
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cardiovasc. Med. , 29 January 2024
Sec. General Cardiovascular Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1349584
This article is part of the Research Topic Insights in Cardiology from Caring for a Diverse Community: Perspectives from Inova Schar Heart and Vascular View all 22 articles
 Eunice Yang1,2*
Eunice Yang1,2* Haroon Rashid1,3
Haroon Rashid1,3
Heart failure with preserved ejection fraction (HFpEF) and atrial fibrillation (AF) have emerged as major age-related epidemics within cardiology. Both conditions carry overlapping symptomatology, and delineating between AF and HFpEF from a diagnostic standpoint is challenging as echocardiographic and biomarker assessments used to diagnose HFpEF may be impacted by AF. Indeed, these two conditions are commonly found in the same individual, so much so that AF has been used in proposed diagnostic criteria for HFpEF. The frequent concomitant presence of these two conditions is associated with poorer quality of life, exertional capacity, as well as increased risk for decompensated heart failure and all-cause mortality. Though these deleterious effects of AF in HFpEF patients are well described, we currently have only a superficial understanding of the complex interplay between these two conditions. Preliminary studies on intervening in AF in HFpEF are very small, with mixed data on whether modifying the natural history of AF can lead to improvement in heart failure (HF) outcomes in HFpEF. In this review, we will describe the clinical implications of carrying both cardiovascular conditions, address recent advances in HFpEF and AF, and highlight preliminary studies targeted at reduction of effects associated with AF burden in HFpEF.
Heart failure with preserved ejection fraction (HFpEF) and atrial fibrillation (AF) have emerged as major age-related epidemics within cardiology (1, 2). Both conditions carry overlapping symptomatology, and delineating between AF and HFpEF from a diagnostic standpoint is challenging as echocardiographic and biomarker assessments used to diagnose HFpEF may be impacted by AF (3). Indeed, these two conditions are commonly found in the same individual, so much so that AF has been used in proposed diagnostic criteria for HFpEF (4). The frequent concomitant presence of these two conditions is associated with poorer quality of life, exertional capacity, as well as increased risk for decompensated heart failure and all-cause mortality (5–11). Though these deleterious effects of AF in HFpEF patients are well described, we currently have only a superficial understanding of the complex interplay between these two conditions. Preliminary studies on intervening in AF in HFpEF are very small, with mixed data on whether modifying the natural history of AF can lead to improvement in heart failure (HF) outcomes in HFpEF. In this review, we will describe the clinical implications of carrying both cardiovascular conditions, address recent advances in HFpEF and AF, and highlight preliminary studies targeted at reduction of effects associated with AF burden in HFpEF.
By 2030, an estimated 12 million Americans will have HF, and at least half of these individuals will have HFpEF (12). HFpEF is a HF condition that leads to HF symptoms similar to individuals with heart failure with reduced ejection fraction (HFrEF) (13). HFpEF patients demonstrate impaired exercise capacity and have poorer projected survival: the median five-year survival rate of HFpEF patients after their first HF hospitalization is 35% (14, 15). At present, there have been limited prospective studies identifying effective treatments that modify the natural course of this disease, with sodium-glucose cotransporter 2 (SGLT2) inhibitors recently identified as the first and only class of drugs offering clinical benefit (16), making HFpEF a major cause of morbidity and mortality with unmet healthcare need.
AF is also a growing cardiovascular epidemic affecting millions of patients worldwide (17, 18). Individuals with AF have increased risk for morbidity and mortality, with a five-fold higher risk for developing HF and cerebrovascular events (15–21). In the general population, the presence of AF, defined in the traditional binary fashion, is independently associated with faster decline in cognitive function with age, a 1.4-fold increased risk of dementia, and a 5-fold increased risk of stroke (7, 22). Failure to diagnose AF and initiate systemic anticoagulation in a timely fashion places these patients at undue risk for cerebrovascular events. Clinical management of AF includes two basic goals: (1) prevention of thromboembolism with systemic anticoagulation when appropriate, and (2) selection of medications and/or interventional procedures to either maintain appropriate heart rate or maintain sinus rhythm.
The association between AF and HF has been well described, with modern HF cohorts reporting concomitant AF diagnoses in 13%–27% of all HF subjects (23–27). In prospective follow-up of Framingham Heart Study participants, 1,470 participants developed either new AF or HF between 1948 and 1995, with 26% of those participants developing both of these cardiovascular conditions (7). The prevalence of AF in patients with HF is correlated with the severity of the HF condition, ranging from just 5% in patients with mild HF to up to 50% in patients with end-stage HF symptoms (28). Large cohort studies reveal that least one-third of all HFpEF patients have AF, which has been associated with significantly reduced exercise tolerance, increased risk for decompensated HF requiring hospitalization, and overall poorer survival (5–10).
The natural history of AF is for progression from paroxysmal to persistent, and ultimately permanent AF (29). While the adverse cardiovascular events have generally been explored using a binary approach of presence or absence of AF, studies on the general population suggest that the rates of death, stroke and worsening HF are higher in individuals with persistent and permanent AF than in those with paroxysmal AF (30). Interestingly, the presence of any type of AF burden, even paroxysmal AF, appears to increase risk for poor clinical outcomes in HF patients, including patients with HFpEF (29, 30).
The presence of AF has been associated with increased right and left-sided atrial and ventricular pressures on right heart catheterization (31). Pathophysiologic postulates on the deleterious interplay between AF and HFpEF include these higher right- and left-sided atrial pressures seen in HFpEF with concomitant AF, which may lead to reduced tolerance for fluctuations in intravascular volume, resulting in reduced exercise capacity and increased risk for HF exacerbation requiring hospitalization. This postulate has led to trials assessing the interventional procedures involving placement of an interatrial shunt with the hopes of improving HF outcomes, which have not proven to demonstrate significant benefit (32). However, as shown in Figure 1, the proposed interaction between AF and HFpEF extends well beyond hemodynamic effects alone: patients with both cardiovascular conditions frequent carry other clinical comorbidities such as obesity, hypertension, diabetes, chronic kidney disease, obstructive sleep apnea, alcohol consumption and smoking. These comorbidities all contribute to systemic inflammation, and are associated with higher levels of pro-inflammatory mediators, and longitudinal observational studies report that individuals with higher levels of pro-inflammatory markers at baseline are at higher risk of developing HFpEF and AF during follow-up (33–36). Coronary microvascular dysfunction, defined as myocardial ischemia in the absence of macrovascular epicardial coronary artery disease, has also been shown to be highly prevalent in HFpEF and AF patients, and can cause subtle aberrations in systolic function despite the presence of normal ejection fraction (37). One study assessing prevalence of coronary microvascular dysfunction in HFpEF reported that the prevalence of AF was over twofold higher in HFpEF patients with coronary microvascular dysfunction (58%) than in those without microvascular dysfunction (25%) (38). Other postulated contributors include deposition of epicardial adipose tissue leading to both myocardial infiltration and paracrine effects promoting local inflammation tissue fibrosis and cardiomyocyte dysfunction (39–42), as well as a pro-fibrotic milieu contributing to the pathogenesis and progression of both conditions.
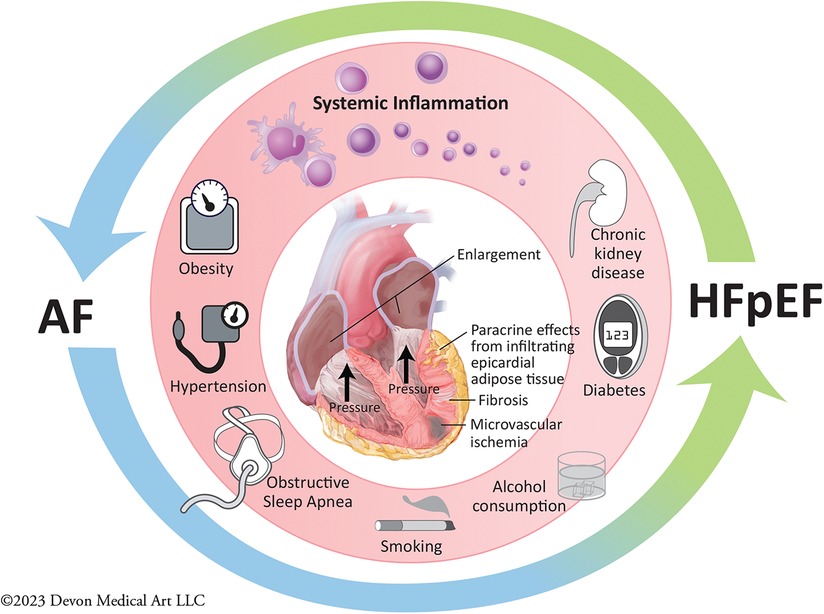
Figure 1. Pathogenesis and progression of atrial fibrillation and heart failure with preserved ejection fraction.
While carrying a concomitant diagnosis of AF portends poorer clinical outcomes in HFpEF, and though many treatments exist to manage AF, no evidence-based treatment guidelines exist for the growing number of patients with HFpEF and AF. Thus, understanding the interplay between AF and HFpEF is vital to guide selection of appropriate therapeutic interventions targeting HFpEF as well as AF to optimize the clinical trajectory of these patients.
Delineating relative timing of diagnosis of AF and HFpEF is particularly helpful as the prognosis of these particular subgroups may differ in response to rhythm control therapy. Amongst these groups are (1) individuals with pre-existing AF and subsequent development of HF; and (2) individuals who have had pre-existing HF before development of clinical AF. The first group with pre-existing AF includes individuals who develop HFrEF with transient or curable etiologies, such as Takotsubo or timely revascularization in ischemic cardiomyopathy, as well as individuals who have HFrEF who have ejection fraction recovery following initiation on goal-directed medial therapy (3, 43). This group also includes individuals with completely reversible ejection fraction after resolution of tachyarrhythmias, for which AF is the most common culprit. In general, the clinical trajectory of individuals diagnosed with AF before developing heart failure who undergo AF control is much more favorable with a higher likelihood of achieving complete recovery from HF symptoms. In contrast, though individuals from the second group who develop AF after already carrying a diagnosis of HF (whether this is HFpEF or HFrEF) may experience clinical benefit in achieving AF rhythm control, these patients typically have poorer long-term clinical projections, including higher thromboembolic risk and increased all-cause mortality (44).
Until recently, extrapolation of medical therapies showing mortality benefit in individuals with systolic HF to HFpEF proved disappointing. Recently, the application of sodium-glucose cotransporter 2 (SGLT2) inhibitors, developed initially for treatment of type 2 diabetes mellitus, have shown major clinical benefits in all HF patients irrespective of diabetes status, including patients with HFpEF (16). The EMPEROR-Preserved and the DELIVER clinical trials evaluated patients with HF with ejection fraction greater than 40%, showing reduction in risk for HF exacerbation, defined as hospitalization or unexpected HF outpatient visit or cardiovascular death (45, 46). The salutary effects of SGLT2 inhibitors was consistent irrespective of whether these patients carried a diagnosis of AF at the time of enrollment, with no reported statistical heterogeneity between the effects of empagliflozin and dapagliflozin. A meta-analysis of these studies demonstrated that the treatment effect noted for the composite endpoint of cardiovascular death or first hospitalization for patients with HFpEF was indeed consistent for patients with AF (HR 0.77, 95% CI 0.69–0.87) and those with no AF (HR 0.83, 95% CI 0.72–0.95), and there was no statistical heterogeneity between empagliflozin and dapagliflozin in the subgroups of patients with AF (47). Analysis of subgroups of patients within the study participants who were at risk for development of AF indicated consistent benefit with SGLT2 inhibitors with no apparent heterogeneity between empagliflozin and dapagliflozin. Dapagliflozin was the only study drug that demonstrated a reduction in incident AF diagnosis compared to placebo, but it was unclear whether this was truly an effect from the drug or a side-effect of improved severity of HF coupled with a higher participant number lending relatively stronger power to identify differences in risk for development of AF following assignment to the treatment arm (48).
Other promising therapies are on the horizon for certain subgroups within the HFpEF population. The STEP-HFpEF trial demonstrated that high-dose weekly administration of semaglutide, a GLP1 receptor agonist, led not only to significant weight loss in obese HFpEF patients, but also resulted in substantial improvement in exertional capacity and reduction in HF symptoms (49). Obesity is a well-described risk factor for AF, and aggressive weight reduction with intensive lifestyle modification has been shown to decrease AF in large randomized control trials (50, 51), so reduction in AF burden should be expected to be seen in obese HFpEF patients who also have AF. Another interesting prospective study demonstrated that HFpEF patients with a permanent pacemaker had significantly improved quality of life, lower N-terminal pro-brain natriuretic peptide levels, and reduced AF burden when their lower rate limit was programmed higher than their underlying resting sinus heart rate, compared against the standard lower rate limit programmatic setting of 60 beats per minute (52). Interplay between these emerging device and medical interventions on AF within the HFpEF population requires further investigation, which is presently under way.
Guidelines for the selection of rate vs. rhythm control strategies has relied on several historical clinical trials, the largest of which was the AFFIRM trial published in 2002 (53–55). Meta-analysis of these studies has not revealed significant differences between pharmacologic rate and rhythm control strategies in risk for all-cause mortality or cerebrovascular events (56). Analysis of the AFFIRM trial, however, did show better outcomes in subjects able to maintain sinus rhythm (53). Following AFFIRM, pharmacologic and procedural advances have led to tremendous advances in our ability to attain rhythm control, with new medications and new therapeutic strategies designed to achieved rhythm control. A central discovery was the identification of ectopic beats originating from the pulmonary veins as the major triggers for initiation of AF has led to an ablation strategy for AF rhythm control that involves pulmonary vein isolation (PVI) (57). AF ablation incorporating PVI, now carries a class I indication for treatment of individuals with symptomatic paroxysmal AF that is refractory to at least one anti-arrhythmic medication, and a class IIa recommendation for individuals with recurrent paroxysmal AF even before therapeutic trials of antiarrhythmic drug therapies (58). Other shifts in AF management noted in comparing AFFIRM with the recently published EAST-AFNET4 study show decreased in use of digoxin, increased availability of newer anti-arrhythmic options, such as dronedarone (59, 60).
Recent studies comparing these current rhythm control strategies to rate control strategies have shown more clinical benefit of pursuit of rhythm control both in the general population as well as in patients with HF, in contrast to earlier landmark trial findings (61–63). As recent studies have highlighted the enhanced likelihood for success of achieving rhythm control with early intervention, pursuit of early intervention on AF in the HFpEF population may mitigate the effects of this rhythm in this population.
The evidence supporting AF ablation that incorporates PVI for AF management in the HFrEF population has grown tremendously over the last 10 years. Following an initial observational study that showed that patients HFrEF and AF tended to perform better after undergoing successful electrical cardioversion (64), a series of small studies were conducted comparing AF ablation with medical management in the HFrEF population—all of which demonstrated significant improvement in ejection fraction and exercise capacity (61, 65, 66). Multicenter randomized control trials following these studies all demonstrated the same improvement in cardiac function as well as small but significant reduction in mortality (63, 67). The mechanism driving improved clinical outcomes in these studies remains unknown, as does the utility of this intervention in the HFpEF population, given that most therapies showing improved outcomes in HFrEF have not demonstrated comparable benefit in HFpEF.
Preliminary single-center exploratory studies have been published describing benefit of AF rhythm control using a catheter ablation strategy compared to a rate control strategy, including ones that demonstrate a reduction of pulmonary capillary wedge measurements following AF ablation in HFpEF patients (68, 69). To our knowledge, no large scale prospective randomized controlled trials have yet evaluated whether AF ablation improves clinical outcomes in the HFpEF population. Pre-specified subgroup analysis of the EAST-AFNET4 study compared early rhythm control of AF and found that it was associated with a lower risk of adverse cardiovascular outcomes in comparison with “usual care” among patients with AF diagnosed within 1 year of study enrollment, which included patients with HF (n = 798), approximately half of whom had HFpEF (56%) (70). The primary outcome of the EAST-AFNET4 study was a composite outcome (including death from cardiovascular causes, stroke, HF hospitalization or hospitalization secondary to acute coronary syndrome) which occurred in 94 of 396 (24%) HF patients assigned to early rhythm control and in 130 of 402 (32%) HF patients randomly assigned to usual care [hazard ratio (HR) 0.74, 95% CI 0.56–0.97, p = 0.03]. As reported in the subgroup analysis, patients with HFpEF demonstrated more improvement in reported NYHA class than patients with HFrEF, and HFpEF participants appeared to have an overall lower risk for time to development of the primary composite outcome in comparison to their HFrEF counterparts. Finally, exploratory analysis of HFpEF patients suggested that treatment with amiodarone, but not treatment with flecainide, propafenone or dronedarone, was associated with early HF hospitalizations (70). This association may be due to patients receiving amiodarone having a higher frequency and severity of clinical comorbidities serving as contraindications for other anti-arrhythmic medications, but serves to temper our complacency in using amiodarone as a rhythm control treatment option in the HF population. Long-term use of amiodarone may require re-examination, particularly in the face of the other rhythm control options available in our current armamentarium of AF therapies in HF patients.
The majority of EAST-AFNET4 patients were prescribed anti-arrhythmic drugs as the first line early rhythm control option, with only a small fraction of these patients undergoing AF ablation. A post-hoc analysis of the CABANA clinical trial compared catheter ablation and antiarrhythmic drug therapy in 778 patients with AF and stable HF at baseline, the vast majority (79%) of whom had HFpEF (71). In this secondary analysis, catheter ablation was associated with a striking 36% relative reduction in the primary composite endpoint of death, disabling stroke, serious bleeding, or cardiac arrest and a 43% relative reduction in all-cause mortality compared to anti-arrhythmic drug therapy. Notably, there was no significant reduction in the frequency of HF hospitalizations and there were limited data to ascertain HFpEF diagnosis. The authors concluded that these results should be reproduced in a confirmatory study dedicated to looking at the HFpEF population.
It should be noted that patients with HFpEF often present with permanent AF with profound left atrial remodeling. These patients may not have a high likelihood of maintaining sinus rhythm despite attempts at rhythm control using catheter ablation and/or antiarrhythmic drug therapy. When ablation and anti-arrhythmic drug strategies can no longer achieve rhythm control, atrioventricular nodal ablation with placement of a biventricular or conduction system pacemaker should be discussed in HFpEF, as the APAF-CRT trial for those with permanent AF and narrow QRS hospitalized for HF demonstrated reduction in all-cause mortality, irrespective of ejection fraction (72).
While the treatments available for HFpEF and AF have improved significantly within the last few years, patients who carry both clinical diagnoses are still faced with limited evidence guiding their clinical management. This unmet need is an opportunity for investigation and improvement of clinical outcomes, and requires not only the cooperation of the HF and electrophysiology teams, but requires a multidisciplinary approach to treatment to target comorbidities and lifestyle modifications (73, 74). We firmly believe that implementation of cross-disciplinary management is essential to success of managing all AF patients and carries the greatest potential for benefit in this patient subgroup.
EY: Writing – review & editing. HR: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
2. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79:1757–80. doi: 10.1016/j.jacc.2021.12.011
3. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Rev Esp Cardiol (Engl Ed). (2022) 75:523. doi: 10.1016/j.recesp.2021.11.027
4. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA, Simple A. Evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. (2018) 138:861–70. doi: 10.1161/CIRCULATIONAHA.118.034646
5. McManus DD, Hsu G, Sung SH, Saczynski JS, Smith DH, Magid DJ, et al. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc. (2013) 2:e005694. doi: 10.1161/JAHA.112.005694
6. Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of left ventricular dysfunction. J Am Coll Cardiol. (1998) 32:695–703. doi: 10.1016/S0735-1097(98)00297-6
7. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham heart study. Circulation. (2003) 107:2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E
8. Kaye DM, Silvestry FE, Gustafsson F, Cleland JG, van Veldhuisen DJ, Ponikowski P, et al. Impact of atrial fibrillation on rest and exercise haemodynamics in heart failure with mid-range and preserved ejection fraction. Eur J Heart Fail. (2017) 19:1690–7. doi: 10.1002/ejhf.930
9. Elshazly MB, Senn T, Wu Y, Lindsay B, Saliba W, Wazni O, et al. Impact of atrial fibrillation on exercise capacity and mortality in heart failure with preserved ejection fraction: insights from cardiopulmonary stress testing. J Am Heart Assoc. (2017) 6(11):e006662. doi: 10.1161/JAHA.117.006662
10. Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the candesartan in heart failure-assessment of reduction in mortality and morbidity (CHARM) program. J Am Coll Cardiol. (2006) 47:1997–2004. doi: 10.1016/j.jacc.2006.01.060
11. Yang E, Vaishnav J, Song E, Lee J, Schulman S, Calkins H, et al. Atrial fibrillation is an independent risk factor for heart failure hospitalization in heart failure with preserved ejection fraction. ESC Heart Fail. (2022) 9(5):2918–27. doi: 10.1002/ehf2.13836
12. Braunwald E. Shattuck lecture–cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. (1997) 337:1360–9. doi: 10.1056/NEJM199711063371906
13. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. (2006) 355:251–9. doi: 10.1056/NEJMoa052256
14. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Get with the guidelines scientific advisory, and investigators, trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. (2012) 126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770
15. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the framingham heart study. Circulation. (1998) 98:946–52. doi: 10.1161/01.CIR.98.10.946
16. Margulies KB. DELIVERing progress in heart failure with preserved ejection fraction. N Engl J Med. (2022) 387:1138–40. doi: 10.1056/NEJMe2210177
17. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics–2015 update: a report from the American heart association. Circulation. (2015) 131:e29–322. doi: 10.1161/circ.131.suppl_2.o29
18. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. (2014) 129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119
19. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the renfrew/paisley study. Am J Med. (2002) 113:359–64. doi: 10.1016/S0002-9343(02)01236-6
20. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. Br Med J. (2016) 354:i4482. doi: 10.1136/bmj.i4482
21. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. (2001) 285:2370–5. doi: 10.1001/jama.285.18.2370
22. Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. (2014) 35:250–6. doi: 10.1093/eurheartj/eht483
23. Middlekauff HR, Stevenson WG, Stevenson LW. Prognostic significance of atrial fibrillation in advanced heart failure. A study of 390 patients. Circulation. (1991) 84:40–8. doi: 10.1161/01.CIR.84.1.40
24. Carson PE, Johnson GR, Dunkman WB, Fletcher RD, Farrell L, Cohn JN. The influence of atrial fibrillation on prognosis in mild to moderate heart failure. The V-HeFT studies. The V-HeFT VA cooperative studies group. Circulation. (1993) 87:VI102–10.8500233
25. Mahoney P, Kimmel S, DeNofrio D, Wahl P, Loh E. Prognostic significance of atrial fibrillation in patients at a tertiary medical center referred for heart transplantation because of severe heart failure. Am J Cardiol. (1999) 83:1544–7. doi: 10.1016/S0002-9149(99)00144-7
26. Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted county, Minnesota, in 1991. Circulation. (1998) 98:2282–9. doi: 10.1161/01.CIR.98.21.2282
27. Deedwania PC, Singh BN, Ellenbogen K, Fisher S, Fletcher R, Singh SN. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). the department of veterans affairs CHF-STAT investigators. Circulation. (1998) 98:2574–9. doi: 10.1161/01.CIR.98.23.2574
28. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. (2003) 91:2D–8D. doi: 10.1016/S0002-9149(02)03373-8
29. Zafrir B, Lund LH, Laroche C, Ruschitzka F, Crespo-Leiro MG, Coats AJS, et al. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14964 patients in the European society of cardiology heart failure long-term registry. Eur Heart J. (2018) 39:4277–84. doi: 10.1093/eurheartj/ehy626
30. Mogensen UM, Jhund PS, Abraham WT, Desai AS, Dickstein K, Packer M, et al. Type of atrial fibrillation and outcomes in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. (2017) 70:2490–500. doi: 10.1016/j.jacc.2017.09.027
31. Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. (2020) 76:1051–64. doi: 10.1016/j.jacc.2020.07.009
32. Shah SJ, Borlaug BA, Chung ES, Cutlip DE, Debonnaire P, Fail PS, et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. (2022) 399:1130–40. doi: 10.1016/S0140-6736(22)00016-2
33. Kalogeropoulos A, Georgiopoulou V, Psaty BM, Rodondi N, Smith AL, Harrison DG, et al. Inflammatory markers and incident heart failure risk in older adults: the health ABC (health, aging, and body composition) study. J Am Coll Cardiol. (2010) 55:2129–37. doi: 10.1016/j.jacc.2009.12.045
34. Patel RB, Colangelo LA, Bielinski SJ, Larson NB, Ding J, Allen NB, et al. Circulating vascular cell adhesion molecule-1 and incident heart failure: the multi-ethnic study of atherosclerosis (MESA). J Am Heart Assoc. (2020) 9:e019390. doi: 10.1161/JAHA.120.019390
35. Patel RB, Colangelo LA, Reiner AP, Gross MD, Jacobs DR Jr, Launer LJ, et al. Cellular adhesion molecules in young adulthood and cardiac function in later life. J Am Coll Cardiol. (2020) 75:2156–65. doi: 10.1016/j.jacc.2020.02.060
36. Prasada S, Rivera A, Nishtala A, Pawlowski AE, Sinha A, Bundy JD, et al. Differential associations of chronic inflammatory diseases with incident heart failure. JACC Heart Fail. (2020) 8:489–98. doi: 10.1016/j.jchf.2019.11.013
37. Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. (2014) 35:1101–11. doi: 10.1093/eurheartj/eht513
38. Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P, et al. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. (2018) 72:29–40. doi: 10.1016/j.jacc.2018.04.039
39. Wong CX, Sun MT, Odutayo A, Emdin CA, Mahajan R, Lau DH, et al. Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ Arrhythm Electrophysiol. (2016) 9(12):e004378. doi: 10.1161/CIRCEP.116.004378
40. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail. (2018) 20:1559–66. doi: 10.1002/ejhf.1283
41. Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab. (2012) 303:E937–49. doi: 10.1152/ajpendo.00061.2012
42. Greulich S, Maxhera B, Vandenplas G, de Wiza DH, Smiris K, Mueller H, et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation. (2012) 126:2324–34. doi: 10.1161/CIRCULATIONAHA.111.039586
43. Arnar DO, Mairesse GH, Boriani G, Calkins H, Chin A, Coats A, et al. Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS). Europace. (2019) 21:844–5. doi: 10.1093/europace/euz046
44. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. (2016) 133:484–92. doi: 10.1161/CIRCULATIONAHA.115.018614
45. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. doi: 10.1056/NEJMoa2107038
46. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387:1089–98. doi: 10.1056/NEJMoa2206286
47. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. (2022) 400:757–67. doi: 10.1016/S0140-6736(22)01429-5
48. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. (2019) 381:1995–2008. doi: 10.1056/NEJMoa1911303
49. Kosiborod MN, Abildstrom SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. (2023) 389(12):1069–84. doi: 10.1056/NEJMoa2306963
50. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. (2014) 64:2222–31. doi: 10.1016/j.jacc.2014.09.028
51. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (LEGACY). J Am Coll Cardiol. (2015) 65:2159–69. doi: 10.1016/j.jacc.2015.03.002
52. Infeld M, Wahlberg K, Cicero J, Plante TB, Meagher S, Novelli A, et al. Effect of personalized accelerated pacing on quality of life, physical activity, and atrial fibrillation in patients with preclinical and overt heart failure with preserved ejection fraction: the myPACE randomized clinical trial. JAMA Cardiol. (2023) 8:213–21. doi: 10.1001/jamacardio.2022.5320
53. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, et al. Atrial fibrillation follow-up investigation of rhythm management, a comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. (2002) 347:1825–33. doi: 10.1056/NEJMoa021328
54. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. (2018) 20:e1–e160. doi: 10.1093/europace/eux274
55. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2023) 389(12):1069–84. doi: 10.1093/eurheartj/ehaa612
56. Sethi NJ, Feinberg J, Nielsen EE, Safi S, Gluud C, Jakobsen JC. The effects of rhythm control strategies versus rate control strategies for atrial fibrillation and atrial flutter: a systematic review with meta-analysis and trial sequential analysis. PLoS One. (2017) 12:e0186856. doi: 10.1371/journal.pone.0186856
57. Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. (1998) 339:659–66. doi: 10.1056/NEJM199809033391003
58. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2014) 64:e1–76. doi: 10.1016/j.jacc.2014.03.022
59. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. (2020) 383:1305–16. doi: 10.1056/NEJMoa2019422
60. Yang E, Tang O, Metkus T, Berger RD, Spragg DD, Calkins HG, et al. The role of timing in treatment of atrial fibrillation: an AFFIRM substudy. Heart Rhythm. (2021) 18:674–81. doi: 10.1016/j.hrthm.2020.12.025
61. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. (2014) 7:31–8. doi: 10.1161/CIRCEP.113.000806
62. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AA, Voskoboinik A, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction (CAMERA-MRI). J Am Coll Cardiol. (2017) 70(16):1949–61. doi: 10.1016/j.jacc.2017.08.041
63. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. (2018) 378:417–27. doi: 10.1056/NEJMoa1707855
64. Massie BM, Fisher SG, Radford M, Deedwania PC, Singh BN, Fletcher RD, et al. Effect of amiodarone on clinical status and left ventricular function in patients with congestive heart failure. CHF-STAT investigators. Circulation. (1996) 93:2128–34. doi: 10.1161/01.CIR.93.12.2128
65. MacDonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M, et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart. (2011) 97:740–7. doi: 10.1136/hrt.2010.207340
66. Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. (2013) 61:1894–903. doi: 10.1016/j.jacc.2013.01.069
67. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, et al. Response by Di Biase et al. To letter regarding article, “Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial”. Circulation. (2016) 134:e189–90. doi: 10.1161/CIRCULATIONAHA.116.024003
68. Chieng D, Sugumar H, Segan L, Tan C, Vizi D, Nanayakkara S, et al. Atrial fibrillation ablation for heart failure with preserved ejection fraction: a randomized controlled trial. JACC Heart Fail. (2023) 11:646–58. doi: 10.1016/j.jchf.2023.01.008
69. Zylla MM, Leiner J, Rahm AK, Hoffmann T, Lugenbiel P, Schweizer P, et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Circ Heart Fail. (2022) 15:e009281. doi: 10.1161/CIRCHEARTFAILURE.121.009281
70. Rillig A, Magnussen C, Ozga AK, Suling A, Brandes A, Breithardt G, et al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation. (2021) 144:845–58. doi: 10.1161/CIRCULATIONAHA.121.056323
71. Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. (2021) 143(14):1377–90. doi: 10.1161/CIRCULATIONAHA.120.050991
72. Brignole M, Pentimalli F, Palmisano P, Landolina M, Quartieri F, Occhetta E, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J. (2021) 42:4731–9. doi: 10.1093/eurheartj/ehab569
73. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. (2017) 14:627–8. doi: 10.1038/nrcardio.2017.153
74. Gopinathannair R, Chen LY, Chung MK, Cornwell WK, Furie KL, Lakkireddy DR, et al. Managing atrial fibrillation in patients with heart failure and reduced ejection fraction: a scientific statement from the American Heart Association. Circ Arrhythm Electrophysiol. (2021) 14:HAE0000000000000078. doi: 10.1161/HAE.0000000000000078
Keywords: atrial fibrillaiton, HFpEF—heart failure with preserved ejection fraction, rhythm control, heart failure, catheter ablation
Citation: Yang E and Rashid H (2024) Heart failure with preserved ejection fraction and atrial fibrillation: clinical management in the context of recent therapeutic advances in heart failure and electrophysiology. Front. Cardiovasc. Med. 11:1349584. doi: 10.3389/fcvm.2024.1349584
Received: 4 December 2023; Accepted: 16 January 2024;
Published: 29 January 2024.
Edited by:
Sana Al-Khatib, Duke University, United StatesReviewed by:
Conrado Roberto Hoffmann Filho, Hospital Regional Hans Dieter Schmidt, Brazil© 2024 Yang and Rashid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eunice Yang RXVuaWNlLllhbmdAaW5vdmEub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.