- 1Department of Cardiology, Asklepeion General Hospital, Athens, Greece
- 2Department of Cardiology, Mitera General Hospital, Hygeia HealthCare Group, Athens, Greece
- 3Department of Cardiology, Catharina Ziekenhuis Eindhoven, Eindhoven, Netherlands
A Commentary on
Guidezilla™ guide extension catheter I for transradial coronary intervention
By Lei XJ, Liang Q, Fang Y, Xiao YH, Wang DQ, Dong MZ, Li JC, Yu T (2022). Front. Cardiovasc. Med. 9:931373. doi: 10.3389/fcvm.2022.931373
We read with great interest the elegantly written article by Lei et al. regarding the use of the Guidezilla I (Boston Scientific) guide catheter extension (GCE) during transradial percutaneous coronary intervention (PCI) (1). The authors report excellent efficacy and safety in the utilization of GCE during PCI, irrespective of the SYNTAX score (1). The authors mention shaft breakage, stent stripping or dislodgement, coronary artery dissection/perforation, and entrapment as possible complications when using GCEs (1). Even though GCEs facilitate the delivery of intracoronary material (stents, balloons), in complex coronary anatomies (calcification, tortuosity, presence of previous stents) stent damage induced by GCE has been reported (2–4). We comment on the underlying mechanisms of stent deformation during its insertion in a GCE while we propose helpful tips to avoid this complication. The frequent use of GCEs in challenging PCIs, mandates for early recognition and timely intervention of possible complications related to GCEs.
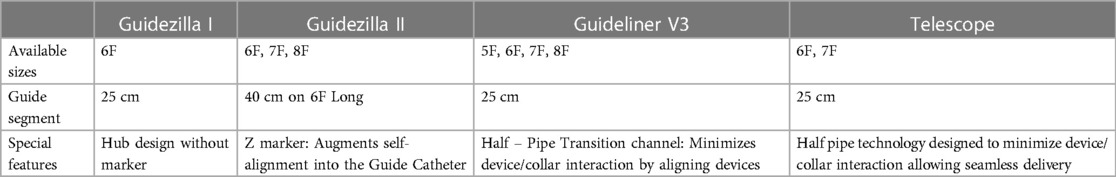
We have noted, in our clinical practice, stent deformation at the entry port of GCEs. Malalignment between the stent and the collar at the entry port of the GCE is the most common cause for encountering resistance to advancement, which can result in stent deformation, if aggressive push persists (3). It is noteworthy that positioning the entry port of the GCE in an arterial bend (anonymous artery for the radial and aortic arch for the femoral approach) may increase friction between the interventional material and the collar (4). The use of a smaller diameter GCE (6-Fr) in a larger diameter GC (7-Fr), may result in an inter-catheter diameter gap which can aggravate malalignment (4). Additionally, the use of two guidewires inside the GCE or twisting between the guidewire and the pushrod may hamper smooth insertion of the stent in the entry port of the GCE. Loading the stent on the guidewire and GCE on the table, outside the patients' body, has been described, as a bailout technique (4). Moreover, newer generation devices are available in larger diameters ensuring improved deliverability and alignment. The Guidezilla II GCE (Boston Scientific) claims to provide self-alignment upon insertion into the GC just by keeping the hub look upwards (Z letter facing up). Furthermore, the incorporation of the novel hydrophilic coating, coupled with the redesigned hub, facilitates smoother delivery, while the shorter hypotube transition length minimizes the device interaction between the stent and the GCE. The Guideliner V3 GCE (Teleflex) claims to minimize device/collar interaction by aligning devices through a half-pipe transition channel. The Telescope (Medtronic) GCE has a half pipe technology designed to minimize device/collar interaction allowing seamless delivery (5–7). Differences between various types of GCEs are illustrated in Table 1.
In conclusion, stent deformation during insertion in a GCE is an infrequent complication. Good alignment between the stent and GCE, 1:1 diameter compatibility, avoidance of locating the transition collar in arterial bends, the use of a single wire inside the GCE, keeping the guidewire and pushrod separated or even loading the stent in the GCE outside the patients' body, and the use of newer generation devices are essential measures to prevent this complication.
Author contributions
SK: Writing – original draft, Writing – review & editing. AK: Writing – review & editing. PT: Writing – review & editing. AT: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lei X, Liang Q, Fang Y, Xiao Y, Wang D, Dong M, et al. Guidezilla™ guide extension catheter I for transradial coronary intervention. Front Cardiovasc Med. (2022) 9:931373. doi: 10.3389/fcvm.2022.931373
2. Theodoropoulos KC, Kouparanis A, Didagelos M, Kassimis G, Ziakas A. Stent damage associated with a guide catheter extension during percutaneous coronary intervention. J Invasive Cardiol. (2023) 35(2):E101–2. 36735871
3. Waterbury TM, Sorajja P, Bell MR, Lennon RJ, Mathew V, Singh M, et al. Experience and complications associated with use of guide extension catheters in percutaneous coronary intervention. Catheter Cardiovasc Interv. (2016) 88(7):1057–65. doi: 10.1002/ccd.26329
4. Shoda M, Yamamoto H, Tsukiyama Y, Kawai H, Takaya T. Rare complications of guideplus guide-extension catheter during complex percutaneous coronary intervention. J Cardiol Cases. (2022) 26(6):399–403. doi: 10.1016/j.jccase.2022.08.006
5. Waggoner T, Desai H, Sanghvi K. A unique complication of the GuideZilla guide extension support catheter and the risk of stent stripping in interventional & endovascular interventions. Indian Heart J. (2015) 67(4):381–4. doi: 10.1016/j.ihj.2015.04.018
6. Chandra S, Tiwari A, Chaudhary G, Yadav R. Guide catheter extension systems: hype or a need? Indian Heart J. (2021) 73(5):535–8. doi: 10.1016/j.ihj.2021.09.011
7. Boston Scientific website, as seen on 17/12/2023. Available online at: https://www.bostonscientific.com/en-EU/products/catheters-guide/guidezilla-II-guide-extension-catheter.html (accessed December 15, 2023).
Keywords: Guidezilla™, complex coronary lesions, transradial, guide extension catheters, tips and tricks, coronary stent deformation
Citation: Kotoulas SC, Kalogeropoulos AS, Tonino PAL and Triantafyllis AS (2024) Commentary: Coronary stent deformation induced by guide catheter extension. Front. Cardiovasc. Med. 11:1357337. doi: 10.3389/fcvm.2024.1357337
Received: 17 December 2023; Accepted: 20 February 2024;
Published: 6 March 2024.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Niya Mileva, Aleksandrovska University Hospital, Bulgaria© 2024 Kotoulas, Kalogeropoulos, Tonino and Triantafyllis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sotirios C. Kotoulas c290ZXI5NkBpY2xvdWQuY29t
 Sotirios C. Kotoulas
Sotirios C. Kotoulas Andreas S. Kalogeropoulos
Andreas S. Kalogeropoulos Pim A. L. Tonino3
Pim A. L. Tonino3