
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 15 February 2024
Sec. Cardiac Rhythmology
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1346414
This article is part of the Research TopicAtrial Fibrillation: Selection of Management Strategy and Evaluation of OutcomesView all 39 articles
 Woo-Hyun Lim1,3,†
Woo-Hyun Lim1,3,† So-Ryoung Lee2,3,†
So-Ryoung Lee2,3,† Eue-Keun Choi2,3*
Eue-Keun Choi2,3* Seung-Woo Lee4
Seung-Woo Lee4 Kyung-Do Han5
Kyung-Do Han5 Seil Oh2,3
Seil Oh2,3 Gregory Y. H. Lip3,6,7
Gregory Y. H. Lip3,6,7
Background: The impact of early rhythm control (ERC) combined with healthy lifestyle (HLS) on the risk of ischemic stroke in elderly patients with atrial fibrillation (AF) remains unaddressed.
Objective: To evaluate the impact of combined ERC and HLS on the risk of stroke in elderly patients with new-onset AF.
Methods: Using the Korean National Health Insurance Service database, we included patients aged ≥75 years with new-onset AF from January 2009 to December 2016 (n = 41,315). Patients who received rhythm control therapy within 2 years of AF diagnosis were defined as the ERC group. Non-smoking, non-to-mild alcohol consumption (<105 g/week), and regular exercise were defined as HLS. Subjects were categorized into four groups: group 1 (without ERC and HLS, n = 25,093), 2 (HLS alone, n = 8,351), 3 (ERC alone, n = 5,565), and 4 (both ERC and HLS, n = 2,306). We assessed the incidence of ischemic stroke as the primary outcome, along with admissions for heart failure, all-cause death, and the composite of ischemic stroke, admission for heart failure, and all-cause death.
Results: Median follow-up duration of the study cohort was 3.4 years. After adjusting for multiple variables, groups 2 and 3 were associated with a lower stroke risk (adjusted hazard ratio [aHR]: 95% confidence interval [CI]: 0.867, 0.794–0.948 and 0.713, 0.637–0.798, respectively) than that of group 1. Compared to Group 1, group 4 showed the lowest stroke risk (aHR: 0.694, 95% CI: 0.586–0.822) among all groups, followed by group 3 (0.713, 0.637–0.798) and group 2 (0.857, 0.794–0.948), respectively. Group 4 was associated with the lowest risk of all-cause death (aHR: 0.680, 95% CI: 0.613–0.754) and the composite outcome (aHR: 0.708, 95% CI: 0.649–0.772).
Conclusion: ERC and HLS were associated with a lower risk of ischemic stroke in elderly patients with new-onset AF. Concurrently implementing ERC and maintaining HLS was associated with the lowest risk of death and the composite outcome, with a modest synergistic effect on stroke prevention.
Atrial fibrillation (AF) is the most prevalent sustained arrhythmia in the elderly (1). Its incidence increases with age, and individuals older than 80 years have a prevalence of AF exceeding 10% (2, 3). Recent data reports that the projected lifetime risk of AF in individuals older than 40 years has increased to 1 in 3 (4). AF is strongly associated with a 5-fold increased risk of ischemic stroke and a 3-fold increase in the risk of heart failure (5, 6). Considering the associations between AF and cardiovascular morbidity and mortality, this arrhythmia imposes a substantial burden on the public health of the elderly.
Guidelines have emphasized the need for an integrated approach to AF management to achieve better clinical outcomes (7, 8). Alongside appropriate anticoagulation therapy and symptom control, the management of cardiovascular risk factors and comorbidities, including lifestyle modification, is crucial (9, 10). Recent studies have demonstrated that early rhythm control (ERC) therapy within 1 year of AF diagnosis can effectively reduce the risk of adverse cardiovascular outcomes compared to conventional care in patients with AF (11, 12). Nevertheless, lifestyle modification is not routinely recommended for elderly patients (13) and older patients with AF are less likely to be offered rhythm control therapy (14). Furthermore, whether ERC therapy and lifestyle modification retain their effectiveness in the elderly remains uncertain.
In this study, we aimed to evaluate the impact of ERC therapy and a healthy lifestyle (HLS) and the combination of both on the risk of ischemic stroke, admission for heart failure, all-cause mortality, and composite clinical outcome in patients with AF aged 75 years and older.
We conducted an observational cohort study using data from a nationwide claims database administered by the Korean National Health Insurance Service (NHIS). The NHIS provides universal coverage, guaranteeing that the extracted data is considered representative of the entire Korean population. To gather information on lifestyle behaviors, we accessed the health check-up database linked to the NHIS database (15, 16). This database is maintained by the National Health Insurance Corporation and includes biennial health screening check-up data for all Korean adults. Integration of this information with the NHIS database is a well-established practice. As of 2016, the adult health examination rate was estimated to be 85%. It is possible to access the data through the NHIS National Health Insurance Sharing Service homepage (http://nhiss.nhis.or.kr). Applications for access to NHIS data are evaluated by the research support inquiry committee. Upon approval, authorized researchers are granted access to the raw data at designated locations. This study was approved by the Institutional Review Board of Seoul National University Hospital (E-2312-019-1488). The requirement for obtaining informed consent from study participants was waived due to the stringent confidentiality guidelines that encrypt personal identification information during cohort generation.
We identified patients who were newly diagnosed with AF between January 1, 2009, and December 31, 2016. For inclusion in this study, we selected patients who had undergone a national health check-up within 2 years of their AF diagnosis because most health check-ups were offered biennially. Figure 1 illustrates the enrollment process and Supplementary Figure S1 shows the time-line graph of data collection.
Rhythm control therapy was defined as the prescription of antiarrhythmic drugs (AAD, class Ic, or class III), direct current cardioversion (DCC), or AF catheter ablation (Supplementary Table S1). Patients who received rhythm control therapy within 2 years of new-onset AF were categorized as the ERC group. Lifestyle behaviors, including smoking, drinking, and physical activity, were assessed using self-reported questionnaires during health check-ups. HLS behaviors were defined as non-current smoking, non-to-mild alcohol consumption (<105 g/week), and performing regular exercise (17–19). Regular exercise was further specified as moderate-intensity physical activity (e.g., brisk-pace walking, tennis doubles, or cycling leisurely for more than 30 min) or vigorous-intensity physical activity (e.g., running, climbing, fast cycling, or performing aerobics for more than 20 min) at least once a week (17). In this study, patients displaying all three HLS behaviors (non-smoker, non-to-mild drinker, and regular exerciser) were categorized as the HLS group. Using a two-by-two factorial design, patients were categorized into the following four groups: group 1, without both ERC and HLS; group 2, HLS alone; group 3, ERC alone; and group 4, both ERC and HLS (Figure 1).
Patient age, sex, and comorbidities were determined using diagnostic codes, prescription records, inpatient and outpatient hospital visits, and health check-up results. Detailed definitions of comorbidities are provided in Supplementary Table S1. Current medications, including AADs, oral anticoagulants (OACs; e.g., warfarin and direct oral anticoagulants), antiplatelet agents, statins, beta-blockers, calcium channel blockers, digoxin, renin–angiotensin system blockers, and diuretics, were identified based on prescription records. The body mass index (BMI), blood pressure, and estimated glomerular filtration rate were obtained from baseline health check-ups. Using these baseline covariates, the CHA2DS2-VASc score and Charlson comorbidity index (CCI) were calculated on the basis of patients' comorbidities and medical history (Supplementary Tables S1, S2) (20).
Lifestyle behaviors were evaluated through self-reported questionnaires during national health check-ups. The HLS behavior score was computed by assigning 1 point each for non-current smoking, non-to-mild drinking, and regular exercise. The bottom 20% of the household income among all NHIS subscribers was classified as low-income.
During the follow-up period, we evaluated outcomes such as ischemic stroke, admission for heart failure, all-cause death, and the composite of these three outcomes. A detailed definition of these outcomes is provided in Supplementary Table S1. Follow-up for study outcomes commenced on the date of the baseline health examination, and patients were monitored until the occurrence of the primary outcome, death, or the end of the study (December 31, 2018), whichever came first. In this study, a detailed investigation into the specific causes of death was not conducted. Nevertheless, we conducted an exploratory analysis for cardiovascular (CV) deaths by defining them arbitrarily as deaths associated with an international classification of disease-10 I code.
Continuous variables are presented as means and standard deviations, whereas categorical variables are presented as numbers and percentages. We used one-way analysis of variance to compare continuous variables and the chi-square test for categorical variables to examine the significance of differences among the four groups. Incidence rates (IR) for ischemic stroke as the primary outcome, along with admissions for heart failure, all-cause death, and the composite of ischemic stroke, admission for heart failure, and all-cause death were calculated as the number of events per 100 person-years (PY). We used Cox proportional hazard regression models to estimate hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs). Initially, unadjusted and age-and sex-adjusted HRs were assessed (model 1 and model 2 respectively). Subsequently, a multivariate Cox analysis was conducted, adjusting for age, sex, all comorbidities, CHA2DS2-VASc score, CCI, all medications, BMI, systolic blood pressure, and income (model 3). The criteria for selecting covariates for Model 3 were determined based on all known factors influencing cardiovascular outcomes in patients with AF, along with baseline covariates included in this study (Table 1) that showed a significant difference (p-value < 0.1) between groups. The evaluation of multicollinearity was conducted using the variance inflation factor, indicating the absence of noteworthy collinearity among the incorporated covariates (Variance inflation factor = 1.00–1.70).
To assess the impact of adding ERC to HLS and adding HLS to ERC, two sets of comparisons were performed as follows: (1) groups 2 vs. 4 to analyse the impact of adding ERC on HLS; and (2) groups 3 vs. 4 to evaluate the impact of adding HLS on ERC. Propensity score (PS) weighting was applied using stabilized weights calculated from the PS to balance differences between the two groups (21). All covariates, including those in the final multivariate Cox analysis model, were used in the PS calculation. After PS weighting, we evaluated the absolute standardized differences (ASD) of covariates for all baseline variables to confirm the balance between the two groups (22). An ASD exceeding 0.1 indicates an imbalance in a covariate. We evaluated weighted IRs (per 100-PY) and weighted cumulative incidence curves using the Kaplan–Meier method with the log-rank test for the study outcomes. The HR for the study outcomes of the two groups was assessed using weighted Cox proportional hazard regression models with PS weighting. The reference groups in the two comparison sets were groups 2 and 3.
Because OAC therapy significantly affects the risk of ischemic stroke, we conducted a subgroup analysis according to the use or non-use of OAC to determine whether there was an interaction in the effect of ERC, HLS, and their combination, depending on OAC use.
All p-values were two-sided, and statistical significance was defined as a p-value < 0.05. We conducted statistical analyses using SAS version 9.4 (SAS Institute, Cary, NC).
A total of 41,315 patients were finally included (Figure 1). The mean age of the patients was 79.8 ± 3.9 years, the mean CHA2DS2-VASc score was 5.5 ± 1.5, and 98.8% of patients had a CHA2DS2-VASc score of ≥3. The baseline characteristics of the total study population are presented in Table 1. Among the total study population, 19% (groups 3 and 4) received ERC therapy. The mean duration from AF diagnosis to rhythm control was 28.0 ± 84.1 days, and 98.0% of these patients received ERC within 1 year of AF diagnosis. Among those receiving ERC, 99.6% were prescribed AAD, 3.8% received DC cardioversion, and 0.1% underwent AF catheter ablation. According to the prespecified definition, 25,093, 8,351, 5,565, and 2,306 patients were classified into groups 1, 2, 3, and 4, respectively (Figure 1). The baseline characteristics of each group are described in Table 1.
During a median 3.4-year follow-up (interquartile range: 2.0–5.4 years), 3,383 patients had ischemic stroke, 3,171 patients were admitted for heart failure, and 10,950 patients died (IR: 2.24, 2.09 and 6.94 per 100 PY, respectively). The crude numbers of events, crude incidence rates, and unadjusted and age- and sex-adjusted HRs for the clinical outcomes of the study groups are shown in Table 2. The Kaplan–Meier curves for the clinical outcomes of the study groups are presented in Figure 2. Figure 3 shows the adjusted HRs using model 3.
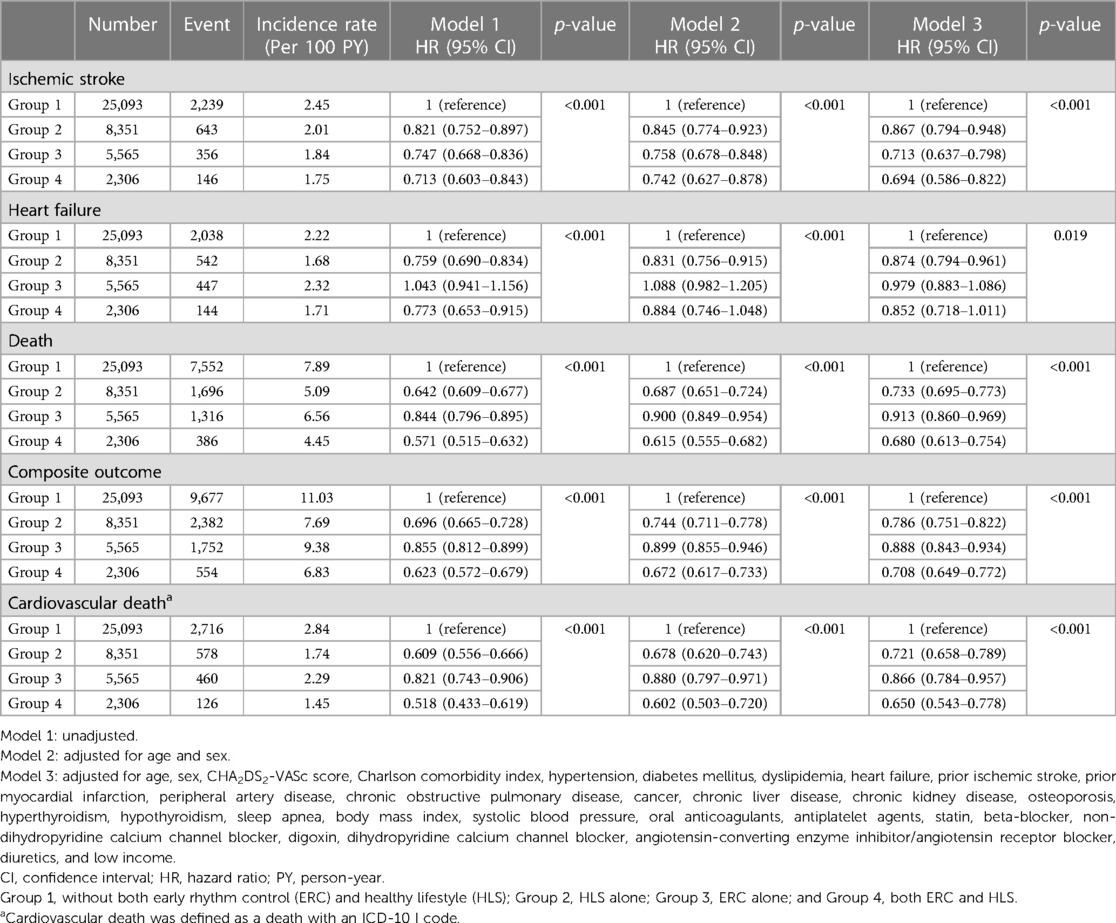
Table 2. Event numbers, incidence rates, unadjusted and adjusted hazard ratios for ischemic stroke, heart failure, death, composite outcome, and cardiovascular death.
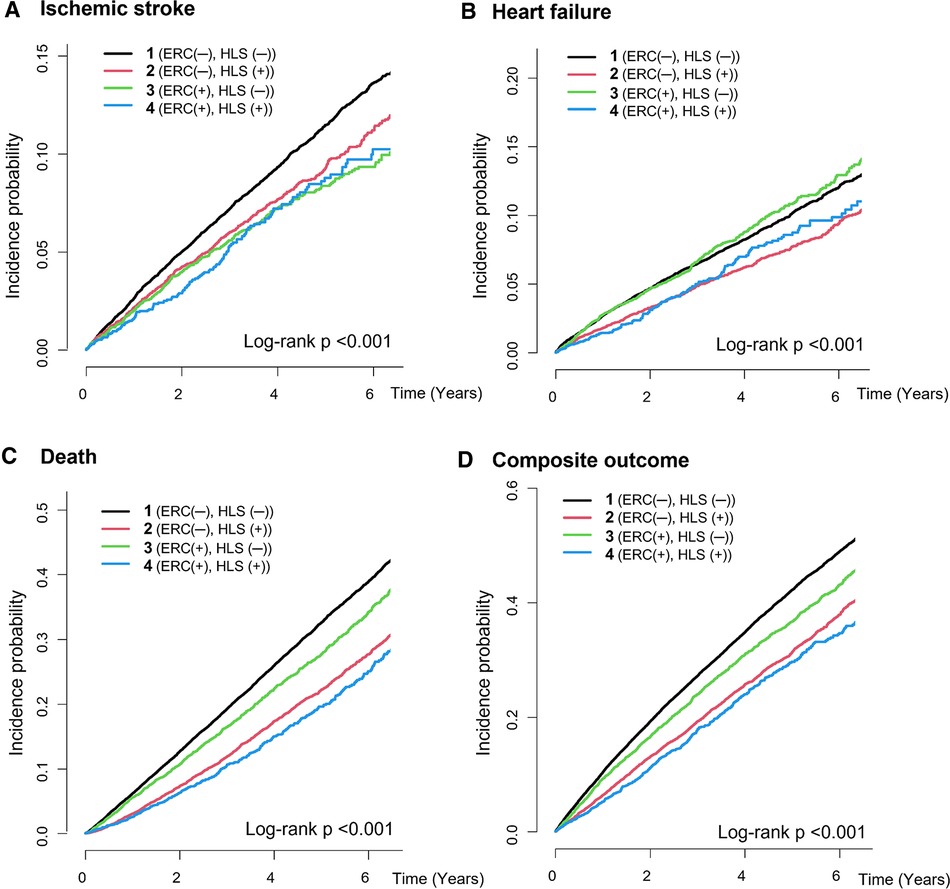
Figure 2. Cumulative incidence curves for the clinical outcomes of the study groups. (A) Ischemic stroke, (B) heart failure, (C) all-cause death, (D) composite outcome. ERC, early rhythm control; HLS, healthy lifestyle.
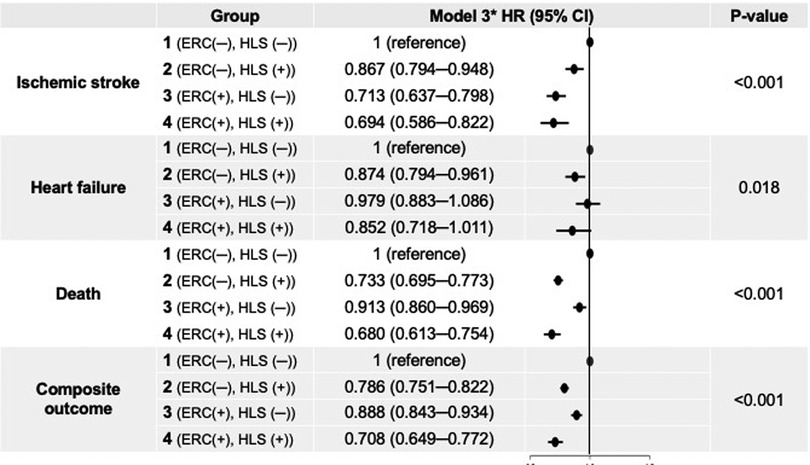
Figure 3. Adjusted hazard ratios for clinical outcomes of the study groups. *Model 3 was adjusted for age, sex, all comorbidities, CHA2DS2-VASc score, Charlson comorbidity index, all medications, body mass index, systolic blood pressure, and income. CI, confidence interval; ERC, early rhythm control; HLS, healthy lifestyle; HR, hazard ratio.
After multivariate adjustment using model 3, group 2 (maintaining HLS alone) was associated with a lower risk of ischemic stroke by 13% compared to group 1. Group 3 (implementing ERC alone) was also associated with a lower risk of ischemic stroke, showing a numerically greater risk reduction than maintaining HLS alone (by 29% compared to group 1). Group 4, patients receiving ERC and maintaining HLS, showed the greatest risk reduction for ischemic stroke by 31% compared to group 1. For admission with heart failure, group 2 was associated with a lower risk than group 1; however, group 3 did not show a significant difference compared to group 1, while group 4 showed only a borderline significant trend toward a lower risk of admission for heart failure compared to group 1.
Groups 2, 3, and 4 were associated with significantly lower risk of all-cause death than group 1 by 27% (adjusted HR, 95% CI; 0.733, 0.695–0.773), 9% (0.913, 0.860–0.969), and 32% (0.680, 0.613–0.754), respectively. For the composite outcome of ischemic stroke, admission for heart failure, and death, group 4 showed the greatest risk reduction by 29% (0.708, 0.649–0.772) compared to group 1. Groups 2 and 3 were also associated with a lower risk of the composite outcome by 21% (0.786, 0.751–0.822) and 11% (0.888, 0.843–0.934), respectively.
Among all deaths, CV deaths accounted for 35.4%. Groups 2, 3, and 4 showed significantly lower risk of CV death compared to group 1, with reduction of 28% (adjusted HR, 95% CI; 0.721, 0.658–0.789), 13% (0.866, 0.784–0.957), and 35% (0.680, 0.543–0.778) (Table 2). The reduction in CV death among the groups was similar to that of all-cause death. In other word, the observed reduction in all-cause death within group 2 and group 4 was primarily driven by a decrease in CV mortality.
To evaluate whether there was an additional association when adding ERC to HLS alone or adding HLS to ERC alone, PS weighting analyses were performed for group 2 vs. group 4 and group 3 vs. group 4.
After PS weighting, the baseline characteristics of the two groups were well balanced with ASD <0.1 in all covariates (Supplementary Tables S3, S4). Compared to group 2, group 4 showed numerically lower weighted IRs for ischemic stroke, admission for heart failure, death, and the composite outcome, but the weighted HRs were not statistically significant (Supplementary Table S5). Compared to group 3, group 4 was associated with a significantly lower risk of composite outcome (weighted HR, 95% CI; 0.857, 0.782–0.940), mainly driven by all-cause death (0.801, 0.719–0.893) (Supplementary Table S6). Regarding the risk of ischemic stroke and admission with heart failure, there were no significant differences between groups 3 and 4.
Overall, trends in association with the risk of clinical outcomes were similarly observed in both subgroups (Supplementary Table S7), and there was no significant interaction among subgroups for admission for heart failure and death. For ischemic stroke, the association of HLS alone was more accentuated in the subgroup without OAC treatment, whereas the association of ERC was more accentuated in the subgroup with OAC treatment. Similar findings were observed for HRs of the composite outcome.
In this large-scale observational study involving elderly patients with new-onset AF, our key findings were as follows: (1) Both ERC alone and HLS alone were associated with a significantly lower risk of ischemic stroke in patients aged ≥75 years than in those without ERC and HLS. The beneficial effect of ERC alone was more pronounced than that of HLS alone; (2) for all-cause death, both ERC alone and HLS alone were associated with a lower risk compared to those without ERC and HLS; however, a greater HR reduction was observed in the HLS alone group than in the ERC alone group, which exhibited a more modest HR reduction; and (3) in elderly patients with new-onset AF, the greatest risk reduction for the composite outcome was observed in those with both ERC and HLS (by 29%), followed by those with HLS alone (by 21%), and those with ERC alone (by 11%) compared to those without ERC and HLS.
The Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4) demonstrated that implementing ERC therapy within 1 year of AF diagnosis reduced the incidence of major cardiovascular adverse events, including stroke, by 35% compared to conventional care (11). Several subsequent observational studies validated the findings of the EAST-AFNET 4 trial (12, 23). However, the clinical benefit of ERC therapy seems to be less obvious in the elderly population with AF. According to the EAST-AFNET 4 trial, the effects of ERC were consistent across all age tertial groups. In contrast, Dickow et al. demonstrated that the effects of ERC were greater in patients aged <75 years (23). Our study showed a clear benefit of ERC therapy in elderly individuals in terms of lowering the risk of ischemic stroke or all-cause death.
Elderly patients with AF often present with multiple comorbidities and clinical complexity, which increase the risk of stroke and other adverse outcomes (24). Moreover, advanced age itself is a major risk factor for stroke (25). However, older patients with AF are less likely to receive optimal treatment. Although there are sufficient data to support the use of oral anticoagulants (OAC) in this population (26), older people are less likely to receive OAC (27). Similarly, older patients with AF tend to receive less rhythm control therapy (14), which can offer benefits such as improved symptom control, enhanced quality of life, and potentially reduced long-term complications, including stroke.
In our study, only 48% of the study subjects received anticoagulation therapy, whereas a mere 19% received ERC therapy. Furthermore, among those receiving ERC therapy, only 0.7% underwent AF catheter ablation. Hence, our study suggests that ERC therapy should be actively considered in elderly patients in addition to appropriate anticoagulation and comorbidities control.
Our study reflects real-world practices in the treatment of elderly patients with AF. Out of the study subjects, only 48% received anticoagulation therapy. Although guidelines strongly recommend anticoagulation for patients with AF aged 75 years and older, it is frequently omitted in those with relatively low CHA2DS2-VASc scores (7, 8). Even with the increasing use of direct oral anticoagulants, up to 50% of Korean patients with AF and an OAC indication are not prescribed OAC (28). Furthermore, a mere 19% received ERC therapy, and within this group, only 0.7% underwent AF catheter ablation. Subgroup analysis revealed that the benefits of ERC alone (group 3) were significant only in the OAC group, not in the no OAC group. Additionally, the advantages of the combining ERC and HLS were more pronounced in the OAC group compared to the no OAC group. Taken together, our study suggests that ERC and HLS should be actively considered in elderly AF patients under the premise of appropriate anticoagulation and comorbidity control.
Increasing evidence suggests that lifestyle modification plays a pivotal role in the comprehensive management of AF. Correcting unhealthy lifestyles such as cigarette smoking cessation, regular exercise, alcohol abstinence, and weight reduction is associated with a significant reduction in AF burden and maintenance of sinus rhythm (29–33). As a result, current guidelines emphasize a more holistic or integrated approach to AF management; this includes lifestyle modification (7, 8, 34), which is associated with improved clinical outcomes (35–37). We observed a significant association between HLS and a lower risk of ischemic stroke, heart failure admissions, and all-cause mortality in the elderly population, which is consistent with the results from several recent observational studies of the general population (17–19), clearly demonstrating the importance of HLS in AF management, even among the elderly.
Based on our results, the synergistic effect of ERC and HLS on the risk of ischemic stroke was relatively modest. Nevertheless, HLS appears to be associated with a more substantial reduction in the risk of all-cause mortality than ERC, and the most significant risk reduction for the composite outcome was observed in elderly patients with AF who both implemented ERC and kept HLS. One possible explanation for this observation is that the population-attributable risk of stroke associated with AF increases exponentially with advancing age (5). In elderly individuals, interventions such as anticoagulation and rhythm control therapy can effectively reduce the risk of stroke in the presence of AF. This interpretation is further supported by the finding that the risk reduction of ischemic stroke in the HLS-alone group was more pronounced in the subgroup without OAC treatment.
This study has several limitations. First, given that the study used the NHIS database, it is important to acknowledge that information not available in the database could act as a confounding factor. Second, we failed to obtain information regarding the subjects' rhythm status and maintenance of sinus restoration as well as symptoms related to AF. Third, the definition of HLS was confined to smoking, drinking, and physical activity, which could be assessed using national health check-up data. We did not include several factors such as weight reduction, a healthy diet, or moderation in caffeine consumption, although we adjusted for BMI in the multivariate analysis. Moreover, while ERC represents a newly implemented treatment, the evaluation of lifestyle was based on each individual's pre-existing healthy lifestyle, which was assessed at the time of the new AF diagnosis. To interpret HLS as an intervention, prospective studies would be necessary. Fourth, although patients' lifestyles could change during follow-up, our analyses did not include the impact of maintaining and changes in components of HLS during the follow-up period on clinical outcomes. We believe that having and consistently maintaining healthier lifestyle will result in favorable outcomes. This aspect requires further investigation in future studies. Finally, based on current guidelines, anticoagulation therapy should be initiated in patients with AF aged >75 years, unless there is a clear contraindication (7, 8). Ideally, the rate of anticoagulation treatment should be close to 100%. However, in our study cohort, it was only 52%, which reflects actual practice in the real world (28). Therefore, we conducted subgroup analyses to examine the use of OAC treatment and identified an interaction between the use of OAC and stroke outcomes. Generalizing our findings to populations with higher anticoagulation rates may be challenging.
ERC and HLS may individually be associated with a lower risk of ischemic stroke in elderly patients with new-onset AF. Concurrently implementing ERC and maintaining HLS was associated with the lowest risk of death and the composite outcome, with a modest synergistic effect on stroke prevention. An integrated approach that includes both ERC and HLS should be considered for achieving better clinical outcomes in elderly patients newly diagnosed with AF.
Publicly available datasets were analyzed in this study. This data can be found here: http://nhiss.nhis.or.kr.
The studies involving humans were approved by the Institutional Review Board of Seoul National University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
WH-L: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft. SR-L: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – original draft. EK-C: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. SW-L: Software, Writing – review & editing. KH: Software, Writing – review & editing. SO: Writing – review & editing, Supervision. GYH-L: Writing – review & editing, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research was supported by a grant from the Patient-Centered Clinical Research Coordinating Center (PACEN) funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC21C0028), and by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: HI20C1662, 1711138358, KMDF_PR_20200901_0173). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
EK-C: Research grants or speaking fees from Abbott, Bayer, BMS/Pfizer, Biosense Webster, Chong Kun Dang, Daewoong Pharmaceutical Co., Daiichi-Sankyo, DeepQure, Dreamtech Co., Ltd., Jeil Pharmaceutical Co. Ltd, Medtronic, Samjinpharm, Seers Technology, and Skylabs. GYH-L: Consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. He is a National Institute for Health and Care Research (NIHR) Senior Investigator and co-principal investigator of the AFFIRMO project on multimorbidity in AF, which has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 899871. No fees are received personally.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1346414/full#supplementary-material
1. Khurshid S, Ashburner JM, Ellinor PT, McManus DD, Atlas SJ, Singer DE, et al. Prevalence and incidence of atrial fibrillation among older primary care patients. JAMA Netw Open. (2023) 6(2):e2255838. doi: 10.1001/jamanetworkopen.2022.55838
2. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (atria) study. JAMA. (2001) 285(18):2370–5. doi: 10.1001/jama.285.18.2370
3. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. (1997) 96(7):2455–61. doi: 10.1161/01.cir.96.7.2455
4. Mou L, Norby FL, Chen LY, O’Neal WT, Lewis TT, Loehr LR, et al. Lifetime risk of atrial fibrillation by race and socioeconomic Status: aric study (atherosclerosis risk in communities). Circ Arrhythm Electrophysiol. (2018) 11(7):e006350. doi: 10.1161/CIRCEP.118.006350
5. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. (1991) 22(8):983–8. doi: 10.1161/01.STR.22.8.983
6. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. (2002) 113(5):359–64. doi: 10.1016/s0002-9343(02)01236-6
7. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the esc. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
8. Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YH, et al. 2021 focused update consensus guidelines of the Asia pacific heart rhythm society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemostasis. (2022) 122(1):20–47. doi: 10.1055/s-0041-1739411
9. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham heart study: a cohort study. Lancet. (2015) 386(9989):154–62. doi: 10.1016/S0140-6736(14)61774-8
10. Magnussen C, Ojeda FM, Wild PS, Sorensen N, Rostock T, Hoffmann BA, et al. Atrial fibrillation manifestations risk factors and sex differences in a population-based cohort (from the Gutenberg health study). Am J Cardiol. (2018) 122(1):76–82. doi: 10.1016/j.amjcard.2018.03.028
11. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. (2020) 383(14):1305–16. doi: 10.1056/NEJMoa2019422
12. Kim D, Yang PS, You SC, Sung JH, Jang E, Yu HT, et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: nationwide cohort study. Br Med J. (2021) 373:n991. doi: 10.1136/bmj.n991
13. Haider S, Grabovac I, Smith L, Stefanac S, Jackson SE, Li Y, et al. Health care providers’ advice on lifestyle modification for older adults. J Am Med Dir Assoc. (2020) 21(3):361–6.e1. doi: 10.1016/j.jamda.2019.07.019
14. Kim D, Yang PS, You SC, Jang E, Yu HT, Kim TH, et al. Age and outcomes of early rhythm control in patients with atrial fibrillation: nationwide cohort study. JACC Clin Electrophysiol. (2022) 8(5):619–32. doi: 10.1016/j.jacep.2022.02.014
15. Seong S C, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data resource profile: the national health information database of the national health insurance service in South Korea. Int J Epidemiol. (2017) 46(3):799–800. doi: 10.1093/ije/dyw253
16. Choi EK. Cardiovascular research using the Korean national health information database. Korean Circ J. (2020) 50(9):754–72. doi: 10.4070/kcj.2020.0171
17. Ahn HJ, Lee SR, Choi EK, Han KD, Jung JH, Lim JH, et al. Association between exercise habits and stroke, heart failure, and mortality in Korean patients with incident atrial fibrillation: a nationwide population-based cohort study. PLoS Med. (2021) 18(6):e1003659. doi: 10.1371/journal.pmed.1003659
18. Lee SR, Choi EK, Jung JH, Han KD, Oh S, Lip GYH. Lower risk of stroke after alcohol abstinence in patients with incident atrial fibrillation: a nationwide population-based cohort study. Eur Heart J. (2021) 42(46):4759–68. doi: 10.1093/eurheartj/ehab315
19. Lee SR, Choi EK, Jung JH, Han KD, Oh S, Lip GYH. Smoking cessation after diagnosis of new-onset atrial fibrillation and the risk of stroke and death. J Clin Med. (2021) 10(11):2238. doi: 10.3390/jcm10112238
20. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. (2005) 43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83
21. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. (2015) 34(28):3661–79. doi: 10.1002/sim.6607
22. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28(25):3083–107. doi: 10.1002/sim.3697
23. Dickow J, Kirchhof P, Van Houten HK, Sangaralingham LR, Dinshaw LHW, Friedman PA, et al. Generalizability of the EAST-AFNET 4 trial: assessing outcomes of early rhythm-control therapy in patients with atrial fibrillation. J Am Heart Assoc. (2022) 11(11):e024214. doi: 10.1161/JAHA.121.024214
24. Romiti GF, Proietti M, Bonini N, Ding WY, Boriani G, Huisman MV, et al. Clinical complexity domains, anticoagulation, and outcomes in patients with atrial fibrillation: a report from the GLORIA-AF registry phase II and III. Thromb Haemostasis. (2022) 122(12):2030–41. doi: 10.1055/s-0042-1756355
25. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. (2010) 137(2):263–72. doi: 10.1378/chest.09-1584
26. Shah SJ, Singer DE, Fang MC, Reynolds K, Go AS, Eckman MH. Net clinical benefit of oral anticoagulation among older adults with atrial fibrillation. Circ Cardiovasc Qual Outcomes. (2019) 12(11):e006212. doi: 10.1161/CIRCOUTCOMES.119.006212
27. Ko D, Lin KJ, Bessette LG, Lee SB, Walkey AJ, Cheng S, et al. Trends in use of oral anticoagulants in older adults with newly diagnosed atrial fibrillation, 2010–2020. JAMA Netw Open. (2022) 5(11):e2242964. doi: 10.1001/jamanetworkopen.2022.42964
28. Lee SR, Choi EK, Han KD, Cha MJ, Oh S, Lip GYH. Temporal trends of antithrombotic therapy for stroke prevention in Korean patients with non-valvular atrial fibrillation in the era of non-vitamin K antagonist oral anticoagulants: a nationwide population-based study. PLoS One. (2017) 12(12):e0189495. doi: 10.1371/journal.pone.0189495
29. Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M, et al. Smoking and incidence of atrial fibrillation: results from the atherosclerosis risk in communities (ARIC) study. Heart Rhythm. (2011) 8(8):1160–6. doi: 10.1016/j.hrthm.2011.03.038
30. Elliott AD, Verdicchio CV, Mahajan R, Middeldorp ME, Gallagher C, Mishima RS, et al. An exercise and physical activity program in patients with atrial fibrillation: the ACTIVE-AF randomized controlled trial. JACC Clin Electrophysiol. (2023) 9(4):455–65. doi: 10.1016/j.jacep.2022.12.002
31. Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. (2020) 382(1):20–8. doi: 10.1056/NEJMoa1817591
32. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, et al. Long-term effect of goal-directed weight management in an atrial fibrillation cohort: a long-term follow-up study (legacy). J Am Coll Cardiol. (2015) 65(20):2159–69. doi: 10.1016/j.jacc.2015.03.002
33. Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, et al. Prevention and regressive effect of weight-loss and risk factor modification on atrial fibrillation: the REVERSE-AF study. Europace. (2018) 20(12):1929–35. doi: 10.1093/europace/euy117
34. Chung MK, Eckhardt LL, Chen LY, Ahmed HM, Gopinathannair R, Joglar JA, et al. Lifestyle and risk factor modification for reduction of atrial fibrillation: a scientific statement from the American heart association. Circulation. (2020) 141(16):e750–72. doi: 10.1161/CIR.0000000000000748
35. Romiti GF, Guo Y, Corica B, Proietti M, Zhang H, Lip GYH, et al. Mobile health-technology-integrated care for atrial fibrillation: a win ratio analysis from the mAFA-II randomized clinical trial. Thromb Haemostasis. (2023) 123(11):1042–8. doi: 10.1055/s-0043-1769612
36. Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D, et al. Adherence to the “atrial fibrillation better care” pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb Haemostasis. (2022) 122(3):406–14. doi: 10.1055/a-1515-9630
Keywords: atrial fibrillation, rhythm control, lifestyle modification, stroke, elderly
Citation: Lim W-H, Lee S-R, Choi E-K, Lee S-W, Han K-D, Oh S and Lip GYH (2024) Combination of early rhythm control and healthy lifestyle on the risk of stroke in elderly patients with new-onset atrial fibrillation: a nationwide population-based cohort study. Front. Cardiovasc. Med. 11:1346414. doi: 10.3389/fcvm.2024.1346414
Received: 29 November 2023; Accepted: 7 February 2024;
Published: 15 February 2024.
Edited by:
Teresa Strisciuglio, University of Naples Federico II, ItalyReviewed by:
Chin-Feng Tsai, Chung Shan Medical University Hospital, Taiwan© 2024 Lim, Lee, Choi, Lee, Han, Oh and Lip. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eue-Keun Choi Y2hvaWVrMTdAc251LmFjLmty
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.