- 1Inova Schar Heart and Vascular, Falls Church, VA, United States
- 2Virginia Heart, Falls Church, VA, United States
- 3Department of Cardiovascular Medicine, Johns Hopkins University, Baltimore, MD, United States
More than 1 million transcatheter-based cardiovascular procedures across the spectrum of interventional cardiology are performed annually in the United States. With the expanded indications for and increased complexities associated with these procedures, interventional cardiologists are expected to possess the requisite expertise to complete these interventions safely and effectively. While the art of vascular access and closure remains a prerequisite and critical skillset in contemporary practice, there remain significant variations in the techniques employed, resulting in the bleeding and vascular complications encountered in clinical practice. With an increasing recognition of the potential merits to standardized approaches to vascular access and closure, cardiovascular societies have put forth recommendations around best practices for performing these procedures in the cardiac catheterization laboratories. In this review, we aim to: (1) Examine the evolving definitions of bleeding and vascular complications; (2) Review best practices for transradial and transfemoral access and closure, including for large bore procedures; and (3) Highlight knowledge gaps and proposed areas of clinical research pertaining to vascular access which may inform clinical practice and potentially optimize the outcomes of patients undergoing transcatheter-based cardiac and vascular interventions.
Introduction
Cardiovascular disease is the leading cause of mortality in the United States, with a prevalence of nearly 50% in all adults over the age of 20, and greater than 2.2 million hospitalizations and 1,000 deaths occurring on a daily basis (1). The population is also increasingly aging with an enhanced burden of cardiac co-morbidities and associated frailty syndromes (2). As a result, more than one million transcatheter-based procedures are performed annually to diagnose and treat cardiac conditions (3). There have also been significant advances in the scope of technologies to perform transcatheter-based cardiac and vascular procedures, covering the broad spectrum of coronary, peripheral and structural heart disease. Patients are also increasingly requiring mechanical circulatory support (MCS) devices to treat cardiogenic shock (CS) and to facilitate high risk percutaneous coronary interventions (PCI) (4, 5). With the expanded indications for and increased complexities associated with cardiac catheterizations, interventional cardiologists are expected to possess the expertise required to perform these procedures safely and effectively. Despite these clinical demands, there remain significant variations in how these techniques are employed, thus contributing to the bleeding and vascular complications encountered in clinical practice (6, 7). As a result of increasing recognition of the potential merits to standardized approaches to vascular access and closure, cardiovascular societies have put forth recommendations around best practices for these techniques (3, 8). In this review, we aim to: (1) Examine the evolving definitions of bleeding and the clinical importance of this complication in daily practice; (2) Review best practices for transradial arterial access (TRA) for coronary angiography and PCI; (3) Assess contemporary techniques for standard and large-bore transfemoral arterial access (TFA) and closure (Figure 1); and (4) Highlight knowledge gaps and proposed areas of clinical research pertaining to vascular access which may potentially optimize the outcomes of patients undergoing transcatheter-based cardiac procedures.
Major bleeding following transcatheter-based cardiac procedures
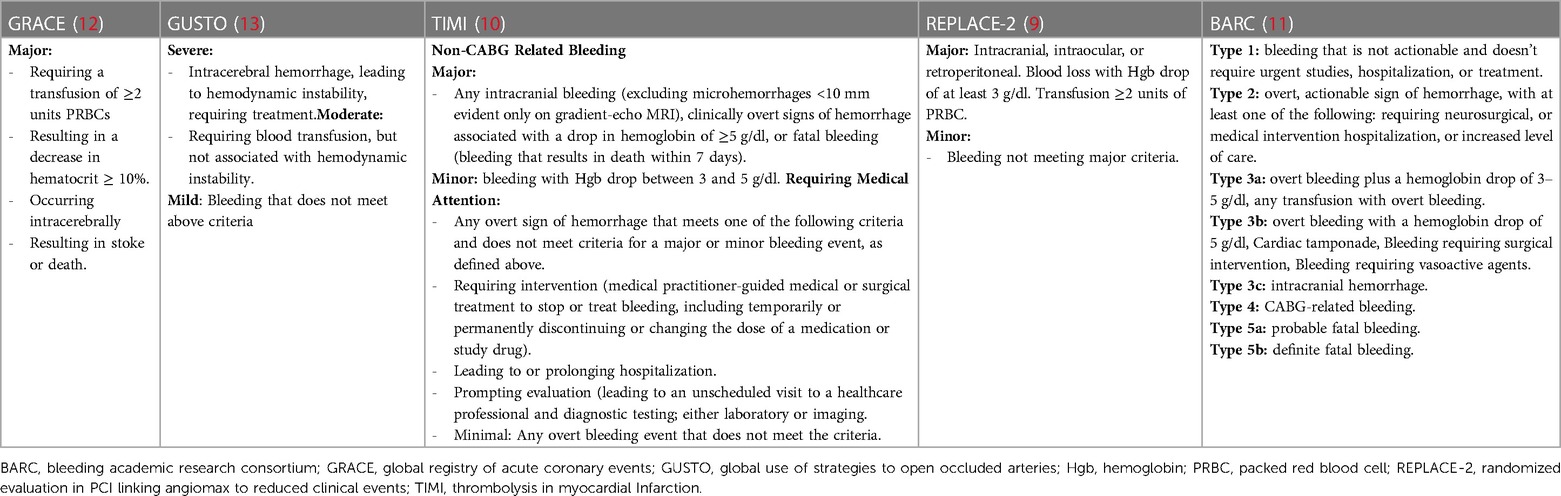
Historically, varying definitions of major bleeding have been studied across different PCI registries and clinical trials, and it has been shown to be a powerful predictor of morbidity and mortality (9–13). A summary of the most commonly used bleeding nomenclatures is highlighted in Table 1. While bleeding following cardiac catheterization may occur from a variety of sources, more than 50% of cases are due to access site complications (14, 15). An analysis of 15,968 patients from the GLOBAL LEADERS study, a contemporary randomized clinical trial comparing 1-month vs. 12-month dual antiplatelet therapy following PCI in an all-comer patient population, reported a 6.6% risk of Bleeding Academic Research Consortium (BARC) 2, 3, or 5 bleeding (16). Overall, 11 percent of patients with a bleeding event died during the two-year follow-up, and the presence of even a minor periprocedural bleed imparted a 1.8-fold increase-risk for mortality beyond 1 year (16). The clinical and economic impacts of periprocedural bleeding are significant, with up to 14% of patients undergoing a blood transfusion and each complication contributing to nearly $12,000 in increased costs (15–17). Notwithstanding the stabilizing effects of increased circulatory volume and oxygen carrying capacity, the practice of blood transfusion is associated with a deleterious impact on clinical outcomes following PCI, with 3-fold increased risk of mortality and major adverse cardiac events (18). This effect is likely multifactorial, due to a combination of increased platelet reactivity, upregulation of plasminogen activator inhibitor proteins, reductions in 2, 3-diphosphoglyceric acid and nitric oxide levels in the stored red blood cells (19–22).
The prognostic implications of major bleeding complications are only further amplified in patients undergoing large bore access for MCS devices or structural heart interventions, often with delivery catheters that are 14 Fr or greater in diameter (23–25). In the case of the former, they are frequently inserted under emergency circumstances when patients are experiencing circulatory collapse (8). These findings were noted in recent observational data from the Cath-PCI and Chest Pain-MI registries which showed that among 1,680 propensity matched pair patients with acute myocardial infarction complicated by CS undergoing PCI, those supported with axial-flow percutaneous ventricular assist devices (pVAD) had double the risk of bleeding with more than 10-fold increased risk of death compared to patients supported with smaller platform intra-aortic balloon pumps (23). Frequently performed under more elective circumstances, patients undergoing structural heart interventions are similarly at risk for access site complications. While there has a notable decline from the nearly 15% incidence rates of periprocedural bleeding and vascular complications seen in the early days of TAVR due to smaller 14 Fr sheaths delivery systems, the frequency of these adverse events and variations in clinical expertise around recognition and management remain significant (26, 27). A contemporary analysis of 34,893 patients undergoing TAVR from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (TVT) Registry from 2011 to 2016 observed a 7.6% incidence of in-hospital bleeding events (28). Significant inter-hospital variations were noted, with complication rates ranging from 0% to 46% among the 345 TVT sites (28). A bleeding complication was associated with significant increased risk for death and hospital readmissions at 1 year (28).
Transradial access
Since its first description by Lucien Campeau in 1989, the adoption of transradial access (TRA) has steadily increased worldwide (29–31). It is associated with reductions in bleeding and vascular complications as well as major adverse cardiac events compared to transfemoral access (TFA) across the spectrum of coronary artery disease (32). It is also associated with improved quality-of-life outcomes, with reductions in bed rest time and bodily pains (33, 34). There are also potential economic benefits to TRA, with a significant reduction in healthcare costs related to not only fewer access site complications, but also shorter hospital lengths of stay, with nearly 20% increased likelihood for same-day discharge following elective PCI (35–37). A contemporary analysis of 279,987 PCI patients observed a nearly $3,700 adjusted savings in hospital costs in patients undergoing TRA for PCI who were discharged home the same day (36). With advances in radial sheaths and associated catheter technologies, TRA is also feasible in high-risk patients with complex coronary anatomy or hemodynamic compromise, where larger sheath sizes may be needed to facilitate intervention. In these circumstances, TRA is feasible without any appreciable increased risk for bleeding/vascular complications, contrast or radiation exposure (38). Given these considerations, TRA is recommended as the default access site for patients undergoing diagnostic coronary angiography and PCI (39–41).
Bleeding and vascular access site complications with TRA
Initial observational studies with TRA demonstrated lower rates of major and minor bleeding compared to TFA, particularly in high-risk subgroups (42, 43). A retrospective cohort study of 2,820,874 PCI procedures from the National Cardiovascular Data Registry from 2007 to 2012 demonstrated that while TRA accounted for only 1 in 6 PCI's, it was associated with a significantly lower adjusted risk of bleeding (aOR 0.51; 95% CI: 0.49–0.54; p < 0.001) and vascular complications (aOR 0.39; 95% CI: 0.31–0.50; p < 0.001) compared to TFA, with the greatest benefit seen in women, patients ≥75 years of age and those with ACS (42). Similar finding were noted in the UK where TRA was adopted earlier, with marked reductions in major adverse cardiac events in patients with both stable ischemic heart disease and acute coronary syndrome (ACS) (35, 44–46).
The Radial vs. Femoral Access for Coronary Intervention (RIVAL) trial initially showed the greatest bleeding reductions among centers with the highest TRA utilization rates, suggesting that there may be volume-outcome relationship with utilizing the radial artery (44). However, subsequent studies including Radial vs. Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome (RIFLE-STEACS) and Minimizing Adverse Haemorrhagic Events by TRansradial Access Site and Systematic Implementation of angioX (MATRIX), further confirmed these findings across the spectrum of TRA-expertise with reductions in composite endpoints encapsulating outcomes such as death, MI, stroke, recurrent MI and target lesion revascularization (35, 46). Subsequent investigations, including a meta-analysis of 24 studies and a post-hoc examination of a high volume North American quaternary care center, have subsequently confirmed these findings with reductions in major adverse cardiac events compared to TFA across the severity spectrum of CAD, including cardiogenic shock (32, 47). Interestingly, the most recently published study in this field, The Safety and Efficacy of Femoral Access Versus Radial for Primary Percutaneous Coronary Intervention in ST-Elevation Myocardial Infarction (SAFARI-STEM) trial, was terminated early due to futility as it failed to demonstrate the superiority of TRA in STEMI with regards to 30-day all-cause mortality, non-surgical TIMI major bleeding, or death/re-infarction/stroke compared to TFA in patients with STEMI (48). While this study has been scrutinized for its limited statistical power and for enrolling patients with lower acuity of illness and bleeding risk than previous trials, its findings do highlight the need for the development of institutional competency pathways in vascular access so that operators may remain facile with both access techniques in contemporary clinical practice. A summary of the pertinent RCT's evaluating TRA is highlighted in Table 2.
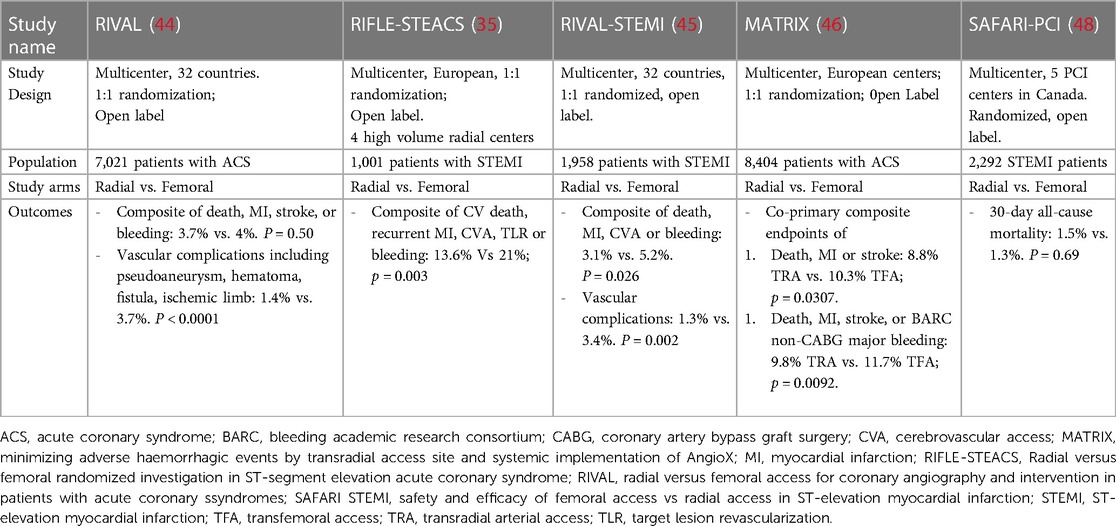
Table 2. Clinical trials comparing radial versus femoral arterial access in acute coronary syndrome.
Access site complications associated with TRA occur infrequently, between 0.2%–1.0% of cases (32). While rare, they can be associated with significant morbidity. Thus it is important to recognize and manage these sequelae in timely fashion. Vascular complications which may be seen following TRA include radial artery spasm, perforation, occlusion due to intimal medical hyperplasia, arteriovenous fistulae and forearm hematomas with compartment syndrome (49). Like most cardiac procedures, there is a learning curve with accessing the radial artery safely and effectively. It is recommended that operators learning TRA perform at least 50 cases initially followed by a minimum of 80 procedures annually to achieve and maintain proficiency, respectively (50). It has also been demonstrated that operators facile with TRA are able to perform both diagnostic cardiac catheterizations and coronary interventions with similar efficacy and safety vs. femoral access, as demonstrated by comparable radiation dosages and total fluoroscopy/procedural times in high-radial volume centers (48, 51).
Best practices for transradial arterial access and hemostasis
A “radial-first” approach is strongly encouraged for all-comers undergoing coronary angiography or PCI (39). The radial artery however is small, typically measuring 2.2 mm–2.6 mm in diameter, and inability to successfully cannulate the vessel is a major contributor to nearly 60% of cases in which there is TRA failure (52). The radial approach can also be challenging in patients with systemic vasculitides, such as Raynaud's disease, as well as in patients with chronic kidney disease due to calcified vessels, brachiocephalic tortuosity, and in those with prior ipsilateral mastectomies with lymph node dissection (39). In the case of the latter, caution against TRA has been borne out of concern for potential risk of access site infection and lymphedema, although this has not been seen in observational studies (53). During TRA, the radial artery is subject to trauma resulting from intimal tears, medial dissection, long-term intimal medial thickness, and impaired flow-mediated vasodilation (54–56). These acute and chronic changes in turn may compromise its utility to serve as a suitable conduit for future coronary artery bypass grafting (CABG) and non-cardiac surgeries, such as arteriovenous fistula formation (56). While not an absolute contraindication to TRA, it is advised to avoid using the ipsilateral radial artery for CABG for at least 3 months given observational data demonstrating reduced radial artery bypass graft patency (3). Historically, confirmation of dual arterial supply to the palmar arch had been required prior to TRA, by examining pulse oximetric waveform patterns following manual occlusion of the ipsilateral radial artery. Commonly referred to as the Barbeau or Allen exams, these pre-procedural tests were once widely performed to predict the risk of hand ischemia. However, given a recent study failing to demonstrate significant differences in thumb capillary lactate, hand grip strength or discomfort between patients with normal and abnormal Barbeau exams, routine pre-procedural assessment of collateral hand circulation is no longer recommended and should not preclude TRA (3, 57). While most interventionalists prefer right TRA primarily because of ergonomic purposes, consideration may be given to left TRA in certain cases. These include patients with brachiocephalic tortuosity and those who have undergone CABG with left internal mammary arteries, although right TRA may still be feasible in these circumstances (58–61). The use of two-dimensional ultrasound may facilitate more timely radial arterial puncture by allowing for direct visual inspection of vessel size, potential anatomic anomalies, needle tip puncture, wire passage and sheath insertion. Several studies have published the benefits associated with ultrasound-guided TRA, including a meta-analysis of 12 studies (n = 1,992) and the Radial Artery Access with Ultrasound Trial (RAUST), a multi-center RCT of 698 patients undergoing TRA who were randomized to manual palpation or ultrasound guidance (62, 63). Both studies showed enhanced efficiency and success with TRA when US was utilized, with the former also reporting reductions in site hematomas, findings which result in reductions radial artery occlusion (RAO) up to 30 days post-procedure (62–64). TRA may be obtained either by way of single- or double-wall puncture, with the latter technique associated with higher rates of successful puncture on the first attempt (65). In cases in which TRA is not successful, the ipsilateral brachial or ulnar arteries may be considered as alternative conduits. Limited studies have demonstrated comparable safety and efficacy between the radial and ulnar access sites, although the ulnar approach was associated with increased access site cross-over (66, 67). While not observed in clinical studies, caution is advised with ulnar access in patients with occluded ipsilateral radial arteries given the risk for hand ischemia with ulnar nerve injury (68).
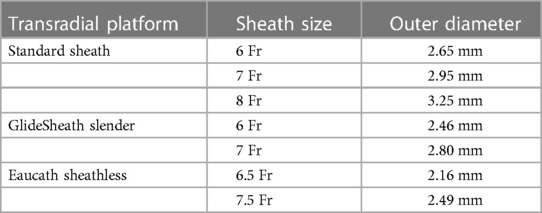
During TRA, concerted efforts should also be made to undertake measures to prevent radial artery occlusion (RAO), a multifactorial phenomenon stemming from vascular injury and inflammatory changes of arterial wall during sheath insertion. Procedural factors associated with RAO include insufficient anticoagulation, sheath-to-arterial diameter ratio >1, multiple puncture attempts, vasospasm and prolonged occlusive hemostasis (64, 69–74). Pharmacologic measures to achieve this goal include adequate administration of moderate sedation and local analgesia, anti-spasmolytic therapy with intra-arterial nitroglycerine (100–200 μg) and verapamil (5 mg), as well as systemic anticoagulation with intra-arterial or intravenous heparin (at least 5,000 U or 50 U/kg bolus) following sheath insertion (69, 70, 75). Iterative advances in TRA technology with reductions in sheath size and hydrophilic coating have also advanced the field, allowing operators to perform complex PCI via TRA with relative impunity. An example of this advancement is the 6 Fr Glidesheath Slender (Terumo, Somerset, NJ), a novel thin-walled sheath design with 5 Fr (2.46 mm) outer diameter while maintaining 6 Fr inner architecture. This technology was compared in a randomized, multi-center non-inferiority trial with standard 5 Fr technology, and no differences were noted with regards to RAO, vasospasm, access-site vascular complications or local bleeding (71). Cordis (Rainsheath) and Merit Medical (Prelude Ideal) similarly have thin-walled sheath platforms which can facilitate complex PCI using 7 Fr guide catheters. An alternative conduit for TRA coronary angiography and PCI are sheathless systems, which are usually 1–2 Fr smaller than standard sheaths and obviate the need for additional mechanical stretch of the radial artery (76). Two commonly used sheathless platforms are the Eucath (Asahi, Irvine, CA) and the Railway (Cordis, Hialeah, Fl) systems. They are introduced using a dilator and without the need for a sheath, and while 5 Fr, 6 Fr, or 7 Fr catheters can be used with these technologies, catheter backup and manipulation may not be as consistent as with traditional sheathed systems. Sheathless guide catheters have been evaluated in non-randomized cohort studies with successful outcomes in patients with calcified and bifurcation lesions (77, 78). While there have not been any randomized clinical trials studying thin-wall sheaths and sheathless platforms for PCI in head-to-head fashion, a recent propensity-matched analysis of 728 patients with acute coronary syndrome, those undergoing transradial PCI using a 7 Fr Glidesheath Slender had lower rates of RAO (OR = 0.32, 95% CI: 0.11–0.93; p = 0.036) (79) Further research is needed to better understand the optimal transradial platform for performing safe and effective higher risk PCI in patients with complex CAD. A summary of currently available transradial sheaths and sheathless systems is described in Table 3 (80).
Upon completion of the cardiac catheterization, steps should be undertaken to ensure safe and effective hemostasis. A number of radial occlusion devices are currently available for commercial use. To date, a strategy of patent hemostasis has been shown to be most effective in reducing the rates of RAO, with the Prevention of Radial Artery Occlusion—Patent Hemostasis Evaluation (PROPHET) study demonstrating reductions in early (<24 h) and late (30 days) incidence of RAO of 59% and 75%, respectively, compared to a strategy of conventional pressure application (73). Shorter durations of hemostatic compression have been associated with reductions in radial artery compromise, with many centers employing protocols which advocate for patent hemostasis times of 2 and 4 h following diagnostic angiography and PCI, respectively (69). Prophylactic ipsilateral ulnar artery compression following completion of transradial cardiac catheterization has also been shown to be efficacious in not only facilitating safe and patent hemostasis of the RA, but also in reducing the risk of RAO. The PROPHET-II (PROPhylactic Hyperperfusion Evaluation Trial), a multicenter randomized clinical trial of 3,000 patients undergoing TRA for cardiac catheterization demonstrated that a strategy of occlusive compression of the ipsilateral ulnar artery at the Guyon's canal at the time of radial band application followed by removal of the radial sheath and patent hemostasis was associated with nearly 3-fold reduction in RAO up to 30-days post-procedure compared to patent hemostasis alone (81). It is recommended that TRA centers develop post-procedural care pathways and quality assurance mechanisms to ensure that multi-modality assessments are employed to ensure timely hemostasis and early ambulation following transradial coronary angiography or PCI (39).
Distal transradial access
More recently, distal transradial access (dTRA) in the “anatomic snuffbox” a triangular depression on the dorsum of the hand that is bordered by the extensor pollicis brevis and abductor pollicis longus tendons laterally and by the extensor pollicis longus tendon medially, has been proposed as an alternative cannulation site to traditional TRA in the wrist (82). In addition to ergonomic benefits and shorter hemostasis times due to the superficial location of the distal RA with a boney basement in the snuffbox, dTRA may also potentially impart greater reductions in RAO compared to traditional TRA (83, 84). The putative mechanism for this hypothesis is the more distal location of the puncture site, which in turn may facilitate greater preservation of flow in the radial artery proximally, due to the rich cascade of adjacent collateral networks from the deep palmar arch. The safety and feasibility of dTRA for coronary angiography and PCI has been demonstrated, with clinical trial data suggesting greater reductions in RAO, as determined by doppler ultrasound, compared to traditional TRA (84, 85). Little is known, however, regarding the pathobiology of radial artery injury and remodeling following dTRA. The PRESERVE Radial study (A PRospEctive Randomized Clinical Study Comparing Radial ArtERy Intimal Hyperplasia Following Distal Vs. ForEarm TransRADIAL Arterial Access for Coronary Angiography) (NCT04801901) will be the first clinical trial to compare intimal medial hyperplasia and other markers of vessel healing following dTRA and traditional TRA using ultrahigh resolution ultrasound. Given the more superficial location and potential tortuosity of the radial artery in the snuffbox, however, patients undergoing dTRA may experience greater number of puncture attempts, time-to-sheath insertion and access-site crossover (84, 86).
Transfemoral access
Despite a significant shift towards TRA for coronary angiography and PCI, the femoral artery remains a necessary conduit for numerous procedures in contemporary clinical practice, such as the insertion of percutaneous MCS devices and TAVR. It is still also required in non-large bore coronary angiogram/PCI cases (<8 Fr) where TRA or ulnar access are not feasible. Contemporary TFA should ideally employ four multimodality techniques: 1. Fluoroscopy; 2. Doppler ultrasound; 3. Micropuncture needles; and 4. Iliofemoral angiography (87). Familiarity with femoral artery anatomy is key to safe and efficient vascular access and closure. The Common Femoral Artery (CFA) is a continuation of the External Iliac Artery and lies below the inferior epigastric artery, the anatomic location of the inguinal ligament, where the vessel dives posteriorly into the retroperitoneal space. Distally, the CFA bifurcates into the Superficial Femoral Artery (SFA) and Profunda Femoris Artery (PFA). The angiographic anatomy of the femoral arteries are shown in Figure 2. The following steps are recommended for optimal TFA (87):
1. The inferomedial edge of the femoral head should be identified under fluoroscopy and its position should be noted either by way of a hemostat or marker.
2. Ultrasound should then be used to scan for the CFA and its tributaries.
3. Local anesthetic should then be applied using ultrasound guidance to the subcutaneous tissue above and adjacent to the CFA.
4. Using ultrasound guidance, a 21-gauge micropuncture needle should then be used to access the CFA approximately 1 cm–3 cm below the probe with direct visualization of the puncture. This is recommended to avoid a steep angle on the needle with consequent risk for high femoral access, and thus a retroperitoneal bleed. A micropuncture needle may be preferrable to standard 18 gauge platform as it can reduce not only the size of the initial puncture, but also the amount of bleeding associated with vascular injury should there be multiple attempts at cannulating the artery (8). Despite these potential advantages, randomized data supporting this strategy is lacking, and it is advised that institutions develop best practices around vessel puncture, regardless of which needle platform is used (88).
5. Once TFA is obtained, a 0.018″ micro access guidewire should then be advanced under fluoroscopy to ensure appropriate passage, avoiding the circumflex iliac artery, as inadvertent perforation of this vessel with a guidewire may lead to an abdominal hematoma, requiring blood transfusion and endovascular intervention (89).
6. A 4 Fr microcatheter dilator should then be advanced over the guidewire. An iliofemoral angiogram may be performed through the microcatheter dilator, typically at 30 degree ipsilateral angulation, to confirm the location of the arteriotomy. If the access site is not felt to be appropriate, the dilator may then be removed followed by 10 min of manual pressure prior to re-access. Low access at the level of the superficial femoral artery is associated with increased risk for arteriovenous fistula formation, pseudoaneurysms and thrombosis. If the arteriotomy site is deemed to be satisfactory, the tract can then be dilated to place the requisite sheath size to perform the respective cardiac procedure.
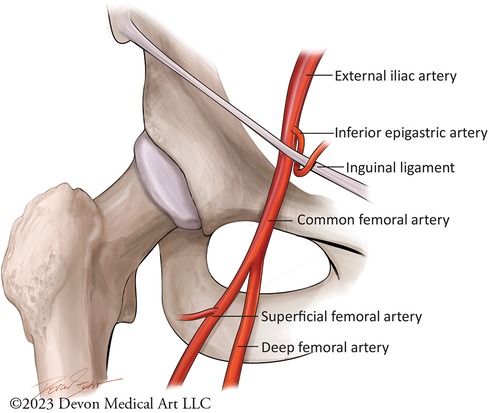
Figure 2. Femoral artery anatomy. This cartoon figure depiction demonstrates the branches of the femoral artery. Safe and effective transfemoral access should ideally rely on fluorscopic and ultrasound-guided landmarks, with micropuncture access of the common femoral artery at the lower edge of the femoral ahead below the inferior epigastric artery.
Ultrasound guided femoral artery access
Routine utilization of ultrasound should be the standard of care for all TFA cases for optimal access and preventing the risk for vascular complications (Figure 3) (87). The merits of ultrasound guidance for femoral access have been studied extensively, including a meta-analysis of 1,422 patients demonstrating nearly 50% reduction in all complications, including bleeding and accidental puncture, as well as 42% improvement in likelihood of successful arterial access on the first attempt (90–93). The Femoral Arterial Access with Ultrasound Trial (FAUST) demonstrated that the use of ultrasound was associated with 80% increased likelihood of first-pass success, 85% reduced risk of accidental venipuncture and nearly 60% reduction vascular complications compared to fluoroscopic guidance (90). The greatest benefit with successful vessel cannulation was noted with common femoral arterial bifurcations above the femoral head, an inherently high risk cohort of patients (90). Interestingly, the routine Ultrasound Guidance for Vascular Access for Cardiac Procedures (UNIVERSAL) Trial, a multi-center prospective study 621 patients undergoing TFA for coronary angiography or PCI, failed to show a decrement in Bleeding Academic Research Consortium 2, 3, or 5 major bleeding or vascular complications with ultrasound-guided access compared with manual palpation, although the former was associated with reduced number of access attempts and accidental venipunctures (94). While the study has been critiqued for not mandating the use of micro puncture catheters and for including BARC 2 minor bleeds which may be unrelated to the procedure, the findings do highlight the need for further research in the form of large multicenter registries and clinical trials evaluating the potential benefits of skilled ultrasound utilization to reduce the risk of adverse periprocedural sequelae during TFA (95).
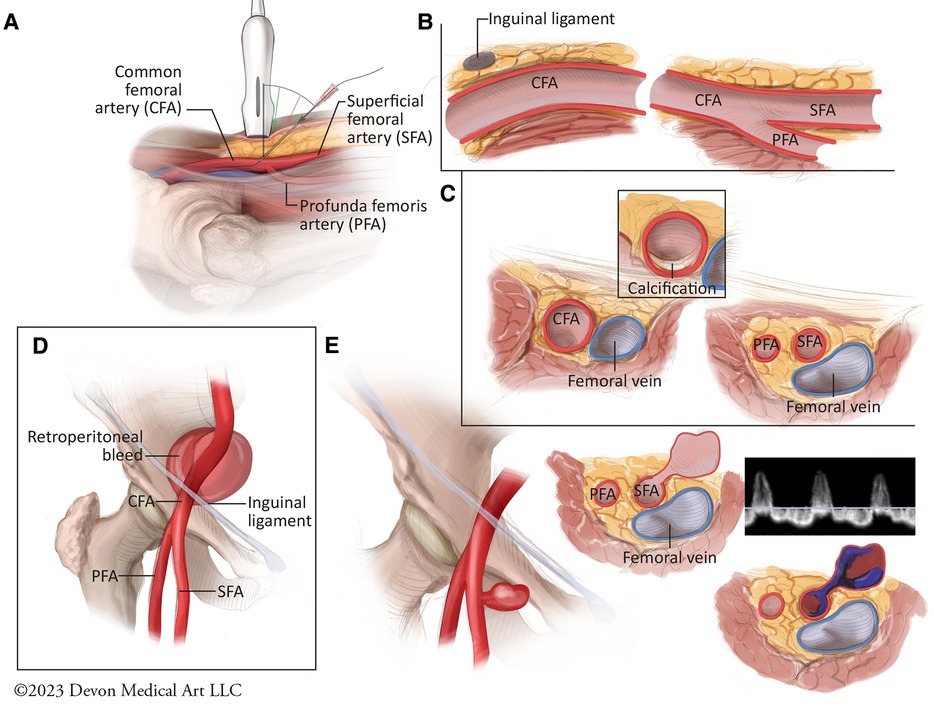
Figure 3. Best practices for femoral arterial access and potential vascular complications. (A) The optimal technique for ultrasound-guided femoral arterial access requires the use of a 5- to 10-MHz linear ultrasound probe which is held such that the vertical marker is facing upward from the femoral artery to facilitate a longitudinal view of the common femoral artery and its branches. The access needle should then be used at a 45 degree angle to access the common femoral artery. (B) Longitudinal view of the femoral vasculature demonstrates the posterior dive that the common femoral artery takes below the inguinal ligament as the probe is advanced cranially. (C) Rotating the ultrasound proble 90 degrees allows for cross sectional view of the femoral bifurcation, including anterior or posterior vessel wall calcification. (D) Vessel puncture cranial to the inferior epigastric artery is associated with increased risk for retroperitoneal bleeds. (E) Vessel puncture below the common femoral artery bifurcation and involving the superfical femoral artery is associated with increased for pseudoaneurysm formation. Pseudoaneurysms can present clinically as pulsatile hematomas, painful ecchmyoses or as frank bleeding. They typically consist of 1 or 2 layers of the vessel wall, and are therefore associated with increased risk for rupture, embolization, and limb ischemia. They typically demonstrate “to-and-fro” patterns of flow with pulsed Doppler assessment.
Vascular closure devices
Historically, manual compression has been the standard of care for achieving hemostasis following femoral arterial sheath removal, usually once activated clotting time (ACT) has been less than160–180 s (96). Given the labor intensive nature of digital compression by catheterization laboratory staff members, a number of mechanical compression devices, such as the Femostop (Radi Medical System) and CompressAR C-clamp (Advanced Vascular Dynamics) were subsequently developed, with some studies suggesting that they may be associated with reduced vascular complications compared with manual hold (97–99). Despite the benefits of allowing staff members to be relieved from the demands of bedside manual compression, these devices do need careful supervision to ensure that they do not migrate and that they are not applied at pressures which could compromise limb flow and result in arterial and/or venous thrombosis.
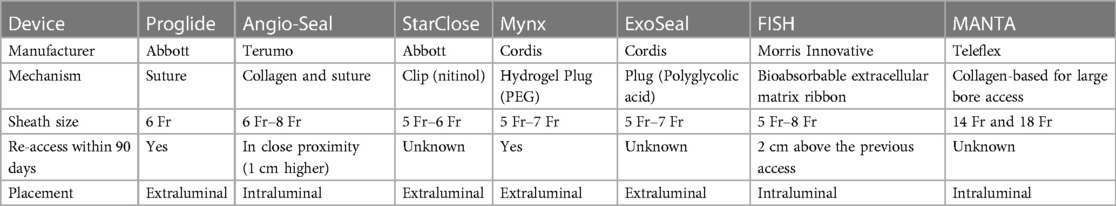
In response to the logistical challenges associated with manual pressure and passive closure platforms, a variety of vascular devices (VCD) have been developed since the mid 1990s which can be deployed at the conclusion of cardiac catheterization, allowing for potentially faster hemostasis, shorter duration of bed rest and reductions in patient discomfort (96). They are used in up to 75% of TFA cases in contemporary clinical practice (100). VCD's have historically been classified into 4 categories: plugs, sutures, glues or topical patches. Despite their potential to improve patient satisfaction and clinical throughput, the evidence supporting their use has been limited to observational studies and modest sized clinical trials (100). These studies have demonstrated the safety and feasibility of VCD's, but they have not provided definitive evidence around efficacy or reductions in bleeding complications compared to manual compression (100–105). As such, caution is advised with the routine deployment of these devices following TFA. Instead, societal guidelines advocate for a patent-centered approach that takes into consideration clinical factors such as body habitus, arteriotomy location and vessel anatomy (size, calcification tortuosity) (101). It is advised that all patients undergoing cardiac procedures via TFA undergo femoral angiography prior to closure to determine whether the arteriotomy site and vessel anatomy are suitable for the deployment of these devices. Potential complications associated with these devices include acute limb ischemia, bleeding, infection, distal embolization, pseudoaneurysm and arteriovenous fistula formation (106, 107). It is recommended that interventionalists have familiarity with all device and that they able to address any potential device-related complications with facile expertise (108). Table 4 provides a summary of the some of the currently Food and Drug Administration approved VCD's employed in clinical practice in the United States.
The Angio-Seal system (Terumo, Somerset, NJ) is one of the most commonly used platforms in the United States, consisting of a co-polymer which is deployed intravascularly and attached by an absorbable Dexon traction suture to an extravascular collagen plug (96). It was first studied against manual compression in a multi-center RCT of 435 patients undergoing cardiac catheterization and was noted to have 85% reduction in time to hemostasis and lower rates of bleeding and hematomas (109). These results were further observed in a subsequent clinical trial of 612 patients undergoing PCI who were randomized to Angio-Seal or manual compression (110). These patients had select high risk features for vascular complications (age >70, previous puncture at the same site, hypertension, treatment with ticlopidine at least 2 days prior, use of abxicimab, 8 Fr access, prolonged heparin treatment after the angioplasty, and use of fibrinolytics) (110). Angio-Seal was associated with significant reductions in time to hemostasis, total bedrest time, and bleeding (110). Available in 6 and 8 Fr platforms, Angio-Seal is rapidly deployable with hemostasis success rates of up to 97% (107).
The ProGlide Perclose device (Abbott Vascular, Abbott Park, IL) is a suture-mediated device that is advanced over an 0.035″ J wire through the arteriotomy after the femoral sheath is removed. Once the return of pulsatile blood is noted from the side arm, the device lever is pulled and two simultaneous needles are deployed through the anterior wall of the femoral artery utilizing the non-biodegradable polypropylene microfilament with preformed knot that is tightened to form the suture loop. The suture is then retracted to the anterior wall of the arteriotomy site and deployed to achieve hemostasis (96) (Figure 4). The Perclose platform is unique among VCDs as it maintains access to the vessel by way of the 0.035 inch guidewire after the needles and suture have been deployed. Therefore if the needles do not capture the sutures and adequate hemostasis is not achieved, the guidewire may be re-introduced into the arteriotomy and ultimately removed following confirmation of satisfactory hemostasis. Similar to the Angio-Seal device, Perclose has been associated with reduced time to hemostasis and ambulation with no significant differences in bleeding complications compared to manual hemostasis (111, 112). In experienced hands, it can also be associated with significant costs savings as it may obviate the need for further manpower and work personnel typically associated with manual compression (113).
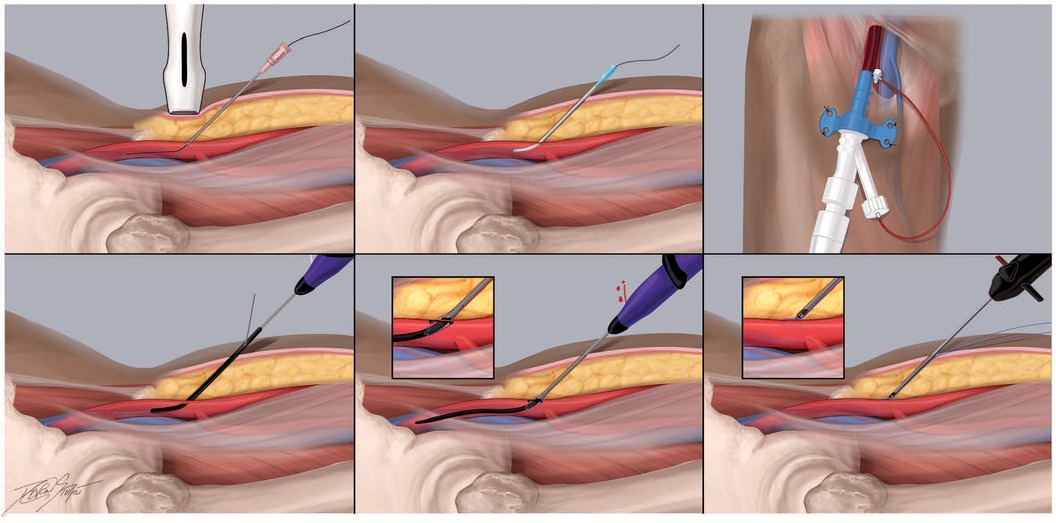
Figure 4. Recommended practices for the perclose proglide vascular closure device. The Perclose Proglide platform (Abbott, Chicago, IL) is a suture-mediated vascular closure device commonly used in contemporary practice to hemostase standard and large bore femoral arterial access sites. It consists of two needles in the proximal compartment and a catheter that house the suture. It is 0.035″ J wire compatible. This cartoon depiction demonstrates the steps of large bore access and closure using this platform. (A) Using ultrasound guidance, the common femoral artery is accessed with a 21 gauge micrpuncture needle 1-3 cm below the probe. (B) Once femoral access is obtained, a.018″ micro access guidewire should then be advanced under fluoroscopy to ensure appropriate passage into the ipsilateral common iliac artery and abdominal aorta. (C) Following confirmation of the femoral access site, the arteriotomy may be “pre-closed” using 1 or 2 Proglide sutures, typically in the 10- and 12-o’clock positions. Here, the percutaneous ventricular assist device is secured in place following dilation of the arteriotomy (D) During deployment of the Proglide system, the catheter is advanced over an 0.035″ J wire through the arteriotomy. (E-F) Once the return of pulsatile blood is noted from the side arm, the device lever is pulled and two simultaneous needles are deployed through the anterior wall of the femoral artery utilizing the non-biodegradable polypropylene microfilament with preformed knot that is tightened to form the suture loop. The suture is then retracted to the anterior wall of the arteriotomy site and deployed to achieve hemostasis.
The MANTA™ vascular closure received pre-market investigational device exemption approval by the Food and Drug Administration in 2019 following multi-center observational data demonstrating safety and feasibility in 263 patients undergoing TAVR and percutaneous endovascular thoracic and abdominal aortic aneurysm repair (114). With an effective sheath outer diameter of 22 Fr or 7.3 mm, rapid closure was able to be achieved, with median time to hemostasis of 24 s and only 4.2% rate of Valve Academic Research Consortium-2 major vascular complications (114). A collagen-based platform, it is currently available in 14 Fr and 18 Fr configurations, and it is 0.035″ guidewire compatible, consisting of a poly-lactic-co-glycolic snap attached to a bovine collagen plug which is tampered down, sandwiching the arteriotomy while a toggle is deployed intravascularly. The toggle and collagen plug usually are fully absorbed within 6 months and the vessel may be re-accessed 2.5 cm cranial or caudal to the MANTA device (96). The safety and efficacy of MANTA was recently compared with Proglide in the Randomized Comparison of Catheter-based Strategies for Interventional Access Site Closure during Transfemoral Transcatheter Aortic Valve Implantation (CHOICE-CLOSURE) study, a multi-center RCT of 516 patients undergoing TAVR (115). The authors reported significantly lower times to hemostasis with MANTA [80 (32–180) vs. 240 (174–316) seconds; p < 0.001) but at the cost of increased rates of access-site vascular complications [relative risk, 1.61 (95% CI: 1.07–2.44), p = 0.029], in particular pseudoaneurysms and hematomas (115). No differences were noted, however, with respect to access site bleeding or device failure. Historically, vascular closure following TAVR and other large bore access procedures has been performed by tightening the knots of 1 or 2 Perclose devices which were partially deployed at the time of initial access (“pre-closure”) (116).Further research is needed in the form of large multi-center registries and nested clinical trials using standardized best-practices to identify closure strategies in patients undergoing large bore access that are the most safe, effective and cost-sensitive.
Large bore vascular access and closure
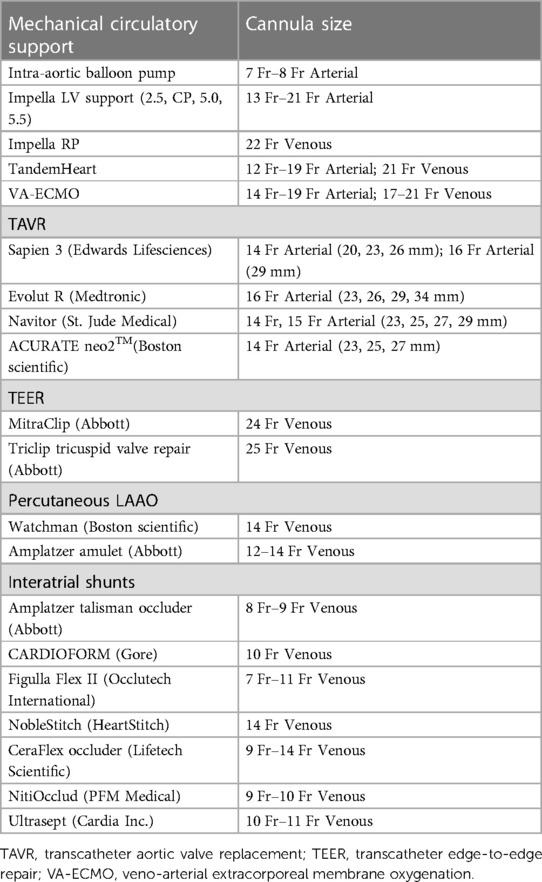
The employment of safe and effective femoral arterial access is of paramount importance in patients requiring large bore sheaths (>8 Fr) for structural heart interventions, endovascular aneurysm repairs and percutaneous MCS placement. Despite advances in device-based therapies to treat these conditions using minimally invasive techniques, there remain significant variations in the technical expertise of large bore access within and among institutions (6, 8). A contemporary analysis of 17,672 patients undergoing large bore access for TAVR, EVAR and percutaneous MCS observed an 18% rate of bleeding complications, which in turn was associated with nearly 3-fold increased risk for in-hospital mortality, 2-fold increase in hospital-length-of-stay and marked increases in total healthcare costs (25). In cases requiring MCS implantation under emergency circumstances, the rates of bleeding and vascular complications can be as high as 40%, with greater than 4-fold increased risk for acute ischemia if advanced platforms such as percutaneous ventricular assist devices or VA-ECMO are utilized (117, 118). A summary of currently available MCS and structural heart platforms with their respective sheath sizes is described in Table 5. Given these considerations, societal recommendations and position statements have been developed around best practices for proper large bore femoral arterial access and closure (8, 119):
1. Similar to standard TFA, multi-modality imaging techniques should be employed to visualize the common femoral artery, including bifurcation, calcification and dimensions, with a minimal diameter of 6 mm typically needed for sheath sizes of up to 18 Fr. In elective circumstances such as TAVR, pre-procedural computed tomography may also be performed to assess iliofemoral anatomy, in addition to other anatomic considerations, such as annular dimensions, valvular calcification and coronary artery height (120).
2. Using fluoroscopic landmarks and doppler ultrasound, a 21 gauge micro-puncture needle should be used to access the CFA, typically 2 cm–3 cm below the mid portion of the inguinal ligament and 1 cm lateral to the lower third of the femoral head (8, 90).
3. Similar to standard TFA, all patients should undergo femoral angiography following micro-puncture access, using standard or digital subtraction angiography (87). Once the femoral access site has been deemed to be suitable, the arteriotomy tract should then dilated for placement of a 6 Fr sheath.
4. Given the clinical ramifications of bleeding and vascular complications associated with large bore access, it is advised that pre-procedural planning occur and contingency measures be developed around closure strategies. While it is generally recognized that VCDs may be preferrable to manual pressure following large bore access, there is no single best practice strategy around a particular device and when to employ it. A “pre-closure” strategy of using 1 or 2 Proglide sutures which are partially deployed, typically in the 10- and 2-o'clock configurations, is commonly employed in contemporary practice, and has been associated with low rates of bleeding and vascular complications (116).
5. Given the enhanced complexities and time intensive nature of large bore procedures, it is advised that operators make concerted effort to mitigate radiation exposure to the patient and staff members at all times. This includes at the time of access when inguinal fluoroscopy is performed to confirm device placement and vessel patency. Strategies include ensuring that the patient is close to the image receptor and away from the x-ray source so as to reduce radiation scatter, maintaining at least 1 cm distance between the operator and the patient, avoiding the use of steep angles with the x-ray beam and placing the C-arm in 0° to 20° angulations, minimizing the number of cineograms and shortening each cine acquisition, utilizing lower frame rates (i.e. 7.5 frames/s) and the application of protective scatter-radiation absorbing shields (121, 122).
6. Once the arteriotomy has been duly upsized and the respective large-bore sheath has been secured, a run-off angiogram should be performed to ensure adequate distal limb perfusion (8). If limb flow is impaired, a distal perfusion catheter consisting of a 5- or 6-Fr braided sheath may be placed using ultrasound-guided antegrade access of the ipsilateral superficial or profunda femoral artery. Distal perfusion can be maintained by utilizing one of three donor strategies: (1) “External” bypass from the ipsilateral common femoral artery by connecting the side-arm of the large-bore sheath to the distal perfusion cannula; (2) “External” bypass from the contralateral common femoral artery by connecting the side-arm of a 5 or 6-Fr retrograde sheath placed in the contralateral vessel to the distal perfusion cannula; and (3) “Internal” contralateral bypass from a 7 Fr retrograde sheath in he the contralateral common femoral artery to the ipsilateral profunda femoris artery through an up-and-over internal 4 Fr sheath which is inserted through the contralateral conduit (8, 123). The radial artery may also be utilized as a donor for distal perfusion, although caution is advised if it prolonged indwelling of the radial sheath is anticipated, given the potential risk for radial artery thrombosis and upper extremity digital ischemia (8). It should be noted that the feasibility and safety for distal perfusion cannula placement is primarily from observational data, and further research in the form of randomized clinical trials is needed to inform the application of this practice on a larger scale (8, 119, 123).
7. While the radial artery has been demonstrated to reduce bleeding and major adverse cardiac events in patients undergoing complex PCI with pVAD support, there is an alternative technique currently employed at many centers in the United which may reduce the number of access sites. Commonly referred to the SHiP (Single access for High-risk PCI) technique, the procedure is facilitated by a micro-puncture needle which is used to pierce the diaphragm of the pVAD peel-away sheath, allowing for placement of a 7 Fr sheath through the hemostatic valve and adjacent to the 9 Fr pVAD catheter shaft, through which complex PCI can be performed (124).
8. Upon completion of the large bore cardiac procedure, it is recommended that vascular closure be performed in the cardiac catheterization laboratory or hybrid operating room with cardiac and vascular surgical back-up capabilities (8). Closure strategies currently employed in clinical practice include: 1) Deployment of the Perclose Proglides sutures (1 or 2) which were used to “pre-close” the arteriotomy at the onset of the case; (2) “Post-Closure” using a double-wire approach to facilitate the deployment of two Perclose Proglide; (3) “Hybrid” approach with the combined use of one Perclose Proglide suture and either one Angioseal or Mynx VCD; (4) “Dry closure” with balloon hemostasis of the ipsilateral external iliac artery via radial or contralateral femoral arterial access; or 5) Deployment of the MANTA device (114, 125–127).
9. Once hemostasis is achieved, final run-off femoral angiography (via the radial artery or contralateral femoral artery) is advised to ensure distal perfusion and recognize any potential vascular complications (8). In the event of vessel injury stemming from perforation, hemostasis can be obtained percutaneously by way of endovascular deployment of covered stents. While both self- and balloon-expandable platforms can be used, the former may be preferred given its more adaptive frame in response to daily physiologic stressors imposed on the iliofemoral vessels from flexion and extension (128).
10. All vascular complications stemming from standard or large bore TFA access should be reviewed at institutional quality assurances forums in standardized fashion by physician and administrative leadership. Direct feedback can then provided to the interventional team around potential opportunities for improvement (129).
Conclusion
Despite technologic advances in transcatheter-based therapies using minimally invasive techniques, major bleeding and vascular complications continue to hinder outcomes across the spectrum of contemporary interventional cardiology. While cardiovascular societies have put forth guidelines around proper management of arterial access and closure, the data supporting these recommendations have either been observational in design or derived from medium-sized clinical trials. As a result, there remains an unmet need for further research in the form of large multi-center registries with nested and pragmatic clinical trials to address the knowledge gaps that remain in this field. Clinical questions that remain unanswered and could potentially optimize catheter-based procedures include the role that short-acting parenteral antiplatelet agents may play in reducing the risk for periprocedural bleeding, the potential merits of pre-emptive atherectomy and/or lithotripsy of calcified iliofemoral vessels in patients with peripheral arterial disease who are undergoing large bore procedures, the development of smaller caliber delivery platforms to facilitate MCS and structural heart interventions, and the creation of novel bleeding detection platforms capable of recognizing hemorrhagic sequelae prior to overt clinical manifestation (130–132). Until then, concerted efforts should be made to minimize variability in clinical practice by developing institutional protocols and competency pathways incorporating best practices for safe and effective vascular access and closure. Coupled with institutional mechanisms for oversight and quality improvement, these measures hold promise in informing multicenter collaborations and clinical research designs aimed at advancing the care of patients undergoing catheter-based cardiovascular procedures.
Author contributions
ME: Writing – original draft. MF: Writing – review & editing. WB: Writing – review & editing. LC: Writing – review & editing. ADu: Writing – review & editing. KEn: Writing – review & editing. KEp: Writing – review & editing. AM: Writing – review & editing. RN: Writing – review & editing. CR: Writing – review & editing. MS: Writing – review & editing. DS: . AT: . CV: Writing – review & editing. ADa: Writing – review & editing. BT: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank Dwight and Martha Schar for their generous support of Inova Schar Heart and Vascular, and the Dudley Family for their continued contributions and support of the Inova Dudley Family Center for Cardiovascular Innovation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. (2022) 145(8):e153–639. doi: 10.1161/CIR.0000000000001052
2. Damluji AA, Forman DE, Wang TY, Chikwe J, Kunadian V, Rich MW, Young BA, et al. Management of acute coronary syndrome in the older adult population: a scientific statement from the American Heart Association. Circulation. (2023) 147(3):e32–62. doi: 10.1161/CIR.0000000000001112
3. Bangalore S, Barsness GW, Dangas GD, Kern MJ, Rao SV, Shore-Lesserson L, et al. Evidence-based practices in the cardiac catheterization laboratory: a scientific statement from the American Heart Association. Circulation. (2021) 144(5):e107–19. doi: 10.1161/CIR.0000000000000996
4. Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support. J Am Coll Cardiol. (2014) 64(14):1407–15. doi: 10.1016/j.jacc.2014.07.958
5. Lansky AJ, Tirziu D, Moses JW, Pietras C, Ohman EM, O'Neill WW, et al. Impella versus intra-aortic balloon pump for high-risk PCI: a propensity-adjusted large-scale claims dataset analysis. Am J Cardiol. (2022) 185:29–36. doi: 10.1016/j.amjcard.2022.08.032
6. Damluji AA, Nelson DW, Vagimigli M, Windecker S, Byrne RA, Cohen F, et al. Transfemoral approach for coronary angiography and intervention: a collaboration of international cardiovascular societies. JACC Cardiovasc Interv. (2017) 10(22):2269–79. doi: 10.1016/j.jcin.2017.08.035
7. Amin AP, Spertus JA, Desai N, Masoudi FA, Bach RG, McNeely C, et al. The evolving landscape of impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. (2020) 141(4):273–84. doi: 10.1161/CIRCULATIONAHA.119.044007
8. Damluji AA, Tehrani B, Sinha SS, Samsky MD, Henry TD, Thiele H, et al. Position statement on vascular access safety for percutaneous devices in AMI complicated by cardiogenic shock. JACC Cardiovasc Interv. (2022) 15(20):2003–19. doi: 10.1016/j.jcin.2022.08.041
9. Feit F, Voeltz MD, Attubato MJ, Lincoff AM, Chew DP, Bittl JA, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 trial. Am J Cardiol. (2007) 100(9):1364–9. doi: 10.1016/j.amjcard.2007.06.026
10. Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, et al. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. (2005) 96(9):1200–6. doi: 10.1016/j.amjcard.2005.06.056
11. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. (2011) 123(23):2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449
12. Moscucci M, Fox KAA, Cannon CP, Klein W, Lopez-Sendon J, Montalescot G, et al. Predictors of major bleeding in acute coronary syndromes: the global registry of acute coronary events (GRACE). Eur Heart J. (2003) 24(20):1815–23. doi: 10.1016/S0195-668X(03)00485-8
13. Sabatine MS, Morrow DA, Giugliano RP, Burton PBJ, Murphy SA, McCabe CH, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. (2005) 111(16):2042–9. doi: 10.1161/01.CIR.0000162477.70955.5F
14. Kwok CS, Rao SV, Myint PK, Keavney B, Nolan J, Ludman PF, et al. Major bleeding after percutaneous coronary intervention and risk of subsequent mortality: a systematic review and meta-analysis. Open Heart. (2014) 1(1):e000021. doi: 10.1136/openhrt-2013-000021
15. Chhatriwalla AK, Amin AP, Kennedy KF, House JA, Cohen DJ, Rao SV, et al. Association between bleeding events and in-hospital mortality after percutaneous coronary intervention. JAMA. (2013) 309(10):1022–9. doi: 10.1001/jama.2013.1556
16. Hara H, Takahashi K, Kogame N, Tomaniak M, Kerkmeijer LS, Ono M, et al. Impact of bleeding and myocardial infarction on mortality in all-comer patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. (2020) 13(9):e009177. doi: 10.1161/CIRCINTERVENTIONS.120.009177
17. Amin AP, Miller S, Rahn B, Caruso M, Pierce A, Sorenson K, et al. Reversing the “risk-treatment paradox” of bleeding in patients undergoing percutaneous coronary intervention: risk-concordant use of bleeding avoidance strategies is associated with reduced bleeding and lower costs. J Am Heart Assoc. (2018) 7(21):e008551. doi: 10.1161/JAHA.118.008551
18. Kwok CS, Sherwood MW, Watson SM, Nasir SB, Sperrin M, Nolan J, et al. Blood transfusion after percutaneous coronary intervention and risk of subsequent adverse outcomes: a systematic review and meta-analysis. JACC Cardiovasc Interv. (2015) 8(3):436–46. doi: 10.1016/j.jcin.2014.09.026
19. Sherwood MW, Wang Y, Curtis JP, Peterson ED, Rao SV. Patterns and outcomes of red blood cell transfusion in patients undergoing percutaneous coronary intervention. JAMA. (2014) 311(8):836–43. doi: 10.1001/jama.2014.980
20. Doyle BJ, Rihal CS, Gastineau EA, Holmes DR. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol. (2009) 53(22):2019–27. doi: 10.1016/j.jacc.2008.12.073
21. Silvain J, Abtan J, Kerneis M, Martin R, Finzi J, Vignalou JB, et al. Impact of red blood cell transfusion on platelet aggregation and inflammatory response in anemic coronary and noncoronary patients: the TRANSFUSION-2 study (impact of transfusion of red blood cell on platelet activation and aggregation studied with flow cytometry use and light transmission aggregometry). J Am Coll Cardiol. (2014) 63(13):1289–96. doi: 10.1016/j.jacc.2013.11.029
22. Hedstrom M, Flordal PA, Ahl T, Svensson J, Dalen N. Autologous blood transfusion in hip replacement. No effect on blood loss but less increase of plasminogen activator inhibitor in a randomized series of 80 patients. Acta Orthop Scand. (1996) 67(4):317–20. doi: 10.3109/17453679609002322
23. Dhruva SS, Ross JS, Mortazavi BJ, Hurley NC, Krumholz HM, Curtis JP, et al. Association of use of an intravascular microaxial left ventricular assist device vs intra-aortic balloon pump with in-hospital mortality and Major bleeding among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. (2020) 323(8):734–45. doi: 10.1001/jama.2020.0254
24. Thiele H, Zeymer U, Akin I, Behnes M, Rassaf T, Mahabadi AA, et al. Extracorporeal life support in infarct-related cardiogenic shock. N Engl J Med. (2023) 389(14):1286–97. doi: 10.1056/NEJMoa2307227
25. Redfors B, Watson BM, McAndrew T, Palisaitis E, Francese DP, Razavi M, et al. Mortality, length of stay, and cost implications of procedural bleeding after percutaneous interventions using large-bore catheters. JAMA Cardiol. (2017) 2(7):798–802. doi: 10.1001/jamacardio.2017.0265
26. Smith CR, Leon MB, Mack MJ, Miller C, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. (2011) 364(23):2187–98. doi: 10.1056/NEJMoa1103510
27. Popma JJ, Reardon MJ, Khabbaz K, Harrison JK, Hughes GC, Kosali S, et al. Early clinical outcomes after transcatheter aortic valve replacement using a novel self-expanding bioprosthesis in patients with severe aortic stenosis who are suboptimal for surgery: results of the evolut R U.S. Study. JACC Cardiovasc Interv. (2017) 10(3):268–75. doi: 10.1016/j.jcin.2016.08.050
28. Sherwood MW, Xiang K, Matsouaka R, Li Z, Vemulapalli S, Vora AN, et al. Incidence, temporal trends, and associated outcomes of vascular and bleeding complications in patients undergoing transfemoral transcatheter aortic valve replacement. Circ: Cardiovasc Interventions. (2020) 13(1): p. e008227. doi: 10.1161/CIRCINTERVENTIONS.119.008227
29. Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn. (1989) 16(1):3–7. doi: 10.1002/ccd.1810160103
30. Rao SV, Ou FS, Wang TY, Roe MT, Brindis R, Rumsfled JS, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. (2008) 1(4):379–86. doi: 10.1016/j.jcin.2008.05.007
31. Masoudi FA, Ponirakis A, de Lemos JA, Gollis JG, Kremers M, Messenger JC, et al. Executive summary: trends in U.S. Cardiovascular care: 2016 report from 4 ACC national cardiovascular data registries. J Am Coll Cardiol. (2017) 69(11):1424–6. doi: 10.1016/j.jacc.2016.12.004
32. Ferrante G, Rao SV, Juni P, Da Costa BR, Reimers B, Condorelli G, et al. Radial versus femoral access for coronary interventions across the entire spectrum of patients with coronary artery disease: a meta-analysis of randomized trials. JACC Cardiovasc Interv. (2016) 9(14):1419–34. doi: 10.1016/j.jcin.2016.04.014
33. Chodor P, Krupa H, Kurek T, Sokal A, Swierad M, Was T, et al. RADIal versus femoral approach for percutaneous coronary interventions in patients with acute myocardial infarction (RADIAMI): a prospective, randomized, single-center clinical trial. Cardiol J. (2009) 16(4):332–40.19653176
34. Cooper CJ, El-Sheikh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J. (1999) 138(3):430–6. doi: 10.1016/S0002-8703(99)70143-2
35. Romagnoli E, Biondi-Zoccai G, Sciahbasi A, Politi L, Rigattieri S, Pendeza G, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (radial versus femoral randomized investigation in ST-elevation acute coronary syndrome) study. J Am Coll Cardiol. (2012) 60(24):2481–9. doi: 10.1016/j.jacc.2012.06.017
36. Amin AP, Patterson M, House JA, Giersiefen H, Spertus JA, Baklanov DV, et al. Costs associated with access site and same-day discharge among medicare beneficiaries undergoing percutaneous coronary intervention: an evaluation of the current percutaneous coronary intervention care pathways in the United States. JACC Cardiovasc Interv. (2017) 10(4):342–51. doi: 10.1016/j.jcin.2016.11.049
37. Bradley SM, Kaltenbach LA, Xiang K, Amin AP, Hess PL, Maddox TM, Poulose A, et al. Trends in use and outcomes of same-day discharge following elective percutaneous coronary intervention. JACC: Cardiovasc Interv. (2021) 14(15):1655–66. doi: 10.1016/j.jcin.2021.05.043
38. Meijers TA, Aminian A, van Wely M, Teeuwen K, Schmitz T, Dirksen MT, et al. Randomized comparison between radial and femoral large-bore access for Complex percutaneous coronary intervention. JACC Cardiovasc Interv. (2021) 14(12):1293–303. doi: 10.1016/j.jcin.2021.03.041
39. Mason PJ, Shah B, Tamis-Holland JE, Bittl JA, Cohen MG, Safirstein J, et al. An update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome: a scientific statement from the American Heart Association. Circ Cardiovasc Interv. (2018) 11(9):e000035. doi: 10.1161/HCV.0000000000000035
40. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Bernedetto U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J, 2018. 40(2): p. 87–165. doi: 10.1093/eurheartj/ehy394
41. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. (2022) 145(3):e18–e114. doi: 10.1161/CIR.0000000000001038
42. Feldman DN, Swaminathan RV, Kaltenbach LA, Baklanov DV, Kim LK, Wong SC, et al. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the national cardiovascular data registry (2007–2012). Circulation. (2013) 127(23):2295–306. doi: 10.1161/CIRCULATIONAHA.112.000536
43. Ratib K, Mamas MA, Anderson SG, Bhatia G, Routledge H, De Belder M, et al. Access site practice and procedural outcomes in relation to clinical presentation in 439,947 patients undergoing percutaneous coronary intervention in the United Kingdom. JACC Cardiovasc Interv. (2015) 8(1 Pt A):20–9. doi: 10.1016/j.jcin.2014.06.026
44. Jolly SSM, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. (2011) 377(9775):1409–20. doi: 10.1016/S0140-6736(11)60404-2
45. Mehta S, Jolly SS, Cairns J, Niemela K, Rao SV, Cheema AN, et al. Effects of radial versus femoral artery access in patients with acute coronary syndromes with or without ST-segment elevation. J Am Coll Cardiol. (2012) 60(24):2490–9. doi: 10.1016/j.jacc.2012.07.050
46. Valgimigli M, Gagnor A, Calabro P, Frigoli E, Leonardi S, Zaro T, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. (2015) 385(9986):2465–76. doi: 10.1016/S0140-6736(15)60292-6
47. Tehrani BN, Damluji AA, Sherwood MW, Rosner C, Truesdell AG, Epps KE, et al. Transradial access in acute myocardial infarction complicated by cardiogenic shock: stratified analysis by shock severity. Catheter Cardiovasc Interv. (2021) 97(7):1354–66. doi: 10.1002/ccd.29098
48. Le May M, Wells G, So D, Chong AY, Dick A, Froeschl M, et al. Safety and efficacy of femoral access vs radial access in ST-segment elevation myocardial infarction: the SAFARI-STEMI randomized clinical trial. JAMA Cardiology. (2020) 5(2):126–34. doi: 10.1001/jamacardio.2019.4852
49. Sandoval Y, Bell MR, Gulati R. Transradial artery access complications. Circ: Cardiovasc Interventions. (2019) 12(11):e007386. doi: 10.1161/CIRCINTERVENTIONS.119.007386
50. Rao SV, Tremmel JA, Gilchrist IC, Shah PB, Gulati R, Shroff AR, et al. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and intervention’s transradial working group. Catheter Cardiovasc Interv. (2014) 83(2):228–36. doi: 10.1002/ccd.25209
51. Georges JL, Belle L, Meunier L, Dechery T, Khalife K, Pecheux M, et al. Radial versus femoral access for coronary angiography and intervention is associated with lower patient radiation exposure in high-radial-volume centres: insights from the RAY'ACT-1 study. Arch Cardiovasc Dis. (2017) 110(3):179–87. doi: 10.1016/j.acvd.2016.09.002
52. Adelaal E, Brousseau-Provencher C, Montminy S, Plourde G, MacHaalany J, Bataile Y, et al. Risk score, causes, and clinical impact of failure of transradial approach for percutaneous coronary interventions. JACC Cardiovasc Interv. (2013) 6(11):1129–37. doi: 10.1016/j.jcin.2013.05.019
53. Yadav PK, Bagur R, Baquero GA, Gilchrist IC. Safety and feasibility of transradial catheterization in breast cancer survivors: a 2-center international experience. JACC Cardiovasc Interv. (2015) 8(4):639–41. doi: 10.1016/j.jcin.2014.08.018
54. Wakeyama T, Ogawa H, Lida H, Takaki A, Iwami T, Mochizuki M, et al. Intima-media thickening of the radial artery after transradial intervention. An intravascular ultrasound study. J Am Coll Cardiol. (2003) 41(7):1109–14. doi: 10.1016/S0735-1097(03)00089-5
55. Yonetsu T, Kakuta T, Lee T, Takayama K, Kakita K, Iwamoto T, et al. Assessment of acute injuries and chronic intimal thickening of the radial artery after transradial coronary intervention by optical coherence tomography. Eur Heart J. (2010) 31(13):1608–15. doi: 10.1093/eurheartj/ehq102
56. Kamiya H, Ushijima T, Kanamori T, Ikeda C, Nakagaki C, Ueyama K, et al. Use of the radial artery graft after transradial catheterization: is it suitable as a bypass conduit? Ann Thorac Surg. (2003) 76(5):1505–9. doi: 10.1016/S0003-4975(03)01018-X
57. Valgimigli M, Campo G, Penzo C, Tebaldi M, Biscaglia S, Ferrari R, et al. Transradial coronary catheterization and intervention across the whole spectrum of allen test results. J Am Coll Cardiol. (2014) 63(18):1833–41. doi: 10.1016/j.jacc.2013.12.043
58. Larsen P, Shah S, Waxman S, Freilich M, Riskalla N, Piemonte T, et al. Comparison of procedural times, success rates, and safety between left versus right radial arterial access in primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Catheter Cardiovasc Interv. (2011) 78(1):38–44. doi: 10.1002/ccd.22843
59. Dehghani P, Mohammad A, Bajaj R, Hong T, Suen CM, Sharieff W, et al. Mechanism and predictors of failed transradial approach for percutaneous coronary interventions. JACC Cardiovasc Interv. (2009) 2(11):1057–64. doi: 10.1016/j.jcin.2009.07.014
60. Kado H, Patel A, Suryadevara S, Zenni M, Box L, Angiolillo D, et al. Operator radiation exposure and physical discomfort during a right versus left radial approach for coronary interventions: a randomized evaluation. JACC Cardiovasc Interv. (2014) 7(7):810–6. doi: 10.1016/j.jcin.2013.11.026
61. Patel T, Shah S, Patel T. Cannulating LIMA graft using right transradial approach: two simple and innovative techniques. Catheter Cardiovasc Interv. (2012) 80(2):316–20. doi: 10.1002/ccd.24321
62. Gu WJ, Wu XD, Wang F, Ma ZL, Gu XP. Ultrasound guidance facilitates radial artery catheterization: a meta-analysis with trial sequential analysis of randomized controlled trials. Chest. (2016) 149(1):166–79. doi: 10.1378/chest.15-1784
63. Seto A, Roberts J, Abu-Fadel M, Czak S, Latif F, Jain S, et al. Real-time ultrasound guidance facilitates transradial access: rAUST (radial artery access with ultrasound trial). JACC Cardiovasc Interv. (2015) 8(2):283–91. doi: 10.1016/j.jcin.2014.05.036
64. Costa F, Van Leeuwen M, Daemen J, Diletti R, Kauer F, Van Geuns R, et al. The rotterdam radial access research. Circ: Cardiovasc Interventions. (2016) 9(2):e003129. doi: 10.1161/CIRCINTERVENTIONS.115.003129
65. Bernat I, Abdelaal E, Plourde G, Bataille Y, Cech J, Pesek J, et al. Early and late outcomes after primary percutaneous coronary intervention by radial or femoral approach in patients presenting in acute ST-elevation myocardial infarction and cardiogenic shock. Am Heart J. (2013) 165(3):338–43. doi: 10.1016/j.ahj.2013.01.012
66. Hahalis G, Tsigkas G, Xanthopoulou I, Deftereos S, Ziakas A, Raisakis K, et al. Transulnar compared with transradial artery approach as a default strategy for coronary procedures: a randomized trial. The transulnar or transradial instead of coronary transfemoral angiographies study (the AURA of ARTEMIS study). Circ Cardiovasc Interv. (2013) 6(3):252–61. doi: 10.1161/CIRCINTERVENTIONS.112.000150
67. Dahal K, Rijal J, Lee J, Korr KS, Azrin M. Transulnar versus transradial access for coronary angiography or percutaneous coronary intervention: a meta-analysis of randomized controlled trials. Catheter Cardiovasc Interv. (2016) 87(5):857–65. doi: 10.1002/ccd.26221
68. Kedev S, Zafirovska B, Dharma S, Petkoska D. Safety and feasibility of transulnar catheterization when ipsilateral radial access is not available. Catheter Cardiovasc Interv. (2014) 83(1):E51–60. doi: 10.1002/ccd.25123
69. Bernat I, Aminian A, Pancholy S, Mamas M, Gaudino M, Nolan J, et al. Best practices for the prevention of radial artery occlusion after transradial diagnostic angiography and intervention: an international consensus paper. JACC Cardiovasc Interv. (2019) 12(22):2235–46. doi: 10.1016/j.jcin.2019.07.043
70. Hahalis G, Leopoulou M, Tsigkas G, Xanthpoulou I, Patsilinakos S, Patsourakos N, et al. Multicenter randomized evaluation of high versus standard heparin dose on incident radial arterial occlusion after transradial coronary angiography. JACC Cardiovasc Interv. (2018) 11(22):2241–50. doi: 10.1016/j.jcin.2018.08.009
71. Aminian A, Saito S, Takahashi A, Bernat I, Jobe R, Kajiya T, et al. Comparison of a new slender 6 fr sheath with a standard 5 fr sheath for transradial coronary angiography and intervention: rAP and BEAT (radial artery patency and bleeding, efficacy, adverse evenT), a randomised multicentre trial. EuroIntervention. (2017) 13(5):e549–56. doi: 10.4244/EIJ-D-16-00816
72. Dharma S, Kedev S, Patel T, Kiemeneij F, Gilchrist IC. A novel approach to reduce radial artery occlusion after transradial catheterization: postprocedural/prehemostasis intra-arterial nitroglycerin. Catheter Cardiovasc Interv. (2015) 85(5):818–25. doi: 10.1002/ccd.25661
73. Pancholy S, Coppola J, Patel T, Roke-Thomas M. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv. (2008) 72(3):335–40. doi: 10.1002/ccd.21639
74. Dangoisse V, Guedes A, Chenu P, Hanet C, Albert C, Robin V, et al. Usefulness of a gentle and short hemostasis using the transradial band device after transradial access for percutaneous coronary angiography and interventions to reduce the radial artery occlusion rate (from the prospective and randomized CRASOC I, II, and III studies). Am J Cardiol. (2017) 120(3):374–9. doi: 10.1016/j.amjcard.2017.04.037
75. Kwok CS, Rashid M, Fraser D, Nolan J, Mamas M. Intra-arterial vasodilators to prevent radial artery spasm: a systematic review and pooled analysis of clinical studies. Cardiovasc Revasc Med. (2015) 16(8):484–90. doi: 10.1016/j.carrev.2015.08.008
76. Raje V, Krathen C, Sanghvi K. Evaluation of railway sheathless access system for transradial coronary and peripheral interventions. Cardiovasc Revasc Med. (2021) 22:91–7. doi: 10.1016/j.carrev.2020.06.016
77. Mamas M, D'souza S, Hendry C, Ali R, Iles-Smith H, Palmer K, et al. Use of the sheathless guide catheter during routine transradial percutaneous coronary intervention: a feasibility study. Catheter Cardiovasc Interv. (2010) 75(4):596–602. doi: 10.1002/ccd.22246
78. Kassimis G, Patel N, Kharbanda RK, Channon KM, Banning AP. High-speed rotational atherectomy using the radial artery approach and a sheathless guide: a single-centre comparison with the “conventional” femoral approach. EuroIntervention. (2014) 10(6):694–9. doi: 10.4244/EIJV10I6A121
79. Isawa T, Horie K, Honda T. Sheathless guiding catheter versus “slender” sheath/guiding catheter combination in acute coronary syndrome: a propensity-matched analysis of two downsized devices. Eur Heart J. (2020) 41(Supplement_2):1739. doi: 10.1093/ehjci/ehaa946.1739
80. Meijers T, Aminian A, Valgimigli M, Dens J, Agostoni P, Iglesias J, et al. Vascular access in percutaneous coronary intervention of chronic total occlusions: a state-of-the-art review. Circ: Cardiovasc Interventions. (2023) 16(8):e013009. doi: 10.1161/CIRCINTERVENTIONS.123.013009
81. Pancholy SB, Bernat I, Bertrand O, Patel TM. Prevention of radial artery occlusion after transradial catheterization. JACC Cardiovasc Interv. (2016) 9(19):1992–9. doi: 10.1016/j.jcin.2016.07.020
82. Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention. (2017) 13(7):851–7. doi: 10.4244/EIJ-D-17-00079
83. Valgimigli M, Landi A. Distal transradial access for coronary procedures. JACC Cardiovasc Interv. (2022) 15(1):33–8. doi: 10.1016/j.jcin.2021.10.032
84. Tsigkas G, Papageorgiou A, Moulias A, Kalogeropoulos A, Papageorgopoulou C, Apostolos A, et al. Distal or traditional transradial access site for coronary procedures: a single-center, randomized study. JACC Cardiovasc Interv. (2022) 15(1):22–32. doi: 10.1016/j.jcin.2021.09.037
85. Feghaly J, Chen K, Blanco A, Pineda AM. Distal versus conventional radial artery access for coronary catheterization: a systematic review and meta-analysis. Catheter Cardiovasc Interv. (2023) 101(4):722–36. doi: 10.1002/ccd.30602
86. Aminian A, Sgueglia G, Wiemer M, Kefer J, Gasparini G, Ruzsa Z, et al. Distal versus conventional radial access for coronary angiography and intervention. JACC Cardiovasc Interv. (2022) 15(12):1191–201. doi: 10.1016/j.jcin.2022.04.032
87. Sandoval Y, Burke M, Lobo A, Lips D, Seto A, Chavez I, et al. Contemporary arterial access in the cardiac catheterization laboratory. JACC Cardiovasc Interv. (2017) 10(22):2233–41. doi: 10.1016/j.jcin.2017.08.058
88. Ambrose J, Lardizabal J, Mouanoutoua M, Buhari C, Berg R, Joshi B, et al. Femoral micropuncture or routine introducer study (FEMORIS). Cardiology. (2014) 129(1):39–43. doi: 10.1159/000362536
89. Moon IT, Shin JH, Sohn YS, Lee JY, Park HC, Choi SI, et al. Abdominal wall hematoma as a rare complication following percutaneous coronary intervention. Korean Circ J. (2016) 46(3):408–11. doi: 10.4070/kcj.2016.46.3.408
90. Seto AH, Abu-Fadel MS, Sparling JM, Zacharias SJ, Daly TS, Harrison AT, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: fAUST (femoral arterial access with ultrasound trial). JACC Cardiovasc Interv. (2010) 3(7):751–8. doi: 10.1016/j.jcin.2010.04.015
91. Gedikoglu M, Oguzkurt M, Gur S, Andic C, Sariturk C, Ozkan U, et al. Comparison of ultrasound guidance with the traditional palpation and fluoroscopy method for the common femoral artery puncture. Catheter Cardiovasc Interv. (2013) 82(7):1187–92. doi: 10.1002/ccd.24955
92. Kalish J, Eslami M, Gillespie D, Schermerhorn M, Rybin D, Doros G, et al. Routine use of ultrasound guidance in femoral arterial access for peripheral vascular intervention decreases groin hematoma rates. J Vasc Surg. (2015) 61(5):1231–8. doi: 10.1016/j.jvs.2014.12.003
93. Sobolev M, Slovut DP, Chang AL, Shiloh AL, Eisen LA. Ultrasound-guided catheterization of the femoral artery: a systematic review and meta-analysis of randomized controlled trials. J Invasive Cardiol. (2015) 27(7):318–23.26136279
94. Jolly SS, AlRashidi S, D'Entremont MA, Alansari O, Brochu B, Heenan L, et al. Routine ultrasonography guidance for femoral vascular access for cardiac procedures: the UNIVERSAL randomized clinical trial. JAMA Cardiology. (2022) 7(11):1110–8. doi: 10.1001/jamacardio.2022.3399
95. Fanaroff AC, Giri J. Fluoroscopic guidance for femoral artery access—pushing patients out of the plane without a parachute? JAMA Cardiology. (2022) 7(11):1118–20. doi: 10.1001/jamacardio.2022.3413
96. Meucci F, Stolcova M, Caniato F, Sarraf M, Mattesini A, Mario CD, et al. The Essentials of Femoral Vascular Access and Closure, in Interventional Cardiology. p. 21-33. (2022).
97. Pracyk JB, Wall TC, Longabaugh JP, Tice FD, Hochrein J, Green C, et al. A randomized trial of vascular hemostasis techniques to reduce femoral vascular complications after coronary intervention. Am J Cardiol. (1998) 81(8):970–6. doi: 10.1016/S0002-9149(98)00074-5
98. Semler HJ. Transfemoral catheterization: mechanical versus manual control of bleeding. Radiology. (1985) 154(1):234–5. doi: 10.1148/radiology.154.1.3880610
99. Bogart MA. Time to hemostasis: a comparison of manual versus mechanical compression of the femoral artery. Am J Crit Care. (1995) 4(2):149–56. doi: 10.4037/ajcc1995.4.2.149
100. Prouse A, Gunzburger E, Yang F, Morrison J, Valle JA, Armstrong EJ, et al. Contemporary use and outcomes of arterial closure devices after percutaneous coronary intervention: insights from the veterans affairs clinical assessment, reporting, and tracking program. J Am Heart Assoc. (2020) 9(4):e015223. doi: 10.1161/JAHA.119.015223
101. Patel MR, Jneid H, Derdeyn CP, Klein LW, Levine GN, Lookstein RA, et al. Arteriotomy closure devices for cardiovascular procedures. Circulation. (2010) 122(18):1882–93. doi: 10.1161/CIR.0b013e3181f9b345
102. Dangas G, Mehran R, Kokolis S, Feldman D, Satler LF, Pichard AD, et al. Vascular complications after percutaneous coronary interventions following hemostasis with manual compression versus arteriotomy closure devices. J Am Coll Cardiol. (2001) 38(3):638–41. doi: 10.1016/S0735-1097(01)01449-8
103. Tavris DR, Gallauresi BA, Lin B, Rich SE, Shaw RE, Weintraub WS, et al. Risk of local adverse events following cardiac catheterization by hemostasis device use and gender. J Invasive Cardiol. (2004) 16(9):459–64.15353824
104. Nikolsky E, Mehran R, Halkin A, Aymong ED, Mintz GS, Lasic Z, et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta-analysis. J Am Coll Cardiol. (2004) 44(6):1200–9. doi: 10.1016/j.jacc.2004.06.048
105. Koreny M, Riedmuller M, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. (2004) 291(3):350–7. doi: 10.1001/jama.291.3.350
106. Sohail MR, Khan AH, Holmes DR, Wilson WR, Steckelberg JM, Baddour LM, et al. Infectious complications of percutaneous vascular closure devices. Mayo Clin Proc. (2005) 80(8):1011–5. doi: 10.4065/80.8.1011
107. Hoffer EK, Bloch RD. Percutaneous arterial closure devices. J Vasc Interv Radiol. (2003) 14(7):865–85. doi: 10.1097/01.RVI.0000071086.76348.8E
108. Warren BS, Warren SG, Miller SD. Predictors of complications and learning curve using the angio-seal closure device following interventional and diagnostic catheterization. Catheter Cardiovasc Interv. (1999) 48(2):162–6. doi: 10.1002/(SICI)1522-726X(199910)48:2%3C162::AID-CCD8%3E3.0.CO;2-2
109. Kussmaul WG 3rd, et al., Rapid arterial hemostasis and decreased access site complications after cardiac catheterization and angioplasty: results of a randomized trial of a novel hemostatic device. J Am Coll Cardiol. (1995) 25(7):1685–92. doi: 10.1016/0735-1097(95)00101-9
110. Chevalier B, Lancelin B, Koning R, Henry M, Gommeaux A, Pilliere R, et al. Effect of a closure device on complication rates in high-local-risk patients: results of a randomized multicenter trial. Catheter Cardiovasc Interv. (2003) 58(3):285–91. doi: 10.1002/ccd.10431
111. Baim DS, Knopf WD, Hinohara T, Schwarten DE, Schatz RA, Pinkerton CA, et al. Suture-mediated closure of the femoral access site after cardiac catheterization: results of the suture to ambulate aNd discharge (STAND I and STAND II) trials. Am J Cardiol. (2000) 85(7):864–9. doi: 10.1016/S0002-9149(99)00882-6
112. Gerckens U, Cattelaens N, Lampe EG, Grube E. Management of arterial puncture site after catheterization procedures: evaluating a suture-mediated closure device. Am J Cardiol. (1999) 83(12):1658–63. doi: 10.1016/S0002-9149(99)00174-5
113. Rickli H, Unterweger M, Sutsch G, La Rocca HP, Sagmeister M, Ammann P, et al. Comparison of costs and safety of a suture-mediated closure device with conventional manual compression after coronary artery interventions. Catheter Cardiovasc Interv. (2002) 57(3):297–302. doi: 10.1002/ccd.10294
114. Wood DA, Krajcer Z, Sathananthan J, Strickman n, Metzger C, Fearon W, et al. Pivotal clinical study to evaluate the safety and effectiveness of the MANTA percutaneous vascular closure device. Circ Cardiovasc Interv. (2019) 12(7):e007258. doi: 10.1161/CIRCINTERVENTIONS.119.007258
115. Abdel-Wahab M, Hartung P, Dumpies O, Obradovic D, Wilde J, Majunke N, et al. Comparison of a pure plug-based versus a primary suture-based vascular closure device strategy for transfemoral transcatheter aortic valve replacement: the CHOICE-CLOSURE randomized clinical trial. Circulation. (2022) 145(3):170–83. doi: 10.1161/CIRCULATIONAHA.121.057856
116. Bazarbashi N, Ahuja K, Gad MM, Sammour YW, Kaur M, Karrthik A, et al. The utilization of single versus double perclose devices for transfemoral aortic valve replacement access site closure: insights from Cleveland clinic aortic valve center. Catheter Cardiovasc Interv. (2020) 96(2):442–7. doi: 10.1002/ccd.28585
117. Pahuja M, Ranka S, Chehab O, Mishra T, Akintoye E, Adegbala O, et al. Incidence and clinical outcomes of bleeding complications and acute limb ischemia in STEMI and cardiogenic shock. Catheter Cardiovasc Interv. (2021) 97(6):1129–38. doi: 10.1002/ccd.29003
118. Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. (2014) 97(2):610–6. doi: 10.1016/j.athoracsur.2013.09.008
119. Shroff A, Pinto D. Vascular access, management, and closure: best practices. Soc Cardiovasc Angiography Interv. (2019).
120. Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H, et al. Computed tomography imaging in the context of transcatheter aortic valve implantation (TAVI)/transcatheter aortic valve replacement (TAVR). JACC Cardiovasc Imaging. (2019) 12(1):1–24. doi: 10.1016/j.jcmg.2018.12.003
121. Gutierrez-Barrios A, Canadas-Pruano D, Noval-Morillas I, Gheorghe L, Zayas-Rueda R, Calle-Perez G, et al. Radiation protection for the interventional cardiologist: practical approach and innovations. World J Cardiol. (2022) 14(1):1–12. doi: 10.4330/wjc.v14.i1.1
122. Vlastra W, Delewi R, Sjauw KD, Beijk MA, Claessen BE, Streekestra GJ, et al. Efficacy of the RADPAD protection drape in reducing Operators’ radiation exposure in the catheterization laboratory: a sham-controlled randomized trial. Circ Cardiovasc Interv. (2017) 10(11):6058–59. doi: 10.1161/CIRCINTERVENTIONS.117.006058
123. Lichaa H. The “lend a hand” external bypass technique: external radial to femoral bypass for antegrade perfusion of an ischemic limb with occlusive large bore sheath—a novel and favorable approach. Catheter Cardiovasc Interv. (2020) 96(6):E614–e620. doi: 10.1002/ccd.29187
124. Wollmuth J, Korngold E, Croce K, Pinto DS. The single-access for hi-risk PCI (SHiP) technique. Catheter Cardiovasc Interv. (2020) 96(1):114–6. doi: 10.1002/ccd.28556
125. Hirst CS, Thayer KL, Harwani N, Kapur NK. Post-Closure technique to reduce vascular complications related to impella CP. Cardiovasc Revasc Med. (2022) 39:38–42. doi: 10.1016/j.carrev.2021.10.008
126. Genereux P, Kodali S, Leon MB, Smith CR, Ben-Gal Y, Kirtane AJ, et al. Clinical outcomes using a new crossover balloon occlusion technique for percutaneous closure after transfemoral aortic valve implantation. JACC Cardiovasc Interv. (2011) 4(8):861–7. doi: 10.1016/j.jcin.2011.05.019
127. Amponash MK, Tayal R, Khakwani Z, Sinclair M, Wasty N. Safety and efficacy of a novel “hybrid closure” technique in large-bore arteriotomies. Int J Angiol. (2017) 26(2):116–20. doi: 10.1055/s-0037-1598252
128. Maurina M, Condello F, Mangieri A, Sanz-Sanchez J, Stefanini GG, Bongiovanni D, et al. Long term follow-up after balloon expandable covered stents implantation for management of transcatheter aortic valve replacement related vascular access complications. Catheter Cardiovasc Interv. (2022) 100(5):903–9. doi: 10.1002/ccd.30385
129. Frey P, Connors A, Resnic FS. Quality measurement and improvement in the cardiac catheterization laboratory. Circulation. (2012) 125(4):615–9. doi: 10.1161/CIRCULATIONAHA.111.018234
130. Genereux P, Nazif TM, George JK, Barker CM, Klodell CT, Slater JP, et al. First-in-human study of the saranas early bird bleed monitoring system for the detection of endovascular procedure-related bleeding events. J Invasive Cardiol. (2020) 32(7):255–61.32507753
131. Sawaya FJ, Bajoras V, Vanhaverbeke M, Wang C, Bieliauskas G, Sondergaard L, et al. Intravascular lithotripsy-assisted transfemoral TAVI: the Copenhagen experience and literature review. Front Cardiovasc Med. (2021) 8:739750. doi: 10.3389/fcvm.2021.739750
Keywords: vascular access and closure, major bleeding, major vascular complication, vacular closure device, large bore access
Citation: Eltelbany M, Fabbri M, Batchelor WB, Cilia L, Ducoffe A, Endicott K, Epps K, McBurnie A, Neville R, Rosner C, Sherwood MW, Spinosa D, Truesdell AG, Vorgang C, Damluji AA and Tehrani BN (2024) Best practices for vascular arterial access and closure: a contemporary guide for the cardiac catheterization laboratory. Front. Cardiovasc. Med. 11:1349480. doi: 10.3389/fcvm.2024.1349480
Received: 4 December 2023; Accepted: 19 February 2024;
Published: 14 March 2024.
Edited by:
Maurice Enriquez-Sarano, Minneapolis Heart Institute Foundation (MHIF), United StatesReviewed by:
Adel Aminian, Centre Hospitalier Universitaire de Charleroi, BelgiumTobias Zeus, University Hospital of Düsseldorf, Germany
© 2024 Eltelbany, Fabbri, Batchelor, Cilia, Ducoffe, Endicott, Epps, McBurnie, Neville, Rosner, Sherwood, Spinosa, Truesdell, Vorgang, Damluji and Tehrani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Behnam N. Tehrani YmVobmFtLnRlaHJhbmlASW5vdmEub3Jn
Abbreviations BARC, bleeding academic research consortium; CS, cardiogenic shock; dTRA, distal transradial access; MCS, mechanical circulatory support; PCI, percutaneous coronary intervention; pVAD, percutaneous ventricular assist device; RAO, radial artery occlusion; TFA, transfemoral arterial access; TRA, transradial arterial access.
 Moemen Eltelbany1
Moemen Eltelbany1 Matteo Fabbri
Matteo Fabbri Wayne B. Batchelor
Wayne B. Batchelor Richard Neville
Richard Neville Carolyn Rosner
Carolyn Rosner Matthew W. Sherwood
Matthew W. Sherwood Behnam N. Tehrani
Behnam N. Tehrani