- 1Department of Medicine, Dow University of Health Sciences, Karachi, Pakistan
- 2Dow Medical College, Dow University of Health Sciences, Karachi, Pakistan
- 3Department of Surgery, Assab Military Hospital, Assab, Eritrea
- 4Department of Medicine, Rahbar Medical and Dental College, Lahore, Pakistan
Introduction: Cardiovascular disease (CVD) is a leading global cause of morbidity and mortality, with high systolic blood pressure (SBP) identified as a major risk factor. Aspirin (Acetylsalicylic acid—ASA) has been considered for CVD prevention, prompting questions about its optimal use in primary and secondary prevention and the ideal dosing time to maximize its impact on circadian blood pressure rhythms. Previous research suggests a potential benefit of bedtime aspirin dosing in reducing blood pressure, attributed to its effects on the renin-angiotensin-aldosterone system and nitric oxide production. This systematic review and meta-analysis aim to further explore the circadian effects of aspirin on blood pressure, focusing on the timing of administration.
Methods: Adhering to PRISMA guidelines, a comprehensive search of PubMed, Cochrane Library, and clinicaltrials.gov was conducted. Randomized controlled trials (RCTs) involving patients aged >18 with cardiovascular history and hypertension were included. The primary objective was to assess the impact of bedtime-dosed and morning-dosed aspirin on systolic and diastolic blood pressure. Low-dose aspirin was administered for primary or secondary prevention. The Cochrane Risk of Bias tool evaluated study quality. Meta-analyses were conducted using RevMan 5.3, with mean deviations and 95% confidence intervals employed for outcomes.
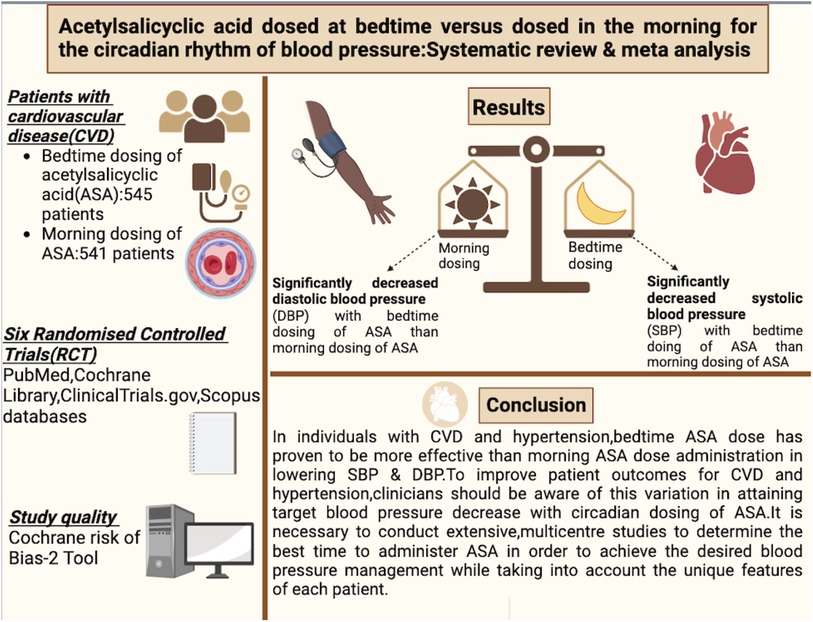
Results: Initial searches yielded 1,181 articles, with six studies meeting the inclusion criteria. These RCTs involved 1,470 patients, with 1,086 completing follow-up. Bedtime aspirin dosing demonstrated a significant reduction in both systolic and diastolic blood pressure compared to morning dosing (p < 0.05). Meta-analysis results for systolic blood pressure revealed a weighted mean difference of approximately 3.65 mmHg in favour of bedtime dosing, with low heterogeneity (I2 = 0%). For diastolic blood pressure, the weighted mean difference was 1.92, again favouring bedtime dosing, with 3% heterogeneity.
Conclusion: This meta-analysis, involving over 1,300 cardiovascular/hypertensive patients, supports the effectiveness of bedtime aspirin in reducing systolic and diastolic blood pressure compared to morning dosing. The results align with previous findings but distinguish themselves by incorporating a more diverse patient population and addressing moderate heterogeneity. While the study's outcomes are promising, further research, including larger sample sizes and longer durations, is warranted for comprehensive clinical implementation. As the study exclusively focused on aspirin timing, future investigations should explore sustained blood pressure effects in patients with clinical indications for aspirin alongside other hypertensive medications.
1 Introduction
Several diseases contribute to the makeup of the term cardiovascular disease (CVD). CVD is a major cause of death, morbidity, and healthcare resource expenditure in the world (1). CVD has a number of prognosis-determining modifiable risk factors. High systolic blood pressure (SBP) is attributed to be the leading risk factor for premature cardiovascular deaths. Globally in 2021, high SBP accounted for 10.8 million CVD-related deaths and 11.3 million overall. It is a particularly strong precipitator of ischemic heart disease, stroke, and intracerebral hemorrhage-related deaths (2). In the United States alone during the 2017–2020 period, 58.4% of adult non-Hispanic Black females had high blood pressure (HBP), the highest prevalence of all race and sex categories. The lowest prevalence is in Hispanic females at 35.3%. Of the HBP mortality population in 2020, 51.3% were females and 48.7% were males (3). Logically, HBP or High SBP tend to be impactful targets for the primary and secondary prevention of cardiovascular and cerebrovascular events in at-risk hypertensive patients. Aspirin or Acetylsalicylic acid (ASA) is one such drug attempting prophylaxis in this regard. Two questions arise for optimal aspirin clinical use. Firstly, whether it is more suited for primary or secondary prevention of CVD and secondly the ideal dose swallow time to cause the greatest beneficial impact on circadian rhythms of blood pressure in humans.
A previous meta-analysis answering the first question indicated that using aspirin for secondary prevention of CVD-related events yielded a greater absolute reduction in serious vascular events (6·7% aspirin vs. 8·2% control per year, p < 0.0001) as compared to the primary prevention group (0·51% vs. 0·57% per year, p = 0·0001). Additionally, there were increased major gastrointestinal and extracranial bleeds (0·10% vs. 0·07% per year, p < 0·0001) in the primary prevention patients on aspirin administration (4). Hence, for primary prevention patient populations the increased chance of bleeding events makes any rationale to use aspirin prophylactically questionable (4, 5).
Several factors such as serum nitric oxide, prostaglandin, angiotensin II, angiotensin-converting enzyme, renin-angiotensin-aldosterone system (RAAS) activity, expression of pro-inflammatory cytokines, and leukocyte adhesives contribute to HBP (6). Many of these influencers of HBP are known to be under the control of circadian clocks (7–9). The HBP-lowering effect of aspirin in the evening can be explained by the effect of inhibiting the otherwise increasing RAAS system activity, as well as production of the vasodilatory nitric oxide (10).
Normal individuals have a 10%–20% decrease in Blood Pressure (BP) at night. Non-dipping individuals with less than 10% dips in BP are linked to activation of the RAAS system (11) and at greater risk of CVD events (12, 13). This suggests the assumption that the night dipping phenomenon may neutralize the relatively higher blood pressures present in the day which are attributed to greater sympathetic system activity unmasking the full extent of aspirin BP lowering effects (14). It is also known that the antiplatelet action of aspirin is suboptimal during the morning while a significant reduction in platelet aggregation was obtained in a relevant randomized trial (15, 16). The multifactorial usefulness of aspirin in lowering BP via its effects on vascular endothelium, inhibiting thromboxane A2 mediated platelet aggregation paired with optimal bedtime administration deals a significant blow to HBP and as a result reoccurrences of CVD events such as Myocardial Infarction (MI) and stroke. Several clinical trials and previous meta-analyses support this evening/bedtime/night BP lowering hypothesis in CVD patients (15–17).
An issue with previous trials and meta-analyses on this subject is that the majority of the data was from populations of Chinese origin. Our updated meta-analysis includes RCTs conducted on populations other than Chinese origin. Additionally, we analyze only high-level evidence from RCTs, as reflected by our exclusion criteria.
This present analysis is intended to further establish the benefits of nocturnally dosed aspirin in countering the major modifiable risk factor of CVD, namely BP. If this quick fact-turning bedtime hypothesis keeping in mind individual patient BP circadian rhythms in mind is adopted widely enough the overall incidence of CVD and many of its various causative factors may visibly reduce worldwide.
2 Methods
2.1 Data sources and search strategy
This comprehensive review adheres to the meticulous reporting standards outlined in the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines 2019 (18) discern studies meeting the criteria for inclusion, we methodically searched three databases, including PubMed, Cochrane Library, and clinicaltrials.gov. These searches were carried out autonomously by two of our study investigators and culminated on August 31, 2020. Our search keywords encompassed terms like “Aspirin,” “ASA,” “acetylsalicylic acid,” “Time,” “Morning,” “Night,” “Before Sleeping,” “Chronotherapy,” “Circadian rhythms,” “Cardiovascular,” “Hypertension,” combined with study-type identifiers such as “RCT,” “Randomized-Controlled trials,” and “trial.” Further details of our search strategy can be found in the Additional file (Supplementary Table S1).
2.2 Inclusion and exclusion criteria
(a) The study design included Randomized Control Trials (RCTs).
(b) The targeted population was patients aged >18 years with a history of cardiovascular and hypertension.
(c) The primary objective of our investigation was to evaluate the impact of bedtime-dosed and morning-dosed aspirin as an intervention and control, respectively, on systolic and diastolic blood pressure.
(d) Low-dose aspirin was administered for primary or secondary cardiovascular event prevention, either as a sole antiplatelet therapy or in combination with non-antiplatelet drugs, such as antihypertensive medications.
(e) Our study excluded abstracts, unpublished materials, reviews, book chapters, non-RCT studies, and studies published in languages other than English, as well as studies lacking clear reporting of primary and secondary outcomes.
2.3 Quality assessment and risk of bias in studies
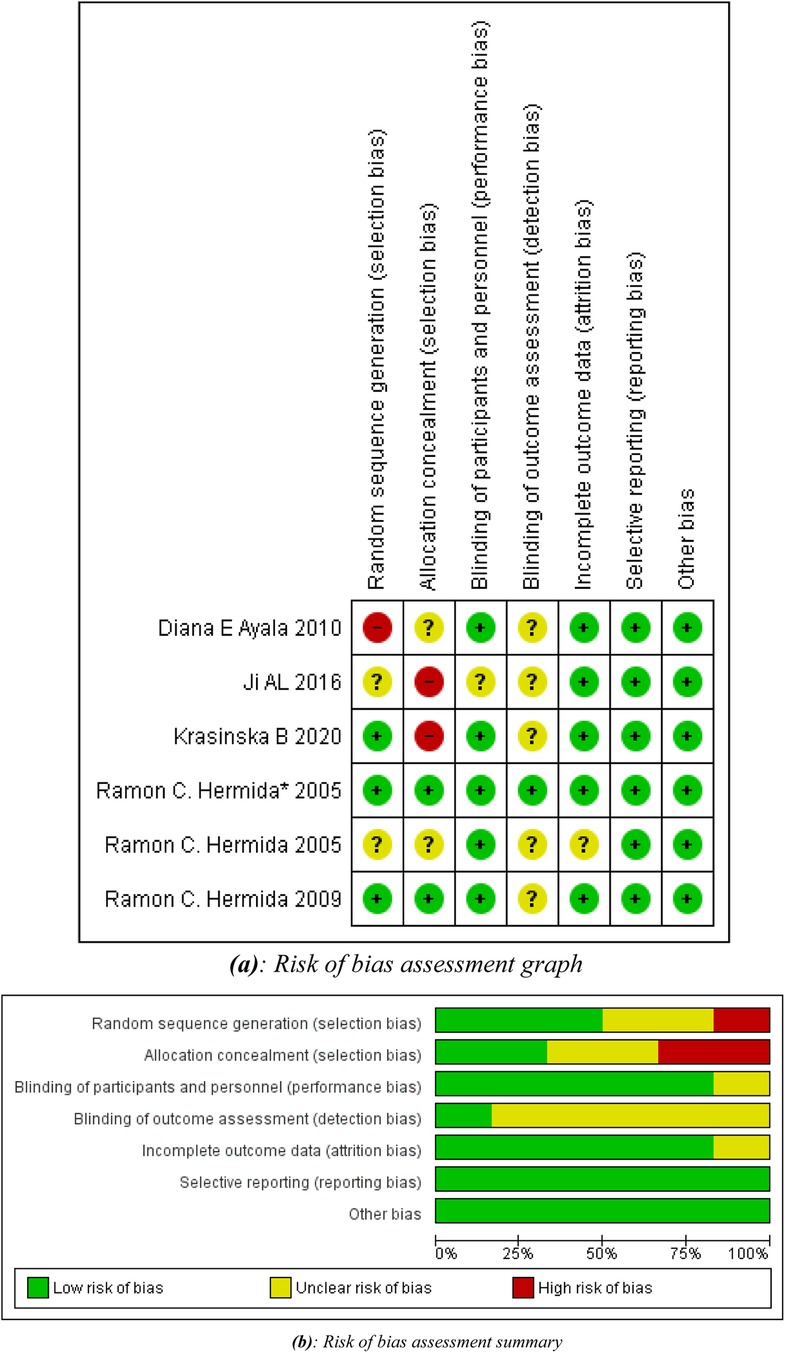
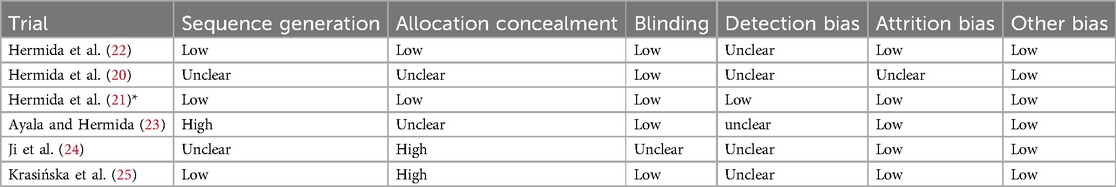
The Cochrane Risk of Bias tool (19) was employed to evaluate the quality of the included RCTs. This assessment encompassed factors such as sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, handling of incomplete outcome data, selective outcome reporting, and other potential sources of bias. Each study was categorized as having a low, high, or unclear risk of bias for each of these factors. If a study was deemed to have a “high risk” of bias in any one category, it was classified as having an overall “high risk of bias”. Two independent assessors conducted the quality assessment, and any discrepancies were resolved through consensus or by consulting a third researcher.
2.4 Data extraction
Two investigators independently collected all pertinent data from the 7 included studies. This information, including general study characteristics (such as first authors, publication years, study type, and sample size), demographic details (covering age and gender), intervention and control specifics (including treatment frequency and duration), and outcome-related features (comprising outcome categories, definitions, and follow-up details), was systematically entered in Google sheets.
2.5 Data analysis
We conducted meta-analyses utilizing Cochrane's Review Manager (RevMan) version 5.3. Mean deviations with 95% confidence intervals (CIs) were employed to assess clinical outcomes, systolic and diastolic blood pressure, in patients with cardiovascular disease and hypertension who were undergoing Aspirin treatment at Morning and Bedtime respectively. We applied a fixed effects model to calculate combined effect estimates for comparing outcomes between the intervention and control groups. Heterogeneity was assessed using the I2 statistic, with a classification of significant heterogeneity (I2 ≥ 50%), interpreted based on study characteristics.
2.6 Ethical consideration
No ethical approval or patient consent was necessary for this meta-analysis since we solely utilized data extracted from RCTs identified in the literature search. All patient data used was entirely anonymized, eliminating the need for ethical clearance or individual patient consent.
3 Result
3.1 Literature search result
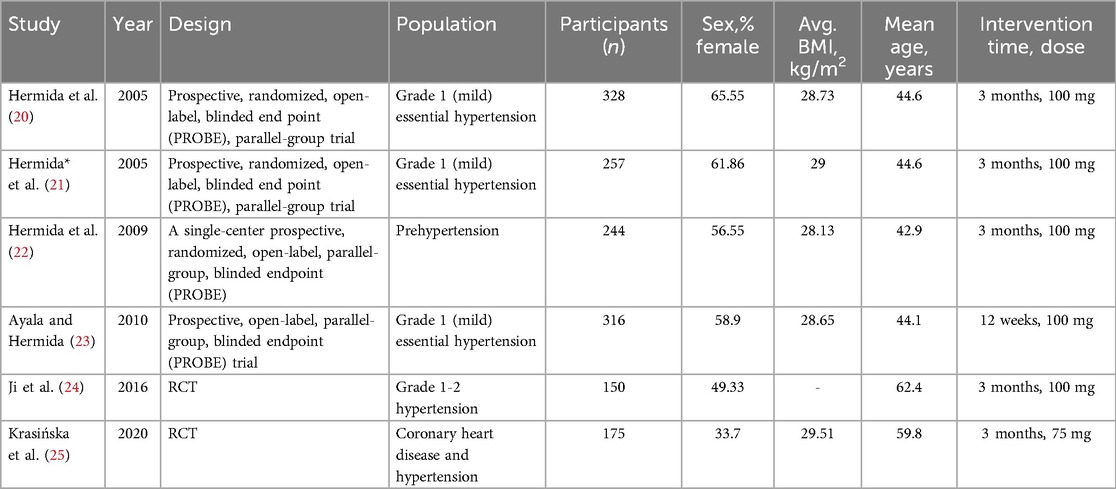
An initial search of the 3 databases i.e., PubMed, Cochrane and clinicaltrials.gov, revealed 1,181 potentially relevant articles, of which 157 remained after excluding duplicates. After applying eligibility criteria, we selected 6 articles for inclusion in the systematic review (20–25). The Preferred Reporting Items for Systematic Reviews and Meta-analyses flowchart shown in Figure 1 summarizes the literature search for this study. All included studies were given 100 mg of aspirin except Krasińska B, (25), in which 75 mg of acetylsalicylic acid was given to the participants.
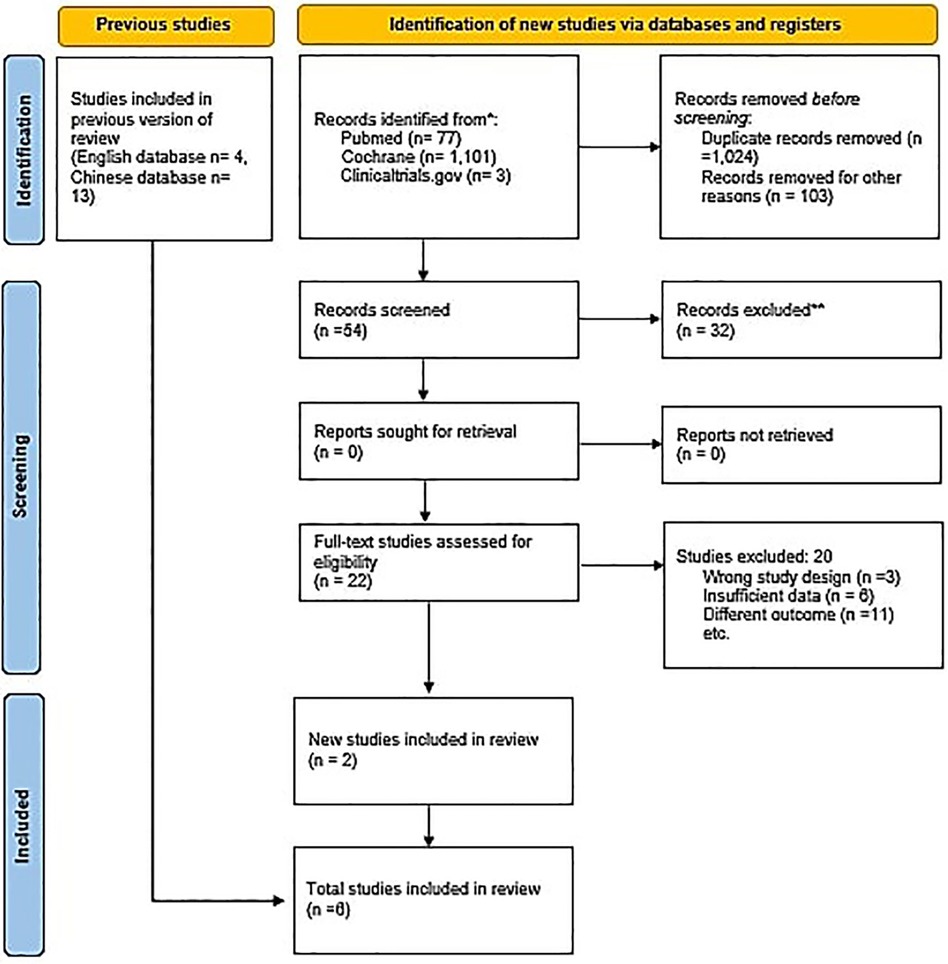
Figure 1. PRISMA flowchart. The PRISMA flow diagram for the systematic review detailing the database searches, the number of abstracts screened, and the full texts retrieved.
3.2 Study characteristics and quality assessment
These trials randomly assigned a total of 1,470 patients, out of which 1,086 completed the follow-up with 545 in the intervention group (bedtime dose) and 541 in the control group (morning dose). Follow-up duration ranged from 12 weeks to 3 months. The baseline characteristics of all trials are summarized in Table 1.
All studies were of considerably high methodological quality as shown in Figures 2a,b. A detailed quality assessment of each study is given in Table 2.
3.3 Change in blood pressure in response to ASA chronotherapy
Seven distinct studies examined the impact of aspirin on blood pressure at various time intervals. The findings indicated that the administration of 75–100 mg of aspirin before bedtime resulted in a noteworthy reduction in both systolic and diastolic blood pressure when compared to morning dosing (both p < 0.05), signifying a significant difference in the outcomes.
3.3.1 Meta-analysis results for systolic blood pressure
Systolic blood pressure represents the maximum force exerted by blood against arterial walls during each cardiac cycle, particularly during the heart's left ventricular contraction. Aspirin's anti-inflammatory properties enhance blood vessel flexibility, reducing resistance to blood flow, while its antiplatelet effects prevent clot formation, promoting improved circulation. These factors collectively contribute to a modest reduction in blood pressure, specifically systolic values.
The analysis, depicted in Figure 3, underscores a more pronounced decrease in systolic blood pressure when aspirin is administered before bedtime. The data reveals a weighted mean difference (WMD) of approximately 3.65 (95 per cent confidence interval, 2.58–4.73) relative to morning dosing. Within the seven studies analyzed, a moderate level of heterogeneity at 0 per cent was observed, with a highly significant p-value of less than 0.00001, indicating substantial and statistically significant diversity in the results. Consequently, these findings substantiate the association between bedtime aspirin administration and a mean systolic blood pressure reduction of 3.65 mmHg in randomized controlled trials.
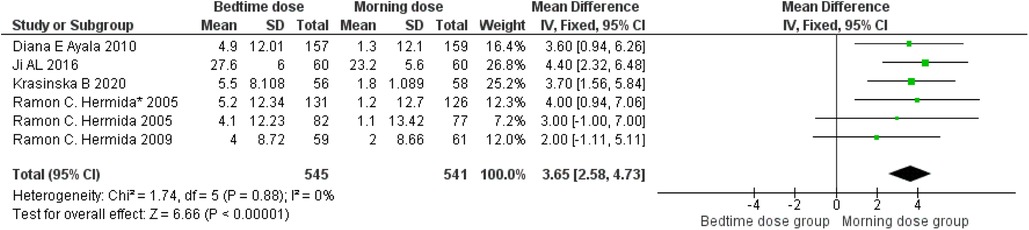
Figure 3. Bedtime vs. Morning dose of ASA on Systolic Blood Pressure (SBP). The diamond indicates the overall MD with an associated 95% CI. SD, standard deviation; IV, inverse variance; CI, confidence interval.
3.3.2 Meta-analysis results for diastolic blood pressure
Diastolic blood pressure, representing arterial pressure during the heart's resting phase, is influenced by aspirin's antiplatelet and anti-inflammatory properties, potentially enhancing blood vessel function. A comparative analysis illustrated in Figure 4 indicates a weighted mean difference of 1.92 (95 per cent confidence interval: 1.41–2.71) between individuals taking aspirin at bedtime and those taking it in the morning. This suggests a modest reduction in diastolic blood pressure for bedtime aspirin users. The observed 3 per cent heterogeneity, along with a remarkably low p-value of 0.00001, signifies statistically significant diversity in the studied populations, emphasizing the need for further exploration in this context.
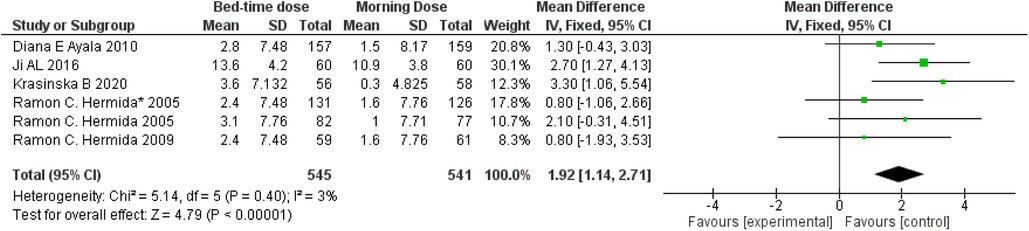
Figure 4. Bedtime vs. Morning dose of ASA on Diastolic Blood Pressure (DBP). The diamond indicates the overall MD with an associated 95% CI. SD, standard deviation; IV, inverse variance; CI, confidence interval.
4 Discussion
Our pooled analysis of six RCTs involving more than 1300 cardiovascular/hypertensive patient populations assessed the effects of different chronotherapy of aspirins over blood pressure and demonstrated that administration of aminosalicylic acid before bedtime resulted in a statistically significant reduction in systolic as well as diastolic blood pressure in comparison to the administration of same dosage of aspirin in the morning (Figures 3,4).
It is particularly noteworthy because this is the only meta-analysis that combined the results of RCTs with relatively moderate heterogeneity in contrast to other studies that reported their results with substantial heterogeneity (17) The findings of our study are consistent with the results reported by previous meta-analyses (17) that included 17 RCTs, however, there are many factors that make our study dissimilar from the previously published study. The prior meta-analysis consisted of 17 RCTs, out of which 13 were Chinese RCTs which could potentially create the bias of application of their reported findings only to the Chinese population. Apart from that, significantly high heterogeneity was reported in its results not only in total effect size but also in subgroup analysis. This substantially high heterogeneity with no resolution with subgroup analysis is another factor that distinguishes our study from previous meta-analyses. Thus, our systematic review and meta-analysis could serve as a source of robust findings owing to its moderate heterogeneity and diverse patient population. Nevertheless, the core outcomes of this and previous study share the same results of reduction in systolic blood pressure and diastolic blood pressure at evening dose rather than in the morning.
While our results show promise, questions linger about its practical implications in real-world clinical settings. The modest magnitude of this reduction prompts a critical consideration of its significance in the broader context of cardiovascular risk management. Thus, our study endeavours to bridge the gap between statistical significance and clinical applicability, aiming to investigate the true impact of bedtime dosing of aspirin on long-term cardiovascular health outcomes.
The probable explanation of such findings could be provided by the study that reported a significant reduction in renin activity of plasma for 24 h when aspirin is taken at bedtime in comparison to its administration in the morning. Furthermore, cortisol, dopamine and norepinephrine were also significantly reduced with their urinary excretion over a 24 h period proving a sound biological rationale for the drop in blood pressure at bedtime dosages of aspirin (26). Similarly, one of the multiple studies conducted by Hermida et al. on Chrono pharmacological effects of aspirin demonstrated the decline in blood pressure at bedtime based on the reason that bedtime-associated use of aspirin induces the nocturnal release of NO, reduces nocturnal peak of plasma renin activity, produced a favorable effect on vascular endothelial cells and blocks alpha- and beta-adrenergic receptors. However patient population (e.g., without co-morbidities) included in this study was very different from those that came for consulting in nephrology and cardiology (22). Although this study is strictly based on the established reporting specification of meta-analysis, we do not claim it to be implemented in clinical practice. While we observed significant outcomes, it is crucial to acknowledge the presence of moderate heterogeneity across the studies. This heterogeneity was effectively addressed through a sensitivity analysis, revealing that the study conducted by Maria V. Ruiz in 2019 was the primary source of maximum heterogeneity in both systolic and diastolic blood pressure. The rationale behind this heightened heterogeneity lies in the fact that the study encompassed a total of 20 primary centres. The intention-to-treat analyses were methodically conducted, comparing outcomes between bedtime and daytime intake periods. The analysis included adjustments for baseline values at the start of each period, utilizing mixed effects repeated measures analysis of covariance models.
Furthermore, the authors of the study extended their analytical models to account for potential confounding or modifying variables. This encompassed adjustments for changes in medication, age, sex, unhealthy habits, risk factors, comorbidities, and the time of study enrollment. This level of comprehensive adjustment was not reported in other trials, contributing to the observed heterogeneity.
In the vast field of medical research, our study stands out for its methodological rigour and clinical insight on aspirin chronotherapy. Although our results align with the previous meta-analysis, they are distinguished by their methodological refinement and the inclusion of diverse patient populations. It is within this context that our study gains significance and offers a nuanced exploration of the timing dynamics of aspirin administration in addition to a synthesis of existing evidence. Our pursuit represents a scholarly contribution aimed at adding valuable insights to the ongoing discussion on managing hypertension.
It is important to note that this meta-analysis has certain limitations. Firstly, the number of randomized controlled trials (RCTs) and the size of the study population were relatively small, which may limit the ability to draw consistent findings. Additionally, the total duration of dose administration was relatively short, which could potentially influence the accuracy of the results.
Certain studies included in this analysis exhibited some moderate bias, which should be taken into consideration when interpreting the results. This study exclusively compared data between individuals taking medicine in the morning and at bedtime, with no data available for those taking medicine in the morning and a placebo. Therefore, it is difficult to conclusively determine whether taking aspirin in the morning can effectively reduce blood pressure.
Before implementation of these findings in clinical practice, there's a need to conduct studies in future to evaluate the sustainability of blood pressure-reducing effects of bedtime aspirin in patients who carry clinical indications for intake of aspirin as they are on many other hypertensive medications as well which can likely dilute or interfere with the time-dependent impact of aspirin on blood pressure. However, if further, large and dedicated trials are conducted to explore the effects of dosage timing of aspirin on blood pressure keeping all the above-mentioned factors together, it could prove to be the most convenient way of improving control of blood pressure in patients who are already under the effect of so many therapeutic interventions since just a shift in the schedule of intake from morning to bedtime would be required to bring fine change in blood pressure in already debilitated patients, preventing the needs of further medicines and without effecting compliance of patients. In conclusion, further research is imperative to extract meaningful insights for clinical implementation. Additional studies with larger sample sizes, longer durations, and a more comprehensive approach are necessary in future to provide a more robust understanding of the effects of aspirin on blood pressure.
5 Conclusion
In conclusion, our systematic review and meta-analysis provide valuable insights into the impact of aspirin chronotherapy on blood pressure in cardiovascular and hypertensive patients. The analysis, based on high-quality randomized controlled trials (RCTs) with diverse populations, reveals a statistically significant reduction in both systolic and diastolic blood pressure when aspirin is administered at bedtime compared to morning dosing. This finding is consistent with previous studies but contributes unique strengths such as moderate heterogeneity and a more varied patient population.
The observed reduction in blood pressure is of clinical significance, considering the crucial role of elevated blood pressure as a major modifiable risk factor for cardiovascular diseases. The bedtime administration of aspirin demonstrates a potential strategy for optimizing the therapeutic effects of aspirin in countering hypertension, thereby reducing the risk of cardiovascular events. The underlying biological mechanisms, including the impact on plasma renin activity, cortisol, dopamine, and norepinephrine, offer a plausible rationale for the observed blood pressure-lowering effects during nighttime dosing.
However, it is essential to acknowledge the limitations of our analysis. The relatively small number of RCTs and study populations, along with the short duration of dose administration, warrant a cautious interpretation of the findings. Additionally, certain studies included in the analysis exhibited moderate bias, emphasizing the need for further dedicated trials.
Future research should focus on assessing the sustainability of bedtime aspirin's blood pressure-reducing effects, particularly in patients with clinical indications for aspirin who are concurrently on other hypertensive medications. Large-scale, long-term studies with a comprehensive approach are crucial for validating and extending the clinical implications of our findings. Before widespread implementation in clinical practice, further investigation is necessary to establish the optimal timing of aspirin administration for blood pressure control, considering patient compliance and potential interactions with other therapeutic interventions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AN: Writing – review & editing, Writing – original draft, Software, Project administration, Methodology, Formal Analysis, Conceptualization. TR: Writing – review & editing, Writing – original draft, Software, Methodology, Investigation, Formal Analysis, Data curation. MA: Writing – review & editing, Writing – original draft, Software, Project administration, Methodology, Formal Analysis. AH: Writing – review & editing, Writing – original draft, Methodology, Formal Analysis, Data curation. TS: Writing – review & editing, Writing – original draft. RK: Writing – review & editing. RM: Writing – review & editing. AH: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1346265/full#supplementary-material
References
1. Iqbal J, Iqbal A, Mukhtar H, Jahangir K, Mashkoor Y, Zeeshan MH, et al. Cardioprotective effects of nanoparticles in cardiovascular diseases: a state-of-the-art review. Curr Problems Cardiol. (2023) 48(8):101713. doi: 10.1016/j.cpcardiol.2023.101713
2. Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk. J Am College Cardiol. (2022) 80(25):2361–71. doi: 10.1016/j.jacc.2022.11.005
3. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation. (2023) 147(8):e93–e621. doi: 10.1161/CIR.0000000000001123
4. Antithrombotic Trialists' (ATT) Collaboration; Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. (2009) 373(9678):1849–60. doi: 10.1016/S0140-6736(09)60503-1
5. Liu S, Eckstein J, Lam A, Cheema AN. Acetylsalicylic acid (Aspirin) for primary prevention of cardiovascularevents in patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Curr Vasc Pharmacol. (2023) 21(2):111–9. doi: 10.2174/1570161121666230131120544
6. Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cífková R, Dominiczak AF, et al. Hypertension. Nat Rev Dis Primers. (2018) 4(1):18014. doi: 10.1038/nrdp.2018.14
7. Felip-Benach À, Sobrino-Martínez J. Cronoterapia en hipertensión arterial. Med Clín. (2006) 126(10):18–9. doi: 10.1157/13086056
8. Kanabrocki EL, George M, Hermida RC, Messmore HL, Ryan MD, Ayala DE, et al. Day-night variations in blood levels of nitric oxide, T-TFPI, and E-selectin. Clin Appl Thromb Hemost. (2001) 7(4):339–45. doi: 10.1177/107602960100700417
9. Cermakian N, Westfall S, Kiessling S. Circadian clocks and inflammation: reciprocal regulation and shared mediators. Arch Immunol Ther Exp. (2014) 62(4):303–18. doi: 10.1007/s00005-014-0286-x
10. Gordon RD, Wolfe LK, Island DP, Liddle GW. A diurnal rhythm in plasma renin activity in man. J Clin Invest. (1966) 45(10):1587–92. doi: 10.1172/JCI105464
11. Pati P, Fulton DJR, Bagi Z, Chen F, Wang Y, Kitchens J, et al. Low-salt diet and circadian dysfunction synergize to induce angiotensin II–dependent hypertension in mice. Hypertension. (2016) 67(3):661–8. doi: 10.1161/HYPERTENSIONAHA.115.06194
12. Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure. J Hypertens. (2002) 20(11):2183–9. doi: 10.1097/00004872-200211000-00017
13. Liu M. Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant. (2003) 18(3):563–9. doi: 10.1093/ndt/18.3.563
14. Sayk F, Becker C, Teckentrup C, Fehm HL, Struck J, Wellhoener JP, et al. To dip or not to dip. Hypertension. (2007) 49(5):1070–6. doi: 10.1161/HYPERTENSIONAHA.106.084343
15. Racca C, van Diemen JJK, Fuijkschot WW, Spit K, Bonten TN, Numans ME, et al. Aspirin intake in the morning is associated with suboptimal platelet inhibition, as measured by serum thromboxane B2, during infarct-prone early-morning hours. Platelets. (2019) 30(7):871–7. doi: 10.1080/09537104.2018.1528347
16. Krasińska B, Paluszkiewicz L, Miciak-Lawicka E, Krasiński M, Rzymski P, Tykarski A, et al. The effect of acetylsalicylic acid dosed at bedtime on the anti-aggregation effect in patients with coronary heart disease and arterial hypertension: a randomized, controlled trial. Cardiol J. (2020) 26(6):727–35. doi: 10.5603/CJ.a2018.0142
17. Zhou B, Wang Q, Zhao Z, Feng X. A systematic review of the efficacy and safety of aspirin when delivered at different medication times for the primary and secondary prevention of cardiovascular and cerebrovascular diseases. Ther Innov Regul Sci. (2020) 54(6):1339–48. doi: 10.1007/s43441-020-00156-w
18. Moher D, Liberati A, Tetzlaff J, Altman DG, Antes G, Atkins D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
19. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 2019(10):ED000142. doi: 10.1002/14651858.ED000142
20. Hermida RC, Ayala DE, Calvo C, López JE. Aspirin administered at bedtime, but not on awakening, has an effect on ambulatory blood pressure in hypertensive patients. J Am Coll Cardiol. (2005) 46(6):975–83. doi: 10.1016/j.jacc.2004.08.071
21. Hermida RC, Ayala DE, Calvo C, López JE, Mojón A, Rodríguez M, et al. Differing administration time-dependent effects of aspirin on blood pressure in dipper and non-dipper hypertensives. Hypertension. (2005) 46(4):1060–8. doi: 10.1161/01.HYP.0000172623.36098.4e
22. Hermida RC, Ayala DE, Mojon A, Fernandez JR. Ambulatory blood pressure control with bedtime aspirin administration in subjects with prehypertension. Am J Hypertens. (2009) 22(8):896–903. doi: 10.1038/ajh.2009.83
23. Ayala DE, Hermida RC. Sex differences in the administration-time-dependent effects of low-dose aspirin on ambulatory blood pressure in hypertensive subjects. Chronobiol Int. (2010) 27(2):345–62. doi: 10.3109/07420521003624662
24. Ji AL, Chen WW, Huang WJ. Clinical study on influences of enteric coated aspirin on blood pressure and blood pressure variability. Eur Rev Med Pharmacol Sci. (2016) 20(23):5017–20.27981528
25. Krasińska B, Paluszkiewicz L, Miciak-Ławicka E, Krasinski M, Rzymski P, Tykarski A, et al. The impact of acetylsalicylic acid dosed at bedtime on circadian rhythms of blood pressure in the high-risk group of cardiovascular patients—a randomized, controlled trial. Eur J Clin Pharmacol. (2021) 77(1):35–43. doi: 10.1007/s00228-020-02997-8
Keywords: cardiovascular disease, aspirin, systolic blood pressure, diastolic blood pressure, hypertension, circadian rhythm, bedtime dosing
Citation: Nadeem A, Rais T, Aamir M, Habte A, Siddiqui T, Karamat RI, Munsab R and Habib A (2024) Acetylsalicylic acid dosed at bedtime vs. dosed in the morning for circadian rhythm of blood pressure- a systematic review and meta-analysis. Front. Cardiovasc. Med. 11:1346265. doi: 10.3389/fcvm.2024.1346265
Received: 29 November 2023; Accepted: 27 September 2024;
Published: 22 October 2024.
Edited by:
Guido Iaccarino, Federico II University Hospital, ItalyReviewed by:
David John Vigerust, Vanderbilt University, United StatesJens Djurhuus, Aarhus University, Denmark
Copyright: © 2024 Nadeem, Rais, Aamir, Habte, Siddiqui, Karamat, Munsab and Habib. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdullah Nadeem, aWFtLmFiZHVsbkBvdXRsb29rLmNvbQ==
†ORCID:
Abdullah Nadeem
orcid.org/0000-0002-1205-5426
Taruba Rais
orcid.org/0000-0001-9323-353X
Alexander Habte
orcid.org/0009-0004-4736-8475
Tasmiyah Siddiqui
orcid.org/0000-0002-1620-5102
Riyan Imtiaz Karamat
orcid.org/0009-0000-1836-0459
Rabbia Munsab
orcid.org/0009-0009-5210-3822
Ashna Habib
orcid.org/0000-0001-5421-0212
 Abdullah Nadeem
Abdullah Nadeem Taruba Rais
Taruba Rais Minahil Aamir2
Minahil Aamir2 Tasmiyah Siddiqui
Tasmiyah Siddiqui Rabbia Munsab
Rabbia Munsab Ashna Habib
Ashna Habib