
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 10 May 2024
Sec. Heart Failure and Transplantation
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1347908
 Filippo Maria Sarullo1*
Filippo Maria Sarullo1* Cinzia Nugara1
Cinzia Nugara1 Silvia Sarullo2
Silvia Sarullo2 Massimo Iacoviello3
Massimo Iacoviello3 Gabriele Di Gesaro4
Gabriele Di Gesaro4 Daniela Miani5
Daniela Miani5 Mauro Driussi5
Mauro Driussi5 Michele Correale6
Michele Correale6 Claudio Bilato7
Claudio Bilato7 Andrea Passantino8
Andrea Passantino8 Erberto Carluccio9
Erberto Carluccio9 Alessandra Villani10
Alessandra Villani10 Luca Degli Esposti11
Luca Degli Esposti11 Chiara D’Agostino12
Chiara D’Agostino12 Elena Peruzzi12
Elena Peruzzi12 Simone Poli12
Simone Poli12 Andrea Di Lenarda13
Andrea Di Lenarda13
Background: Heart failure (HF) significantly affects the morbidity, mortality, and quality of life of patients. New therapeutic strategies aim to improve the functional capacity and quality of life of patients while controlling HF-related risks. Real-world data on both the functional and cardiopulmonary exercise capacities of patients with HF with reduced ejection fraction upon sacubitril/valsartan use are lacking.
Methods: A multicenter, retrospective, cohort study, called REAL.IT, was performed based on the data collected from the electronic medical records of nine specialized HF centers in Italy. Cardiopulmonary exercise testing was performed at baseline and after 12 months of sacubitril/valsartan therapy, monitoring carbon dioxide production (VCO2) and oxygen consumption (VO2).
Results: The functional capacities of 170 patients were evaluated. The most common comorbidities were hypertension and diabetes (i.e., 53.5 and 32.4%, respectively). At follow-up, both the VO2 peak (from 15.1 ± 3.7 ml/kg/min at baseline to 17.6 ± 4.7 ml/kg/min at follow-up, p < 0.0001) and the predicted % VO2 peak (from 55.5 ± 14.1 to 65.5 ± 16.9, p < 0.0001) significantly increased from baseline. The VO2 at the anaerobic threshold (AT-VO2) increased from 11.5 ± 2.6 to 12.5 ± 3.3 ml/kg/min (p = 0.021), and the rate ratio between the oxygen uptake and the change in work (ΔVO2/Δwork slope) improved from 9.1 ± 1.5 to 9.9 ± 1.6 ml/min/W (p < 0.0001).
Conclusions: Sacubitril/valsartan improves the cardiopulmonary capacity of patients with HFrEF in daily clinical practice in Italy.
Heart failure (HF) affects approximately 64 million people worldwide (1) and is recognized as a global pandemic. HF imposes a significant burden on the morbidity and mortality of patients, showing reductions in their functional capacities and quality of life and requiring high societal and healthcare costs (2). The HF prevalence is reported to be approximately 1.7% in Italy and increases sharply with age (3, 4). Reducing the social and economic burdens of HF has become a major public health concern worldwide. Ongoing studies prioritize innovative therapeutic approaches to enhance the clinical condition, functional capacity, and quality of life of patients while minimizing the likelihood of HF-related hospitalization and mortality (5).
However, the underuse and underdosing of guideline-directed medical therapy in daily clinical practice continue to hinder the achievement of optimized HF management (6). Sacubitril/valsartan therapy simultaneously inhibits neprilysin and blocks the AT1 receptor via sacubitril and valsartan, respectively, and has been shown to provide substantial clinically relevant cardiovascular (CV) benefits. It also improves the symptoms and physical constraints of HF when compared to the standard treatment that uses enalapril in patients experiencing symptomatic HF with reduced ejection fraction (HFrEF) (7). The improvement in the exercise tolerance and the VO2 peak observed during sacubitril/valsartan therapy is likely attributed to the combined effects of the AT1 receptor antagonist and the neprilysin inhibitor. Through the action on AT1, valsartan causes vasodilation and volume reduction, while the sacubitril inhibition of neprilysin reduces the breakdown of vasoactive peptides, including natriuretic peptides, bradykinin, and adrenomedullin. The increased levels of these substances promote vasodilation and natriuresis, amplifying the system of vasoactive substances, such as bradykinin, adrenomedullin, endothelin-1, substance P, and angiotensin II. This amplification of the vasodilatory and natriuretic effects likely contributes to the observed improvement in exercise tolerance during sacubitril/valsartan therapy (8).
The most recent guidelines of the European Society of Cardiology (ESC) for the treatment of HFrEF include sacubitril/valsartan in the recommended sequence of different classes of medical therapies for HF (5).
Although sacubitril/valsartan is an established mainstay of HFrEF therapy and supported by strong evidence-based clinical trial data, evidence for its use and the characteristics and resource utilization of patients treated in real-world settings of routine practice are more limited, as are the effects of sacubitril/valsartan on treatment outcomes. Patients in real-world clinical settings are likely to be older and frailer than those included in randomized clinical trials because the latter usually present more stringent inclusion and exclusion criteria. Therefore, real-world data are needed to provide valuable support for the efficacy and safety findings of Phase 3 clinical studies; however, data regarding the use of sacubitril/valsartan in clinical routine in Italy are currently limited (9).
In light of this, a multicenter, retrospective, cohort study, called the REAL.IT, was performed by utilizing data gathered from Italian outpatient specialist clinics to define the clinical characteristics of and outcomes for Italian patients with HFrEF upon sacubitril/valsartan therapy (10, 11). The exploratory objective of REAL.IT, which was the focus of this study, was to describe the outcome of using sacubitril/valsartan on the functional capacity and cardiopulmonary exercise capacity of the Italian real-world cohort of patients with HFrEF.
For the retrospective REAL.IT study, the patients' data were retrieved from the electronic medical records (EMR) and administrative databases of nine hospitals in Italy that specialize in the management of HF. Appendix 1 lists the principal investigators involved in the study with the centers they belong to.
The study participants were adults aged ≥18 who were diagnosed with HF and were attending the outpatient clinic at any of the nine centers involved in this work. The patients had to be prescribed sacubitril/valsartan at least once in the time window of 1 October 2016 (time of launch in Italy)–30 June 2019 (inclusion period). The rationale for the 30 June 2019 cut-off date was to allow for a follow-up period of ≥1 year at the time of data extraction on 30 June 2020.
The index date and the characterization period were defined as the date of the first prescription of sacubitril/valsartan during the inclusion period and the 6 months before the index date, respectively. The baseline characteristics of the patient population were evaluated during the characterization period. A comparison between the characterization and follow-up periods was conducted only for patients with available information on the variables of interest in both periods. If there was missing information regarding age or sex, the patients were excluded from the analysis.
The study adhered to the principles of the Declaration of Helsinki and was submitted for review to the ethics committee of each participating center in accordance with the Italian regulations governing observational studies.
As previously reported, data were collected not earlier than 6 months before the index date. The demographic features, previous clinical history, functional parameters, comorbidities, and pharmacological treatments for HF of the general study population have already been reported (10). Given the use of secondary data, safety monitoring was not performed.
Cardiopulmonary exercise testing (CPET) was performed at baseline before the initiation of the therapy and after 12 months. All CPET sessions were conducted following a previously reported protocol (8) using the Vmax 2900 device (SensorMedics, Yorba Linda, CA, USA). All throughout the examination, the test continuously monitored every individual's 12-lead electrocardiogram (ECG) and oxygen saturation levels with a pulse oximeter. The patients were advised to persist with physical activity until they experienced muscle exhaustion and/or shortness of breath. The output parameters included the anaerobic threshold (AT) determined through the V-slope analysis, the increased VO2 per watt of work (ΔVO2/Δwork), the slope of the relationship between minute ventilation and CO2 production (VE/VCO2 slope), and the ratio between the dead space volume and the tidal volume (RV/VT) calculated using Jones' prediction equation (12), VO2, ventilation, tidal volume, and RV/VT ratio at peak exercise (8).
The data were analyzed as already reported (10, 11). Briefly, χ2 and paired t-tests were used to compare categorical and continuous variables, respectively. A p-value of <0.05 was considered statistically significant. STATA SE version 12.0 (StataCorp LLC, College Station, TX, USA) was used for the statistical analyses. Microsoft SQL Server 2012 was used for data management.
The overall patient population of REAL.IT consisted of 948 adult HF patients. The baseline demographic, clinical characteristics, and clinical outcomes of the overall population have already been reported (10).
The functional capacities of 170 patients were evaluated. Table 1 reports their demographic and clinical features. In summary, 85.9% of the patients were males with a mean [standard deviation (SD)] age of 60.1 (10.2) years. The mean LV ejection fraction (LVEF) was 28 ± 5.7%. The major comorbidities reported were hypertension (53.5%) and diabetes (32.4%). Of the 126 patients with available data, 94 (74.6%) had an implantable device, and 30/48 patients (62.5%) had ischemic heart disease. As for drug therapy, 83% of the patients were taking furosemide; 70%, ACEIs; 30%, ARBs; 83%, mineralocorticoid antagonists; 94%, β-blockers; 18%, ivabradine; and 5%, digoxin. The baseline characteristics of the subgroup considered for this analysis were similar to those of the whole sample described in the related publication (10).
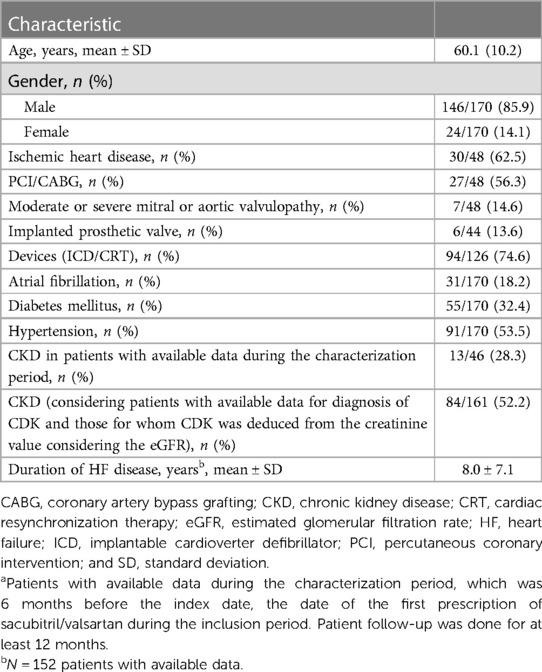
Table 1. Baseline demographic and clinical characteristics of patients included in the functional capacity analyses (N = 170)a.
When the baseline data were compared to those from a 12-month follow-up, treatment with sacubitril/valsartan improved the cardiopulmonary functional capacity of patients (Table 2). There were highly significant improvements in the peak and predicted peak oxygen consumption (VO2 peak) and the VE/VCO2 slope between the baseline and the 12-month follow-up. The mean peak VO2 improved from 15.1 ± 3.7 ml/kg/min at the baseline to 17.6 ± 4.7 ml/kg/min at the follow-up (p < 0.0001). The mean predicted % peak VO2 improved from 55.5 ± 14.1 to 65.5 ± 16.9 (p < 0.0001). Lastly, the mean minute ventilation per unit carbon dioxide production (VE/VCO2) slope decreased from 33.2 ± 6.1 to 30.7 ± 6.1 (p = 0.0009). Correspondingly, there were improvements observed in the VE/VCO2 slope > 34 (p = 0.015); the AT at the oxygen consumption peak (AT-VO2) increased from 11.5 ± 2.6 to 12.5 ± 3.3 ml/kg/min (p = 0.021); and the changes in the oxygen uptake and work rate ratio (ΔVO2/Δwork) improved from 9.1 ± 1.5 to 9.9 ± 1.6 ml/min/W (p < 0.0001). There were also corresponding improvements in the oxygen pulse, peak ventilation, tidal peak volume, and ventilatory oscillation (Table 2).
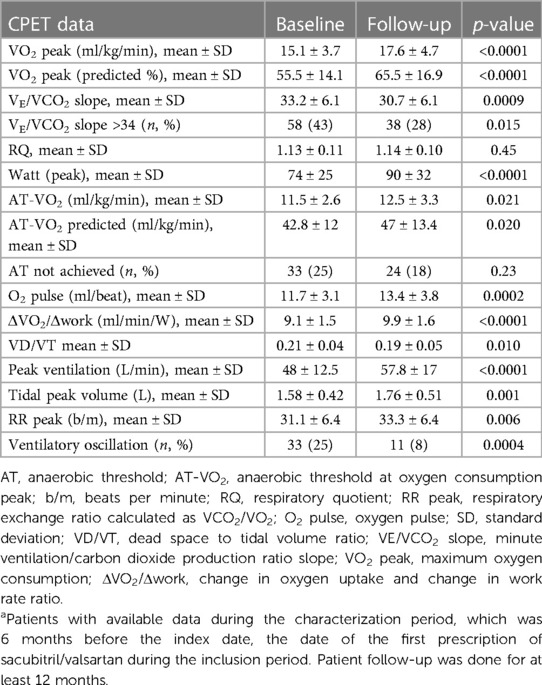
Table 2. Cardiopulmonary exercise stress testing (CPET) data at the baseline and after a 12-month follow-up (N = 170)a.
REAL.IT was a real-world, multicentric study that used the EMR of HF patients who started therapy with sacubitril/valsartan in nine specialist centers in Italy. The study aimed to provide insights into the utilization of sacubitril/valsartan and its changing clinical patterns. A total of 924 patients were evaluated, with their demographic (mean age, 65 years; 85% male) and clinical characteristics similar to those of the population of other international studies reporting patients' experience with sacubitril/valsartan. For example, the mean age of the patients who participated in REAL.IT was similar to those enrolled in the pivotal PARADIGM-HF study (13) and slightly lower than those of the participants in real-world studies of sacubitril/valsartan utilization (14). The full baseline demographic, clinical characteristics, and clinical outcomes of the REAL.IT study have recently been published, reporting that the therapy improved the New York Heart Association (NYHA) class in 37.5% of the patients after 5 months (10).
In line with this improvement in the clinical characteristics, this study observed improvements in the functional capacity of patients initiating treatment with sacubitril/valsartan.
A number of cardiopulmonary exercise tests have been developed to objectively assess the physical functional capacity of patients, including those with chronic HF. These include the measures utilized in REAL.IT: peak VO2, which is a measure of maximal exercise capacity; and VE/VCO2 slope, which is a measure of ventilatory efficiency (15–19). The improvements in these parameters have been shown to bear a prognostic value in HF (15, 17, 18, 20–22).
Several studies have shown improvements in the objective exercise capacity and tolerance measures of patients with symptomatic HF after the sacubitril/valsartan initiation (23–33). Palau et al. (23) and Vitale et al. (24) showed improvements in the peak VO2 and VE/VCO2 slope in HFrEF patients after follow-up durations between 1 and 6 months after the sacubitril/valsartan initiation (i.e., the peak VO2 improved from 14.6 ± 3.3 to 17.2 ± 4.7 ml/kg/min; p < 0.0001, and the VE/VCO2 slope decreased from 34.1 ± 6.3 to 31.7 ± 6.1; p = 0.006). Similarly, a study in a smaller Italian cohort showed that sacubitril/valsartan treatment improved the cardiopulmonary response to exercise in HF patients (i.e., the peak VO2 increased from 15.8 ± 3.4 to 17.0 ± 4.0 ml/kg/min) (28). Cacciatore et al. (34) also demonstrated that the main effect of sacubitril/valsartan is the improvement of functional performance, including PF together with 6MWT and VO2 max, and a reduction in the PAPs, E/E′, VE/VCO2 slope, and NT-proBNP. Correspondingly, in REAL.IT, sacubitril/valsartan significantly improved the peak VO2, which, together with a decrease in the VE/VCO2 slope, indicated an overall improvement in the functional and cardiopulmonary capacities of the patients. Sacubitril/valsartan has also been shown to benefit a 6-min walk distance (25–27, 35–37).
The measures of cardiopulmonary testing, namely, the peak VO2, the VE/VCO2 slope, and the 6-min walk test, provided valuable prognostic information for HF mortality and morbidity (15, 22); therefore, we propose herein that they all be included as routine components of clinical studies designed to assess the impact of therapeutic interventions on patients with HFrEF. These measures could help enhance management options and optimize outcomes in HF patients.
This study was a retrospective observational study that is reliant on anonymized data sourced from EMR. The analyses were subject to limitations inherent in this study design, particularly relying on precise patient data registration. Although steps were taken to account for this in the analyses, some patients had incomplete available data (10, 11). For example, underestimated events could be because they were not routinely registered in the electronic records. This could also lead to a potential underreporting of the outcomes analyzed in the study. However, these data on prescription and adherence to therapy cannot be referred to the entire national territory. The patients included in the analysis were referred to highly specialized HF centers in Italy. In this setting, the prescription of sacubitril/valsartan is likely more widespread, and cardiologists could have initiated this treatment earlier than under different circumstances. Therefore, our patient population may not completely represent patients under typical primary care settings, and the applicability of our findings to the broader population may somewhat be limited (10, 11). Nevertheless, this was a multicenter study that involved several Italian centers with relevant expertise in the management of HF.
Another limitation of this work is the unavailability of a correlation analysis between CPET and other clinical functional and laboratory variables, such as LVEF, NYHA classification, and NT-proBNP levels, for the subpopulation analyzed.
Overall, however, the data along with the observed protective effects of sacubitril/valsartan in the initial year of follow-up indicate that the initiation of this treatment was an effective management approach for patients with HFrEF. This strategy demonstrated notable benefits in terms of functional and cardiopulmonary capacities.
The retrospective nature of this analysis was indeed the main limitation of this study, and the analysis of the functional capacity was conducted on only 170 patients. Another limitation is the lack of a control group. However, following the current guidelines, it would have been ethically unjustifiable to withhold treatment from patients with HFrEF. Selecting a control group with contraindications to sacubitril/valsartan therapy is also considered methodologically incorrect because the two populations under examination would have been highly heterogeneous.
Finally, a further limitation of this study is the follow-up duration of approximately 12 months. It would be desirable to observe the effects of the drug on a patient's long-term functional capacity.
The practical, real-world data presented in this study demonstrate that the initiation of sacubitril/valsartan in patients with HF under daily clinical settings in Italy leads to enhanced functional and cardiopulmonary capacities, which suggests effective therapeutic management.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study involving humans was approved by U.O.S.D. di Riabilitazione Cardiovascolare Ospedale Buccheri La Ferla Fatebenefratelli, Palermo. This study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
FMS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CN: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MI: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. GDG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. DM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CB: Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Conceptualization, Data curation. AP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. EC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. AV: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. LDE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CDA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. EP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. ADL: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article.
The REAL.IT study was funded by Novartis Farma.
Medical writing support was provided by Ray Hill, an independent medical writer, on behalf of Health Publishing & Services Srl. This support was funded by Novartis Farma SpA, Italy.
MI: Lectures or consultant for Novartis, Vifor Pharma, Boehringer, Lilly, Bayer, AstraZeneca, Roche Diagnostics, Neopharmed Gentili. ADL: Lectures for Novartis, Vifor Pharma, Boheringer, Daiichi, Bayer, Pfizer, AstraZeneca, Research Funds from Novartis, Amgen, AstraZeneca, Vifor Pharma, Bayer. CDA, EP, and SP are employees of Novartis. LDE was employed by company CliCon S.r.l.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The authors declare that this study received funding from Novartis Farma. The funder had the following involvement in the study: study design, data collection and analysis, supporting manuscript preparation.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. GBD 2017 Disease Injury and Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392(10159):1789–858. doi: 10.1016/S0140-6736(18)32279-7
2. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. (2021) 23(3):352–80. doi: 10.1002/ejhf.2115
3. Seferović PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinković I, et al. The Heart Failure Association Atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail. (2021) 23(6):906–14. doi: 10.1002/ejhf.2143
4. Piccinni C, Antonazzo IC, Simonetti M, Mennuni MG, Parretti D, Cricelli C, et al. The burden of chronic heart failure in primary care in Italy. High Blood Press Cardiovasc Prev. (2017) 24(2):171–8. doi: 10.1007/s40292-017-0193-4
5. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
6. Savarese G, Bodegard J, Norhammar A, Sartipy P, Thuresson M, Cowie MR, et al. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden). Eur J Heart Fail. (2021) 23(9):1499–511. doi: 10.1002/ejhf.2271
7. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. (2014) 371(11):993–1004. doi: 10.1056/NEJMoa1409077
8. Nugara C, Giallauria F, Vitale G, Sarullo S, Gentile G, Clemenza F, et al. Effects of sacubitril/valsartan on exercise capacity in patients with heart failure with reduced ejection fraction and the role of percentage of delayed enhancement measured by cardiac magnetic resonance in predicting therapeutic response. Card Fail Rev. (2023) 8:e07. doi: 10.15420/cfr.2022.13
9. Mapelli M, Salvioni E, de Martino F, Mattavelli I, Bonomi A, Sassi V, et al. Sacubitril/valsartan use in a real-world population of patients with heart failure and reduced ejection fraction. J Cardiovasc Med. (2020) 21(11):882–8. doi: 10.2459/JCM.0000000000001018
10. Di Lenarda A, Di Gesaro G, Sarullo FM, Miani D, Driussi M, Correale M, et al. Sacubitril/valsartan in heart failure with reduced ejection fraction: real-world experience from Italy (the REAL.IT study). J Clin Med. (2023) 12(2):699. doi: 10.3390/jcm12020699
11. Iacoviello M, Di Gesaro G, Sarullo FM, Miani D, Driussi M, Correale M, et al. Pharmacoutilization and adherence to sacubitril/valsartan in real world: the REAL.IT study in HFrEF. ESC Heart Fail. (2023).
12. Guazzi M, Marenzi G, Assanelli E, Perego GB, Cattadori G, Doria E, et al. Evaluation of the dead space/tidal volume ratio in patients with chronic congestive heart failure. J Card Fail. (1995) 1(5):401–8. doi: 10.1016/S1071-9164(05)80009-0
13. Yandrapalli S, Andries G, Biswas M, Khera S. Profile of sacubitril/valsartan in the treatment of heart failure: patient selection and perspectives. Vasc Health Risk Manag. (2017) 13:369–82. doi: 10.2147/VHRM.S114784
14. Proudfoot C, Studer R, Rajput T, Jindal R, Agrawal R, Corda S, et al. Real-world effectiveness and safety of sacubitril/valsartan in heart failure: a systematic review. Int J Cardiol. (2021) 331:164–71. doi: 10.1016/j.ijcard.2021.01.061
15. Corrà U, Agostoni PG, Anker SD, Coats AJS, Crespo Leiro MG, de Boer RA, et al. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2018) 20(1):3–15. doi: 10.1002/ejhf.979
16. Maldonado-Martin S, Brubaker PH, Eggebeen J, Stewart KP, Kitzman DW. Association between 6-min walk test distance and objective variables of functional capacity after exercise training in elderly heart failure patients with preserved ejection fraction: a randomized exercise trial. Arch Phys Med Rehabil. (2017) 98(3):600–3. doi: 10.1016/j.apmr.2016.08.481
17. Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J. (2004) 147(2):354–60. doi: 10.1016/j.ahj.2003.07.014
18. Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2). Eur Heart J. (2000) 21(2):154–61. doi: 10.1053/euhj.1999.1863
19. Ingle L, Goode K, Carroll S, Sloan R, Boyes C, Cleland JG, et al. Prognostic value of the VE/VCO2 slope calculated from different time intervals in patients with suspected heart failure. Int J Cardiol. (2007) 118(3):350–5. doi: 10.1016/j.ijcard.2006.07.105
20. Du H, Wonggom P, Tongpeth J, Clark RA. Six-minute walk test for assessing physical functional capacity in chronic heart failure. Curr Heart Fail Rep. (2017) 14(3):158–66. doi: 10.1007/s11897-017-0330-3
21. Ingle L, Cleland JG, Clark AL. The long-term prognostic significance of 6-min walk test distance in patients with chronic heart failure. Biomed Res Int. (2014) 2014:505969. doi: 10.1155/2014/505969
22. Alba AC, Adamson MW, MacIsaac J, Lalonde SD, Chan WS, Delgado DH, et al. The added value of exercise variables in heart failure prognosis. J Card Fail. (2016) 22(7):492–7. doi: 10.1016/j.cardfail.2016.01.012
23. Palau P, Mollar A, Dominguez E, Sanchis J, Bayes-Genis A, Nunez J. Early sacubitril/valsartan-driven benefit on exercise capacity in heart failure with reduced ejection fraction: a pilot study. Rev Esp Cardiol. (2019) 72(2):167–9. doi: 10.1016/j.recesp.2017.11.019
24. Vitale G, Romano G, Di Franco A, Caccamo G, Nugara C, Ajello L, et al. Early effects of sacubitril/valsartan on exercise tolerance in patients with heart failure with reduced ejection fraction. J Clin Med. (2019) 8(2):262. doi: 10.3390/jcm8020262
25. Rodil Fraile R, Malafarina V, Tiberio Lopez G. Sacubitril-valsartan in heart failure and multimorbidity patients. ESC Heart Fail. (2018) 5(5):956–9. doi: 10.1002/ehf2.12338
26. Sgorbini L, Rossetti A, Galati A. Sacubitril/valsartan: effect on walking test and physical capability. Cardiology. (2017) 138(Suppl 1):17–20. doi: 10.1159/000484879
27. Beltrán P, Palau P, Domínguez E, Faraudo M, Núñez E, Guri O, et al. Sacubitril/valsartan and short-term changes in the 6-min walk test: a pilot study. Int J Cardiol. (2018) 252:136–9. doi: 10.1016/j.ijcard.2017.10.074
28. Malfatto G, Ravaro S, Caravita S, Baratto C, Sorropago A, Giglio A, et al. Improvement of functional capacity in sacubitril–valsartan treated patients assessed by cardiopulmonary exercise test. Acta Cardiol. (2020) 75(8):732–6. doi: 10.1080/00015385.2019.1669317
29. Mapelli M, Mattavelli I, Paolillo S, Salvioni E, Magri D, Galotta A, et al. Effects of sacubitril/valsartan on exercise capacity: a prognostic improvement that starts during uptitration. Eur J Clin Pharmacol. (2023) 79(9):1173–84. doi: 10.1007/s00228-023-03527-y
30. Mapelli M, Mattavelli I, Salvioni E, Banfi C, Ghilardi S, De Martino F, et al. Impact of sacubitril/valsartan on surfactant binding proteins, central sleep apneas, lung function tests and heart failure biomarkers: hemodynamic or pleiotropism? Front Cardiovasc Med. (2022) 9:971108. doi: 10.3389/fcvm.2022.971108
31. Mapelli M, Mattavelli I, Salvioni E, Bonomi A, Capra N, Palermo P, et al. Looking into the kinetics of NT-proBNP and sST2 changes in patients with heart failure treated with sacubitril/valsartan: a hint to different therapeutic pathways. Drugs R D. (2023) 23(4):397–402. doi: 10.1007/s40268-023-00438-2
32. Mantegazza V, Volpato V, Mapelli M, Sassi V, Salvioni E, Mattavelli I, et al. Cardiac reverse remodelling by 2D and 3D echocardiography in heart failure patients treated with sacubitril/valsartan. Diagnostics. (2021) 11(10):1845. doi: 10.3390/diagnostics11101845
33. Brioschi M, D’Alessandra Y, Mapelli M, Mattavelli I, Salvioni E, Eligini S, et al. Impact of sacubitril/valsartan on circulating microRNA in patients with heart failure. Biomedicines. (2023) 11(4):1037. doi: 10.3390/biomedicines11041037
34. Cacciatore F, Amarelli C, Maiello C, Mattucci I, Salerno G, Di Maio M, et al. Sacubitril/valsartan in patients listed for heart transplantation: effect on physical frailty. ESC Heart Fail. (2020) 7(2):757–62. doi: 10.1002/ehf2.12610
35. Bunsawat K, Ratchford SM, Alpenglow JK, Park SH, Jarrett CL, Stehlik J, et al. Sacubitril-valsartan improves conduit vessel function and functional capacity and reduces inflammation in heart failure with reduced ejection fraction. J Appl Physiol. (2021) 130(1):256–68. doi: 10.1152/japplphysiol.00454.2020
36. Lelonek M, Wiśniowska-Śmiałek S, Rubiś P, Nowakowska I, Pawlak A. Sacubitril/valsartan for heart failure with reduced ejection fraction: a first real-life observational study in Poland. Adv Clin Exp Med. (2021) 30(1):67–75. doi: 10.17219/acem/128230
37. Dattilo G, Bitto R, Correale M, Morabito C, Vaccaro V, Laterra G, et al. Trend of perceived quality of life and functional capacity in outpatients with chronic heart failure and in treatment with sacubitril/valsartan: a real-life experience. Minerva Cardiol Angiol. (2022) 70(5):555–62. doi: 10.23736/S2724-5683.20.05494-8
Appendix 1 Principal investigators of the REAL.IT study.
Dr. Andrea Di Lenarda, SC Cardiovascolare e Medicina dello sport Azienda Sanitaria Universitaria Giuliano Isontina (ASU GI), Trieste, Italy;
Dr. Andrea Passantino, U.O. Cardiologia ICS Maugeri Bari SpA SB IRCCS Istituto di Bari, Bari, Italy;
Dr. Alessandra Villani, U.O. Day Hospital—MAC Cardiologia Acuto/Riab Istituto Auxologico Italiano—Ospedale S. Luca, Milano, Italy;
Dr. Claudio Bilato, U.O.C. Di Cardiologia Azienda ULSS 8 Berica—Ospedali dell'Ovest Vicentino, Arzignano, Italy;
Dr. Michele Correale, SC Universitaria di Cardiologia AOU “Ospedali Riuniti” Foggia, Foggia, Italy;
Dr. Gabriele Di Gesaro, U.O. Cardiologia IRCCS ISMETT Palermo, Palermo, Italy;
Dr. Daniela Miani, SOC Cardiologia, Dipartimento Cardiotoracico Azienda Sanitaria Universitaria Friuli Centrale Ospedale S Maria della Misericordia, Udine, Italy;
Dr. Filippo Maria Sarullo, U.O.S.D. di Riabilitazione Cardiovascolare Ospedale Buccheri La Ferla Fatebenefratelli, Palermo, Italy;
Prof. Erberto Carluccio, Cardiologia e Fisiopatologia Cardiovascolare Azienda Ospedaliera Universitaria “Santa Maria della Misericordia”, Perugia, Italy.
Keywords: functional capacity, cardiopulmonary exercise testing, heart failure with reduced ejection fraction, real-world practice, sacubitril/valsartan
Citation: Sarullo FM, Nugara C, Sarullo S, Iacoviello M, Di Gesaro G, Miani D, Driussi M, Correale M, Bilato C, Passantino A, Carluccio E, Villani A, Degli Esposti L, D’Agostino C, Peruzzi E, Poli S and Di Lenarda A (2024) Effects of sacubitril/valsartan on the functional capacity of real-world patients in Italy: the REAL.IT study on heart failure with reduced ejection fraction. Front. Cardiovasc. Med. 11:1347908. doi: 10.3389/fcvm.2024.1347908
Received: 1 December 2023; Accepted: 12 April 2024;
Published: 10 May 2024.
Edited by:
Matteo Cameli, University of Siena, ItalyReviewed by:
Francesco Cacciatore, University of Naples Federico II, Italy© 2024 Sarullo, Nugara, Sarullo, Iacoviello, Di Gesaro, Miani, Driussi, Correale, Bilato, Passantino, Carluccio, Villani, Degli Esposti, D'Agostino, Peruzzi, Poli and Di Lenarda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Filippo Maria Sarullo c2FydWxsby5maWxpcHBvQGZiZnBhLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.