- 1Department of Cardiology, Inova Schar Heart and Vascular, Falls Church, VA, United States
- 2Department of Cardiology, Virginia Heart, Falls Church, VA, United States
Heart failure (HF) represents a worldwide health burden and the annual per patient cost to treat HF in the US is estimated at $24,383, with most of this expense driven by HF related hospitalizations. Decompensated HF is a leading cause for hospital admissions and is associated with an increased risk of subsequent morbidity and mortality. Many hospital admissions for decompensated HF are considered preventable with timely recognition and effective intervention.Systems of care that include interventions to facilitate early recognition, timely and appropriate intervention, intensification of care, and optimization to prevent recurrence can help successfully manage decompensated HF in the ambulatory setting and avoid hospitalization.
Introduction
Heart failure (HF) represents a massive health burden worldwide, with approximately 6 million adults in the United States carrying the diagnosis (1). As the annual per patient cost to treat HF is estimated at $24,383, the financial impact is significant, and most of this expense is due to HF related hospitalizations (2). Decompensated HF is a leading cause for hospital admissions and carries negative prognostic implications, as hospitalization is associated with an increased risk of subsequent morbidity and mortality (3–5).
Many hospital admissions for decompensated HF are considered preventable with timely recognition and effective intervention (6). Interventions targeting preventing hospitalization for decompensated HF are increasingly patient-centered, with both an episodic focus and longitudinal perspective (7–9). Four key concepts are consistently associated with successful systems of care for ambulatory management of decompensated HF: (1) Early recognition of decompensation; (2) Timely and appropriate intervention to address decompensation; (3) Intensification of care until stabilization; and (4) Optimization to prevent recurrence (Figure 1).
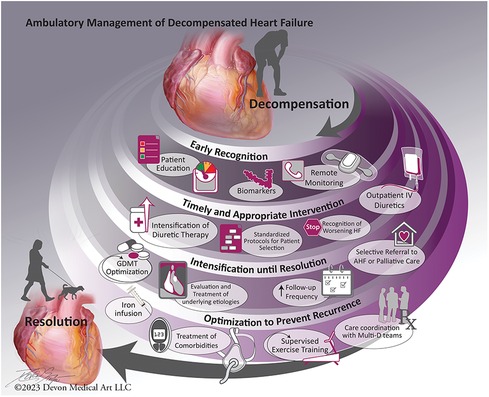
Figure 1. Main concepts in systems of care for ambulatory management of acute decompensated heart failure.
We will present current evidence for interventions corresponding with each of these elements, the residual clinical challenges, and future directions for care improvement (Table 1).
Early recognition of decompensation
The first key element of a successful system of care for ambulatory management of decompensated HF is early recognition of an episode of active or impending decompensation. The concept of “decompensated” HF describes an exacerbation or abrupt worsening of symptoms in individuals with pre-existing HF (10). While typically considered a rapid onset of severe symptoms requiring immediate attention and frequently resulting in hospitalization, decompensation can also occur gradually (10). Symptoms of decompensation are often noted after a patient has been in the state for several days and prompt recognition is critical to facilitate appropriate treatment (11). Interventions shown to facilitate early recognition of decompensated HF include patient education, clinician driven remote patient management, remote monitoring, and biomarkers.
Patients themselves play a critical role in recognizing and responding to indicators of decompensation, and they should be routinely taught to check weight, heart rate (HR) and blood pressure (BP) regularly and report significant alterations to their healthcare provider (12). Several studies of this approach produced variable results in improvements in clinical outcomes and quality of life (QOL), but may have been confounded by poor adherence and lack of clear comprehensive management algorithm (12–15). In contrast, the TIM-HF2 trial showed reduction in the number of days hospitalized and all-cause mortality in patients with HF with EF <45%, using a structured approach to remote patient management, and self-care and monitoring that are widely used and incorporated in HF management guidelines (16–19).
Advances in technology have led to increased utilization of medical devices to improve early detection of alterations in health (11, 18). The CardioMEMS™ device is approved by the US Food and Drug Administration to wirelessly monitor pulmonary artery (PA) pressure and HR in patients with HF who were recently hospitalized or have elevated naturetic peptides (NP) with NYHA class II or III symptoms and is an important tool for early detection of decompensation (20). The readings from CardioMEMS™ devices are transmitted to patients' clinicians who can adjust HF therapy, specifically intensify diuretic regimens, as needed and often before symptoms of decompensation are present. In the CHAMPION trial, remote monitoring via PA pressure monitoring was associated with a 37% reduction in HF hospitalizations over 15 months for patients with symptomatic HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF), as compared to usual care (21, 22). Some of the benefits of remote PA pressure monitoring are that it requires minimal effort from patients, yielding a high rate of adherence with daily pressure transmissions in trials, and facilitates appropriate and safe adjustment of medical therapy and guideline directed medical therapy (GDMT) optimization (21–23). In addition to the clinical benefits, the GUIDE-HF trial showed that the CardioMEMS device was associated with improved QOL (24). Some potential challenges in implementation include the need for adequate internet for data transmission, clinic infrastructure required to manage often large volumes of readings from patients in a timely and effective way, and occasional need to repeat right heart catheterization and recalibration to ensure accurate readings (25, 26).
Most contemporary implantable defibrillators and pacemakers include technology that evaluate physiologic variables and can provide diagnostic information to aid in management of patients with HF (20). Data provided from these devices includes heart rate variability, activity, thoracic impedance, as well as atrial and ventricular arrhythmias (20). The use of multiple parameters from these devices has great potential for clinical utility though has had limited evidence supporting improved HF outcomes, likely related to lack of standardization in the response to the data (27–29).
Lastly, biomarkers are another tool for early detection of decompensation with established utility in diagnosing HF and assessing prognosis (17, 30). The most frequently utilized biomarkers in HF care are NP, which have excellent specificity for diagnosing HF (30). Beyond their role in establishing diagnosis, the Val-HeFT study demonstrated that changes in NT-proBNP values over time were closely associated with changes in prognosis, including prediction of mortality (31). While GUIDE-IT did not demonstrate benefit in utilizing NP to guide HF therapies, more recently, STRONG-HF demonstrated that a strategy of rapid up titration of GDMT, as compared to those in the usual care, resulted in more patients with HF reaching target doses of these therapies and also lower NT-proBNP levels, and also incorporated modifications to GDMT titration schedule based on elevated NT-proBNP levels (32, 33).
Timely and appropriate intervention to address decompensation
Once a patient with HF shows signs and symptoms of decompensation, it is imperative to act quickly to implement the appropriate treatment (34). A key factor in determining if outpatient management is safe and likely to be effective involves determining if the episode is consistent with “decompensated HF” or “worsening HF”. This distinction has been a recent point of focus in care guidance, with decompensating HF connoting the potential for outpatient management, but with worsening HF typically indicating the need for inpatient care (10).
Assessment of end organ function, including renal and hepatic parameters, should be used to augment risk assessment in decompensated HF and to guide management (17). The COACH trial demonstrated that providing decision-making support for clinicians in the emergency department facilitated accurate identification of low risk patients, and that initial treatment there, followed by 30-days of transitional care, yielded a low rate of hospitalization (35). This study contained several key elements to optimize outcomes for patients with acute HF, including a risk-score to guide real-time decision-making, transitional care, and the use of a standardized, dedicated HF clinic (36).
While there are unique differences in the etiologies, treatments, and clinical course of patients with HFrEF as compared to those with HFpEF, both are susceptible to decompensation with volume overload and assessing presence and degree of congestion is an important aspect of determining appropriate interventions (2). Loop diuretics are the mainstay therapy and escalation of oral diuretic regimens should be done as soon as decompensation is identified (37). However, oral diuretics may not be able to achieve adequate diuresis due to interstitial and bowel edema, kidney dysfunction and decreased organ perfusion, and parenteral administration may be needed (38).
Historically, most patients with HF have not had an option for IV diuretics outside of an emergency department or hospital admission (39). However, multiple recent studies have suggested that the use of outpatient IV diuretics is safe and effective in treatment of volume overload and reducing hospitalization (38, 40–44). The main tenets of clinics that offer outpatient IV diuretics includes same or next-day availability, careful patient selection, the use of standardized protocols to guide diuretic dosing, and ability to replace electrolytes and monitor BP to safely achieve large volume diuresis. Other best practices clarified by these studies include close follow up, either with additional visits or return to the primary cardiologist and adjusting outpatient regimens to achieve euvolemia and avoid admission particularly in the next 30 days (38, 40–44).
The use of subcutaneous furosemide for ambulatory management of decompensated HF is a promising recent development, particularly given the challenge of access to outpatient IV diuretics and it overcomes the suboptimal efficacy of oral medications. It has a favorable safety and efficacy profile and represents another outpatient option for the treatment of volume overload (39).
Intensification of care until stabilization
Intensification of care amidst a HF exacerbation is another important element present in systems of care developed to successfully manage outpatient decompensation. Increasing the frequency of outpatient monitoring, with focus on achieving clinical stabilization and identifying and addressing a reversible or modifiable cause is key in preventing hospitalization and reducing the risk of future exacerbations (35, 38). In addition to ensuring patients with decompensated HF have ongoing appropriate titration of diuretic doses, specific activities in this area include evaluation and treatment of underlying etiologies, selective referral for advanced HF therapies and palliative care consultation and optimization of GDMT.
While an ischemic evaluation is warranted for patients with a new HF diagnosis, the role of coronary revascularization in patients with known HF in a decompensated state is less clear. Some observational studies showed improved outcomes with percutaneous coronary intervention (PCI) in patients with HF and high-grade CAD, however recent data from REVIVED-BCIS2 showed no reduction in all-cause mortality or HF hospitalization for patients with HFrEF related to CAD who underwent PCI, and further investigation is warranted to determine appropriate timing and patient selection (45, 46).
Patients with decompensated HF episode may warrant investigation and treatment of atrial and ventricular arrhythmias, as uncontrolled and persistent arrhythmias can cause HF or trigger decompensation (17, 47). Recent studies have shown that catheter ablation for atrial fibrillation can be superior to medical therapy in select patients with HFrEF in improving survival and reducing HF hospitalizations, and suppression of premature ventricular contractions, either with medication or ablation, way be warranted in the setting of HFrEF (47–50). Additionally, for patients with HF and significant arrhythmias, testing for infiltrative cardiomyopathies should considered (17).
Failure to respond to above mentioned interventions and recurrent HF hospitalizations despite intensifying medical care are important triggers for referral to advanced HF specialists (51). Certain patients may benefit from advanced HF therapies including inotropes, mechanical circulatory support devices, and heart transplantation, and all patients with HF refractory to GDMT merit palliative care consultation to address symptoms and map out goals of care (51).
Lastly and arguably most importantly, intensification of care should focus on prescription and titration of GDMT, which has been repeatedly demonstrated to improve HF symptoms and improve morbidity and mortality (17, 52–55). Despite the weight of evidence, most patients with HFrEF are not currently at optimal doses of all four pillars of GDMT (54). Initiation and rapid up-titration of GDMT has a significant impact on reducing HF hospitalizations, regardless of baseline EF or initial biomarkers, as demonstrated in STRONG-HF (32). This rapid up titration model, wherein most of the GDMT adjustment was delivered in the outpatient setting, has been previously described and encouraged and the positive findings are unsurprising, given the significant and additive benefits of each of the 4-components of GDMT for HFrEF, including reductions in future HF hospitalizations and cardiovascular death (9, 53–56).
Optimization to prevent recurrence
The final element of systems of care for outpatient management of decompensated HF is a comprehensive focus on optimizing HF care to prevent recurrence. Interventions demonstrated to improve outcomes for patients with HF include iron infusion, supervised exercise training, and treatment of comorbidities (17, 57–62). These activities are often coordinated by multidisciplinary care teams, including social workers and RN navigators, who have been demonstrated to be valuable partners to help address non-clinical barriers to GDMT optimization and facilitate more patients achieving target doses of GDMT (17, 63, 64).
There is a growing interest in the use of IV ferric carboxymaltose (FCM) in the treatment of iron deficiency anemia in patients with HFrEF, with its use associated with improvement in QOL and six-minute walk distance in CONFIRM-HF and FAIR-HF (57, 59). Most recently, AFFIRM-AHF demonstrated that when used in patients hospitalized for acute HF, IV FCM was associated with a decrease in HF hospitalizations, but did not reduce CV death; and HEART-FID noted no significant difference in their primary outcome- a composite of death, hospitalizations for HF, or change in 6-minute walk distance- with the use of IV FCM in ambulatory patients with HFrEF (58, 65). Both studies note the challenge of interpreting results given the inherent heterogeneity of HF patient populations included, and the need to clarify criteria for iron-deficiency to identify which patients may benefit most (58, 65). Nevertheless, based on the demonstrated safety and potential to improve functional status and quality of life, IV iron supplementation is a Class IIa recommendation for use in patients with HF with iron deficiency (17, 66).
Another important intervention shown to improve quantity and QOL in patients with HF is exercise training, which carries a Class Ia level of recommendation for management of patients with Stage C HF regardless of EF (17). Led by the HF-ACTION trial, which demonstrated that exercise training was safe and carried modest but significant reductions in all-cause mortality or hospitalization and cardiovascular mortality or HF hospitalization, there are numerous additional cardiac rehabilitation studies demonstrating improvement in functional capacity and QOL in the HF population (60–62). Most relevant studies focused on the HFrEF population, but available data suggest a benefit in the HFpEF subset as well, and exercise training should be prescribed for all HF patients who lack a contraindication (60–62, 67).
In addition to these HF-focused interventions, it is important to take a broader and holistic approach to optimization of the care of patients with HF, including consideration of socioeconomic factors and comorbidities that may contribute to HF exacerbations and hospitalizations (17, 68, 69). Consideration of access to food that is low in sodium, transportation to visits, access to medications that are part of GDMT for HF, and evaluating and supporting health literacy are all essential components of reducing disparities in HF care and outcomes that are based in social determinants of health (70). Counseling patients on smoking and alcohol cessation, screening for and treating sleep disorders including obstructive sleep apnea, achieving optimal glycemic control, and enhancing BP management are all important components to prevent recurrence of decompensation in patients with HF (17). Lastly, depression is a comorbid condition commonly plaguing patients with HF and increasing the risk for rehospitalization and all-cause mortality (71). The prevalence of depression in patients with HF is higher than in the general population and limits the ability to perform needed self-care activities (71). Trials have produced little evidence of benefits of psychoactive medications on HF outcome (71). However, in contrast, a meta-analysis of the use of exercise training in HF showed it to be consistently associated with improvement in symptoms of depression, thus providing another reason to encourage increased activity for patients with HF (72).
Future directions
Despite numerous advancements in treatment strategies and therapies, episodes of decompensation remain a detriment to patients with HF and a significant healthcare burden. The goals of outpatient treatment of decompensated HF are to prevent hospitalization and return patients to previous or better clinical status (38). Systems of care that successfully manage decompensated HF in the ambulatory setting include interventions that promote early identification of an episode of decompensation, facilitate timely and appropriate intervention, provide intensification of HF care until resolution of the episode, and then focus on optimization of patients with HF to reduce the risk of future decompensation.
While there have been transformational trials yielding dramatically improved prognosis associated with the use of GDMT and other areas of HF care, significant work is needed to improve access to these therapies and reduce disparities in HF care. Cost, insurance approval, disparities in accessing care related to other social determinants of health, as well as logistics of navigating the healthcare system present barriers to utilization of GDMT, IV FCM, outpatient IV diuretics, and exercise training for patients with HFpEF, despite their potential to improve care and reduce the burden of disease (9, 38, 53, 56, 69, 70, 73). Addressing these factors is of utmost importance given the growing body of evidence demonstrating the impact of socioeconomic status and social determinants of health on cardiovascular outcomes in general and HF outcomes specifically (68–70).
Additionally, sex and race-based disparities with underrepresentation in trials for GDMT and RPM has led to gaps in understanding of potential unique risks and benefits and there has also been under-referral for RPM noted in these populations as well, despite the benefit noted specifically for these populations (24, 74).
Some of the potential interventions aimed at reducing disparities and increasing utilization of therapies for HF include utilizing integrated electronic medical record alerts, multidisciplinary teams with workforce and leadership diversity and clinician education and feedback, which have shown promise in increasing utilization of best practices in HF care and warrant ongoing investigation (26, 63, 64, 75, 76). Ensuring policies are in place that encourage enrollment of women and historically underrepresented populations is critical to improve understanding of the unique benefit of medications, invasive and noninvasive remote monitoring and other interventions aimed at improving outcomes for patients with HF (70, 74).
There is an ongoing opportunity to harvest the benefits of novel technology to improve HF care. The area of artificial intelligence holds great promise with the ability to create multiparameter models with input from invasive and noninvasive sources, that will allow HF care to evolve from identifying decompensation when it occurs to being able to predict and intervene earlier and with greater success (20, 26, 77, 78). Improved understanding of a patient's HF risk profile would allow low risk patients to be successfully managed in the ambulatory setting and would facilitate appropriate allocation of resources to those at highest risk. Related to this is the opportunity to optimize integration of relevant data from RM devices, wearable devices, and testing into the EMR, to allow efficient, standardized and appropriate care for patients with HF, particularly those in evolving decompensation (20, 79, 80).
Lastly, there is continued focus on providing acute HF care outside the hospital environment. While some aspects of this are increasingly available, including outpatient IV and SQ diuretics, and point of care tools like ultrasound and laboratory tests to augment diagnosis and decision making, continued work is needed to determine optimal timing and patient selection and also ensure these important events are recognized with the same prognostic weight as HF hospitalizations (10, 35, 38, 81).
Author contributions
NB: Writing – original draft, Writing – review & editing. SS: Writing – review & editing. CR: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to acknowledge the Dudley Family for their continued contributions and support of the Inova Dudley Family Center for Cardiovascular Innovation and Devon Stuart for her illustrations.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. (2021) 143(8):e254–743. doi: 10.1161/CIR.0000000000000950
2. Urbich M, Globe G, Pantiri K, Heisen M, Bennison C, Wirtz HS, et al. A systematic review of medical costs associated with heart failure in the USA (2014–2020). Pharmacoeconomics. (2020) 38(11):1219–36. doi: 10.1007/s40273-020-00952-0
3. Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. (2007) 154(2):260–6. doi: 10.1016/j.ahj.2007.01.041
4. Blumer V, Mentz RJ, Sun JL, Butler J, Metra M, Voors AA, et al. Prognostic role of prior heart failure hospitalization among patients hospitalized for worsening chronic heart failure. Circ Heart Fail. (2021) 14(4):e007871. doi: 10.1161/CIRCHEARTFAILURE.120.007871
5. Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002–2004: findings from acute decompensated heart failure national registry (ADHERE). Am Heart J. (2007) 153(6):1021–8. doi: 10.1016/j.ahj.2007.03.012
6. van Walraven C, Jennings A, Forster AJ. A meta-analysis of hospital 30-day avoidable readmission rates. J Eval Clin Pract. (2012) 18(6):1211–8. doi: 10.1111/j.1365-2753.2011.01773.x
7. Driscoll A, Meagher S, Kennedy R, Hay M, Banerji J, Campbell D, et al. What is the impact of systems of care for heart failure on patients diagnosed with heart failure: a systematic review. BMC Cardiovasc Disord. (2016) 16(1):195. doi: 10.1186/s12872-016-0371-7
8. Chan WV, Pearson TA, Bennett GC, Cushman WC, Gaziano TA, Gorman PN, et al. ACC/AHA special report: clinical practice guideline implementation strategies: a summary of systematic reviews by the NHLBI implementation science work group: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. (2017) 69(8):1076–92. doi: 10.1016/j.jacc.2016.11.004
9. Ellrodt AG, Fonarow GC, Schwamm LH, Albert N, Bhatt DL, Cannon CP, et al. Synthesizing lessons learned from get with the guidelines: the value of disease-based registries in improving quality and outcomes. Circulation. (2013) 128(22):2447–60. doi: 10.1161/01.cir.0000435779.48007.5c
10. Bozkurt B. Differentiation between worsening heart failure and decompensated heart failure. JACC Heart Fail. (2023) 11(7):859–61. doi: 10.1016/j.jchf.2023.06.001
11. Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. (2007) 116(14):1549–54. doi: 10.1161/CIRCULATIONAHA.107.690768
12. Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, et al. Telemonitoring in patients with heart failure. N Engl J Med. (2010) 363(24):2301–9. doi: 10.1056/NEJMoa1010029
13. Rahimi K, Nazarzadeh M, Pinho-Gomes AC, Woodward M, Salimi-Khorshidi G, Ohkuma T, et al. Home monitoring with technology-supported management in chronic heart failure: a randomised trial. Heart. (2020) 106(20):1573–8. doi: 10.1136/heartjnl-2020-316773
14. Ong MK, Romano PS, Edgington S, Aronow HU, Auerbach AD, Black JT, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition— heart failure (BEAT-HF) randomized clinical trial. JAMA Intern Med. (2016) 176(3):310–8. doi: 10.1001/jamainternmed.2015.7712
15. Mohebali D, Kittleson MM. Remote monitoring in heart failure: current and emerging technologies in the context of the pandemic. Heart. (2021) 107(5):366–72. doi: 10.1136/heartjnl-2020-318062
16. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet. (2018) 392(10152):1047–57. doi: 10.1016/S0140-6736(18)31880-4
17. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79(17):e263–421. doi: 10.1016/j.jacc.2021.12.012
18. Moser DK, Dickson V, Jaarsma T, Lee C, Stromberg A, Riegel B. Role of self-care in the patient with heart failure. Curr Cardiol Rep. (2012) 14(3):265–75. doi: 10.1007/s11886-012-0267-9
19. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Winkler S, et al. Telemedical interventional management in heart failure II (TIM-HF2), a randomised, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalisations and mortality in heart failure patients: study design and description of the intervention. Eur J Heart Fail. (2018) 20(10):1485–93. doi: 10.1002/ejhf.1300
20. Mastoris I, Gupta K, Sauer AJ. The war against heart failure hospitalizations: remote monitoring and the case for expanding criteria. Cardiol Clin. (2023) 41(4):557–73. doi: 10.1016/j.ccl.2023.06.001
21. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. (2011) 377(9766):658–66. doi: 10.1016/S0140-6736(11)60101-3
22. Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, Yadav C, et al. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. (2014) 7(6):935–44. doi: 10.1161/CIRCHEARTFAILURE.113.001229
23. Cowie MR, Simon M, Klein L, Thokala P. The cost-effectiveness of real-time pulmonary artery pressure monitoring in heart failure patients: a European perspective. Eur J Heart Fail. (2017) 19(5):661–9. doi: 10.1002/ejhf.747
24. Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, et al. Haemodynamic-guided management of heart failure 622 (GUIDE-HF): a randomised controlled trial. Lancet. (2021) 398(10304):991–1001. doi: 10.1016/S0140-6736(21)01754-2
25. Harvey M, Seiler A. Challenges in managing a remote monitoring device clinic. Heart Rhythm O2. (2022) 3(1):3–7. doi: 10.1016/j.hroo.2021.12.002
26. Mastoris I, DeFilippis EM, Martyn T, Morris AA, Van Spall HG, Sauer AJ. Remote patient monitoring for patients with heart failure: sex- and race-based disparities and opportunities. Card Fail Rev. (2023) 9:e02. doi: 10.15420/cfr.2022.22
27. Boehmer JP, Hariharan R, Devecchi FG, Smith AL, Molon G, Capucci A, et al. A multisensor algorithm predicts heart failure events in patients with implanted devices: results from the MultiSENSE study. JACC Heart Fail. (2017) 5(3):216–25. doi: 10.1016/j.jchf.2016.12.011
28. Hernandez AF, Albert NM, Allen LA, Ahmed R, Averina V, Boehmer JP, et al. Multiple cardiac sensors for management of heart failure (MANAGE-HF)—phase I evaluation of the integration and safety of the heartlogic multisensor algorithm in patients with heart failure. J Card Fail. (2022) 28(8):1245–54. doi: 10.1016/j.cardfail.2022.03.349
29. D'Onofrio A, Solimene F, Calò L, Calvi V, Viscusi M, Melissano D, et al. Combining home monitoring temporal trends from implanted defibrillators and baseline patient risk profile to predict heart failure hospitalizations: results from the SELENE HF study. EP Europace. (2021) 24(2):234–44. doi: 10.1093/europace/euab170
30. Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1,256 patients: the international collaborative of NT-proBNP study. Eur Heart J. (2006) 27(3):330–7. doi: 10.1093/eurheartj/ehi631
31. Masson S, Latini R, Anand IS, Barlera S, Angelici L, Vago T, et al. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in val-HeFT (valsartan heart failure trial). J Am Coll Cardiol. (2008) 52(12):997–1003. doi: 10.1016/j.jacc.2008.04.069
32. Mebazaa A, Davison B, Chioncel O, Cohen-Solal A, Diaz R, Filippatos G, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. (2022) 400(10367):1938–52. doi: 10.1016/S0140-6736(22)02076-1
33. Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, et al. Effect of natriuretic peptide–guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. (2017) 318(8):713–20. doi: 10.1001/jama.2017.10565
34. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion—a position statement from the heart failure association of the European society of cardiology. Eur J Heart Fail. (2019) 21(2):137–55. doi: 10.1002/ejhf.1369
35. Lee DS, Straus SE, Farkouh ME, Austin PC, Taljaard M, Chong A, et al. Trial of an intervention to improve acute heart failure outcomes. N Engl J Med. (2023) 388(1):22–32. doi: 10.1056/NEJMoa2211680
36. Lee DS, Ross HJ, C.T. Investigators. Trial of an intervention to improve acute heart failure outcomes. Reply. N Engl J Med. (2023) 388(13):e46. doi: 10.1056/NEJMc2301251
37. Nguyen T, Chu H, Miah R, Zeng D. Safe use of loop diuretics in the management of acute decompensated heart failure in the setting of worsening renal function. Am J Ther. (2021) 28(6):e708–21. doi: 10.1097/MJT.0000000000001328
38. Girerd N, Mewton N, Tartière JM, Guijarro D, Jourdain P, Damy T, et al. Practical outpatient management of worsening chronic heart failure. Eur J Heart Fail. (2022) 24(5):750–61. doi: 10.1002/ejhf.2503
39. Khan WJ, Arriola-Montenegro J, Mutschler MS, Bensimhon D, Halmosi R, Toth K, et al. A novel opportunity to improve heart failure care: focusing on subcutaneous furosemide. Heart Fail Rev. (2023) 28(6):1315–23. doi: 10.1007/s10741-023-10331-4
40. Wierda E, Dickhoff C, Handoko ML, Oosterom L, Kok WE, de Rover Y, et al. Outpatient treatment of worsening heart failure with intravenous and subcutaneous diuretics: a systematic review of the literature. ESC Heart Fail. (2020) 7(3):892–902. doi: 10.1002/ehf2.12677
41. Wierda E, van Maarschalkerwaart WWA, van Seumeren E, Dickhoff C, Montanus I, de Boer D, et al. Outpatient treatment of worsening heart failure with intravenous diuretics: first results from a multicentre 2-year experience. ESC Heart Fail. (2023) 10(1):594–600. doi: 10.1002/ehf2.14168
42. Buckley LF, Carter DM, Matta L, Cheng JW, Stevens C, Belenkiy RM, et al. Intravenous diuretic therapy for the management of heart failure and volume overload in a multidisciplinary outpatient unit. JACC Heart Fail. (2016) 4(1):1–8. doi: 10.1016/j.jchf.2015.06.017
43. Buckley LF, Stevenson LW, Cooper IM, Knowles DM, Matta L, Molway DW, et al. Ambulatory treatment of worsening heart failure with intravenous loop diuretics: a four-year experience. J Card Fail. (2020) 26(9):798–9. doi: 10.1016/j.cardfail.2019.10.015
44. Rosner CM, Lee SB, Badrish N, Maini AS, Young KD, Vorgang CM, et al. Advanced practice provider urgent outpatient clinic for patients with decompensated heart failure. J Card Fail. (2023) 29(4):536–9. doi: 10.1016/j.cardfail.2022.11.015
45. Perera D, Clayton T, O'Kane PD, Greenwood JP, Weerackody R, Ryan M, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. (2022) 387(15):1351–60. doi: 10.1056/NEJMoa2206606
46. Parikh PB, Bhatt DL, Bhasin V, Anker SD, Skopicki HA, Claessen BE, et al. Impact of percutaneous coronary intervention on outcomes in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol. (2021) 77(19):2432–47. doi: 10.1016/j.jacc.2021.03.310
47. Panizo JG, Barra S, Mellor G, Heck P, Agarwal S. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. (2018) 7(2):128–34. doi: 10.15420/aer.2018.23.2
48. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. (2018) 378(5):417–27. doi: 10.1056/NEJMoa1707855
49. Parkash R, Wells GA, Rouleau J, Talajic M, Essebag V, Skanes A, et al. Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation: results from the RAFT-AF trial. Circulation. (2022) 145(23):1693–704. doi: 10.1161/CIRCULATIONAHA.121.057095
50. Sohns C, Fox H, Marrouche NF, Crijns HJGM, Costard-Jaeckle A, Bergau L, et al. Catheter ablation in end-stage heart failure with atrial fibrillation. N Engl J Med. (2023) 389(15):1380–9. doi: 10.1056/NEJMoa2306037
51. Allen LA, Stevenson LW, Grady KL, Goldstein NE, Matlock DD, Arnold RM, et al. Decision making in advanced heart failure. Circulation. (2012) 125(15):1928–52. doi: 10.1161/CIR.0b013e31824f2173
52. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. (2018) 72(4):351–66. doi: 10.1016/j.jacc.2018.04.070
53. Greene SJ, Butler J, Fonarow GC. Simultaneous or rapid sequence initiation of quadruple medical therapy for heart failure—optimizing therapy with the need for speed. JAMA Cardiol. (2021) 6(7):743–4. doi: 10.1001/jamacardio.2021.0496
54. Luo N, Fonarow GC, Lippmann SJ, Mi X, Heidenreich PA, Yancy CW, et al. Early adoption of sacubitril/valsartan for patients with heart failure with reduced ejection fraction: insights from get with the guidelines-heart failure (GWTG-HF). JACC Heart Fail. (2017) 5(4):305–9. doi: 10.1016/j.jchf.2016.12.018
55. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. (1999) 341(10):709–17. doi: 10.1056/NEJM199909023411001
56. Fiuzat M, Ezekowitz J, Alemayehu W, Westerhout CM, Sbolli M, Cani D, et al. Assessment of limitations to optimization of guideline-directed medical therapy in heart failure from the GUIDE-IT trial: a secondary analysis of a randomized clinical trial. JAMA Cardiol. (2020) 5(7):757–64. doi: 10.1001/jamacardio.2020.0640
57. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. (2009) 361(25):2436–48. doi: 10.1056/NEJMoa0908355
58. Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, et al. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. (2020) 396(10266):1895–904. doi: 10.1016/S0140-6736(20)32339-4
59. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, Ertl G, Komajda M, Mareev V, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency†. Eur Heart J. (2015) 36(11):657–68. doi: 10.1093/eurheartj/ehu385
60. Haykowsky MJ, Timmons MP, Kruger C, McNeely M, Taylor DA, Clark AM. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am J Cardiol. (2013) 111(10):1466–9. doi: 10.1016/j.amjcard.2013.01.303
61. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. (2009) 301(14):1439–50. doi: 10.1001/jama.2009.454
62. Kitzman DW, Whellan DJ, Duncan P, Pastva AM, Mentz RJ, Reeves GR, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. (2021) 385(3):203–16. doi: 10.1056/NEJMoa2026141
63. Heidenreich PA, Fonarow GC, Breathett K, Jurgens CY, Pisani BA, Pozehl BJ, et al. 2020 ACC/AHA clinical performance and quality measures for adults with heart failure: a report of the American college of cardiology/American heart association task force on performance measures. J Am Coll Cardiol. (2020) 76(21):2527–64. doi: 10.1016/j.jacc.2020.07.023
64. Driscoll A, Currey J, Tonkin A, Krum H. Nurse-led titration of angiotensin converting enzyme inhibitors, beta-adrenergic blocking agents, and angiotensin receptor blockers for people with heart failure with reduced ejection fraction. Cochrane Database Syst Rev. (2015) 2015(12):Cd009889. doi: 10.1002/14651858.CD009889.pub2
65. Mentz RJ, Garg J, Rockhold FW, Butler J, De Pasquale CG, Ezekowitz JA, et al. Ferric carboxymaltose in heart failure with iron deficiency. N Engl J Med. (2023) 389(11):975–86. doi: 10.1056/NEJMoa2304968
66. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
67. Sachdev V, Sharma K, Keteyian SJ, Alcain CF, Desvigne-Nickens P, Fleg JL, et al. Supervised exercise training for chronic heart failure with preserved ejection fraction: a scientific statement from the American heart association and American college of cardiology. Circulation. (2023) 147(16):e699–715. doi: 10.1161/CIR.0000000000001122
68. Mathews L, Ding N, Mok Y, Shin JI, Crews DC, Rosamond WD, et al. Impact of socioeconomic status on mortality and readmission in patients with heart failure with reduced ejection fraction: the ARIC study. J Am Heart Assoc. (2022) 11(18):e024057. doi: 10.1161/JAHA.121.024057
69. Teng TK, Tay WT, Richards AM, Chew TSM, Anand I, Ouwerkerk W, et al. Socioeconomic status and outcomes in heart failure with reduced ejection fraction from Asia. Circ Cardiovasc Qual Outcomes. (2021) 14(4):e006962. doi: 10.1161/CIRCOUTCOMES.120.006962
70. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, et al. Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American heart association. Circulation. (2015) 132(9):873–98. doi: 10.1161/CIR.0000000000000228
71. Sbolli M, Fiuzat M, Cani D, O'Connor CM. Depression and heart failure: the lonely comorbidity. Eur J Heart Fail. (2020) 22(11):2007–17. doi: 10.1002/ejhf.1865
72. Tu RH, Zeng ZY, Zhong GQ, Wu WF, Lu YJ, Bo ZD, et al. Effects of exercise training on depression in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Eur J Heart Fail. (2014) 16(7):749–57. doi: 10.1002/ejhf.101
73. Halatchev IG, Wu WC, Heidenreich PA, Djukic E, Balasubramanian S, Ohlms KB, et al. Inpatient versus outpatient intravenous diuresis for the acute exacerbation of chronic heart failure. Int J Cardiol Heart Vasc. (2021) 36:100860. doi: 10.1016/j.ijcha.2021.100860
74. Markson F, Abe TA, Adedinsewo D, Olanipekun T, Shamaki GR, Kesiena O, et al. Sex differences in CardioMEMS utilization and impact on readmissions and mortality in heart failure patients. JACC Heart Fail. (2023) 11(12):1760–2. doi: 10.1016/j.jchf.2023.08.021
75. Psotka MA, Fiuzat M, Solomon SD, Chauhan C, Felker GM, Butler J, et al. Challenges and potential improvements to patient access to pharmaceuticals: examples from cardiology. Circulation. (2020) 142(8):790–8. doi: 10.1161/CIRCULATIONAHA.119.044976
76. Ghazi L, Yamamoto Y, Riello RJ, Coronel-Moreno C, Martin M, O'Connor KD, et al. Electronic alerts to improve heart failure therapy in outpatient practice: a cluster randomized trial. J Am Coll Cardiol. (2022) 79(22):2203–13. doi: 10.1016/j.jacc.2022.03.338
77. Averbuch T, Sullivan K, Sauer A, Mamas MA, Voors AA, Gale CP, et al. Applications of artificial intelligence and machine learning in heart failure. Eur Heart J Digit Health. (2022) 3(2):311–22. doi: 10.1093/ehjdh/ztac025
78. Sherrod CF, Farr SL, Sauer AJ. Overcoming treatment inertia for patients with heart failure: how do we build systems that move US from rest to motion? Eur Heart J. (2023) 44(22):1970–2. doi: 10.1093/eurheartj/ehad169
79. Al-Alusi MA, Khurshid S, Wang X, Venn RA, Pipilas D, Ashburner JM, et al. Trends in consumer wearable devices with cardiac sensors in a primary care cohort. Circ Cardiovasc Qual Outcomes. (2022) 15(7):e008833. doi: 10.1161/CIRCOUTCOMES.121.008833
80. Petek BJ, Al-Alusi MA, Moulson N, Grant AJ, Besson C, Guseh JS, et al. Consumer wearable health and fitness technology in cardiovascular medicine: JACC state-of-the-art review. J Am Coll Cardiol. (2023) 82(3):245–64. doi: 10.1016/j.jacc.2023.04.054
Keywords: heart failure, decompensated heart failure, ambulatory, systems of care, remote monitoring
Citation: Badrish N, Sheifer S and Rosner CM (2024) Systems of care for ambulatory management of decompensated heart failure. Front. Cardiovasc. Med. 11:1350846. doi: 10.3389/fcvm.2024.1350846
Received: 6 December 2023; Accepted: 25 January 2024;
Published: 21 February 2024.
Edited by:
Lauren Cooper, Northwell Health, United StatesReviewed by:
Afsana Rahman, Columbia University, United States© 2024 Badrish, Sheifer and Rosner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolyn M. Rosner Y2Fyb2x5bi5yb3NuZXJAaW5vdmEub3Jn
 Narotham Badrish
Narotham Badrish Stuart Sheifer1,2
Stuart Sheifer1,2 Carolyn M. Rosner
Carolyn M. Rosner