- 1Core Diagnostics, Abbott Laboratories, Abbott Park, IL, United States
- 2Inova Schar Heart and Vascular, Falls Church, VA, United States
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that block immune checkpoints and therefore activate immune cells, allowing them to recognize and attack cancer cells. ICIs have revolutionized oncology practice, but their use has been complicated by immune-related adverse events (irAEs). Of cardiovascular (CV) irAEs, ICI-related myocarditis has received significant attention due to high mortality rates, ranging from 25% to 50%, despite its overall low incidence. Establishing the early diagnosis of ICI-myocarditis is important for early initiation of steroids and consideration of hospitalization in patients who are at risk for hemodynamic compromise and need high acuity care in a tertiary setting. In this review, we summarize the diagnostic and prognostic tools for ICI-myocarditis, including electrocardiography, echocardiography, cardiac magnetic resonance imaging, with emphasis on circulating biomarkers. Cardiac troponins (cTns) are an essential component of the diagnosis of ICI-myocarditis, and we provide a summary of the recent studies that utilized different assays (cTnI vs. cTnT) and outcomes (diagnosis vs. prognosis including major adverse cardiac outcomes). With the exponential increase in ICI use across different oncology indications, there is a major need to include biomarkers in risk stratification to guide diagnosis and treatment. Our review proposes a framework for future multisite registries, including cTn evaluation at baseline and at the time of irAE suspicion, with development of central biobanking to allow head-to-head evaluation and clinical validation of different biomarker assays in ICI-myocarditis. This approach, with the inclusion of CV biomarkers into clinical and pragmatic oncology trials, holds promise to improve the early recognition and management of ICI-myocarditis and CV irAEs, thus leading to better outcomes.
1 Immune checkpoint inhibitors in oncology and immune related adverse events
Immuno-oncology (IO) is a form of cancer treatment that utilizes the body's own immune system to recognize and target cancer cells. One of the key IO approaches involves the use of immune checkpoint inhibitors (ICIs). Immune checkpoints (ICs) are molecules present on the immune cells that regulate responses to antigens and in physiologic situations prevent immune system overactivation. Many cancers have the ability of binding to ICs to decrease the immune response and evade immune surveillance. ICIs are monoclonal antibodies that block the ICs and therefore activate immune cells, allowing them to recognize and attack cancer cells. Currently approved ICIs target two prominent IC pathways: (1) Programmed Cell Death Protein 1 (PD-1) signaling by binding and blocking PD-1 receptors (e.g., nivolumab, pembrolizumab, and cemiplimab) or PD-ligand 1 [(PD-L1), e.g., atezolizumab, avelumab, and durvalumab] and (2) Cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA-4) pathways by binding and blocking CTLA-4 (e.g., ipilimumab).
ICIs have revolutionized oncology practice as multiple agents have been approved in treatment of different cancers in early, advanced, and metastatic settings (1). In 2022 there were more than 85 oncology indications for the 7 Food and Drug Administration (FDA)-approved antibodies targeting PD-1/PD-L11 pathways (2) and an analysis in 2019 indicated that more than a third of all patients with invasive cancer diagnoses in the US would be eligible to receive an ICI (3). The use of ICIs has been complicated by immune-related adverse events (irAEs) which result from overactivation of the immune system and may affect any organ and/or system. While irAEs differ widely in their clinical presentations, rapidly evolving and severe symptoms have been reported requiring prompt recognition and urgent treatment most often with steroids (4). Of cardiovascular (CV) irAEs, myocarditis has received the most attention due to its very high morbidity and reported mortality of 25%–50% in clinically symptomatic patients (5). The incidence of ICI-(related) myocarditis is low, ranging from 0.6% to 2.1% depending on the immunotherapy combination used, cancer type, and study design (5). The underlying mechanisms remain to be fully elucidated, but lymphocytic infiltrates in the myocardium point to T-cell mediated processes (6). Other CV events reported in ICI clinical trials have included pericardial disease, acute coronary syndrome (ACS), arrhythmias, and non-myocarditis related cardiac dysfunction (7) suggesting that different mechanisms may be underlying these clinical presentations. In this review we focus on ICI-myocarditis but emphasize the importance of the differential diagnoses and recognition of all irAEs.
2 Clinical presentation and diagnosis of ICI-myocarditis
Patients may present with a variety of symptoms including chest pain, dyspnea, fatigue, and/or palpitations, often mimicking ACS and/or heart failure (HF). Clinical features favoring ICI-myocarditis include recent initiation of ICI, most often within 30–60 days prior, and presence of other irAEs, (e.g., myositis, myasthenia gravis, pneumonitis, and/or hepatitis). The co-existence of severe myocarditis with myositis and/or myasthenia gravis has been reported (8, 9) and is recognized as clustered toxicity with recommendations for comprehensive evaluation when any one of the three conditions is found (10).
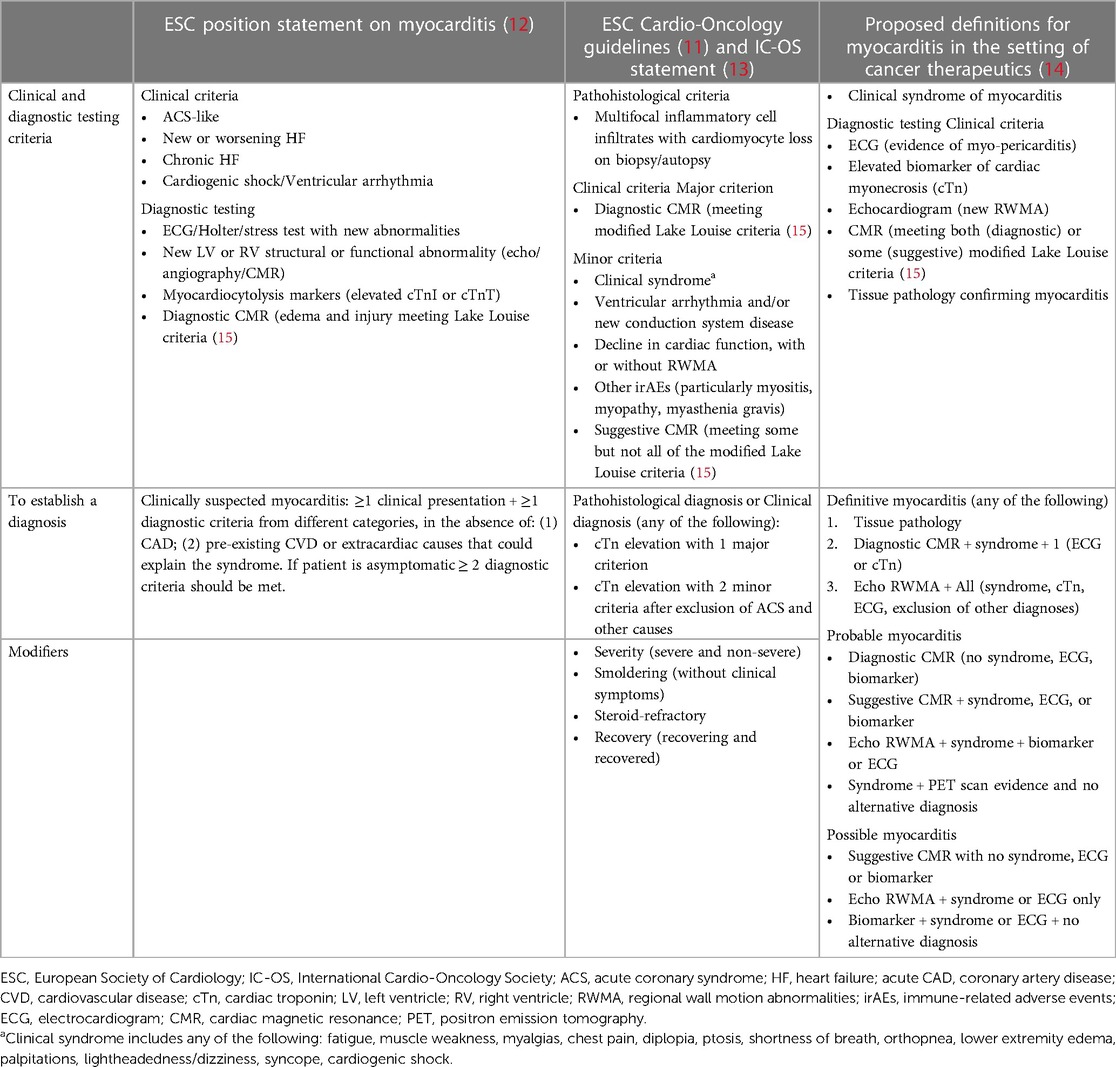
Currently recommended initial tests in patients with suspected ICI-myocarditis include electrocardiography (ECG), cardiac troponins (cTns), natriuretic peptides (NPs), and echocardiography (11). While there is general agreement about inclusion of these key clinical measures for the diagnosis of ICI-myocarditis (Table 1), there are significant variations in the definitions, reflecting the lack of high-quality data as well as the rapidly evolving field (10). Key characteristics of these diagnostic tests are summarized below followed by detailed discussion of circulating biomarkers.
3 Electrocardiography
A variety of ECG findings have been reported in patients with ICI-myocarditis, varying from life-threatening heart block, ventricular and atrial arrythmias and ST-elevation, to nonspecific ST-T wave abnormalities. ECG is usually the first test performed in a symptomatic patient and ECG abnormalities may overlap with those of ACS, requiring investigation of ischemia prior to being attributed to myocarditis. With regards to its prognostic value, retrospective analyses found associations between pathological Q-waves and mortality (16) and between QRS prolongation and major adverse CV events (MACE) (17) in patients with ICI-myocarditis.
4 Echocardiography
While reduced left ventricular systolic function and regional wall motion abnormalities (RWMA) on the echocardiogram can occur, a normal left ventricular ejection fraction (LVEF) has been demonstrated in more than 50% of patients with confirmed ICI-myocarditis, indicating that the presence of normal LVEF cannot exclude the diagnosis (18). In a retrospective analysis (19) including 140 patients with ICI-myocarditis, the presence of decreased global longitudinal strain (GLS) was a predictor of MACE regardless of LVEF (19); similar findings have been reported using global radial and circumferential strain (20). Finally, in a surveillance study among 129 patients who received ICIs, a decline in GLS correlated with elevation in high sensitivity (hs) cTnI suggesting that GLS is associated with myocyte injury (21). Abnormal GLS is associated with multiple cardiac conditions (15) and echocardiography is not consistently performed in the baseline evaluation of patients receiving ICIs, thus assessing an interval decline in GLS may be challenging when toxicity is suspected. Therefore, further research into the role of GLS for risk stratification and diagnosis of ICI-myocarditis is needed.
5 Cardiac magnetic resonance (CMR) imaging
CMR is the gold standard imaging methodology for diagnosis of myocarditis, providing visualization of edema and inflammation. The modified Lake Louise criteria (22) require confirmation of an abnormality in T2-weighted images indicating myocardial edema (T2-based criterion), and T1-based criterion indicating myocardial injury (e.g., increased myocardial T1 map value, increased extracellular volume, or positive late gadolinium enhancement) to establish the CMR diagnosis of acute myocarditis. These cardiac imaging criteria have been incorporated into the International Cardio-Oncology Society (IC-OS) consensus statement on definitions of CV toxicities (13) and included in the European Society of Cardiology (ESC) Guidelines on cardio-oncology (11) as well as other documents (12, 14) (Table 1). In the ESC algorithm, diagnostic CMR constitutes a major clinical criterion and its presence in addition to elevation of cTn with an appropriate clinical scenario is diagnostic of ICI-myocarditis (11). However, the sensitivity of CMR criteria has been questioned in a study demonstrating that less than 30% of patients with confirmed ICI-myocarditis met Lake Louise criteria (23) leading to a recommendation that endomyocardial biopsy should be pursued in patients with negative CMR and clinical suspicion for ICI-myocarditis (10, 11). Among patients diagnosed with ICI-myocarditis, abnormal T1-values, quantitated by T1-mapping, were predictive of subsequent MACE, pointing to its potential role in risk stratification of these patients (24).
6 Circulating biomarkers
6.1 Cardiac troponins (cTn)
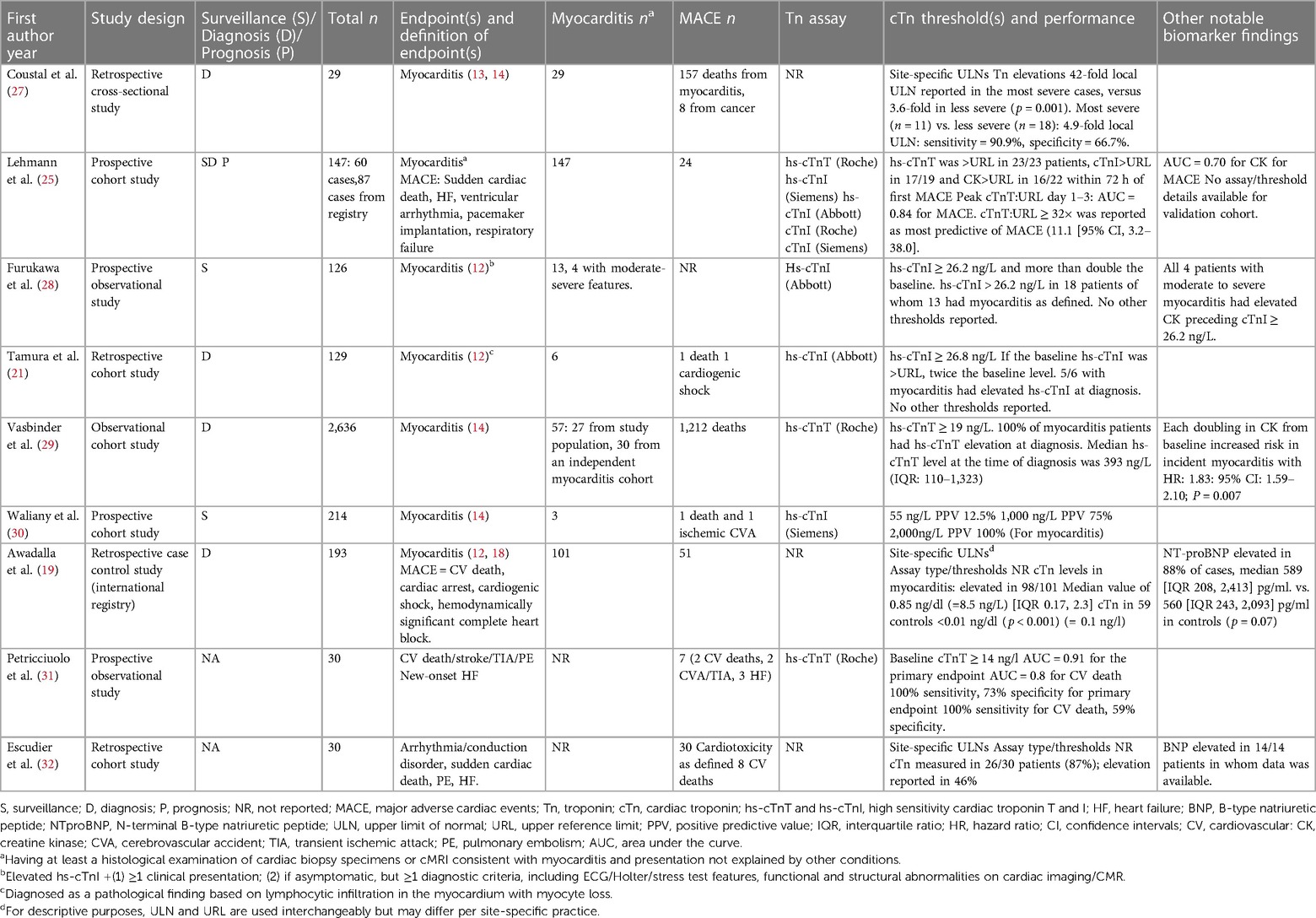
Though elevated cTn levels are considered necessary for the diagnosis of myocarditis, other etiologies that require immediate investigation must also be considered. The degree of elevation and presence or absence of a rising/falling pattern of cTnI and T provide important insights, as persistently elevated cTn is typically seen in ICI-related myocarditis, but rapid rising may be related to an ACS (25). Furthermore, cTnI and T levels are often many folds higher than the upper reference limit [(URL), typically defined by the manufacturer as the 99th percentile of a healthy general population]. Substantial variability has been noted based on the specific cTn assay and timing of sample procurement (26), however these issues have been difficult to reconcile given the low frequency myocarditis and heterogeneity of assays used in practice. Unique cut-offs still need to be validated to optimize negative predictive value (NPV) and higher thresholds that optimize positive predictive value (PPV) both at baseline and with serial assessments. Table 2 summarizes the literature by different clinical scenarios highlighted below.
6.1.1 cTn surveillance in asymptomatic patients
Screening for cardiac injury and assessing risk for subsequent symptomatic ICI-myocarditis could be feasible, but there are unique issues to this patient population. For example, a single institution study of prospective surveillance in 214 patients demonstrated the need to test 72 patients receiving ICI therapies to detect 1 case of myocarditis based on the hs-cTnI URL (30). The PPV at the URL (55 ng/L) was only 12.5% and a PPV of 75% required hs-cTnI threshold value of 1000 ng/L.
6.1.2 cTn for diagnosis in symptomatic patients
cTn elevations of any extent have been reported in over 94% of patients with ICI-myocarditis (18, 21). A case series of 29 patients with ICI myocarditis reported elevations 42-fold the URL in severe cases, vs. 3.6-fold in less severe (27). In another study, cTn values correlated with myocardial histopathology and hs-cTnT values exceeding 300 ng/L (URL 19 ng/L) were found more frequently among patients with higher degrees of T-cell infiltration (33).
6.1.3 cTn for prognosis of MACE in patients with ICI-myocarditis
In a multicenter study that investigated 35 patients with ICI-myocarditis, higher cTnT was associated with MACE, and 10-fold higher median cTnT values were reported in patients with MACE compared to patients without (1,450 vs. 140 ng/L, respectively) (18). In another study of patients with ICI-myocarditis, presence of elevated hs-cTnT:URL ratio of >32 within 3 days of presentation, was associated with a hazard ratio of 11 (95% CI, 3–38) for MACE (25). In this investigation, MACE definition included all myotoxicity with respiratory failure reported in 50% of patients with MACE, raising a question whether the prognostic value of cTnT may reflect its sensitivity to detect myotoxicity in addition to cardiotoxicity (25, 34). Supporting this hypothesis, mRNA expression of cTnT, but not cTnI, was found in the skeletal muscle in patients with ICI myositis (25) indicating a need for further research of clinical significance of cTnT in detecting and monitoring systemic myotoxicity.
6.1.4 Clinical caveats for applying cTns in surveillance, diagnosis and prognostication of ICI-myocarditis
In general population cohorts, differences in associations with outcomes have been reported for low-grade elevations of hs-cTns: cTnI was associated with myocardial infarction (MI) and elevated cTnT was more strongly associated with all-cause mortality and non-CV death (35). Increased cTnT has also been found in the presence of skeletal muscle damage (36–38), which is similar to the observations in cardio-oncology literature where elevations in cTnT correlated with concomitant ICI-related myositis and myocarditis (25).
In patients with ICI-myocarditis, hs-cTnI has been noted to rise and fall more rapidly than cTnT (39), leading to recommendations for its preferential use in the initial assessment and diagnosis of ICI-myocarditis (10, 11), with cTnT having additional prognostic and potentially diagnostic utility for skeletal muscle myotoxicity.
The phenomenon of macrotroponin (macroTn) indicating formation of immunoglobulin-troponin complexes, is also coming to attention in the ICI population, where immune activation may result in autoantibody binding to circulating cTn, forming a macrocomplex. Initially considered a spurious cTn result finding, macroTn has been reported with all cTn assays and has been associated with myocarditis and cardiomyopathy (40–42). While clinical implications of macroTn in ICI-myocarditis remain an area of active investigation, elevated cTn must always be interpreted in conjunction with clinical context. If inconsistent, the laboratory should be consulted, as further analytic methodologies can be applied to investigate for macrocomplexes, and verification sought with another cTn assay.
6.2 Natriuretic peptides
Concomitant elevation of natriuretic peptides (B-type natriuretic peptide [BNP] and amino terminal proBNP [NT-proBNP]) is common in ICI-myocarditis. Elevated NT-proBNP was present in 88% of 83 patients with ICI-related myocarditis in one study (19), however NT-proBNP values were not significantly different in patients with subsequent MACE compared to patients without MACE. In another small surveillance study of 126 patients receiving ICI, BNP was elevated in 11 patients, in some possibly reflecting presence of baseline cardiomyopathy (28).
6.3 Creatine kinase
Elevations in CK and CK-MB have been utilized for diagnosis (29) and surveillance of ICI-myocarditis (28). Rising CK levels that predate elevations in cTns in ICI-myocarditis have been noted (28, 29), although CK and CK-MB are generally less sensitive and specific for myocardial injury. When peak biomarker levels measured within 3 days of admission in 57 patients with ICI-myocarditis were compared, hs-cTnT:URL was found superior to CK:URL ratio in predicting MACE 24.
7 Future directions
With expanding indications for IO therapies, the need for accurate diagnosis of ICI-myocarditis and irAEs will continue to increase. Current diagnostic criteria rely on the detection of new diagnostic test abnormalities (e.g., new cTn increase, new RWMA, and/or new T1/T2 abnormality on CMR) which may be difficult to ascertain in absence of baseline values. The relevance of pre-treatment assessment is further emphasized in older individuals many of whom may have prior CV conditions, including MI or HF, and in whom differentiation of acute from chronic myocardial injury creates a particular challenge. At the present time baseline cardiac testing is not included in routine oncology practice (43) although it has been recommended in the ESC guidelines (11). Prospective studies evaluating circulating biomarkers at baseline (pre-treatment) and at the time of clinical suspicion are needed to further refine the diagnostic criteria and provide insight about the extent of myocardial injury in an individual patient (Figure 1). Adoption of different biomarker thresholds to identify risk for CV irAE may ultimately be needed for each assay, similar to requirements for the diagnosis of acute MI. This approach will also allow us to identify predictors of risk that should guide further diagnostic and treatment steps. In addition to cTns, CK and natriuretic peptides, novel biomarkers will be needed to elucidate the role of inflammation, metabolic and immune system derangement in pathogenesis of myocardial injury and other irAEs (44). Beyond ICI-myocarditis and irAEs, prospective investigations are needed to understand the association between ICI use and progression of atherosclerosis and plaque vulnerability which have been reported in the retrospective studies (45). Central biobanking of multisite registries incorporating baseline and serial sampling would allow more reliable, head-to-head evaluation of different cTn assays as well as investigation and clinical validation of novel biomarkers. Finally, inclusion of CV biomarker investigations into IO clinical and pragmatic trials holds promise to improve the early recognition and management of cardiotoxicity and lead to better outcomes.
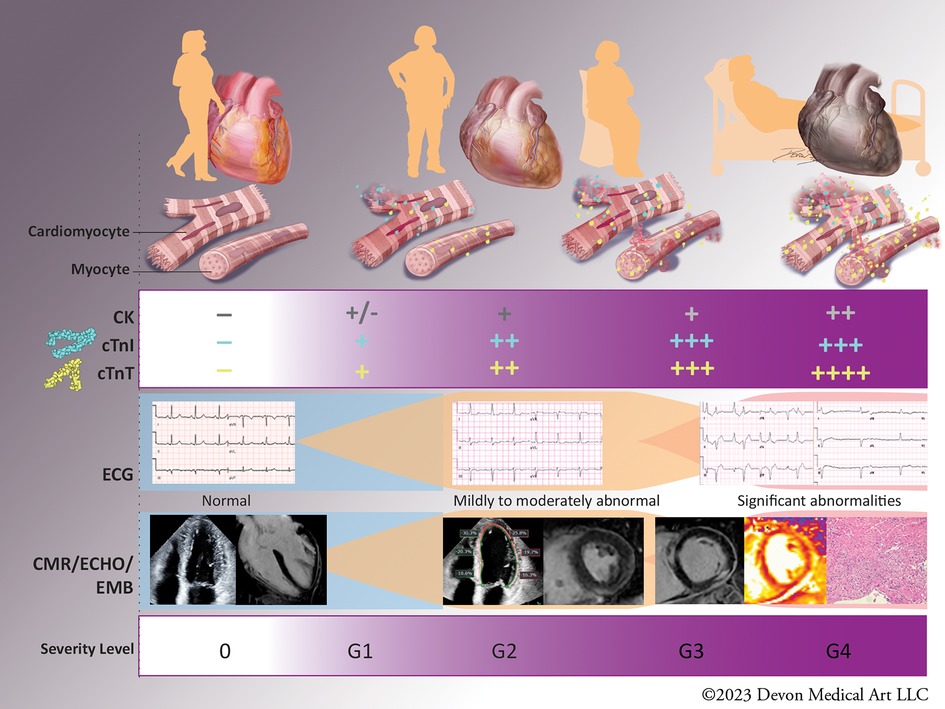
Figure 1. Biomarkers in diagnosis and severity of immune checkpoint inhibitor (ICI)-myocarditis. The diagnosis of ICI-myocarditis relies on clinical presentation, circulating biomarkers, cardiac imaging and endomyocardial biopsy in select cases. Mild elevations of cardiac troponins (cTns) have been described in asymptomatic patients without abnormalities in cardiac imaging (Grade 1, subclinical ICI-myocarditis), while patients with Grade 2 or mild ICI-myocarditis have abnormal cTns and some abnormalities on electrocardiogram (ECG), echocardiogram (ECHO), and/or cardiac magnetic resonance (CMR). Patients with moderate (Grade 3) or severe (Grade 4) ICI-myocarditis have clinical symptoms and often present with concomitant myositis reflected in increase in creatine kinase (CK) and cTnT. Severity of biomarker abnormalities has been shown to correlate with the adverse outcomes, however the exact cut-off values remain to be determined. “-”: values below the upper reference limit“; “+”: increments above the upper reference limit; CK, creatine kinase; hs-cTnI, high sensitivity cardiac troponin I; hs-cTnT, high sensitivity cardiac troponin T; ECG, electrocardiogram; ECHO, echocardiogram; CMR, cardiac magnetic resonance; EMB, endomyocardial biopsy; G1-4, Grade1-4.
Author contributions
GM: Conceptualization, Writing – review & editing. CF: Writing – review & editing. QZ: Writing – review & editing. AB: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank Dwight and Martha Schar for their generous support of Inova Schar Heart and Vascular, and the Dudley Family for their continued contributions and support of the Inova Dudley Family Center for Cardiovascular Innovation.
Conflict of interest
GM: Fulltime employee and shareholder of Abbott Laboratories. CF: Consulting: Abbott, FujiRebio, Quidel:Ortho, Roche Diagnostics, Siemens. Speaker: Pathfast. Grants/contracts as PI: Abbott, FujiRebio, Quidel:Ortho, Roche Siemens, NIH. Adjudication committee: Siemens, Tosoh. Patent: #10509044, Methods for assessing differential risk for developing heart failure. AB: Advisory board: Astra-Zeneca, DSMB: CTI Biopharma.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tan S, Day D, Nicholls SJ, Segelov E. Immune checkpoint inhibitor therapy in oncology: current uses and future directions: JACC: CardioOncology state-of-the-art review. JACC CardioOncol. (2022) 4:579–97. doi: 10.1016/j.jaccao.2022.09.004
2. Beaver JA, Pazdur R. The wild west of checkpoint inhibitor development. N Engl J Med. (2022) 386:1297–301. doi: 10.1056/NEJMp2116863
3. Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open. (2020) 3:e200423. doi: 10.1001/jamanetworkopen.2020.0423
4. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. (2021) 9:e002435. doi: 10.1136/jitc-2021-002435
5. Thuny F, Naidoo J, Neilan TG. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur Heart J. (2022) 43:4458–68. doi: 10.1093/eurheartj/ehac456
6. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. (2020) 9:e013757. doi: 10.1161/JAHA.119.013757
7. Rubio-Infante N, Ramírez-Flores YA, Castillo EC, Lozano O, García-Rivas G, Torre-Amione G. Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis. Eur J Heart Fail. (2021) 23:1739–47. doi: 10.1002/ejhf.2289
8. Pathak R, Katel A, Massarelli E, Villaflor VM, Sun V, Salgia R. Immune checkpoint inhibitor-induced myocarditis with myositis/myasthenia gravis overlap syndrome: a systematic review of cases. Oncologist. (2021) 26:1052–61. doi: 10.1002/onco.13931
9. Johnson DB, Manouchehri A, Haugh AM, Quach HT, Balko JM, Lebrun-Vignes B, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer. (2019) 7:134. doi: 10.1186/s40425-019-0617-x
10. Naidoo J, Murphy C, Atkins MB, Brahmer JR, Champiat S, Feltquate D, et al. Society for immunotherapy of cancer (SITC) consensus definitions for immune checkpoint inhibitor-associated immune-related adverse events (irAEs) terminology. J Immunother Cancer. (2023) 11:e006398. doi: 10.1136/jitc-2022-006398
11. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA). Eur Heart J (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. (2022) 43:4229–361. doi: 10.1093/eurheartj/ehac244
12. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. (2013) 34:2636–48. doi: 10.1093/eurheartj/eht210
13. Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. (2022) 43:280–99. doi: 10.1093/eurheartj/ehab674
14. Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. (2019) 140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497
15. Asch FM, Miyoshi T, Addetia K, Citro R, Daimon M, Desale S, et al. Similarities and differences in left ventricular size and function among races and nationalities: results of the world alliance societies of echocardiography normal values study. J Am Soc Echocardiogr. (2019) 32:1396–1406.e2. doi: 10.1016/j.echo.2019.08.012
16. Power JR, Alexandre J, Choudhary A, Ozbay B, Hayek S, Asnani A, et al. Electrocardiographic manifestations of immune checkpoint inhibitor myocarditis. Circulation. (2021) 144:1521–3. doi: 10.1161/CIRCULATIONAHA.121.055816
17. Zlotoff DA, Hassan MZO, Zafar A, Alvi RM, Awadalla M, Mahmood SS, et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J Immunother Cancer. (2021) 9:e002007. doi: 10.1136/jitc-2020-002007
18. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. (2018) 71:1755–64. doi: 10.1016/j.jacc.2018.02.037
19. Awadalla M, Mahmood SS, Groarke JD, Hassan MZO, Nohria A, Rokicki A, et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol. (2020) 75:467–78. doi: 10.1016/j.jacc.2019.11.049
20. Quinaglia T, Gongora C, Awadalla M, Hassan MZO, Zafar A, Drobni ZD, et al. Global circumferential and radial strain among patients with immune checkpoint inhibitor myocarditis. JACC Cardiovasc Imaging. (2022) 15:1883–96. doi: 10.1016/j.jcmg.2022.06.014
21. Tamura Y, Tamura Y, Takemura R, Yamada K, Taniguchi H, Iwasawa J, et al. Longitudinal strain and troponin I elevation in patients undergoing immune checkpoint inhibitor therapy. JACC CardioOncol. (2022) 4:673–85. doi: 10.1016/j.jaccao.2022.10.007
22. Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. (2018) 72:3158–76. doi: 10.1016/j.jacc.2018.09.072
23. Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. (2020) 41:1733–43. doi: 10.1093/eurheartj/ehaa051
24. Thavendiranathan P, Zhang L, Zafar A, Drobni ZD, Mahmood SS, Cabral M, et al. Myocardial T1 and T2 mapping by magnetic resonance in patients with immune checkpoint inhibitor-associated myocarditis. J Am Coll Cardiol. (2021) 77:1503–16. doi: 10.1016/j.jacc.2021.01.050
25. Lehmann LH, Heckmann MB, Bailly G, Finke D, Procureur A, Power JR, et al. Cardiomuscular biomarkers in the diagnosis and prognostication of immune checkpoint inhibitor myocarditis. Circulation. (2023) 148:473–86. doi: 10.1161/CIRCULATIONAHA.123.062405
26. Jaffe AS. Biomarkers for immune checkpoint inhibitor-induced myocarditis: caution needed. JACC CardioOncol. (2022) 4:701–3. doi: 10.1016/j.jaccao.2022.11.014
27. Coustal C, Vanoverschelde J, Quantin X, Lesage C, Michot JM, Lappara A, et al. Prognosis of immune checkpoint inhibitors-induced myocarditis: a case series. J Immunother Cancer. (2023) 11:e004792. doi: 10.1136/jitc-2022-004792
28. Furukawa A, Tamura Y, Taniguchi H, Kawamura A, Nagase S, Hayashi A, et al. Prospective screening for myocarditis in cancer patients treated with immune checkpoint inhibitors. J Cardiol. (2023) 81:63–7. doi: 10.1016/j.jjcc.2022.07.009
29. Vasbinder A, Chen Y, Procureur A, Gradone A, Azam TU, Perry D, et al. Biomarker trends, incidence, and outcomes of immune checkpoint inhibitor-induced myocarditis. JACC CardioOncol. (2022) 4(5):689–700. eCollection 2022 Dec. doi: 10.1016/j.jaccao.2022.11.004
30. Waliany S, Neal JW, Reddy S, Wakelee H, Shah SA, Srinivas S, et al. Myocarditis surveillance with high-sensitivity troponin I during cancer treatment with immune checkpoint inhibitors. JACC CardioOncol. (2021) 3:137–9. doi: 10.1016/j.jaccao.2021.01.004
31. Petricciuolo S, Delle Donne MG, Aimo A, Chella A, De Caterina R. Pre-treatment high-sensitivity troponin T for the short-term prediction of cardiac outcomes in patients on immune checkpoint inhibitors. Eur J Clin Invest. (2021) 51:e13400. doi: 10.1111/eci.13400
32. Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. (2017) 136:2085–7. doi: 10.1161/CIRCULATIONAHA.117.030571
33. Champion SN, Stone JR. Immune checkpoint inhibitor associated myocarditis occurs in both high-grade and low-grade forms. Mod Pathol. (2020) 33:99–108. doi: 10.1038/s41379-019-0363-0
34. deFilippi C, Barac A. Cardiac troponins for diagnosis and prognostic assessment of immune checkpoint inhibitor myocarditis and myositis: the emerging importance of peripheral vision. Circulation. (2023) 148(15):1135–7. doi: 10.1161/CIRCULATIONAHA.123.065988.37812655
35. Welsh P, Preiss D, Hayward C, Shah ASV, McAllister D, Briggs A, et al. Cardiac troponin T and troponin I in the general population. Circulation. (2019) 139:2754–64. doi: 10.1161/CIRCULATIONAHA.118.038529
36. Wu AHB, Christenson RH, Greene DN, Jaffe AS, Kavsak PA, Ordonez-Llanos J, et al. Clinical laboratory practice recommendations for the use of cardiac troponin in acute coronary syndrome: expert opinion from the academy of the American association for clinical chemistry and the task force on clinical applications of cardiac bio-markers of the international federation of clinical chemistry and laboratory medicine. Clin Chem. (2018) 64:645–55. doi: 10.1373/clinchem.2017.277186
37. Jaffe AS, Vasile VC, Milone M, Saenger AK, Olson KN, Apple FS. Diseased skeletal muscle: a noncardiac source of increased circulating concentrations of cardiac troponin T. J Am Coll Cardiol. (2011) 58:1819–24. doi: 10.1016/j.jacc.2011.08.026
38. du Fay de Lavallaz J, Prepoudis A, Wendebourg MJ, Kesenheimer E, Kyburz D, Daikeler T, et al. Skeletal muscle disorders: a noncardiac source of cardiac troponin T. Circulation. (2022) 145:1764–79. doi: 10.1161/CIRCULATIONAHA.121.058489
39. Rossi VA, Gawinecka J, Dimitriou F, von Eckardstein A, Dummer R, Ruschitzka F, et al. Value of troponin T versus I in the diagnosis of immune checkpoint inhibitor-related myocarditis and myositis: rechallenge? ESC Heart Fail. (2023) 10:2680–5. doi: 10.1002/ehf2.14360
40. Vylegzhanina AV, Kogan AE, Katrukha IA, Antipova OV, Kara AN, Bereznikova AV, et al. Anti-cardiac troponin autoantibodies are specific to the conformational epitopes formed by cardiac troponin I and troponin T in the ternary troponin complex. Clin Chem. (2017) 63:343–50. doi: 10.1373/clinchem.2016.261602
41. Akhtar Z, Dargan J, Gaze D, Firoozi S, Collinson P, Shanmugam N. False-positive troponin elevation due to an immunoglobulin-G-cardiac troponin T complex: a case report. Eur Heart J Case Rep. (2020) 4:1–5. doi: 10.1093/ehjcr/ytaa082
42. Park JY, Jaffe AS. Troponin autoantibodies: from assay interferent to mediator of cardiotoxicity. Clin Chem. (2017) 63:30–2. doi: 10.1373/clinchem.2016.268920
43. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. (2021) 39:4073–126. doi: 10.1200/JCO.21.01440
44. Axelrod ML, Meijers WC, Screever EM, Qin J, Carroll MG, Sun X, et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature. (2022) 611:818–26. doi: 10.1038/s41586-022-05432-3
Keywords: cardiotoxicity, biomarkers, myocarditis, immune checkpoint inhibitors, immuno-oncology
Citation: Murtagh G, deFilippi C, Zhao Q and Barac A (2024) Circulating biomarkers in the diagnosis and prognosis of immune checkpoint inhibitor-related myocarditis: time for a risk-based approach. Front. Cardiovasc. Med. 11:1350585. doi: 10.3389/fcvm.2024.1350585
Received: 5 December 2023; Accepted: 16 January 2024;
Published: 12 February 2024.
Edited by:
Maurice Enriquez-Sarano, Minneapolis Heart Institute Foundation (MHIF), United StatesReviewed by:
Tobias Graf, Luebeck University of Applied Sciences, Germany© 2024 Murtagh, deFilippi, Zhao and Barac. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Barac YW5hLmJhcmFjQGlub3ZhLm9yZw==
 Gillian Murtagh1
Gillian Murtagh1 Christopher deFilippi
Christopher deFilippi Ana Barac
Ana Barac