- 1Department of Pediatrics and Adolescent Medicine, The American University of Beirut Medical Center (AUBMC), Beirut, Lebanon
- 2Department of Pediatric Cardiac Surgery, Heart Center of Excellence, Al Jalila Children’s Specialty Hospital, Dubai, United Arab Emirates
- 3Division of Pediatric Cardiology, Department of Pediatrics and Adolescent Medicine, Children’s Heart Center, AUBMC, Beirut, Lebanon
Background: Hypoplastic left heart syndrome (HLHS) accounts for 2.6% of congenital heart disease and is an invariably fatal cardiac anomaly if left untreated. Approximately 33,750 babies are born annually with HLHS in developing countries. Unfortunately, the majority will not survive due to the scarcity of resources and the limited availability of surgical management.
Aim: To describe and analyze our experience with the hybrid approach in the management of HLHS in a developing country.
Methods: We performed a retrospective single-center study involving all neonates born with HLHS over five years at the Children's Heart Center at the American University of Beirut. The medical records of patients who underwent the hybrid stage 1 palliation were reviewed, and data related to baseline characteristics, procedure details and outcomes were collected to describe the experience at a tertiary care center in a developing country.
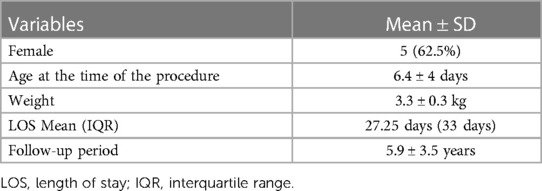
Results: A total of 18 patients were diagnosed with HLHS over a five-year period at our institution, with male to female ratio of 1:1. Of those, eight patients underwent the hybrid stage I procedure. The mean weight at the time of the procedure was 3.3 ± 0.3 kg with an average age of 6.4 ± 4 days. The mean hospital length of stay was 27.25 days, with an interquartile range of 33 days. The cohort's follow-up duration averaged 5.9 ± 3.5 years. The surgical mortality was zero. Only one mortality was recorded during the interstage period between stage I and II and was attributed to sepsis. Notably, all surviving patients maintained preserved and satisfactory cardiac function with good clinical status.
Conclusion: Our limited experience underscores the potential of developing countries with proper foundations to adopt the hybrid procedure for HLHS, yielding outcomes on par with those observed in developed countries. This demonstrates the viability of establishing a more balanced global landscape for children with congenital heart disease.
1 Introduction
Globally, approximately 1.5 million babies are born with congenital heart defects (CHD) annually (1, 2). Unfortunately, their survival prospects are closely linked to their geographical location and available medical resources. Tragically, 90% of CHD cases in low- and middle-income countries (LMICs) lack access to vital cardiac care (2–4), resulting in significantly elevated mortality and disability rates compared to their counterparts in high-income countries. The burden of CHD is particularly pronounced in regions with higher fertility rates and lower per capita income, representing 90% of births in the world's most economically disadvantaged areas (5).
Hypoplastic left heart syndrome (HLHS) constitutes 2.6% of all congenital heart diseases (6, 7), and thus, the estimated number of babies born with HLHS in LMICs each year is around 33,750. Sadly, most of these infants will not survive infancy due to the bleak prospects of receiving treatment.
HLHS is characterized by the underdevelopment of structures on the left side of the heart, including the mitral valve, left ventricle, aortic valve, and aortic arch. Before the 1980s, this complex malformation was linked to a staggering 95% mortality rate within the first month of life (8).
The introduction of the Norwood procedure in the early 1980s revolutionized the prognosis for these children. The majority now undergo a series of three surgical stages, enabling survival beyond the neonatal phase and into early adulthood. Neonatal treatment options for this condition include the Norwood operation or the hybrid procedure (9, 10). The hybrid procedure involves stenting the ductus arteriosus and banding the pulmonary branches to regulate pulmonary flow.
Managing infants diagnosed with HLHS in LMICs continues to face challenges due to the condition's complexity, poor surgical outcomes, and the necessity for specialized medical care. The management of HLHS raises multifaceted ethical, social, and economic concerns. Striking a balance between local health priorities, weighing the potential benefits of intervention against the risks, and allocating resources for one condition over others become intricate.
This study aims to elucidate the experience of employing the hybrid procedure for HLHS at a tertiary center in a developing nation. It encompasses the procedure's outcomes, patient follow-up, and associated mortality and morbidity rates. It also compares these findings to those in developed countries.
2 Materials and methods
This is a retrospective study that includes all neonates diagnosed with HLHS at the Children's Heart Center (CHC) at the American University of Beirut-Medical Center (AUBMC) in Lebanon between October 2013, and January 2018, inclusive. The CHC is a tertiary center in a developing country, and a major referral center in the Middle East and North Africa. The center treats annually around 2,000 patients, performs around 150–200 congenital heart surgeries and about 120–150 cardiac catheterizations. After institutional review board (IRB) approval, patients' data were collected from the AUBMC medical records. Collected data included demographics, surgical procedures, progress notes, imaging, cause of mortality, morbidity, and follow-up information.
The hybrid procedure is performed in the catheterization laboratory and includes the collaboration of a pediatric cardiac surgeon and a pediatric interventional cardiologist. Under general anesthesia, a median sternotomy is made, and the thymus is resected. Bilateral pulmonary artery (PA) banding is achieved using a 3.5 mm polytetrafluoroethylene graft, divided longitudinally, and wrapped around the branch pulmonary arteries for a width of approximately 3 mm. The bands are then secured to the adventitia of the main PA with 5-0 or 6-0 non-absorbable polypropylene sutures to avoid distal dislodging. The patient is usually partially heparinized, a purse-string suture is placed on the proximal main pulmonary artery, and a 6-Fr sheath is inserted. The ductal stent is deployed through the sheath under fluoroscopic guidance, achieving stage I palliation (Figure 1). We use standard-size balloon-expandable stents, 18 mm long and 8–9 mm in diameter, depending on availability. The used stents are either the Scuba™ Stent System or the Visio-Pro Stent System. The stent is placed in an appropriate position, confirmed by angiography. The saturation usually decreases by 8%–10% following the procedure to achieve a final saturation of 80%–85%, and the systolic BP increases by about 10 mm Hg. Balloon atrial septostomy is performed under echo guidance in a different setting. Figure 2 depicts a schematic representation of the hybrid procedure.
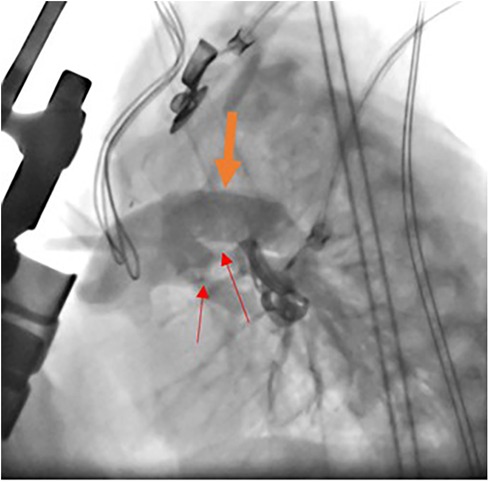
Figure 1. Catheterization during hybrid procedure. Angiographic study during stent deployment in hybrid stage I palliation. The modified lateral view shows the stent into the arterial duct (thick organ arrow) and bilateral banding of the PAs (thin red arrow).
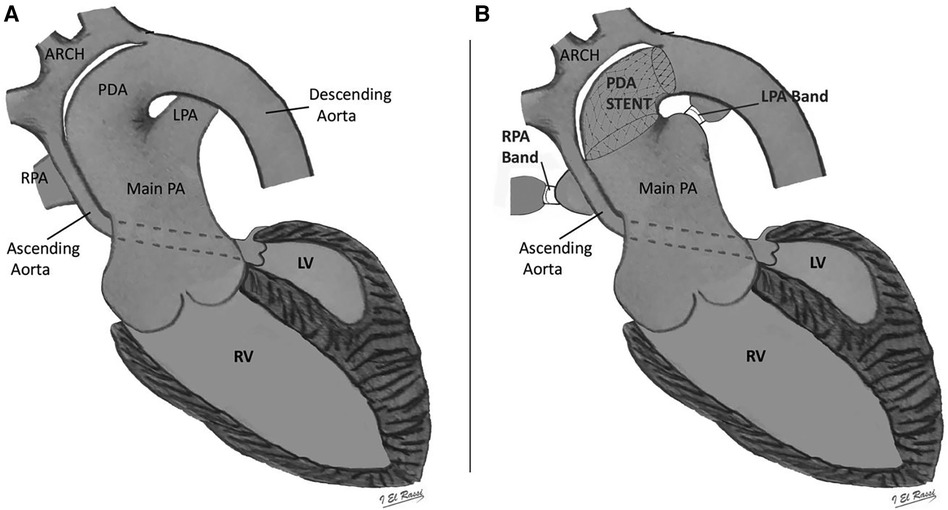
Figure 2. Schematic representation of the hybrid procedure. Panel A depicts hypoplastic left heart syndrome; Panel B is a schematic image of Hybrid stage 1 that shows stent into the arterial duct and bilateral banding of the LPA and RPA. PDA, patent ductus arteriosus; RPA, right pulmonary artery; LPA, left pulmonary artery; LV, left ventricle; RV, right ventricle.
Patients underwent the second stage at an average age of 8.6 months (range 5–11 months), following clinical, echocardiographic and catheterization assessment. A repeat median sternotomy is performed under cardiopulmonary bypass and hypothermia. This stage includes the removal of the branch pulmonary arteries bands, resection of the PDA stent and dissection of the ductus arteriosus, creation of neoaorta through anastomosis of the ascending aorta and the main pulmonary artery, plasty of the pulmonary artery branches when needed, transection of the superior vena cava from the right atrium, and creation of the cavo-pulmonary connection.
Stage 3, completed at 3–5 years of age, is performed with an extracardiac fenestrated conduit connecting the inferior vena cava to the pulmonary arteries via a Gore-Tex conduit to form total cavo-pulmonary circulation.
3 Results
A total of 18 patients with the diagnosis of HLHS in the CHC at the AUBMC between October 2013 and January 2018 were identified and included in this retrospective cohort study.
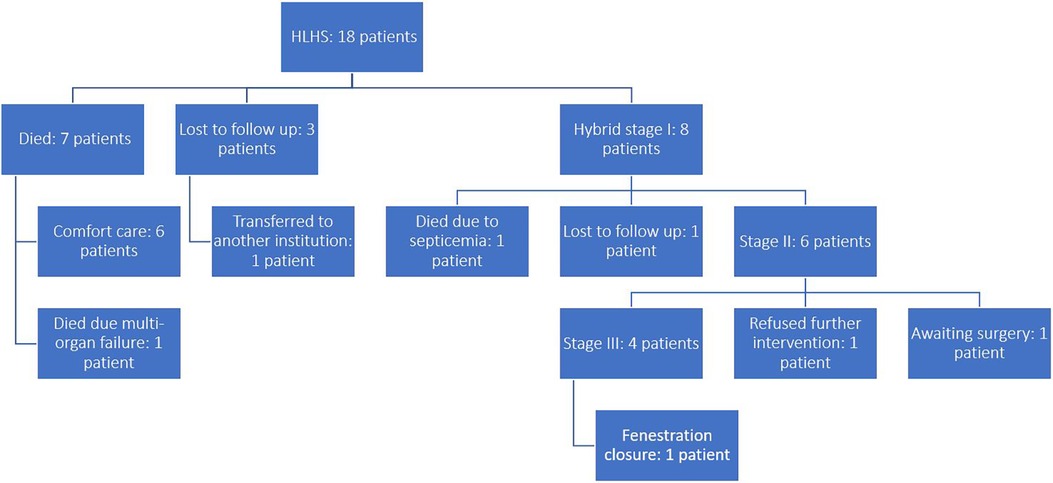
Females constituted 50% of the population. Only eight (44%) patients were operated on. Six (33%) patients were placed on comfort care after discussion with the parents regarding their medical condition, treatment options, risks and long-term prognosis; They survived on average until day of life eight. One patient (5.5%) underwent urgent bedside atrial septostomy, however later developed acute kidney injury, disseminated intravascular coagulation and shock, and eventually died at day of life 7. Another patient (5.5%) was transferred to another institution after being placed in comfort care and was lost to follow up thereafter. Two (11%) more patients presented to the outpatient clinic at day of life 10 and 38 respectively, however parents refused hospitalization and were lost to follow up. Figure 3 depicts the flow of patients in this study.
Among patients who underwent the hybrid stage I procedure, females constituted 62.5% of the cohort. The patients’ mean weight was 3.3 ± 0.3 kg, and the mean age was 6.4 ± 4 days at the time of the procedure. The mean length of stay at the hospital during the hybrid procedure was 27.25 days (IQR = 33 days). The average follow-up period for this cohort was 5.9 ± 3.5 years. Table 1 represents the demographic characteristics of these patients. Among the eight patients undergoing the hybrid procedure, two had mitral atresia along with hypoplastic aortic valve and ascending aorta, one patient had hypoplasia of mitral valve, aortic valve and ascending aorta with good left ventricular size, five patients had severe hypoplasia of the mitral valve, aortic valve, left ventricle and ascending aorta, and the last patient had hypoplastic aortic valve and ascending aorta.
Of the eight patients who completed the hybrid procedure, four underwent atrial septostomy, while two had atrial septectomy. Half of the patients (n = 4) of the patients had insertion of a 9 × 18 mm stent, while the other half had an 8 × 18 mm stent.
Three patients (37.5%) developed complications, which might be secondary to ductal steal. One patient developed bowel perforation of undetermined etiology and was managed surgically. Another patient developed transient renal failure with no other issues. The third patient developed unspecified nephrotic syndrome and did not require any intervention. These three patients had a mean length of stay of 44.3 ± 22.1 days, more than the mean of the collective cohort. The three patients left the hospital in good condition. None of the three patients died later.
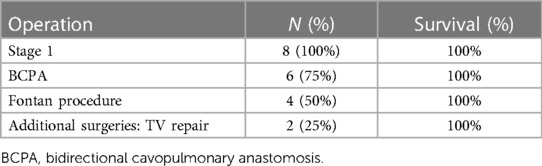
Overall, the mortality rate for the hybrid procedure was zero. All patients (100%) were followed up with a preserved and good cardiac function. Only one death (12.5%) was recorded due to non-cardiac complications. This patient developed septicemia in the setting of an underlying metabolic disorder two months following his discharge from the hospital. Overall, the total mortality of the group at one month was 0%, at two months was 12.5%, and remained unchanged after following up for an average of 5 years. The survival rate after each operation is presented in Table 2.
Six of the eight patients had completed the Glenn procedure (75%) with no associated mortality. 25% (n = 2) of the patients underwent valvular repair of the tricuspid because of regurgitation, and none died. 50% of the patients (n = 4) underwent the Fontan procedure with 18 mm Gor-TexR and fenestration. One patient underwent successful device closure of a fenestrated Fontan. The rate of intervention involving the pulmonary arteries was high. Six patients required pulmonary artery plasty for single or bilateral pulmonary artery branches. Four patients had the plasty performed during stage 2. No stents were used.
Four patients did not undergo the Fontan procedure. One patient died two months after the hybrid procedure. Another patient did not undergo surgery since the parents were reluctant to do so after the benefits and risks were explained; the third patient is awaiting the Fontan surgery. The last patient was lost for follow-up after four months from the hybrid procedure and probably left for a neighboring country.
The surgical mortality rate for stage 2 and stage 3 palliation was 0%. Since the onset of the hybrid procedure, all patients were seen periodically. On their latest follow-up, all the patients demonstrated good ventricular systolic function and tricuspid valve competence as assessed by echocardiography.
4 Discussion
In this study we share our experience with the hybrid approach in palliating patients with HLHS. It is important to note that before 2013, no interventions were provided for patients with HLHS in our country. Therefore, our findings demonstrate the potential of the hybrid approach to be utilized in the management of neonates with HLHS, particularly in developing countries with restricted resources and limited surgical and peri-operative expertise.
Of the 18 patients with HLHS, eight patients successfully completed hybrid stage I. The long-term outcomes of our relatively small cohort, tracked over several years, unveiled a 5-year survival rate of 87.5% after completing the Norwood 2 comprehensive operation and the Fontan procedure. Remarkably, all surviving patients maintained a good functional cardiac status. Our eldest patient is a 10-year-old male, with echocardiographic findings revealing normal right ventricular function, alongside mild tricuspid regurgitation, during the latest follow-up. Five other patients are still being followed up at the pediatric cardiology outpatient clinic. Three patients, who completed stage 3, have good right ventricular systolic function, mild tricuspid regurgitation, and patent connections. One of them had device closure of the fenestrations, and another patient has mild to moderate aortic regurgitation. The patient, whose parents opted not to proceed to the third stage, exhibits mildly diminished right ventricular function, moderate tricuspid regurgitation, and mild neoaortic regurgitation. The last patient, who is currently awaiting the third stage, has good right ventricular function with mild to moderate tricuspid regurgitation.
Our current experience shows a 0% mortality rate of stage 2 and 3, surpassing the survival rates described in the literature. However, these findings may be influenced by the limited size of the study population. Nevertheless, the mortality rates of comprehensive stage 2 reported by large studies in the literature are still reassuring and promising, estimated at 4%–9% (11). Additionally, other variables, including the low birth weight, the presence of associated congenital anomalies, and concomitant non-cardiac morbidities, which might contribute to procedural failure and increased complications during stage 2, are absent in our population. Most importantly, our high success rate is attributed to the effective communication and coordination between the interventional and surgical teams. Furthermore, it is crucial to highlight that our center performs around 200 congenital heart surgeries annually, necessitating an effective multidisciplinary approach involving surgical, pediatric, cardiology, and intensive care teams and requiring solid peri-operative care. Thus, our robust and impactful experience in post-operative and pediatric intensive care management significantly contributed to improved peri-operative and overall outcomes.
Therefore, despite operating with a modest volume, our surgical mortality and length of stay align with those reported in developed countries. Our experience underscores that successful HLHS surgical programs can be established in LMICs if a suitable foundation is in place, yielding favorable outcomes. Our findings offer valuable insights into the applicability and viability of these hybrid procedures in developing nations.
Therefore, despite operating with a modest volume, our surgical mortality and length of stay align with those reported in developed countries. Our experience underscores that successful HLHS surgical programs can be established in LMICs if a suitable foundation is in place, yielding favorable outcomes. Our findings offer valuable insights into the applicability and viability of these hybrid procedures in developing nations.
HLHS presents as a uniformly lethal cardiac anomaly if left untreated. The introduction of the Norwood procedure in the early 1980s marked a pivotal moment in HLHS treatment. The hybrid procedure later emerged as a groundbreaking strategy to manage neonates with high-risk HLHS. The concept was first introduced in 1993 by Gibbs et al., when bilateral pulmonary artery bands were placed surgically, followed by the placement of a stent in the ductus arteriosus (12). This has opened the door for additional innovative interventions to address neonates with HLHS who are not eligible for the Norwood procedure. Subsequently, Akintuerk et al. modified the procedure and highlighted its cardinal role in providing a stepwise approach to palliate HLHS (13). However, the technique was still performed in two different settings: the operating room and the catheterization lab. This hybrid strategy continued to evolve until it was first introduced as a single procedure consisting of the placement of PA bands and PDA stent simultaneously, introducing the first true hybrid procedure (14). Following these milestones, the experience with hybrid procedure has expanded and improved significantly (15–17). However, despite all the technical development and the fact that outcomes for Hybrid I and Norwood procedures have demonstrated comparability, the Norwood procedure remains more prevalent in developing countries.
In our context, adopting the hybrid approach stemmed from local expertise, involving collaboration between the local pediatric cardiac surgeon and the pediatric interventional cardiology team. Moreover, the hybrid procedure was favored as it circumvents open-heart surgery and cardiopulmonary bypass and is feasible within a cardiac catheterization suite. By avoiding cardioplegia and cardiopulmonary bypass, the hybrid approach reduces procedural duration and hospital stay, and holds significantly lower financial burden (2). Notably, when evaluating risk-adjusted outcomes, there was no significant disparity between the primary Norwood and bilateral pulmonary artery banding groups in higher-volume institutions (4).
Besides, surgical outcomes have shown notable improvement over subsequent decades. The mortality rate for stage 1 palliation decreased from 40% to 30% within a decade. A study by Mascio et al. elucidated that a total of 1,663 Norwood procedures had a survival rate demonstrating a positive trajectory, with mortality rates dropping from 40.4% in the mid-80s to a plateau of 15.7% in the mid-2010s (18). According to the 2020 Harvest STS CHSD feedback report, a mortality rate of 12.4% with an interquartile range of 6.5%–16.2%, accompanied by average postoperative hospital stay of 58.7 days and an interquartile range of 43.7–66.9 days was reported in patients following the Norwood procedure (19).
Surgical treatment for HLHS in limited-resource countries has remained uncommon, with only a handful of centers in LMICs undertaking HLHS surgery. These efforts have primarily focused on Norwood stage 1 surgery, demonstrating a 41% survival rate after the initial stage. A few instances of utilizing the hybrid stage 1 procedure are documented in the Philippines (20) and India (21).
The hybrid stage I palliative procedure is a logical alternative to traditional Norwood or Sano operations for neonates with HLHS in LMICs. Challenges such as limited experience with cardiopulmonary bypass, circulatory arrest in neonates, and elevated costs contribute additional risks to surgeries in resource-constrained settings. Therefore, hybrid approaches yield good outcomes when resources permit, irrespective of geographical location. HLHS imposes a substantial financial burden, entailing a series of approximately three procedures: Norwood, Glenn, and Fontan. Dean et al. revealed mean operation costs of $214,680, $82,174, and $79,549, respectively (22). Adopting the hybrid 1 procedure could alleviate the expense of the initial operation. Nevertheless, challenging stages and interstage periods following the hybrid procedure should be expected. Indeed, the risks associated with palliation are not eradicated but are rather moved from stage 1 to later stages. Choosing the hybrid approach holds high rates of reintervention along with their associated risk of complications and mortality (16, 17). This is particularly important during comprehensive stage 2 in the setting of elevated pulmonary pressure or the presence of poorly confluent pulmonary vessels. Besides, heart transplant might be unavoidable in several patients. Close monitoring during the interstage period is also essential, however, might not always be feasible. Several patients were reported to experience sudden death during the interstage period while at home (16). Other significant concerns include retrograde aortic arch obstruction and the fate of the branch pulmonary arteries which might face significant distortion. The reconstruction of pulmonary arteries is challenging, as achieving reasonable confluence is crucial for subsequent successful Fontan. The need for interventions in the branch pulmonary artery is variable and can reach high rates, reported at 18%–86%, which exceeds the rate of intervention required after Norwood (11, 16, 23, 24).
Our experience at a developing country's tertiary center is encouraging, showing outcome measures comparable to those in developed countries. Specifically, our in-house surgical mortality for stage I hybrid procedures was zero, and our survival rate aligned with that of developed nations. Although limited by the humble population size, this experience with the hybrid approach in managing HLHS can pave the way for further utilization of hybrid interventions in developing countries. As discussed by our colleagues, the hybrid approach can provide the best out of transcatheter and surgical techniques in treating several congenital heart lesion, ranging from defect closure to managing valvulopathy (25). Therefore, the hybrid management of HLHS can be used as an example to manage other conditions in developing countries that suffer from the lack of expertise and resources. This partnership can shape and influence the future of pediatric and congenital cardiology.
It's important to acknowledge the limitations of our cohort study, including its small sample size and limited patient pool. Another limitation is attributed to the inherent retrospective nature of the study. Besides, one of the patients was lost to follow-up after undergoing the hybrid procedure, leaving 12.5% of the study population with an unknown fate. In addition, only 50% of the study population have completed the Fontan, placing restrictions on the feasibility of addressing stage 3 outcomes and long-term follow-up data. However, one patient is expected to complete stage 3 in the coming few months.
While the aspiration to provide optimal care for HLHS patients in developing or resource-limited countries is admirable, challenges tied to medical expertise, facilities, postoperative care, and long-term follow-up must be considered. The feasibility, safety, and ethical considerations of treating intricate conditions like HLHS in resource-constrained settings warrant careful assessment.
Parents and physicians confront arduous decisions regarding appropriate therapeutic interventions for infants with HLHS (8). To foster a more equitable landscape for children with congenital heart disease worldwide and address global disparities in pediatric cardiac care, we share our experience treating HLHS infants at a tertiary center in a developing country.
This limited experience showcases that adequately staffed developing countries can opt for the modified hybrid procedure for HLHS, yielding outcomes on par with those in developed countries. It underscores the feasibility of establishing a more equitable landscape for children with congenital heart disease worldwide.
5 Conclusion
The hybrid palliation stage 1 Norwood is a promising alternative for open heart surgery in the setting of a tertiary center in a developing country with no in-hospital mortality. Its implementation heavily depends on the developing country and its institutions, whether it has solid infrastructure alongside a collaborative group of medical professionals to deliver the appropriate care. To our knowledge, very few studies explore the matter of HLHS in developing countries. Additional studies are needed to understand better the status of the management of HLHS in developing countries.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Institutional Review Board (IRB) at the American University of Beirut. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
FoB: Data curation, Methodology, Writing – original draft. IE-R: Writing – original draft, Visualization. RZ: Writing – original draft, Data curation. YJ: Writing – original draft. JA: Writing – original draft. ZB: Writing – original draft, Supervision, Validation, Writing – review & editing. FaB: Supervision, Validation, Writing – review & editing, Conceptualization. MA: Conceptualization, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu Y, Chen S, Zuhlke L, Black GC, Choy MK, Li N, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. (2019) 48(2):455–63. doi: 10.1093/ije/dyz009
2. Murala JSK, Karl TR, Pezzella AT. Pediatric cardiac surgery in low-and middle-income countries: present status and need for a paradigm shift. Front Pediatr. (2019) 7:214. doi: 10.3389/fped.2019.00214
3. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. (2011) 58(21):2241–7. doi: 10.1016/j.jacc.2011.08.025
4. Dearani JA, Neirotti R, Kohnke EJ, Sinha KK, Cabalka AK, Barnes RD, et al. Improving pediatric cardiac surgical care in developing countries: matching resources to needs. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. (2010) 13(1):35–43. doi: 10.1053/j.pcsu.2010.02.001
5. Hoffman J. The global burden of congenital heart disease. Cardiovasc J Afr. (2013) 24(4):141–5. doi: 10.5830/CVJA-2013-028
6. Ohman A, El-Segaier M, Bergman G, Hanseus K, Malm T, Nilsson B, et al. Changing epidemiology of hypoplastic left heart syndrome: results of a national Swedish cohort study. J Am Heart Assoc. (2019) 8(2):e010893. doi: 10.1161/JAHA.118.010893
7. Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, et al. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. (2019) 111(18):1420–35. doi: 10.1002/bdr2.1589
8. Fruitman DS. Hypoplastic left heart syndrome: prognosis and management options. Paediatr Child Health. (2000) 5(4):219–25. doi: 10.1093/pch/5.4.219
9. Brescia AA, Jureidini S, Danon S, Armbrecht E, Fiore AC, Huddleston CB. Hybrid versus Norwood procedure for hypoplastic left heart syndrome: contemporary series from a single center. J Thorac Cardiovasc Surg. (2014) 147(6):1777–82. doi: 10.1016/j.jtcvs.2014.02.066
10. Tabbutt S, Ghanayem N, Ravishankar C, Sleeper LA, Cooper DS, Frank DU, et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: a report from the pediatric heart network single ventricle reconstruction trial. J Thorac Cardiovasc Surg. (2012) 144(4):882–95. doi: 10.1016/j.jtcvs.2012.05.019
11. Schranz D, Bauer A, Reich B, Steinbrenner B, Recla S, Schmidt D, et al. Fifteen-year single center experience with the “giessen hybrid” approach for hypoplastic left heart and variants: current strategies and outcomes. Pediatr Cardiol. (2015) 36(2):365–73. doi: 10.1007/s00246-014-1015-2
12. Gibbs JL, Wren C, Watterson KG, Hunter S, Hamilton JR. Stenting of the arterial duct combined with banding of the pulmonary arteries and atrial septectomy or septostomy: a new approach to palliation for the hypoplastic left heart syndrome. Br Heart J. (1993) 69(6):551–5. doi: 10.1136/hrt.69.6.551
13. Akintuerk H, Michel-Behnke I, Valeske K, Mueller M, Thul J, Bauer J, et al. Stenting of the arterial duct and banding of the pulmonary arteries: basis for combined Norwood stage I and II repair in hypoplastic left heart. Circulation. (2002) 105(9):1099–103. doi: 10.1161/hc0902.104709
14. Galantowicz M, Cheatham JP. Lessons learned from the development of a new hybrid strategy for the management of hypoplastic left heart syndrome. Pediatr Cardiol. (2005) 26(3):190–9. doi: 10.1007/s00246-004-0962-4
15. Bacha EA, Daves S, Hardin J, Abdulla RI, Anderson J, Kahana M, et al. Single-ventricle palliation for high-risk neonates: the emergence of an alternative hybrid stage I strategy. J Thorac Cardiovasc Surg. (2006) 131(1):163–71.e2. doi: 10.1016/j.jtcvs.2005.07.053
16. Erek E, Aydin S, Temur B, Onalan MA, Suzan D, Iyigun M, et al. Outcomes of hybrid and Norwood stage I procedures for the treatment of hypoplastic left heart syndrome and its variants. Turk Gogus Kalp Damar Cerrahisi Derg. (2019) 28(2):282–93. doi: 10.5606/tgkdc.dergisi.2020.18605
17. Ho AB, Hribernik I, Shillaker D, Thomson J, Salam A, Dedieu N, et al. Hybrid palliation for hypoplastic left heart syndrome: association with contemporary outcomes. Circulation. (2021) 144(14):1189–91. doi: 10.1161/CIRCULATIONAHA.121.055183
18. Mascio CE, Irons ML, Ittenbach RF, Gaynor JW, Fuller SM, Kaplinski M, et al. Thirty years and 1663 consecutive Norwood procedures: has survival plateaued? J Thorac Cardiovasc Surg. (2019) 158(1):220–9. doi: 10.1016/j.jtcvs.2018.12.117
19. Kumar SR, Mayer JE Jr, Overman DM, Shashidharan S, Wellnitz C, Jacobs JP. The society of thoracic surgeons congenital heart surgery database: 2021 update on outcomes and research. Ann Thorac Surg. (2021) 112(6):1753–62. doi: 10.1016/j.athoracsur.2021.10.002
20. Cuaso CC, Cheng DD, Rosario JD, Plucena LP, Pasimio R, Reyes KG. Successful hybrid stage I palliation for hypoplastic left heart syndrome in a developing nation. World J Pediatr Congenit Heart Surg. (2012) 3(1):139–41. doi: 10.1177/2150135111424295
21. Anuradha S, Subramanyan R, Agarwal R, Pezzella AT, Cherian KM. Hybrid stage I palliation for hypo-plastic left heart condition without a hybrid suite: suggestions for developing nations. Indian Heart J. (2012) 64(4):333–7. doi: 10.1016/j.ihj.2012.05.001
22. Dean PN, Hillman DG, McHugh KE, Gutgesell HP. Inpatient costs and charges for surgical treatment of hypoplastic left heart syndrome. Pediatrics. (2011) 128(5):e1181–6. doi: 10.1542/peds.2010-3742
23. Dave H, Rosser B, Knirsch W, Hubler M, Pretre R, Kretschmar O. Hybrid approach for hypoplastic left heart syndrome and its variants: the fate of the pulmonary arteries. Eur J Cardiothorac Surg. (2014) 46(1):14–9. doi: 10.1093/ejcts/ezt604
24. Baba K, Kotani Y, Chetan D, Chaturvedi RR, Lee KJ, Benson LN, et al. Hybrid versus Norwood strategies for single-ventricle palliation. Circulation. (2012) 126(11 Suppl 1):S123–31. doi: 10.1161/CIRCULATIONAHA.111.084616
Keywords: hypoplastic left heart syndrome, congenital heart disease, hybrid procedure, hybrid stage I, developing country
Citation: Bitar F, El-Rassi IM, Zareef R, Jassar Y, Abboud J, Bulbul Z, Bitar F and Arabi M (2024) Hybrid stage 1 palliation for HLHS: the experience of a tertiary center in a developing country. Front. Cardiovasc. Med. 11:1355989. doi: 10.3389/fcvm.2024.1355989
Received: 14 December 2023; Accepted: 22 February 2024;
Published: 7 March 2024.
Edited by:
Salvatore Agati, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Vladislav Vukomanović, University of Belgrade, SerbiaFabrizio De Rita, Newcastle upon Tyne Hospitals NHS Foundation Trust, United Kingdom
Juan-Miguel Gil-Jaurena, Gregorio Marañón Hospital, Spain
Lilia Oreto, Mediterranean Pediatric Cardiology Center, Italy
© 2024 Bitar, El-Rassi, Zareef, Jassar, Abboud, Bulbul, Bitar and Arabi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fadi Bitar ZmJpdGFyQGF1Yi5lZHUubGI= Mariam Arabi bWE4MUBhdWIuZWR1Lmxi
†ORCID Mariam Arabi orcid.org/0000-0001-6895-1580
 Fouad Bitar
Fouad Bitar Issam M. El-Rassi2
Issam M. El-Rassi2 Rana Zareef
Rana Zareef Ziad Bulbul
Ziad Bulbul Fadi Bitar
Fadi Bitar Mariam Arabi
Mariam Arabi