, however, as the morphological characteristics were not typical for a fibroma, and could not exclude thrombus or vegetation in this case, it was difficult to make a confirming diagnosis before surgical excision and pathology. Rhabdomyoma, which originates from cardiac fibroblasts, is a hamartoma formed during the development of heart muscle cells and accounts for more than 60% of primary heart tumors in children. It is more likely to occur in the left and right ventricular wall or septum (11). Fibroma, which is derived from connective tissue fibroblasts, is the second most common benign primary cardiac tumor in children and is more common in infants under 1 year of age. The most common location is the ventricular septum and free wall of the ventricle, rarely the atrium (12, 13). Cardiac myxoma, another common type of cardiac tumors, consists of large numbers of stellate or polygonal myxoma cells with myxoid stroma. It is the most common cardiac tumor in adults, but rare in children. It is often found in the left atrium and rarely in the heart valves and ventricles (14). Malignant cardiac tumors in children are rare, accounting for about 10% of all cardiac tumors in children. Most of these are metastatic malignancies and the incidence is 10–20 times that of primary malignancies. For thrombus, echocardiography often shows sessile masses, enlarged atria, low cardiac output. Clinical signs include congestion and response to thrombolytic therapy. For vegetations, Echocardiography often reveals vegetations with irregular mobility that are adherent to valves, findings that are highly associated with infective endocarditis (15–17).
Whatever it is, the management is typically determined by the severity of symptoms, the size and location of the mass, and the overall health condition of the patient. Surgical resection is required for cases involving severe symptoms or masses that impede heart function (18–22). The aim of surgical removal is to completely excise the mass while preserving normal heart tissue. Care must be taken in order to preserve the adjacent cardiac structures and function. The tumor may affect valvular apparatus, making valve repair necessary. However, this can be achieved with satisfactory outcome as shown in this case.
In summary, cardiac fibromas are uncommon tumors of the heart, particularly in children. The diagnosis and treatment of cardiac fibromas in children require a comprehensive evaluation of symptoms, imaging, and laboratory test results. Sometimes, it may be difficult to differentiate it with other tumors, vegetation or thrombus. This case emphasizes the varying morphology of the cardiac fibroma and significance of diagnosing and surgically treating this condition in children and contributes to the overall body of knowledge and promoting additional research in this area.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by institutional review board of Hunan Children's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
YT: Writing – original draft, Writing – review & editing. JL: Investigation, Writing – original draft, Writing – review & editing. XY: Writing – review & editing. DZ: Investigation, Writing – review & editing. YH: Data curation, Investigation, Methodology, Visualization, Writing – review & editing. JC: Investigation, Methodology, Validation, Writing – review & editing. ZW: Supervision, Validation, Writing – review & editing. XD: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The author(s) declare that financial support was received from Health Commission of Hunan Province (C202304028427) and Hunan Provincial Science and Technology Department (2021SK50521) for the research, authorship, and/or publication of this article.
We would like to thank our colleague Weijian Chen, MD as a pathologist for his assistance in pathology related content of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Asakai H. Ventricular fibromas in children: surgical resection can be a cure to lethal arrhythmias. Heart Rhythm. (2018) 15(10):1513–4. doi: 10.1016/j.hrthm.2018.06.042
2. Padalino MA, Vida VL, Boccuzzo G, Tonello M, Sarris GE, Berggren H, et al. Surgery for primary cardiac tumors in children. Circulation. (2012) 126(1):22–30. doi: 10.1161/CIRCULATIONAHA.111.037226
3. Freedom RM, Lee KJ, MacDonald C, Taylor G. Selected aspects of cardiac tumors in infancy and childhood. Pediatr Cardiol. (2014) 21(4):299–316. doi: 10.1007/s002460010070
4. Padalino MA, Reffo E, Cerutti A, Favero V, Biffanti R, Vida V, et al. Medical and surgical management of primary cardiac tumours in infants and children. Cardiol Young. (2013) 24(2):268–74. doi: 10.1017/S104795111300022X
5. Sheng C, Yang C, Cheng Y, Li Y-M. Current status of diagnosis and treatment of primary benign cardiac tumors in children. Front Cardiovasc Med. (2022) 9:947716. doi: 10.3389/fcvm.2022.947716
6. Morka A, Kohut J, Radzymińska-Chruściel B, Mroczek T, Gładki M, Weryński P, et al. Echocardiography and newer imaging techniques in diagnosis and long-term follow-up of primary heart tumors in children. Int J Environ Res Public Health. (2020) 17(15):1547. doi: 10.3390/ijerph17155471
7. Marshall AS, Dabal RJ, Law MA. A large ventricular fibroma requiring surgical resection in a symptomatic 3-month-old infant. Cardiol Young. (2019) 30(1):129–30. doi: 10.1017/S1047951119002877
8. Ren M, Huang L, Ye X, Xv Z, Ouyang C, Han Z, et al. Evaluation of cardiac space-occupying lesions by myocardial contrast echocardiography and transesophageal echocardiography. J Healthc Eng. (2022) 2022:1–10. doi: 10.1155/2022/2066033
9. Porter TR, Xie F. Contrast echocardiography: latest developments and clinical utility. Curr Cardiol Rep. (2015) 17(3):569. doi: 10.1007/s11886-015-0569-9
10. Wang D, Zhang J, Liu Y, Sun R, Tian J, Zhao W, et al. Diagnostic value of transthoracic echocardiography combined with contrast-enhanced ultrasonography in mediastinal masses. J Ultrasound Med. (2018) 38(2):415–22. doi: 10.1002/jum.14704
11. Amonkar GP, Kandalkar BM, Balasubramanian M. Cardiac rhabdomyoma. Cardiovasc Pathol. (2009) 18(5):313–4. doi: 10.1016/j.carpath.2008.02.002
12. Fan Q, Ling Y, An Q. Huge right ventricle cardiac fibroma in a child patient. J Card Surg. (2021) 36(8):2939–40. doi: 10.1111/jocs.15579
13. Vougiouklakis T, Goussia A, Ioachim E, Peschos D, Agnantis N. Cardiac fibroma. A case presentation. Virchows Arch. (2001) 438(6):635–6. doi: 10.1007/s004280100442
14. Markel ML, Waller BF, Armstrong WF. Cardiac myxoma. A review. Medicine (Baltimore). (1987) 66(2):114–25. doi: 10.1097/00005792-198703000-00003
15. Bussani R, Castrichini M, Restivo L, Fabris E, Porcari A, Ferro F, et al. Cardiac tumors: diagnosis, prognosis, and treatment. Curr Cardiol Rep. (2020) 22(12):169. doi: 10.1007/s11886-020-01420-z
16. L'Angiocola PD, Donati R. Cardiac masses in echocardiography: a pragmatic review. J Cardiovasc Echogr. (2020) 30(1):5–14. doi: 10.4103/jcecho.jcecho_2_20
17. Li W, Jin M, Ding W, Cheng P, Liang Y, Li Q, et al. Clinical analysis of 21 cases of primary cardiac tumors in infants and children. Chin J Appl Clin Pediatr. (2021) 36(3):195–8. doi: 10.3760/cma.j.cn101070-20190523-00446
18. Becker AE. Primary heart tumors in the pediatric age group: a review of salient pathologic features relevant for clinicians. Pediatr Cardiol. (2014) 21(4):317–23. doi: 10.1007/s002460010071
19. Borodinova O, Ostras O, Raad T, Yemets I. Successful surgical excision of a large cardiac fibroma in an asymptomatic child. World J Pediatr Congenit Heart Surg. (2016) 8(2):235–8. doi: 10.1177/2150135116634287
20. Bu H, Gong Y, Xie L. Partial resection of a huge left ventricle cardiac fibroma in an asymptomatic child. Ann Thorac Surg. (2019) 108(6):e393–5. doi: 10.1016/j.athoracsur.2019.04.035
21. Liu X, Hong H, Zhang H, Xu Z, Liu J, Qiu L. Treatment strategies for primary tumors of the heart in children: a 10-year experience. Ann Thorac Surg. (2015) 100(5):1744–9. doi: 10.1016/j.athoracsur.2015.06.030
Keywords: cardiac fibroma, pediatric, echocardiography, surgery, cardiac mass
Citation: Tian Y, Lin J, Yang X, Zeng D, Hu Y, Chen J, Wu Z and Deng X (2024) A rare morphology of the cardiac fibroma in a child: a case report. Front. Cardiovasc. Med. 11:1357747. doi: 10.3389/fcvm.2024.1357747
Received: 18 December 2023; Accepted: 8 March 2024;
Published: 27 March 2024.
Edited by:
Jaspal Dua, Liverpool Heart and Chest Hospital NHS Trust, United KingdomReviewed by:
Pradeep Vaideeswar, King Edward Memorial Hospital and Seth Gordhandas Sunderdas Medical College, India© 2024 Tian, Lin, Yang, Zeng, Hu, Chen, Wu and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xicheng Deng anVzdGluZHhjQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.