- 1Department of Anesthesiology, The Ohio State University Wexner Medical Center, Columbus, OH, United States
- 2Department of Anesthesiology, St Elizabeth’s Medical Center, Brighton, MA, United States
- 3Department of Anesthesiology & Perioperative Medicine, UCLA Medical Center, Los Angeles, CA, United States
- 4University of Toledo, College of Medicine and Life Sciences, Toledo, OH, United States
- 5Touro College of Osteopathic Medicine, New York, NW, United States
Background: Cannabis is one of the most widely used psychoactive substances. Its components act through several pathways, producing a myriad of side effects, of which cardiovascular events are the most life-threatening. However, only a limited number of studies address cannabis's perioperative impact on patients during noncardiac surgery.
Methods: Studies were identified by searching the PubMed, Medline, EMBASE, and Google Scholar databases using relevant keyword combinations pertinent to the topic.
Results: Current evidence shows that cannabis use may cause several cardiovascular events, including abnormalities in cardiac rhythm, myocardial infarction, heart failure, and cerebrovascular events. Additionally, cannabis interacts with anticoagulants and antiplatelet agents, decreasing their efficacy. Finally, the interplay of cannabis with inhalational and intravenous anesthetic agents may lead to adverse perioperative cardiovascular outcomes.
Conclusions: The use of cannabis can trigger cardiovascular events that may depend on factors such as the duration of consumption, the route of administration of the drug, and the dose consumed, which places these patients at risk of drug-drug interactions with anesthetic agents. However, large prospective randomized clinical trials are needed to further elucidate gaps in the body of knowledge regarding which patient population has a greater risk of perioperative complications after cannabis consumption.
1 Introduction
Cannabis, an ancient plant that was originally used as a medicinal herb but also as a recreational drug, has been used since the third millennium BC and is known to have many therapeutic effects (1). It was originally cultivated in central Asia but is now grown all over the world (1). However, until recently, the cardinal use of cannabis has been as a recreational drug for pleasurable psychological effects, mainly from delta-9-tetrahydrocannabinol (THC), the principal psychoactive component of the cannabis plant (1, 2). Nevertheless, the wide therapeutic potential of cannabis has led to much usage and research, although additional studies to determine the safety and efficacy of natural cannabis and synthetic cannabinoids are still needed. Cannabis species possess multiple functions and have been utilized as prospective relief agents for epilepsy, neurodegenerative diseases, posttraumatic stress disorder, rheumatic diseases, chemotherapy-induced nausea and vomiting (CINV), and chronic pain (3–10).
Although caffeine is the most commonly used and accessible drug among the general population, cannabis is the most frequently used of all psychoactive substances, following alcohol and tobacco (11). Cannabis use has been legalized in 24 states (including Guam, the Northern Mariana Islands, and DC). In comparison, 38 states and DCs allow the medical use of cannabis products, and 10 states in the U.S. (and the U.S. Virgin Islands) have decriminalized its use (12), which has increased the availability and diversity of cannabis-containing products. Moreover, in the last decade, the average concentration of THC cannabis has sharply increased from 2% to 3% in the 1970s to more than 20% in 2017, largely due to the growth of more potent strains (13, 14). The “shatter” or “butane hash oil”, also known as “the crack of marijuana”, hits the streets very rapidly in the U.S. It is a cannabis oil concentrate produced by butane solvent that allows the extraction of a cannabis product with a very high concentration (90%–95%) of delta-9-tetrahydrocannabinol (THC) (15).
The most well-known cannabinoids derived from the cannabis plant are delta-9-tetrahydrocannabinol (THC), which has potent psychotropic properties; cannabinol (CBN), which has weak psychoactive effects; and cannabidiol (CBD), which is known for its non-psychotropic properties (16). These chemicals have complicated interactions with endogenous receptors in the endocannabinoid system and the autonomic nervous system and with additional pathways, which can cause unwanted and dangerous physiological responses (11). Synthetic cannabinoids are 10–200 times more potent than naturally grown cannabis, and their increasing availability has correlated with an upsurge in serious adverse events, including renal toxicity, respiratory depression, hyperemesis syndrome, cardiovascular events, and effects on brain function (11, 2). The recreational use of cannabis and synthetic cannabinoids is more frequent in the young population, while medicinal cannabis is more common in the middle-aged population and older individuals (17).
Cardiovascular events are among the risks of cannabis and cannabinoid consumption, as both natural and synthetic cannabinoids induce changes in the cardiovascular system of humans and animals (18). Due to the cardiovascular effects of cannabis, surgeons, and anesthesiologists will be required to take precautionary measures when cannabis users require surgery. The most common route of consumption of cannabis for recreational usage is by inhalation (smoking and vaping), but the oral route in the form of edibles such as “grass” brownies, marijuana tea, and marijuana tincture is increasing (19). Perioperative cardiovascular events resulting from the consumption of cannabis products may differ according to the dose and time of consumption, time of last exposure, potency of the product, composition of the products, route of administration, and concomitant use of other drugs or medications.
There is little data regarding the impact of cannabis use combined with anesthetic drugs and its correlation with an increased risk of perioperative complications in patients enduring noncardiac surgery (20). Consequently, we conducted a literature review to first identify the possible mechanisms underlying the harmful cardiovascular effects of cannabinoids; second, to review the pharmacological interactions of cannabinoids with commonly prescribed drugs, as well as anesthetic agents, in patients with heart disease; and third, to highlight the perioperative cardiovascular consequences associated with cannabis and cannabinoid consumption based on the most recently published evidence.
2 Materials and methods
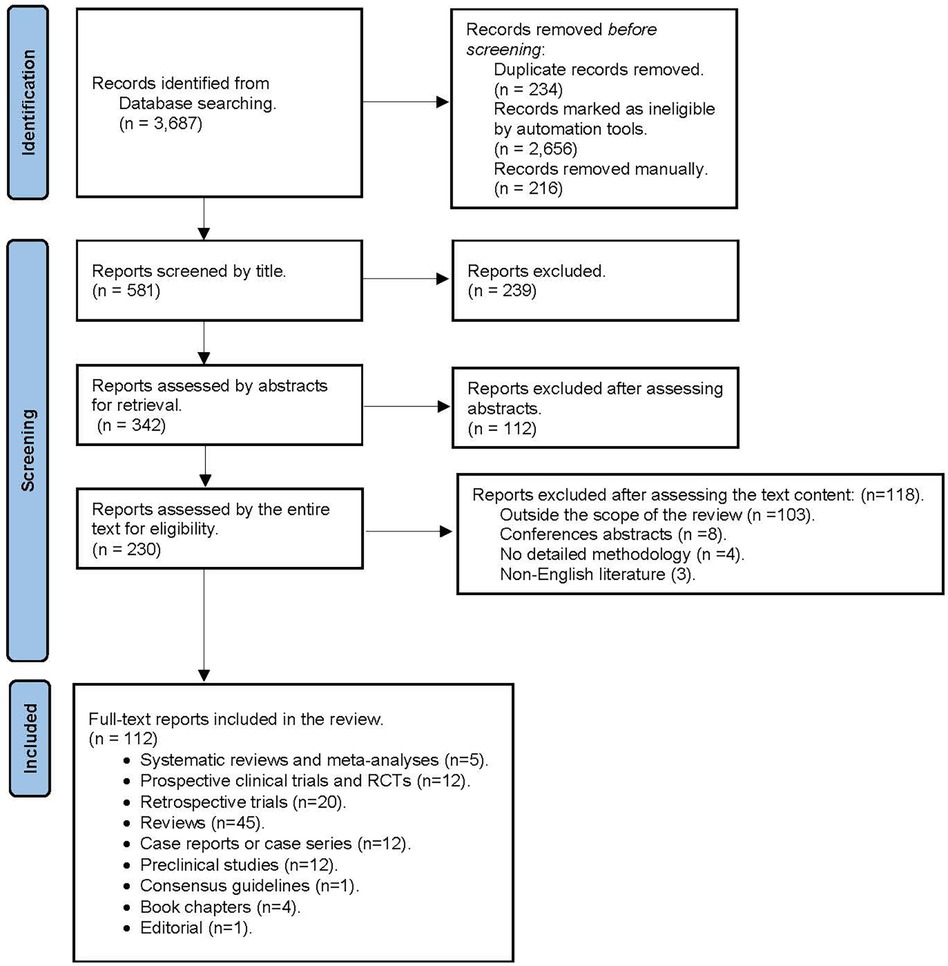
A broad search of the current literature on the perioperative cardiovascular effects of cannabis and cannabinoids was performed via PubMed, Medline, EMBASE, Google Scholar, and Web of Science to identify articles published in the English language between January 2015 and June 2022. We also searched for cardiovascular effects and perioperative cardiovascular outcomes in cannabis or cannabinoid users undergoing surgery using the following terms to search for the listed keywords: “(“cannabis”/exp OR cannabis OR “cannabinoids”/exp OR cannabinoids) AND {“perioperative cardiovascular outcomes” OR [perioperative AND (“cardiovascular”/exp OR cardiovascular) AND (“outcomes”/exp OR outcomes)]} OR“postoperative cardiovascular outcomes” OR [postoperative AND (“cardiovascular”/exp OR cardiovascular) AND (“outcomes”/exp OR outcomes)]”. Manuscripts disclosing no methodology or with no full-text availability were excluded from our narrative review (Figure 1 PRISMA diagram). The authors also searched the reference lists of the included studies.
3 Discussion
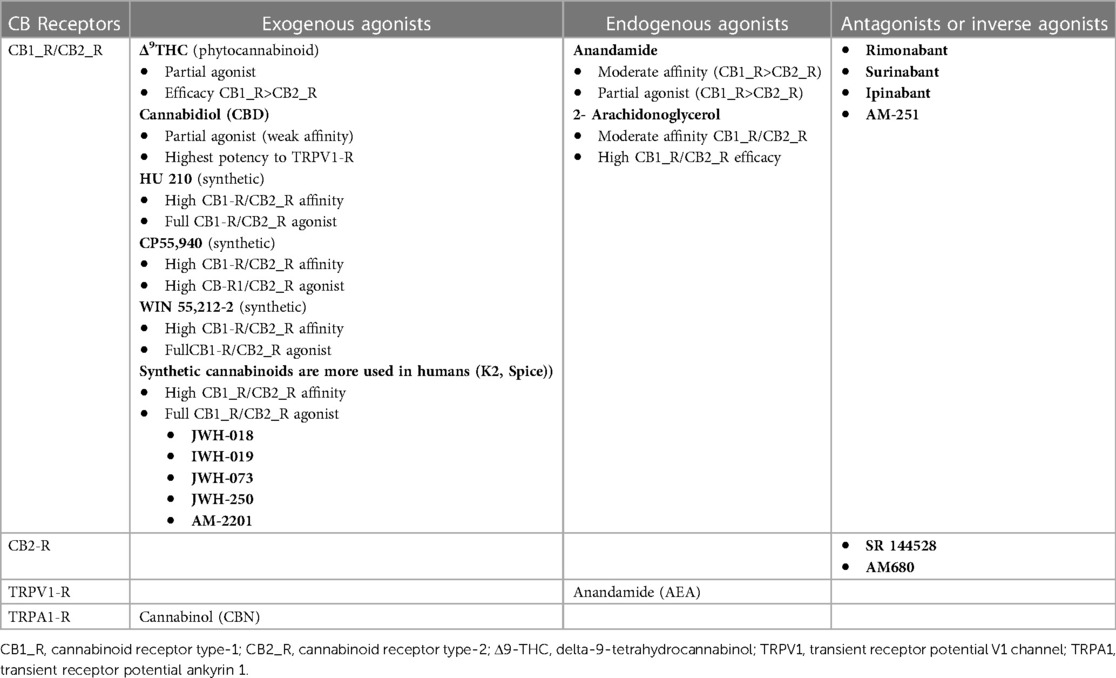
The endocannabinoid system is a complex cell signaling network composed of cell receptors that respond to endogenously produced endocannabinoid ligands and exogenous natural and synthetic agonists and the enzymes responsible for their synthesis and degradation (21). Cannabinoid receptors (CB-Rs) are G protein-coupled receptors (GPCRs) that are extensively distributed in the central and peripheral nervous systems, as well as in other organs and systems. CB1 receptors (CB1-Rs) are predominantly expressed in the brain cortex, hippocampus, dentate gyrus, striatum, amygdala, basal ganglia outflow, nucleus accumbens, thalamus, hypothalamus, periaqueductal gray, brainstem, and cerebellum (22). In the central nervous system (CNS), CB1_Rs are located primarily on neurons [e γ-aminobutyric acid (GABA)ergic, CCK-positive interneurons] and axons (presynaptic terminals), where they modulate neurotransmission (21). CB1_Rs are also found in the peripheral nerves, spinal cord, and enteric nervous system (ENS) (22). CB2_Rs are expressed mainly in leucocytes, the spleen, the thymus, tonsils, and mast cells and modulate cytokine release and the immune response of T cells (23). However, some studies in animals and human tissue have revealed the presence of CB2_Rs in glial cells and neurons, as they function as modulators of differentiation and proliferation of neuronal and nonneuronal cells (24). A list of the exogenous and endogenous ligands, agonists, and antagonists is provided in Table 1.
Unfortunately, there is a paucity of well-designed prospective randomized trials with large sample sizes to assess the safety of preoperative use of cannabis and cannabinoids more accurately. In the following sections of this review, we will present evidence of the potential cardiovascular events triggered in the perioperative period by the use of cannabis and cannabinoids.
3.1 Cardiovascular effects of cannabis and cannabinoids
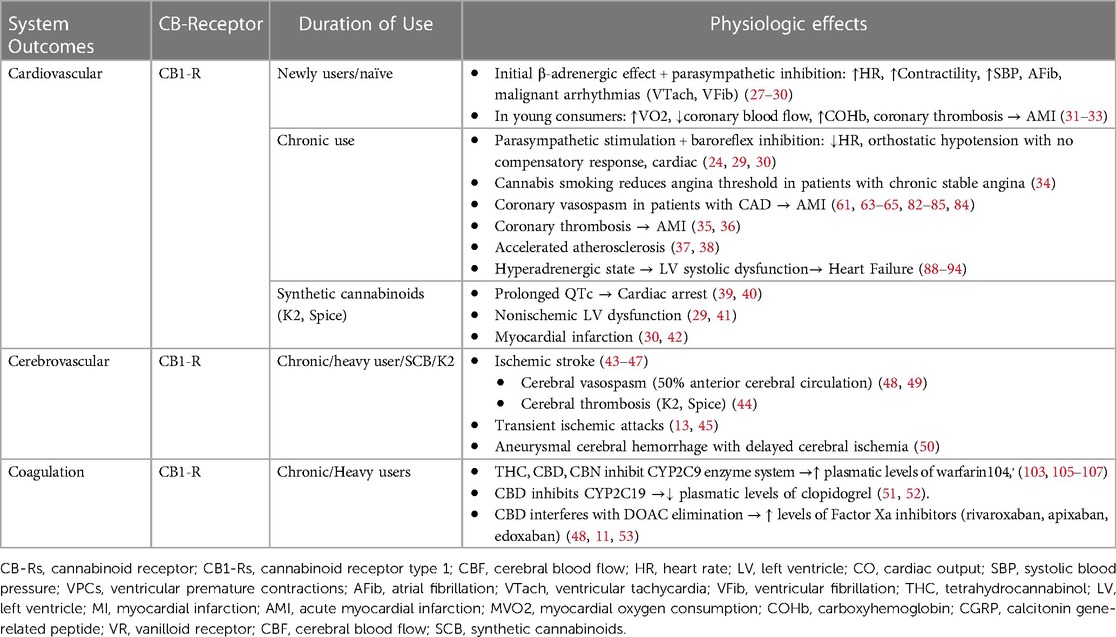
The first scientific publications on the use of cannabis suggested that the use of THC caused adverse cardiovascular events only in individuals with cardiac comorbidities and that, on the contrary, its use was safe in healthy people (25). Since then, several adverse cardiovascular events have been reported related to the recreational use of these substances, to the point that cannabis use has been included in the list of risk factors for myocardial infarction (MI) in young individuals without previous cardiac disease (26). The reported perioperative cardiovascular effects associated with the consumption of cannabis and synthetic cannabinoids are related not only to the consumed dose and route of administration of these drugs but also to their pharmacological interactions with cardiovascular medications, platelet antiaggregants, and anesthetic drugs. The most reported cardiovascular effects of cannabinoids include cardiac arrhythmias, coronary vasospasm, myocardial infarction, cerebrovascular ischemic events, stress cardiomyopathy, and sudden death (Table 2).
Below, we describe the cardiovascular effects elicited by the main cannabinoid components of the cannabis plant, as well as the most commonly used synthetic cannabinoids.
3.2 Phytocannabinoids
3.2.1 Delta-9 tetrahydrocannabinol (Δ9-THC)
Δ9-THC is a partial agonist of CB1_R and CB2_R. Its CB1_R partial agonism is responsible for its psychoactive effects due to the extensive-expression of these cannabinoid receptors in the brain (54). THC can increase the expression and activity of PPARα, improving endothelial cell function and protecting them against ischemia and high intraventricular pressure (27, 28). The most reported cardiovascular outcomes of THC in humans are related to enhanced sympathetic activity or blunted parasympathetic tone, with subsequent increases in heart rate, contractility myocardial oxygen consumption, and peripheral vasoconstriction (55, 38).
3.2.2 Cannabidiol (CBD)
The phytocannabinoid CBD does not exhibit psychoactive effects; it is a CB1_R negative allosteric modulator and CB2_R partial agonist. CBD has cardioprotective effects through its ability to modulate the balance of intracellular Ca2+ and myocyte contraction, especially under ischemic conditions or in patients with arrhythmias (56, 57). Through PPARγ activation, CBD increases the concentration of nitric oxide (NO) with subsequent systemic and pulmonary vasodilation (58). CBD blockade of the GPR55 receptor contributes to regulating myocardial homeostasis during myocardial ischemia (59, 60).
3.2.3 Cannabinol (CBN)
Cannabinol is a phytocannabinoid produced by the oxidation of THC and is found at very low levels in plants. CBN is also a partial agonist of CB1_R and CB2_R, which can produce mild psychoactive reactions in large doses (61). Preclinical studies in rats have reported a reduced heart rate in response to CBN; however, this effect has not been found in humans (62, 63). The potential cardioprotective effects of CBNs could be related to agonistic interactions with CB2_Rs, TRPV1, and TRPV2 (64, 65).
3.2.4 Cannabigerol (CBG)
It is a phytocannabinoid compound that is also a substrate for the synthesis of Δ9-THC and cannabidiol CBD. CBG shows some similarities with CBD. CBG is a partial agonist for CB1_R and CB2_R; however, it also has agonist effects on other receptors, such as TRPA1, TRPV1, TRPV2, TRPV3, TRPV4, PPARγ, and PPARα (66). CBG also has agonist effects on the α2-adrenoceptor endothelial receptor and antagonistic effects on presynaptic 5H T1A receptors, favoring peripheral vasodilation and lowering blood pressure (67, 68). A study by Willoughby et al. showed that CBG inhibits platelet aggregation (43).
3.3 Synthetic cannabinoids
Synthetic cannabinoids are full CB1_Rs and CB2_Rs agonists. They were first produced in the 1970s as a new therapeutic alternative to treat cancer pain (44). These compounds are functionally similar to Δ9-tetrahydrocannabinol (THC) but bind more than 100 times more tightly to the CB1 receptor than to THC, and have long half-lives, therefore its psychotropic effects are more prolonged. Although they are labeled as “not for human consumption”, it has been reported a progressive increase in the consumption of synthetic cannabinoids, especially in the form of “herbal smoking blends” or “herbal incense “ under trade names or brands like K2 or “Spice”, particularly in high schoolers and college students (39). Synthetic cannabinoids are generally sold blended with finely cut plants with no psychotropic effects to give the impression of being a natural product, wrapped in brightly colored metal-foil packages. The most used synthetic cannabinoids or K2 are JWH-018, AM-2201, JWH-019, JWH-073, and JWH-250. Several cardiovascular events have been reported, the most common being serious ischemic stroke of thrombotic etiology (40, 41); cardiac arrest, probably resulting from abnormalities in cardiac conduction (prolonged QTc interval) (29, 30); nonischemic left ventricular dysfunction (42, 69); and myocardial infarction (70, 71). Importantly, synthetic cannabinoids are not detected by the common cannabis urine test. Most cannabinoid receptor antagonists are synthetic cannabinoids.
3.4 Medical cannabinoids
The term medical marijuana is used to design any cannabis product regardless of processing method, dose, or route of administration. The increasing knowledge about the molecular pharmacology of the endocannabinoid system has led to an exponential increase in the use of cannabinoids as a therapeutic agent in the last 20 years, especially in the form of natural and synthetic analogs of THC, and natural CBD (72, 73).
Nevertheless, the body of evidence in the literature on serious cardiovascular outcomes among medical cannabis users with potential or established heart disease is scant and weak. Most publications refer to observational studies, case reports, and case series in patients receiving natural THC or synthetic THC analogs due to their direct cardiovascular effects as well as their interaction with some of the medications they receive for their heart condition (74–77). A recent meta-analysis conducted by Watanabe et al, which included 47 studies (2,800 patients), to assess the existing evidence on cardiovascular events associated with medical cannabinoid consumption revealed that there was an increasing risk of orthostatic hypotension (RR 3.55), hypotension (RR 3.55), and tachycardia (RR 1.94), while no serious adverse outcomes were reported. Also, they reported that the oral route of administration posed a higher risk for hypotension compared with those administered by another route (oromucosal spray, anal). Another important finding of this meta-analysis is that compounds based on THC alone tend to produce adverse cardiovascular effects (hypotension, orthostatic hypotension, syncope) than those containing a balanced combination of THC and CBD (76). Below are the cannabis products authorized for medicinal use by the FDA and the most important hemodynamic effects that have been reported.
3.4.1 Dronabinol (marinol, syndros)
Is a synthetic formulation of 9ΔTHC for the treatment of anorexia in patients with AIDS, as well as for nausea and vomiting associated with chemotherapy. Dronabinol-induced sympathomimetic activity may result in tachycardia, but subjects can also experience orthostatic hypotension and/or syncope upon abrupt standing (31, 76).
3.4.2 Nabilone (cesamet)
A synthetic analog of THC with partial agonistic actions on CB1 and CB2 receptors, is used for treating chemotherapy-associated nausea and vomiting. Nabilone has been shown to produce a dose-dependent increase in heart rate and a decrease in systolic blood pressure (31).
3.4.3 Cannabidiol (epidiolex)
A purified form of CBD, is currently used in the management of epilepsy resistant to standard treatment, such as Lennox-Gastaut syndrome and Dravet syndrome in patients 2 years of age and older, and for patients ≥1-year-old with Tuberous Sclerosis Complex (TS) (78–32). Although there are no reports of serious cardiovascular adverse events in patients medicated with Epidiolex, cannabidiol has the potential to decrease resting systolic blood pressure (33, 37).
3.4.4 Nabiximols (sativex)
A formulation of ethanol cannabis extract with a 1:1 concentration of THC/CBD, used as an oromucosal spray in the treatment of multiple sclerosis spasticity and chronic pain. It has been reported the occurrence of postural and orthostatic hypotension, especially with the initial doses (81).
A systematic review recently published by Jouanjus et al. showed strong evidence linking the use of cannabis with a high risk of cardiovascular events, particularly cerebrovascular ischemic stroke, in young males (31 years and 82% more than females) (74). A retrospective cohort study from a nationwide dataset that included 379,843 patients revealed that the rate of admission for acute myocardial infarction (AMI) in young cannabis users increased by 32%, predominantly in males (79.1%), with an increasing trend in in-hospital mortality of 60% over 4 years (82). A recent cross-sectional study of pooled data from the Behavioral Risk Factor Surveillance System showed that frequent marijuana use was associated with significantly greater probabilities of stroke (81%) and myocardial infarction or coronary artery disease (88%) (83).
Myocardial infarction (MI) and cerebrovascular events can occur in otherwise healthy individuals after heavy cannabis smoking (26, 34, 82). A cohort study conducted by Ladha et al. revealed that in 4,610 respondents (out of 33,173 young adults) who reported recent marijuana use, MI was more frequent among recent cannabis users (1.3%) than among nonusers (0.8%) (34). Likewise, there is evidence that in patients with preexisting coronary artery disease, the use of cannabis may increase the chances of MI recurrence (84). A retrospective study performed by Desai et al. using records from the National Inpatient Sample (NIS) reported a higher rate of MI admissions in cannabis users than in noncannabis users (67% vs. 41%), revealing the same trend in admissions for arrhythmias (5% vs. 2.8%), with the aggravating factor that cannabis users were younger and had a lower incidence of cardiovascular comorbidities (84).
Cannabinoids hamper nuclear receptors in hepatic microsomes, leading to changes in the activity of the P450 enzymatic system (CPY) and the UDP-glucuronosyltransferase (UGT) family of enzymes. Nasrin et al. recently demonstrated the inhibitory effect of major cannabinoids and their active metabolites on several CPY enzymes, especially CYP2B6, CYP2C9, CYP2D6, and CYP3A4, as well as on several UDP-glucuronosyltransferase families of enzymes (UGT1A6, UGT1A9, UGT2B4, and UGT2B7) (35, 85). The inhibition of these important enzymatic systems for metabolism and clearance plays a vital role in the pharmacologic interactions of cannabinoids with cardiac drugs, anticoagulants, platelet antiaggregants, hypnotics, and inhalational anesthetics.
3.5 Proposed mechanisms for cardiovascular outcomes in cannabinoid users
Next, we will examine the most important mechanisms proposed to explain the cardiovascular effects related to the use of cannabis and cannabinoids.
3.5.1 Cannabinoids and arrhythmias
In the 1970s, Beaconsfield et al. described for the first time that marijuana smoking in healthy volunteers who were non-cannabis users was associated with increased heart rate and electrocardiographic changes such as a decreased amplitude of the P wave—which relates to atrial alterations—and inversion of the T wave (36). Since then, a broad range of abnormal electrocardiographic patterns, such as atrial fibrillation, flutter, atrioventricular block, ventricular tachycardia, and Brugada phenocopy, have been reported after marijuana use (86). Tachycardia is the most relevant cardiovascular effect of THC and induces complex hemodynamic consequences, such as a decrease in stroke volume and an imbalance between myocardial oxygen supply and demand.
The mechanisms underlying arrhythmias after cannabis use are not entirely known, but researchers have proposed different theories through animal model studies (86). Thus, cannabis-induced arrhythmias might be caused by concurrent mechanisms rather than solely by sources. Next, we address the potential mechanisms involved in the development of arrhythmias as a consequence of cannabis use.
3.5.1.1 Autonomic dysregulation
Cannabinoids can cause bradyarrhythmia or tachyarrhythmia in a dose-dependent manner and determine the balance of sympathetic-parasympathetic nervous system activation. Low to moderate doses stimulate the sympathetic system and the release of norepinephrine, increasing sinus automaticity and facilitating conduction through the sinoatrial and atrioventricular nodes, favoring tachyarrhythmia. In contrast, high doses of cannabinoids stimulate the parasympathetic system and cause bradyarrhythmia (87). However, a role for CB1_R activation in the brain has recently been suggested. THC can activate inhibitory presynaptic CB1_Rs located in neurons of the autonomous nervous system (ANS) in animals and humans. In animals, THC elicits bradycardia most likely through the inhibitory action of presynaptic CB1_Rs, whereas in humans, stimulation of central CB1_Rs can offset the inhibition of presynaptic CB1-Rs in sympathetic neurons (26). The reported dissimilar cardiac effects of THC in animals and humans can be explained by the differences in parasympathetic-sympathetic tone predominance between species (88).
3.5.1.2 Dysfunction of voltage and ligand-gated ion channels
Cannabinoids interact directly with Na + - and L-type Ca2+ channels in ventricular myocytes (89). In a study with rats, the authors found that the endogenous ligand anandamide (AEA) caused a reduction in the maximal amplitudes of Na + - and Ca2+ currents and, subsequently, the amplitude of the action potential (89). Barana et al. demonstrated that endocannabinoids and cannabinoid analogs block human cardiac potassium (Kv1.5) channels, which are crucial for modulating the duration of the atrial action potential (90).
3.5.2 Cannabinoids and myocardial infection
Data have suggested that marijuana smoke triggers myocardial ischemia, which can range from temporary ischemia to ST-elevation myocardial infarction (STEMI). In addition to marijuana, other synthetic cannabinoid preparations, including MI, are associated with adverse cardiovascular effects (91, 92). Apart from the prototypical mechanisms of MI, such as intravascular thrombus formation and coronary constriction with altered coronary flow, other mechanisms, including autonomic overstimulation, toxic smoke compounds, platelet dysfunction, accelerated atherosclerosis, and increased ROS formation, have been proposed for cannabis-induced MI (38, 93).
3.5.2.1 Sympathetic stimulation
Any modality of cannabis consumption increases heart rate and blood pressure via stimulation of the sympathetic nervous system (36, 94). THC-induced tachycardia is the most relevant cardiovascular effect of cannabis compounds in humans (95). Tachycardia leads to more complex hemodynamic consequences, such as a decrease in stroke volume, which, paired with a reduction in diastolic coronary flow and high levels of carboxyhemoglobin from inhaling combustion products, causes a mismatch between myocardial oxygen supply and demand (26, 96). Several studies in humans have revealed that inhaled THC is accompanied by a rise in blood pressure that could be related to an elevated heart rate, while a study conducted by Crawford and Merrit reported moderate hypotension and tachycardia with cannabis smoking.
3.5.2.2 Myocardial endothelial dysfunction
The activation of CB1-Rs expressed on human endothelial cells has a pro-inflammatory effect, causing oxidative stress through the accumulation of intracellular ROS and the stimulation of mitogen-activated protein kinase (MAPK), ultimately leading to endothelial cell injury and the migration of vascular muscle smooth cells, which stimulates endothelial cell-mediated coronary vasoconstriction and elicits a vasodilatory response (38, 86, 97).
However, activation of CB2_R has the opposite effect, inhibiting endothelial cell adhesion and vascular smooth muscle cell migration (98). Aronow and Cassidy performed a study to evaluate the effect of marijuana on cardiovascular function and exercise-induced angina in 10 men with underlying severe coronary disease, none of whom had previously consumed marijuana. The participants reported a decreased exercise duration (48%) until the onset of angina pectoris after smoking one marijuana cigarette compared to after smoking one placebo cigarette (8.6%) (96). Another study in animal models showed that even only one minute of secondhand marijuana smoke exposure could impair vascular endothelial function; rat blood vessels took at least 90 min to recover function, whereas when breathing secondhand tobacco smoke, their vascular function recovered within 30 min (99). Angiographic studies in patients presenting with ischemic angina or MI after cannabis consumption have corroborated the findings of preclinical studies showing prolonged impairment in arterial flow-mediated dilation (FMD) after short marijuana exposure, correlating the state of low coronary flow with the appearance of ventricular tachycardia (44, 99). In contrast to those of THC, the results of a preclinical study in a rat model showed that CBD has significant cardioprotective effects by reducing the postmyocardial infarction inflammatory response and infarct size (100). Cannabidiol-mediated cardioprotection is also related to its ability to modulate the enzymatic activity of glutathione reductase and glutathione peroxidase, which inhibit the production of ROS, thus protecting myocardial endothelial cells from peroxide-related damage (101). Other in vitro, ex vivo, and in vitro studies have demonstrated the anti-remodeling, ventricular function restoring, and vasorelaxant effects of CBD (33, 102–105).
3.5.2.3 Platelet dysfunction and potential for bleeding
Several authors have described the association between cannabinoids and coronary thrombosis as a potential cause of myocardial infarction even in patients without previous cardiac comorbidities (106). CB1 and CB2 receptors are present on the platelet surface, and THC exposure can cause changes in membrane phospholipids and increase the surface expression of glycoprotein IIb/IIIa and P selectin, which might contribute to a receptor-dependent pathway of THC-induced platelet activation (107). Conversely, in an animal study, De Angelis et al. reported that the endocannabinoid AEA decreases platelet aggregation by reducing α-granule secretion and decreasing glycoprotein IIB/Ia activation (108). One study in which rhesus macaques consumed daily THC edibles also showed a significant reduction in platelet aggregation (109). Despite contradictory evidence regarding the pro- or anticoagulation effects of cannabinoids, there is evidence supporting the modulatory effect of cannabinoids on platelet function and the coagulation system, and multiple factors, such as dosing, route, frequency, and individual sensitivity, might play a role (110). On the other hand, several authors have demonstrated that the endocannabinoid ligands AEA and 2-AG can induce platelet activation and promote aggregation in human platelets (111–113).
3.5.2.4 Vasospasm and altered coronary flow
Case reports of patients with myocardial infarction after marijuana use and a normal angiogram without evidence of underlying atherosclerosis suggest altered coronary flow due to vasospasm as the possible cause of myocardial infarction (114–116). This theory could be supported by the findings of one study in rats, which showed that intra-arterial administration of THC produced increased perfusion pressure, suggesting vasoconstriction (117). Furthermore, cannabis use is believed to cause a mismatch of blood flow supply and demand in the myocardium, but its role in the development of ischemic cardiomyopathy requires additional study (51, 55).
3.5.2.5 Accelerated coronary atherogenesis
CBR1 receptors found within the vascular endothelium are linked to atherogenesis. ROS plays a critical role in atherosclerosis by causing LDL oxidation, apoptosis, and plaque rupture (100). Human studies have shown that CB1_Rs are expressed on macrophages within coronary atheroma (118). The current evidence from preclinical studies is insufficient to conclude that platelet aggregation promoted by THC and synthetic cannabinoids is a leading cause of intracoronary thrombus formation and MI. However, atheromatous plaque formation and rupture secondary to CB1_R activation could be predisposing factors for thrombus formation.
3.5.3 Cannabis and heart failure
Although an association has been identified between marijuana consumption and the onset or worsening of heart failure (HF), additional research studies are needed. Kalla et al. demonstrated via retrospective analysis of a national inpatient database that marijuana usage was an independent predictor of heart failure (52). Although the incidence of hypertension appears to be much greater in marijuana users than in nonusers, the direct effect of marijuana on hypertensive heart failure has not been well established (52).
The inconsistency observed in the data linking marijuana to the development of heart failure may be due to the diverse cardiovascular effects of cannabinoids. As mentioned above, THC increases ROS production, while cannabidiol inhibits ROS production (119). Therefore, the contribution of cannabis products to the development and progression of heart failure is related to ROS production inside the myocardium.
Cannabis has been implicated in left ventricular systolic dysfunction and myocardial stunning, which are the hallmarks of stress cardiomyopathy and Takotsubo cardiomyopathy, respectively (120). Stress cardiomyopathy is a clinical syndrome characterized by acute left ventricular (LV) systolic and diastolic dysfunction secondary to a hyperadrenergic state (48). The proposed deleterious remodeling of the heart muscle in response to a cannabis-induced catecholamine surge is believed to be mediated by CB1-Rs (11, 121). Through CB1_R activation, cannabinoids have been shown to decrease cardiac contractility, possibly leading to heart failure (53–123). Although several mechanisms are implicated in cannabis-induced heart failure, clinical evidence is still inconsistent, and additional studies are needed.
3.6 Cannabis interaction with anticoagulants and antiplatelet agents
Anticoagulant and antiplatelet medications are commonly prescribed to high-risk patients with cardiac conditions as prophylaxis for myocardial infarction or stroke (124). Cannabis can be used for recreational or medicinal purposes in combination with commonly used anticoagulants and antiplatelet agents (125). These drug-drug interactions can lead to decreased efficacy of the medications and increase the risk of recurrent adverse cardiovascular and cerebrovascular events (126). This interplay is mediated by the inhibition of several CYP450 enzymes by cannabinoids (125, 126). One of the most studied relationships is between cannabis and warfarin, which can be separated into two stereoisomers, S-warfarin and R-warfarin (127–131). CYP2C9, CYP2C19, and CPY3A are the enzymes responsible for the breakdown of S-warfarin, while R-warfarin is metabolized by CYP1A2 and CYP3A (46); all these enzymes are moderately inhibited by the three major cannabinoids (THC, CBD, and CBN) (49, 127, 130, 131). CBD is a potent inhibitor of all the CYP enzymes involved in the metabolism of warfarin and can impact the degradation of warfarin and lead to higher plasmatic concentrations than needed (131, 132). R-warfarin, the least potent stereoisomer, is metabolized mainly by CYP3A4 (127, 132). This enzyme is involved in the metabolism of THC and CBD, as shown in clinical trial interaction studies of an oromucosal spray with Cannabis sativa extract (Sativex® U.K. SPC 7/2012). Several case studies have reported an increased international normalized ratio (INR) in cannabis users, which can place patients at risk for epistaxis, bruising, or intracranial hemorrhage (45, 49, 127, 128, 130). Clopidogrel is a popular antiplatelet agent that is used for the treatment of acute coronary syndrome, the prevention of heart attack, and cerebrovascular accidents (47, 133). The metabolism of clopidogrel by the isoenzyme CYP2C19 is inhibited by CBD, which could lead to lower levels of the active metabolite and increase the risk for transient ischemic attacks (47, 131, 134). There is no evidence of interactions between cannabis products and heparin or fondaparinux because they are cleared by the kidneys and have no apparent interactions with CYP enzymes (126, 131, 134).
Direct oral anticoagulants (DOACs) include Factor Xa inhibitors (rivaroxaban, apixaban, and edoxaban) and thrombin inhibitors (dabigatran) (135). DOACs have the potential to replace warfarin as the anticoagulant of choice due to their effectiveness and safety profile (136). DOACs act as substrates of the P-glycoprotein efflux system, which expels substances from cells to favor their metabolization (137). Several researchers have shown that prolonged exposure to cannabis can reduce the expression of P-pg and that CBD may be responsible for these effects (50, 137). Since P-glycoproteins are involved in the absorption of DOACs, the inhibitory effect of CBD on P-glycoproteins interferes with DOAC elimination and increases the plasma concentrations of the drug, increasing the risk of bleeding (50, 131). Additionally, other interaction mechanisms, such as rivaroxaban and apixaban, which are metabolized by the CYP450 enzyme system, similar to CBD, have been proposed for some DOACs. Therefore, the presence of CBD may alter the metabolism of these anticoagulants (138).
The anticoagulant effects of cannabis, as well as interactions with other drugs, are still being studied. In a study on rat models, THC and CBN inhibited thrombin activity and displayed anticoagulant activity (139). More clinical studies on the pharmacological interactions of cannabis in relevant patient populations are needed to understand the relative risk when using anticoagulants and antiplatelet medications (134).
3.7 Cerebrovascular outcomes
3.7.1 Acute cerebral ischemia
The most common adverse cerebrovascular outcomes associated with cannabis and synthetic cannabinoids are acute cerebral ischemia and transient ischemic attacks, particularly in young individuals. (18–47 years) (140–142) Most case series and case reports show that more than 50% of ischemic strokes involve the anterior circulation, but they can also occur in the posterior circulation (143, 144). A case series showed that in 81% of ischemic stroke patients, cannabis consumption was related to the duration of the event; moreover, 22% of patients experienced recurrent cerebral ischemia after re-exposure to cannabis (143). A longitudinal study by Hemachandra et al. reported a 4.7-fold greater risk of cerebral ischemia in chronic cannabis users than in healthy individuals (145). Recently, Parekh et al. conducted a retrospective cohort study with data from the Behavioral Risk Factor Surveillance System Survey Analysis (2016–2017) and reported that recent cannabis use in young subjects (18–44 years of age) was associated with a 1.82 odds ratio (OR) of ischemic stroke compared with that in non-cannabis users; however, for heavy and frequent users, the risk of stroke increased 2.45-fold, suggesting a dose-effect relationship (146).
Multiple mechanisms have been proposed to explain the occurrence of cannabis-induced cerebral ischemia. Two prospective studies showed that young individuals presented with ischemic stroke and positive cannabis urine test results. angiographic studies revealed multiple intracranial vascular spasms, which reverted in some patients with total suppression of cannabis use (140, 147). Early preclinical studies in rats have shown a vasoconstrictor effect of THC (148). Moreover, Herning et al. conducted a prospective cohort study in which cerebral blood flow velocity was measured via transcranial Doppler (TCD) in 46 cannabis users (light, moderate, and heavy) and controls (noncannabis users) within three days of admission and up to 30 days of monitored abstinence. Their findings showed that cannabis users had significantly greater indexes of cerebrovascular resistance (>in heavy users) than did control subjects, which persisted after a month of monitored abstinence, suggesting a long-lasting cerebral vasoconstrictor effect of cannabis (149). The authors suggested that changes in cerebrovascular resistance with sustained cannabis use might be explained by the increased density of CB1-Rs in the brain vessels. Other studies in rat models have demonstrated that THC provokes dose-dependent impairment of mitochondrial function with the subsequent production of ROS and hydrogen peroxide, which leads to endothelial dysfunction in the cerebral vasculature (141). Other contributing factors are myocardial infarction and atrial fibrillation. Filbey et al. conducted a prospective study of 74 abstinent cannabis users for more than 72 h and 101 nonusers, aiming to compare intergroup differences in several neurophysiologic indicators, such as global and regional resting cerebral blood flow (CBF), oxygen extraction, and cerebral metabolic rate of oxygen (CMRO2). They reported that cannabis users had higher oxygen extraction and CMRO2 values as well as greater global and resting CBF values that correlated with THC levels. These findings demonstrated the residual effects of prolonged cannabis use (150).
3.7.2 Cerebral hemorrhage
There is expanding evidence linking prolonged cannabis exposure to aneurysmal subarachnoid hemorrhage (aSAH) (151) and delayed ischemic stroke (152, 153); however, the pathophysiology of this association remains unclear. Nonetheless, some of the aforementioned neurophysiological events may contribute to multifocal intracranial stenosis, increased oxidative stress, and cerebral mitochondrial dysfunction. Recent retrospective cohort studies from secondary databases have shown a significantly greater incidence of hemorrhagic stroke in cannabis users than in nonusers (154, 155). In a retrospective study of consecutive patients admitted with aneurysmal subarachnoid hemorrhage, the neurologic outcomes were compared between patients with cannabis-positive urine test results and patients with negative drug screening results. Delayed cerebral ischemia was diagnosed in 50% of patients with CB-positive urine tests and 23.8% of those with negative drug screens (P = 0.01), and the incidence of poor neurologic outcome was greater in cannabis users (35.7% vs. 13.8%; P = 0.01) (151).
3.8 Pharmacological interactions of cannabinoids with anesthetic drugs
Pharmacological interactions of cannabis and cannabinoids with anesthetic drugs can lead to perioperative cardiovascular complications, although there is a paucity of clinical research studies showing robust evidence regarding the mechanisms involved in these interactions in humans. Most of the current knowledge on these interactions comes from preclinical studies in animal models (156–165), small clinical trials, and case reports (166–171). Interestingly, most of the described effects of cannabinoids and anesthetic agents, intravenous hypnotics, and opiates on animal models are the opposite of what has been described in case reports and clinical studies.
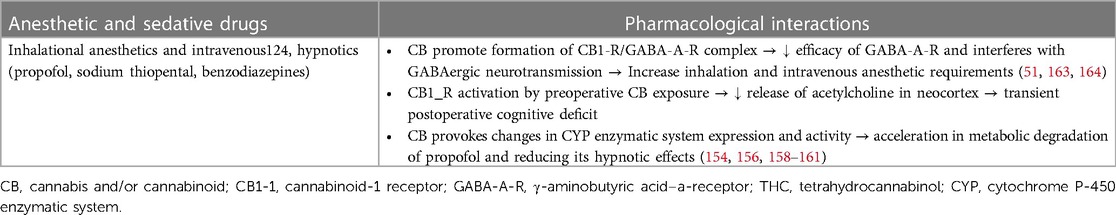
In clinical practice, the most prevalent outcome of these interactions is an increase in the need for inhalation and GABAergic intravenous anesthetics (propofol, thiopental, and benzodiazepines), manifested by resistance to achieve and maintain an adequate level of clinical anesthesia (monitored by the EEG bispectral index) (168, 170, 172–175). Cannabinoids interact with several receptors that are intimately involved in the mechanisms of general anesthesia, such as γ-amino-butyric acid (GABA), N-methyl-D-aspartate (NMDA), and vanilloid receptor 1 (TRPV1) (176). The interaction of THC, other cannabinoid compounds, and endocannabinoid ligands with GABA-A receptors leads to the formation of a CB1/GABA-A complex, impeding neurotransmission in GABAergic neurons and reducing the efficacy of the GABA-A receptor (126, 177, 178). Consequently, this interaction reduces the efficacy of GABAergic anesthetics (inhalation anesthetics and intravenous hypnotics). Additionally, upregulation of the hepatic cytochrome microsomal P-450 (CYP) enzymatic system occurs in heavy cannabis users, causing an increase in the expression and activity of the CYP system, which subsequently accelerates the metabolic degradation of propofol (179). These two pharmacological interactions affect the pharmacodynamic effects of inhalation and intravenous anesthetics, causing an increase in the required doses of these drugs to achieve sufficient drug levels in plasma and at the effector site (brain) to ensure adequate anesthesia depth (166, 169, 174, 180, 181) (Table 3).
3.9 Preoperative considerations of Cannabis users
Most of the evidence related to perioperative cardiovascular complications in cannabis users comes from case reports and small studies showing a low incidence of serious adverse events (181, 182). Nonetheless, recent evidence from retrospective studies from large national healthcare databases contradicts the findings of previous studies and highlights the importance of obtaining accurate information about cannabis use during preoperative assessments and discussing the potential risks of perioperative complications (183–187). In addition to cardiovascular complications, potential airway, and respiratory adverse outcomes can occur perioperatively in heavy users, such as upper airway obstruction by rhinopharyngitis and uveal edema, hyperreactive airways, high levels of carboxyhemoglobin, necrotizing alveolitis (188, 189).
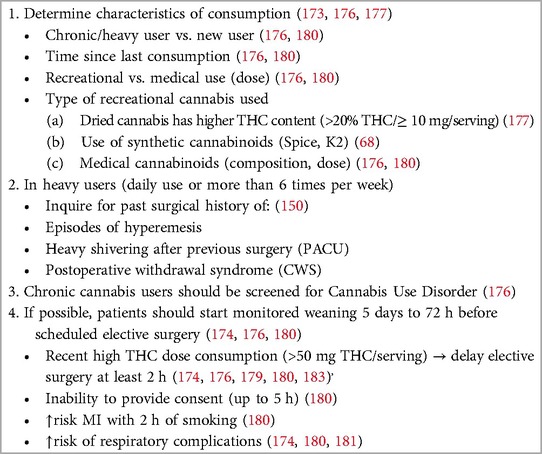
During the preanesthetic evaluation, it is essential to determine whether the patient was a recent or chronic user if cannabis was used for medical or recreational purposes if the dose was most recently consumed if the time elapsed since the most recent exposure if the route or method of administration was used, if the type of cannabis or cannabinoid used was used, or if the THC: CBD ratio was used; if possible (190, 191) (Table 4). If the patient reports recreational use, the anesthesiologists should inquire about the use of the dried product, which usually contains a very heterogeneous mixture of its components, especially THC, whose concentration has increased progressively over time (192). It is also important to obtain information about the use of synthetic cannabinoids such as “K2” or “spice”. If the patient is taking medical formulations, it is imperative to know which product and dosage are in use; however, some cannabis products for medical use are marketed in an unregulated manner and may be labeled inaccurately (193). The combination of cannabis with other drugs, such as amphetamines and paroxetine, is becoming common, but usually, its presence may not be known to the user. A past medical history of hyperemesis episodes or severe “shivering” during a previous surgery should alert patients to the chronic use of cannabis (188).
Recently, a consensus of a panel of experts from the Perioperative Pain and Addiction Interdisciplinary Network (PAIN) (194), as well as the ASRA Guidelines for Perioperative Management of Cannabinoid Users (190), made several recommendations related to the perioperative management of cannabis and cannabinoid users. Current evidence remains uncertain regarding the weaning period of recreational cannabis consumption, particularly in heavy users. Early studies on cannabis users undergoing general anesthesia and recent reviews suggested avoiding anesthesia in any patient who has used cannabis in the last 72 h (188, 189, 195, 196). ASRA guidelines recommend that “all surgical and procedural patients requiring anesthesia should be screened for cannabinoids preoperatively”, and patients who are referred for daily use of cannabis should be screened for cannabinoid use disorder (190, 194). If cannabis use is confirmed, patients must be evaluated clinically for acute intoxication. Drug testing for cannabis especially in urine, saliva, and hair detects evidence of use, not current intoxication or addiction, while blood samples allow to measure quantitative levels of cannabinoids (190, 197). Anesthesiologists and perioperative physicians should discourage the preoperative use of cannabis, except for medical indications. Recent or chronic heavy cannabis consumption poses a high risk of MI within two hours of smoking but also impairs cognitive functions and performance, including memory, and compromises the ability to provide informed consent for up to 5 h (198). ASRA guidelines recommend postponing elective surgery for at least 2 h for patients who exhibit symptoms of acute cannabis intoxication (190). According to the panel of experts, the consumption of 1.5 g/day of smoked cannabis, 300 mg/day of CBD oil, or 20 mg/day of THC oil should be considered significant. If assessed more than 7 days before surgery, monitored weaning should be considered; however, cannabis should not be stopped abruptly if the elapsed time for surgery is more than 24 h to avoid postoperative cannabis withdrawal syndrome (CWS) (190, 194).
3.10 Potential intraoperative cardiovascular adverse events in Cannabis users
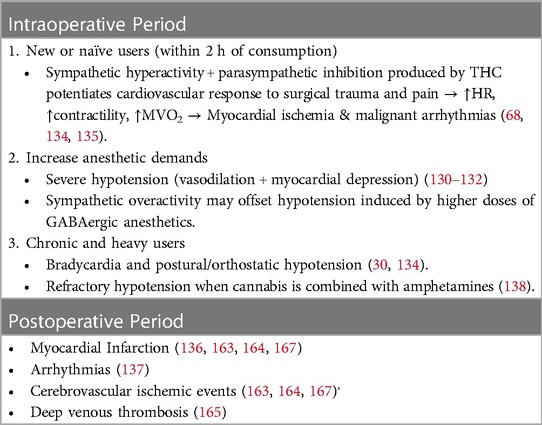
An increase in the dose of anesthetics can lead to severe hypotension due to vasodilation and myocardial depression produced by agents such as propofol, isoflurane, sevoflurane, and desflurane (199–201).
New or naïve users are prone to tachycardia, systolic hypertension, and malignant arrhythmias (AFib, VFib, VTach, and Brugada pattern) within 2 h of consumption, depending on the dose administered due to sympathetic activation and parasympathetic inhibition (87, 202, 203). This sympathetic hyperactivity can potentiate the cardiovascular response to surgical stress, enhancing the increase in heart rate and contractility that some anesthetics, such as ketamine and other cardioactive drugs used intraoperatively (atropine, epinephrine), normally produce and, consequently, myocardial oxygen demand (95, 204). On the other hand, this sympathetic overactivity also counteracts the vasodilation and cardiac depression provoked by higher doses of GABAergic anesthetics.
Although the increase in heart rate following marijuana consumption ranges between 20% and 100% from baseline and reaches its maximum at 10–30 min after smoking, its high liposolubility may prolong tachycardia for up to 72 h (55). This initial response to inhaled cannabis could be followed by an increased parasympathetic tone characterized by bradycardia and hypotension (94, 202). The combination of high myocardial oxygen demands, coronary spasms, and elevated carboxyhemoglobin levels places patients at high risk for intraoperative myocardial infarction or malignant arrhythmias (Table 5) (205).
Conversely, chronic use of cannabis can produce bradycardia and postural and orthostatic hypotension; therefore, precautions should be taken with the use of neuraxial anesthesia, although no evidence of this interaction has been published to date (194). A recently reported case described an incident in which the combination of marijuana with amphetamines and paroxetine caused severe hypotension refractory to treatment with vasopressors such as phenylephrine shortly after the start of sevoflurane anesthesia (206).
3.11 Postoperative cardiovascular outcomes in cannabis users
Until recently, the reported incidence of postoperative cardiovascular events in cannabis consumers was considered very low or similar to that in noncannabis users; however, most of these data came from case reports and small-size prospective and retrospective studies (181, 182). In this subsection, we present findings from recent retrospective cohort-matched studies comparing the incidence of postoperative cardiovascular complications, such as myocardial infarction, arrhythmias, and ischemic cerebrovascular disease, in patients who used cannabis preoperatively with those who did not use cannabis (Table 5) (184, 185, 207–211).
A retrospective cohort study conducted by Goel et al. from a gross sample of 4,186,622 patients who underwent major elective surgery yielded a final cohort of 27,206 patients, with 13,603 patients in each group. Their results showed that chronic cannabis consumption was associated with an increased incidence of postoperative myocardial infarction [adjusted OR 1.88 (95% CI, 1.31–2.69; P < 0.001)] (P < 0.001]) (184). Chiu et al. conducted a study on 2,393 cannabis-using patients from a total of 423,978 patients who underwent elective spine surgery. Cannabis use was associated with a greater frequency of thromboembolic events (OR: 2.2; 95% CI 1.2–4.0; P = 0.005) and ischemic stroke (OR 2.9; 95% CI 1.2–7.5; P = 0.007) and an increased length of hospitalization compared to the control group (211). Zhang et al. studied 524 cannabis users who were undergoing surgery and compared them with a matched-pair cohort of 1,152 patients who were non-Cannabis users as controls. They found that cannabis users had a greater rate of arrhythmias than non-cannabis users (2.7% vs. 1.6%) (185). In another study, 3,842 cannabis users were identified from a total sample of 23,030 patients who underwent THA and were matched with 19,188 individuals in the control group. Cannabis users had greater postoperative cardiovascular complications, such as MI and CVA (23.0 vs. 9.8%, OR 1.6; p < 0.0001), and longer hospital stays than did the control group (208). A recent propensity score-matched cohort study of patients who underwent lumbar fusion from the secondary database (PearlDiver) reported that CBU patients had more myocardial infarction (P < .0001) and deep venous thrombosis (P < 0.0001) than did the control cohort (209). McGuinness et al., from a propensity score matching cohort of 4,684 patients with CUD from a total sample of 510,000 vascular surgery patients, reported that cannabis users had a greater incidence of perioperative MI (3.3% vs. 2.1%; P = .016) and perioperative stroke (5.5% vs. 3.5%; P = .0013) than patients without CUD (207).
4 Conclusion
The results of our search in the most recent publications on cardiovascular complications associated with the consumption of natural and synthetic cannabis show that its use may have important implications for the perioperative care of patients who are cannabis users and who require surgery under general anesthesia or sedation. Cannabis users, whether for recreational or medical purposes, may represent a challenge for the surgical team (anesthesiologists, surgeons, and hospitalists). In addition to the increase in THC concentration, the consumption of THC-containing products continues to increase, most noticeably among adolescents and young adults. The chronic and recent use of cannabis triggers a myriad of cardiovascular reactions that range from tachycardia, bradycardia, hypertension, and hypotension to malignant arrhythmias, myocardial infarction, and ischemic stroke throughout the entire perioperative period. However, the severity of the cardiovascular effects of cannabis may differ according to the duration of consumption and dose. Cannabis consumption also places patients at risk of pharmacological interactions with anesthetic agents, resulting in increased requirements for these drugs to maintain adequate levels of anesthesia. Cannabis also interacts with anticoagulants and antiplatelet drugs, modifying their efficacy.
This review highlights the potential for perioperative cardiovascular complications in surgical patients who consume cannabinoids. We acknowledge several weaknesses in this review due to several factors like (a) the literature relevant to this topic is of low quality as scientific evidence, especially because it is made up mostly of prospective and retrospective studies of small sample size, reports, or case series, which they generally do not clearly describe whether the adverse cardiovascular reaction is caused by the use of cannabis alone, without the influence of other drugs for recreational or medical purposes; (b) except for medical cannabis users, most patients with cannabis use disorder also consume other drugs that contribute to the appearance of adverse reactions mainly in the cardiovascular, respiratory, and nervous systems, therefore, knowing that the patient is an active, recent or chronic consumer, must alert the surgical team of the potential events that may occur acutely during the perioperative period. On the other hand, despite the legalization of the use of cannabis in 24 states in the United States and in other countries, the frequency with which the user manifests itself as such remains very low as shown by most studies (4%–5%), including recent epidemiological studies using repositories that contain data from millions of patients such as INS, Medicare/Medicaid, MarketScan, Cosmos-Epic, etc.
Despite the continued increase in the use of cannabis, both recreationally and medically, there is still high underreporting in our medical system of cannabinoid-consuming patients since the percentage of patients who report being consumers is still very low. Therefore, there is a need for additional large-size, prospective, and observational cohort clinical trials to characterize the perioperative and long-term consequences of cannabis use and the impact of preexisting comorbidities more accurately. The results of these studies will provide a wider knowledge to anesthesiologists and surgeons to more clearly identify the subset of patients most likely to experience severe postoperative adverse events.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ME-V: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. YG: Formal Analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. JM: Investigation, Validation, Writing – original draft, Writing – review & editing, Formal Analysis. DR: Investigation, Validation, Writing – original draft, Writing – review & editing. JC: Investigation, Validation, Writing – original draft, Writing – review & editing. MB: Investigation, Validation, Writing – original draft, Writing – review & editing. LP: Investigation, Validation, Writing – original draft, Writing – review & editing. AU: Formal Analysis, Project administration, Resources, Supervision, Validation, Conceptualization, Writing – original draft, Writing – review & editing. TW: Conceptualization, Formal Analysis, Resources, Supervision, Validation, Methodology, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, or publication of this article.
Acknowledgments
The authors gratefully acknowledge Ms. McKenna Carr, BS, for their editing collaboration (Ms. Carr provided authorization to be named on this publication).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pisanti S, Bifulco M. Medical cannabis: a premillennial history of an evergreen. J Cell Physiol. (2019) 234(6):8342–51. doi: 10.1002/jcp.27725
2. Bukke VN, Archana M, Villani R, Serviddio G, Cassano T. Pharmacological and toxicological effects of phytocannabinoids and recreational synthetic cannabinoids: increasing risk of public health. Pharmaceuticals (Basel, Switzerland). (2021) 14(10):965. doi: 10.3390/ph14100965
3. Zaheer S, Kumar D, Khan MT, Giyanwani PR, Kiran F. Epilepsy and cannabis: a literature review. Cureus. (2018) 10(9):e3278. doi: 10.7759/cureus.3278
4. Zafar R, Schlag A, Phillips L, Nutt DJ. Medical cannabis for severe treatmentresistant epilepsy in children: a case-series of 10 patients. BMJ Paediatr Open. (2021) 5(1):e001234. doi: 10.1136/bmjpo-2021-001234
5. Bhunia S, Kolishetti N, Arias AY, Vashist A, Nair M. Cannabidiol for neurodegenerative disorders: a comprehensive review. Front Pharmacol. (2022) 13:989717. doi: 10.3389/fphar.2022.989717
6. Abizaid A, Merali Z, Anisman H. Cannabis: a potential efficacious intervention for PTSD or simply snake oil? J Psychiatry Neurosci. (2019) 44(2):75–8. doi: 10.1503/jpn.190021
7. Gonen T, Amital H. Cannabis and cannabinoids in the treatment of rheumatic diseases. Rambam Maimonides Med J. (2020) 11(1):e0007. doi: 10.5041/RMMJ.10389
8. Bains S, Mukhdomi T. Medicinal Cannabis for Treatment of Chronic Pain. Treasure Island, FL: StatPearls Publishing (2024).
9. Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S. Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev. (2015) 2015(11):CD009464. doi: 10.1002/14651858.CD009464.pub2
10. Wallace RB. The 2017 cannabis report of the national academy of medicine: a summary of findings and directions for research addressing cannabis use among older persons. Pub Pol Aging Rep. (2019) 29(3):85–7. doi: 10.1093/ppar/prz016
11. Singh A, Saluja S, Kumar A, Agrawal S, Thind M, Nanda S, et al. Cardiovascular complications of marijuana and related substances: a review. Cardiol Ther. (2018) 7(1):45–59. doi: 10.1007/s40119-017-0102-x
12. Lancione S, Wade K, Windle S, Filion K, Thombs B, Eisenberg M. Non-medical cannabis in North America: an overview of regulatory approaches. Public Health. (2020) 178:7–14. doi: 10.1016/j.puhe.2019.08.018
13. Smart R, Caulkins JP, Kilmer B, Davenport S, Midgette G. Variation in cannabis potency and prices in a newly legal market: evidence from 30 million cannabis sales in Washington State. Addiction. (2017) 112(12):2167–77. doi: 10.1111/add.13886
14. Chandra S, Radwan MM, Majumdar CG, Church JC, Freeman TP, ElSohly MA. New trends in cannabis potency in the USA and Europe during the last decade (2008–2017). Eur Arch Psychiatry Clin Neurosci. (2019) 269:5–15. doi: 10.1007/s00406-019-00983-5
15. Bidwell LC, Martin-Willett R, Karoly HC. Advancing the science on cannabis concentrates and behavioural health. Drug Alcohol Rev. (2021) 40(6):900–13. doi: 10.1111/dar.13281
16. Kicman A, Toczek M. The effects of cannabidiol, a non-intoxicating compound of cannabis, on the cardiovascular system in health and disease. Int J Mol Sci. (2020) 21(18):6740. doi: 10.3390/ijms21186740
17. Abuse S. Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health. (2020).
18. Adamson M, Di Giovanni B, Delgado DH. The positive and negative cardiovascular effects of cannabis. Expert Rev Cardiovasc Ther. (2020) 18(12):905–17. doi: 10.1080/14779072.2020.1837625
19. Russell C, Rueda S, Room R, Tyndall M, Fischer B. Routes of administration for cannabis use–basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy. (2018) 52:87–96. doi: 10.1016/j.drugpo.2017.11.008
20. Bakshi C, Barrett AM. Impact of recreational and medicinal marijuana on surgical patients: a review. Am J Surg. (2019) 217(4):783–6. doi: 10.1016/j.amjsurg.2018.10.053
21. Svíženská I, Dubový P, Šulcová A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—a short review. Pharmacol Biochem Behav. (2008) 90(4):501–11. doi: 10.1016/j.pbb.2008.05.010
22. Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Cannabinoids. (2005) 168:299–325. doi: 10.1007/3-540-26573-2_10
23. Pertwee RG, Howlett A, Abood ME, Alexander S, Di Marzo V, Elphick M, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. (2010) 62(4):588–631. doi: 10.1124/pr.110.003004
24. Fernandez-Ruiz J, Romero J, Velasco G, Tolon RM, Ramos JA, Guzman M. Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci. (2007) 28(1):39–45. doi: 10.1016/j.tips.2006.11.001
26. Weresa J, Pędzińska-Betiuk A, Mińczuk K, Malinowska B, Schlicker E. Why do marijuana and synthetic cannabimimetics induce acute myocardial infarction in healthy young people? Cells. (2022) 11(7):1142. doi: 10.3390/cells11071142
27. Tabernero A, Schoonjans K, Jesel L, Carpusca I, Auwerx J, Andriantsitohaina R. Activation of the peroxisome proliferator-activated receptor α protects against myocardial ischaemic injury and improves endothelial vasodilatation. BMC Pharmacol. (2002) 2:1–10. doi: 10.1186/1471-2210-2-10
28. Brigadeau F, Gelé P, Wibaux M, Marquié C, Martin-Nizard F, Torpier G, et al. The PPARα activator fenofibrate slows down the progression of the left ventricular dysfunction in porcine tachycardia-induced cardiomyopathy. J Cardiovasc Pharmacol. (2007) 49(6):408–15. doi: 10.1097/FJC.0b013e3180544540
29. Ahmed T, Khan A, See VY, Robinson S. Cardiac arrest associated with synthetic cannabinoid use and acquired prolonged QTc interval: a case report and review of the literature. HeartRhythm Case Rep. (2020) 6(5):283–6. doi: 10.1016/j.hrcr.2020.02.002
30. Waheed SH, Rai D, Baibhav B, Chuprun D, Parikh V. A rare case of synthetic marijuana use leading to cardiac arrest. J Am Coll Cardiol. (2021) 77(18_Supplement_1):2335. doi: 10.1016/S0735-1097(21)03690-1
31. Bedi G, Cooper ZD, Haney M. Subjective, cognitive and cardiovascular dose-effect profile of nabilone and dronabinol in marijuana smokers. Addict Biol. (2013) 18(5):872–81. doi: 10.1111/j.1369-1600.2011.00427.x
32. Abu-Sawwa R, Scutt B, Park Y. Emerging use of epidiolex (cannabidiol) in epilepsy. J Pediatr Pharmacol Ther. (2020) 25(6):485–99. doi: 10.5863/1551-6776-25.6.485
33. Wheal AJ, Jadoon K, Randall MD, O’Sullivan SE. Cannabidiol treatment improves endothelium-dependent vasorelaxation in mesenteric arteries of zucker diabetic fatty rats. Front Pharmacol. (2017) 8:248. doi: 10.3389/fphar.2017.00248
34. Ladha KS, Mistry N, Wijeysundera DN, Clarke H, Verma S, Hare GM, et al. Recent cannabis use and myocardial infarction in young adults: a cross-sectional study. CMAJ. (2021) 193(35):E1377–E84. doi: 10.1503/cmaj.202392
35. Nasrin S, Watson CJ, Bardhi K, Fort G, Chen G, Lazarus P. Inhibition of UDP-glucuronosyltransferase enzymes by major cannabinoids and their metabolites. Drug Metab Dispos. (2021) 49(12):1081–9. doi: 10.1124/dmd.121.000530
36. Beaconsfield P, Ginsburg J, Rainsbury R. Marihuana smoking. N Engl J Med. (1972) 287(5):209–12. doi: 10.1056/NEJM197208032870501
37. Jadoon KA, Tan GD, O’Sullivan SE. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight. (2017) 2(12):e93760. doi: 10.1172/jci.insight.93760
38. DeFilippis EM, Bajaj NS, Singh A, Malloy R, Givertz MM, Blankstein R, et al. Marijuana use in patients with cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. (2020) 75(3):320–32. doi: 10.1016/j.jacc.2019.11.025
39. Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, Huestis MA. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. (2014) 144:12–41. doi: 10.1016/j.drugalcdep.2014.08.005
40. Freeman MJ, Rose DZ, Myers MA, Gooch CL, Bozeman AC, Burgin WS. Ischemic stroke after use of the synthetic marijuana “spice”. Neurology. (2013) 81(24):2090–3. doi: 10.1212/01.wnl.0000437297.05570.a2
41. Freeman WD, Gooch CL, Louh IK, Freeman MJ, Rose DZ, Burgin WS. Ischemic stroke after use of the synthetic marijuana “spice” author response. Neurology. (2014) 83(8):772–3. doi: 10.1212/01.wnl.0000453555.25241.99
42. Orsini J, Blaak C, Tam E, Rajayer S, Morante J, Yeh A, et al. The wide and unpredictable scope of synthetic cannabinoids toxicity. Case Rep Crit Care. (2015) 2015:542490. doi: 10.1155/2015/54249026788376
43. Willoughby S, Holmes A, Loscalzo J. Platelets and cardiovascular disease. Eur J Cardiovasc Nurs. (2002) 1:273–88. doi: 10.1016/S1474-51510200038-5
44. Rezkalla SH, Sharma P, Kloner RA. Coronary no-flow and ventricular tachycardia associated with habitual marijuana use. Ann Emerg Med. (2003) 42(3):365–9. doi: 10.1016/S0196-0644(03)00426-8
45. Nadkarni A, Oldham MA, Howard M, Berenbaum I. Drug-drug interactions between warfarin and psychotropics: an updated review of the literature. Pharmacotherapy. (2012) 32(10):932–42. doi: 10.1002/j.1875-9114.2012.01119
46. Kim S-Y, Kang J-Y, Hartman JH, Park S-H, Jones DR, Yun C-H, et al. Metabolism of R-and S-warfarin by CYP2C19 into four hydroxywarfarins. Drug Metab Lett. (2012) 6(3):157–64. doi: 10.2174/1872312811206030002
47. Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol. (2015) 12(1):30–47. doi: 10.1038/nrcardio.2014.156
48. Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D, et al. Stress cardiomyopathy diagnosis and treatment: JACC state-of-the-art review. J Am Coll Cardiol. (2018) 72(16):1955–71. doi: 10.1016/j.jacc.2018.07.072
49. Yamaori S, Koeda K, Kushihara M, Hada Y, Yamamoto I, Watanabe K. Comparison in the in vitro inhibitory effects of major phytocannabinoids and polycyclic aromatic hydrocarbons contained in marijuana smoke on cytochrome P450 2C9 activity. Drug Metab Pharmacokinet. (2012) 27(3):294–300. doi: 10.2133/dmpk.DMPK-11-RG-107
50. Paduch M, Thomason AR. Potential drug interactions between cannabinoids and its derivatives and oral anticoagulants. Hosp Pharm. (2022) 57(1):188–92. doi: 10.1177/0018578720985438
51. Jones RT. Cardiovascular system effects of marijuana. J Clin Pharmacol. (2002) 42(S1):58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x
52. Kalla A, Krishnamoorthy PM, Gopalakrishnan A, Figueredo VM. Cannabis use predicts risks of heart failure and cerebrovascular accidents: results from the national inpatient sample. J Cardiovasc Med (Hagerstown). (2018) 19(9):480–4. doi: 10.2459/JCM.0000000000000681
53. Mukhopadhyay P, Bátkai S, Rajesh M, Czifra N, Harvey-White J, Haskó G, et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. (2007) 50(6):528–36. doi: 10.1016/j.jacc.2007.03.057
54. Gülck T, Møller BL. Phytocannabinoids: origins and biosynthesis. Trends Plant Sci. (2020) 25(10):985–1004. doi: 10.1016/j.tplants.2020.05.005
55. Latif Z, Garg N. The impact of marijuana on the cardiovascular system: a review of the most common cardiovascular events associated with marijuana use. J Clin Med. (2020) 9(6):1925. doi: 10.3390/jcm9061925
56. Ali RM, Al Kury LT, Yang K-HS, Qureshi A, Rajesh M, Galadari S, et al. Effects of cannabidiol on contractions and calcium signaling in rat ventricular myocytes. Cell Calcium. (2015) 57(4):290–9. doi: 10.1016/j.ceca.2015.02.001
57. Walsh SK, Hepburn CY, Kane KA, Wainwright CL. Acute administration of cannabidiol in vivo suppresses ischaemia-induced cardiac arrhythmias and reduces infarct size when given at reperfusion. Br J Pharmacol. (2010) 160(5):1234–42. doi: 10.1111/j.1476-5381.2010.00755.x
58. Stanley CP, Hind WH, O’Sullivan SE. Is the cardiovascular system a therapeutic target for cannabidiol? Br J Clin Pharmacol. (2013) 75(2):313–22. doi: 10.1111/j.1365-2125.2012.04351.x
59. Puhl S-L. Cannabinoid-sensitive receptors in cardiac physiology and ischaemia. Biochim Biophys Acta Mol Cell Res. (2020) 1867(3):118462. doi: 10.1016/j.bbamcr.2019.03.009
60. Puhl S-L, Hilby M, Kohlhaas M, Keidel LM, Jansen Y, Hristov M, et al. Haematopoietic and cardiac GPR55 synchronize post-myocardial infarction remodelling. Sci Rep. (2021) 11(1):14385. doi: 10.1038/s41598-021-93755-y
61. Huestis M. Pharmacokinetics and metabolism of the plant cannabinoids, Δ 9 tetrahydrocannabinol, cannabidiol and cannabinol. Cannabinoids. (2005) 168:657. doi: 10.1007/3-540-26573-2_23
62. Graham J, Li D. Cardiovascular and respiratory effects of cannabis in cat and rat. Br J Pharmacol. (1973) 49(1):1. doi: 10.1111/j.1476-5381.1973.tb08262.x
63. Karniol IG, Shirakawa I, Takahashi RN, Knobel E, Musty RE. Effects of Δ9-tetrahydrocannabinol and cannabinol in man. Pharmacology. (1975) 13(6):502–12. doi: 10.1159/000136944
64. Morales P, Hurst DP, Reggio PH. Molecular targets of the phytocannabinoids: a complex picture. In: Kinghorn A, Falk H, Gibbons S, Kobayashi J, editors. Phytocannabinoids: Unraveling the complex Chemistry and Pharmacology of Cannabis sativa. Wien: Springer (2017). p. 103–31.
65. Dziemitko S, Harasim-Symbor E, Chabowski A. How do phytocannabinoids affect cardiovascular health? An update on the most common cardiovascular diseases. Ther Adv Chronic Dis. (2023) 14:20406223221143239. doi: 10.1177/20406223221143239
66. Nachnani R, Raup-Konsavage WM, Vrana KE. The pharmacological case for cannabigerol. J Pharmacol Exp Ther. (2021) 376(2):204–12. doi: 10.1124/jpet.120.000340
67. Cascio MG, Gauson LA, Stevenson LA, Ross RA, Pertwee RG. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist. Br J Pharmacol. (2010) 159(1):129–41. doi: 10.1111/j.1476-5381.2009.00515.x
68. Ramage AG, Villalón CM. 5-hydroxytryptamine and cardiovascular regulation. Trends Pharmacol Sci. (2008) 29(9):472–81. doi: 10.1016/j.tips.2008.06.009
69. Elsheshtawy M, Sriganesh P, Virparia V, Patel F, Khanna A. Synthetic marijuana induced acute nonischemic left ventricular dysfunction. Case Rep Cardiol. (2016) 2016:542490. doi: 10.1155/2015/542490
70. Haq EU, Shafiq A, Khan A, Awan A, Ezad S, Minteer W, et al. “Spice”(synthetic marijuana) induced acute myocardial infarction: a case series. Case Rep Cardiol. (2017) 2017:9252463. doi: 10.1155/2017/9252463
71. Clark BC, Georgekutty J, Berul CI. Myocardial ischemia secondary to synthetic cannabinoid (K2) use in pediatric patients. J Pediatr. (2015) 167(3):757–61.e1. doi: 10.1016/j.jpeds.2015.06.001
72. Marzo VD, Petrocellis LD. Plant, synthetic, and endogenous cannabinoids in medicine. Annu Rev Med. (2006) 57:553–74. doi: 10.1146/annurev.med.57.011205.135648
73. Mendizabal VE, Adler-Graschinsky E. Cannabinoids as therapeutic agents in cardiovascular disease: a tale of passions and illusions. Br J Pharmacol. (2007) 151(4):427–40. doi: 10.1038/sj.bjp.0707261
74. Jouanjus E, Raymond V, Lapeyre-Mestre M, Wolff V. What is the current knowledge about the cardiovascular risk for users of cannabis-based products? A systematic review. Curr Atheroscler Rep. (2017) 19(6):1–15. doi: 10.1007/s11883-017-0663-0
75. Benowitz NL. Managing cannabis use in patients with cardiovascular disease. Can J Cardiol. (2019) 35(2):138–41. doi: 10.1016/j.cjca.2018.12.033
76. Watanabe AH, Navaravong L, Sirilak T, Prasitwarachot R, Nathisuwan S, Page RL, et al. A systematic review and meta-analysis of randomized controlled trials of cardiovascular toxicity of medical cannabinoids. J Am Pharm Assoc (2003). (2021) 61(5):e1–e13. doi: 10.1016/j.japh.2021.03.013
77. Pasha AK, Clements CY, Reynolds CA, Lopez MK, Lugo CA, Gonzalez Y, et al. Cardiovascular effects of medical marijuana: a systematic review. Am J Med. (2021) 134(2):182–93. doi: 10.1016/j.amjmed.2020.09.015
78. Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N Engl J Med. (2017) 376(21):2011–20. doi: 10.1056/NEJMoa1611618
79. O'Connell BK, Gloss D, Devinsky O. Cannabinoids in treatment-resistant epilepsy: a review. Epilepsy Behav. (2017) 70:341–8. doi: 10.1016/j.yebeh.2016.11.012
80. Laux LC, Bebin EM, Checketts D, Chez M, Flamini R, Marsh ED, et al. Long-term safety and efficacy of cannabidiol in children and adults with treatment-resistant lennox-gastaut syndrome or dravet syndrome: expanded access program results. Epilepsy Res. (2019) 154:13–20. doi: 10.1016/j.eplepsyres.2019.03.015
81. Überall MA. A review of scientific evidence for THC: CBD oromucosal spray (nabiximols) in the management of chronic pain. J Pain Res. (2020) 13:399–410. doi: 10.2147/JPR.S240011
82. Patel RS, Katta SR, Patel R, Ravat V, Gudipalli R, Patel V, et al. Cannabis use disorder in young adults with acute myocardial infarction: trend inpatient study from 2010 to 2014 in the United States. Cureus. (2018) 10(8):e3241. doi: 10.7759/cureus.3241
83. Shah S, Patel S, Paulraj S, Chaudhuri D. Association of marijuana use and cardiovascular disease: a behavioral risk factor surveillance system data analysis of 133,706 US adults. Am J Med. (2021) 134(5):614–20.e1. doi: 10.1016/j.amjmed.2020.10.019
84. Desai R, Fong HK, Shah K, Kaur VP, Savani S, Gangani K, et al. Rising trends in hospitalizations for cardiovascular events among young cannabis users (18–39 years) without other substance abuse. Medicina (B Aires). (2019) 55(8):438. doi: 10.3390/medicina55080438
85. Nasrin S, Watson CJ, Perez-Paramo YX, Lazarus P. Cannabinoid metabolites as inhibitors of major hepatic CYP450 enzymes, with implications for cannabis-drug interactions. Drug Metab Dispos. (2021) 49(12):1070–80. doi: 10.1124/dmd.121.000442
86. Kariyanna PT, Wengrofsky P, Jayarangaiah A, Haseeb S, Salciccioli L, Hegde S, et al. Marijuana and cardiac arrhythmias: a scoping study. Int J Clin Trials. (2019) 4(1):132. doi: 10.15344/2456-8007/2019/132
87. Richards JR. Mechanisms for the risk of acute coronary syndrome and arrhythmia associated with phytogenic and synthetic cannabinoid use. J Cardiovasc Pharmacol Ther. (2020) 25(6):508–22. doi: 10.1177/1074248420935743
88. Zandstra TE, Notenboom RG, Wink J, Kiès P, Vliegen HW, Egorova AD, et al. Asymmetry and heterogeneity: part and parcel in cardiac autonomic innervation and function. Front Physiol. (2021) 12:665298. doi: 10.3389/fphys.2021.665298
89. Al Kury LT, Voitychuk OI, Yang K-HS, Thayyullathil FT, Doroshenko P, Ramez AM, et al. Effects of the endogenous cannabinoid anandamide on voltage-dependent sodium and calcium channels in rat ventricular myocytes. Br J Pharmacol. (2014) 171(14):3485–98. doi: 10.1111/bph.12734
90. Barana A, Amorós I, Caballero R, Gómez R, Osuna L, Lillo MP, et al. Endocannabinoids and cannabinoid analogs block cardiac hKv1.5 channels in a cannabinoid receptor-independent manner. Cardiovasc Res. (2009) 85(1):56–67. doi: 10.1093/cvr/cvp284
91. Jafry AH, LaGrow A, Akhtar KH, Hacker E, Russell S, Kliewer B, et al. Synthetic cannabinoids and ST-elevation myocardial infarction: a case report and systematic review of the literature. Am J Med Sci. (2022) 364(4):481–91. doi: 10.1016/j.amjms.2022.05.001
92. Yılmaz S, Ünal S, Kuyumcu MS, Balcı KG, Balcı MM. Acute anterior myocardial infarction after “bonzai” use. Anatol J Cardiol. (2015) 15(3):265–6. doi: 10.5152/akd.2015.5994
93. Chetty K, Lavoie A, Deghani P. A literature review of Cannabis and myocardial infarction—what clinicians may not be aware of. CJC Open (Online). (2021) 3(1):12–21. doi: 10.1016/j.cjco.2020.09.001
94. Benowitz NL, Rosenberg J, Rogers W, Bachman J, Jones RT. Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: autonomic nervous mechanisms. Clin Pharmacol Ther. (1979) 25(4):440–6. doi: 10.1002/cpt1979254440
95. Goyal H, Awad HH, Ghali JK. Role of cannabis in cardiovascular disorders. J Thorac Dis. (2017) 9(7):2079. doi: 10.21037/jtd.2017.06.104
96. Aronow WS, Cassidy J. Effect of marihuana and placebo-marihuana smoking on angina pectoris. N Engl J Med. (1974) 291(2):65–7. doi: 10.1056/NEJM197407112910203
97. Wei TT, Chandy M, Nishiga M, Zhang A, Kumar KK, Thomas D, et al. Cannabinoid receptor 1 antagonist genistein attenuates marijuana-induced vascular inflammation. Cell. (2022) 185(10):1676–93.e23. doi: 10.1016/j.cell.2022.04.005
98. Seif El Dahan K, Machtoub D, Massoud G, Nasser SA, Hamam B, Kobeissy F, et al. Cannabinoids and myocardial ischemia: novel insights, updated mechanisms, and implications for myocardial infarction. Curr Med Chem. (2022) 29(11):1990–2010. doi: 10.2174/0929867328666210608144818
99. Wang X, Derakhshandeh R, Liu J, Narayan S, Nabavizadeh P, Le S, et al. One minute of marijuana secondhand smoke exposure substantially impairs vascular endothelial function. J Am Heart Assoc. (2016) 5(8):e003858. doi: 10.1161/JAHA.116.003858
100. Booz GW. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic Biol Med. (2011) 51(5):1054–61. doi: 10.1016/j.freeradbiomed.2011.01.007
101. Lu X, Zhang J, Liu H, Ma W, Yu L, Tan X, et al. Cannabidiol attenuates pulmonary arterial hypertension by improving vascular smooth muscle cells’ mitochondrial function. Theranostics. (2021) 11(11):5267–78. doi: 10.7150/thno.55571
102. Garza-Cervantes JA, Ramos-González M, Lozano O, Jerjes-Sánchez C, García-Rivas G. Therapeutic applications of cannabinoids in cardiomyopathy and heart failure. Oxid Med Cell Longev. (2020) 2020:4587024. doi: 10.1155/2020/4587024
103. Stanley CP, Wheal AJ, Randall MD, O’Sullivan SE. Cannabinoids alter endothelial function in the zucker rat model of type 2 diabetes. Eur J Pharmacol. (2013) 720(1-3):376–82. doi: 10.1016/j.ejphar.2013.10.002
104. Remiszewski P, Jarocka-Karpowicz I, Biernacki M, Jastrząb A, Schlicker E, Toczek M, et al. Chronic cannabidiol administration fails to diminish blood pressure in rats with primary and secondary hypertension despite its effects on cardiac and plasma endocannabinoid system, oxidative stress and lipid metabolism. Int J Mol Sci. (2020) 21(4):1295. doi: 10.3390/ijms21041295
105. Fouda MA, Ghovanloo MR, Ruben PC. Cannabidiol protects against high glucose-induced oxidative stress and cytotoxicity in cardiac voltage-gated sodium channels. Br J Pharmacol. (2020) 177(13):2932–46. doi: 10.1111/bph.15020
106. Marchetti D, Spagnolo A, De Matteis V, Filograna L, De Giovanni N. Coronary thrombosis and marijuana smoking: a case report and narrative review of the literature. Drug Test Anal. (2016) 8(1):56–62. doi: 10.1002/dta.1898
107. Deusch E, Kress HG, Kraft B, Kozek-Langenecker SA. The procoagulatory effects of Delta-9-tetrahydrocannabinol in human platelets. Anesth Analg. (2004) 99(4):1127–30. doi: 10.1213/01.ANE.0000131505.03006.74
108. De Angelis V, Koekman AC, Weeterings C, Roest M, de Groot PG, Herczenik E, et al. Endocannabinoids control platelet activation and limit aggregate formation under flow. PLoS One. (2014) 9(9):e108282. doi: 10.1371/journal.pone.0108282
109. Reitsma SE, Lakshmanan HHS, Johnson J, Pang J, Parra-Izquierdo I, Melrose AR, et al. Chronic edible dosing of Δ9-tetrahydrocannabinol (THC) in nonhuman primates reduces systemic platelet activity and function. American Journal of Physiology-Cell Physiology. (2022) 322(3):C370–C81. doi: 10.1152/ajpcell.00373.2021
110. Khayat W, Lehmann C. The endocannabinoid system: a potential therapeutic target for coagulopathies. Metabolites. (2022) 12(6):541. doi: 10.3390/metabo12060541
111. Signorello MG, Giacobbe E, Leoncini G. Activation by 2-arachidonoylglycerol of platelet p38MAPK/cPLA2 pathway. J Cell Biochem. (2011) 112(10):2794–802. doi: 10.1002/jcb.23194
112. Keown OP, Winterburn TJ, Wainwright CL, Macrury SM, Neilson I, Barrett F, et al. 2-arachidonyl glycerol activates platelets via conversion to arachidonic acid and not by direct activation of cannabinoid receptors. Br J Clin Pharmacol. (2010) 70(2):180–8. doi: 10.1111/j.1365-2125.2010.03697.x
113. Brantl SA, Khandoga AL, Siess W. Mechanism of platelet activation induced by endocannabinoids in blood and plasma. Platelets. (2014) 25(3):151–61. doi: 10.3109/09537104.2013.803530
114. Rezkalla S, Kloner RA. Cardiovascular effects of marijuana. Trends Cardiovasc Med. (2019) 29(7):403–7. doi: 10.1016/j.tcm.2018.11.004
115. Mehta JJ, Mahendran AK, Bajaj RK, Doshi AR. Myocardial ischemia with cannabinoid use in an adolescent. Cureus. (2017) 9(11):e1899. doi: 10.7759/cureus.1899
116. Karabulut A, Cakmak M. ST segment elevation myocardial infarction due to slow coronary flow occurring after cannabis consumption. Kardiol Pol. (2010) 68(11):1266–8.21108208
117. Adams MD, Earnhardt JT, Dewey WL, Harris LS. Vasoconstrictor actions of delta8- and delta9-tetrahydrocannabinol in the rat. J Pharmacol Exp Ther. (1976) 196(3):649–56.4606
118. Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation. (2009) 119(1):28–36. doi: 10.1161/CIRCULATIONAHA.108.811992
119. García-Rivas G, Lozano O, Ramos M, Silva-Platas C, Bernal-Ramirez J, Alves-Figueiredo H, et al. Cannabidiol therapy for chronic heart failure prevents cardiac hypertrophy and contractile dysfunction by reducing mitochondrial ros generation in A murine model. J Card Fail. (2022) 28(5):S103. doi: 10.1016/j.cardfail.2022.03.263
120. Ting JY. Reversible cardiomyopathy associated with acute inhaled marijuana use in a young adult. Clin Toxicol. (2007) 45(4):432–4. doi: 10.1080/15563650601073587
121. Rajesh M, Mukhopadhyay P, Bátkai S, Haskó G, Liaudet L, Drel VR, et al. Cannabidiol attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption. Am J Physiol Heart Circ Physiol. (2007) 293(1):H610–H9. doi: 10.1152/ajpheart.00236.2007
122. Bátkai S, Mukhopadhyay P, Harvey-White J, Kechrid R, Pacher P, Kunos G. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol Heart Circ Physiol. (2007) 293(3):H1689–95. doi: 10.1152/ajpheart.00538.2007
123. Bonz A, Laser M, Küllmer S, Kniesch S, Babin-Ebell J, Popp V, et al. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol. (2003) 41(4):657–64. doi: 10.1097/00005344-200304000-00020
124. Larson EA, German DM, Shatzel J, DeLoughery TG. Anticoagulation in the cardiac patient: a concise review. Eur J Haematol. (2019) 102(1):3–19. doi: 10.1111/ejh.13171
125. Greger J, Bates V, Mechtler L, Gengo F. A review of cannabis and interactions with anticoagulant and antiplatelet agents. J Clin Pharmacol. (2020) 60(4):432–8. doi: 10.1002/jcph.1557
126. Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. (2014) 46(1):86–95. doi: 10.3109/03602532.2013.849268
127. Damkier P, Lassen D, Christensen MMH, Madsen KG, Hellfritzsch M, Pottegård A. Interaction between warfarin and cannabis. Basic Clin Pharmacol Toxicol. (2019) 124(1):28–31. doi: 10.1111/bcpt.13152
128. Hsu A, Painter NA. Probable interaction between warfarin and inhaled and oral administration of cannabis. J Pharm Pract. (2020) 33(6):915–8. doi: 10.1177/0897190019854958
129. Thomas TF, Metaxas ES, Nguyen T, Bennett W, Skiendzielewski KV, Quinn DH, et al. Case report: medical cannabis—warfarin drug-drug interaction. Journal of Cannabis Research. (2022) 4(1):1–6. doi: 10.1186/s42238-021-00112-x
130. Yamreudeewong W, Wong HK, Brausch LM, Pulley KR. Probable interaction between warfarin and marijuana smoking. Ann Pharmacother. (2009) 43(7-8):1347–53. doi: 10.1345/aph.1M064
131. Polson G, Chung M, Hirani S, Le-Short C. Cannabis drug interactions. Cannabinoids and Pain. New York: Springer Publishing (2021). p. 93–101.
132. Grayson L, Vines B, Nichol K, Szaflarski JP. An interaction between warfarin and cannabidiol, a case report. Epilepsy Behav Case Rep. (2018) 9:10–1. doi: 10.1016/j.ebcr.2017.10.001
133. Beavers CJ, Naqvi IA. Clopidogrel. In: Shumway K, editor. Treasure Island, FL: StatPearls Publishing (2024).
134. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. (2018) 84(11):2477–82. doi: 10.1111/bcp.13710
136. Di Minno A, Frigerio B, Spadarella G, Ravani A, Sansaro D, Amato M, et al. Old and new oral anticoagulants: food, herbal medicines and drug interactions. Blood Rev. (2017) 31(4):193–203. doi: 10.1016/j.blre.2017.02.001
137. Holland M, Panetta J, Hoskins J, Bebawy M, Roufogalis B, Allen J, et al. The effects of cannabinoids on P-glycoprotein transport and expression in multidrug-resistant cells. Biochem Pharmacol. (2006) 71(8):1146–54. doi: 10.1016/j.bcp.2005.12.033
138. Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K. Identification of cytochrome P450 enzymes responsible for the metabolism of cannabidiol by human liver microsomes. Life Sci. (2011) 89(5-6):165–70. doi: 10.1016/j.lfs.2011.05.018
139. Coetzee C, Levendal R-A, Van de Venter M, Frost C. Anticoagulant effects of a Cannabis extract in an obese rat model. Phytomedicine. (2007) 14(5):333–7. doi: 10.1016/j.phymed.2006.02.004
140. Wolff V, Lauer V, Rouyer O, Sellal F, Meyer N, Raul JS, et al. Cannabis use, ischemic stroke, and multifocal intracranial vasoconstriction: a prospective study in 48 consecutive young patients. Stroke. (2011) 42(6):1778–80. doi: 10.1161/STROKEAHA.110.610915
141. Wolff V, Schlagowski A-I, Rouyer O, Charles A-L, Singh F, Auger C, et al. Tetrahydrocannabinol induces brain mitochondrial respiratory chain dysfunction and increases oxidative stress: a potential mechanism involved in cannabis-related stroke. BioMed Res Int. (2015) 2015:323706. doi: 10.1155/2015/323706
142. Rumalla K, Reddy AY, Mittal MK. Recreational marijuana use and acute ischemic stroke: a population-based analysis of hospitalized patients in the United States. J Neurol Sci. (2016) 364:191–6. doi: 10.1016/j.jns.2016.01.066
143. Hackam DG. Cannabis and stroke: systematic appraisal of case reports. Stroke. (2015) 46(3):852–6. doi: 10.1161/STROKEAHA.115.008680
144. Finsterer J, Christian P, Wolfgang K. Occipital stroke shortly after cannabis consumption. Clin Neurol Neurosurg. (2004) 106(4):305–8. doi: 10.1016/j.clineuro.2004.02.001
145. Hemachandra D, McKetin R, Cherbuin N, Anstey KJ. Heavy cannabis users at elevated risk of stroke: evidence from a general population survey. Aust N Z J Public Health. (2016) 40(3):226–30. doi: 10.1111/1753-6405.12477
146. Parekh T, Pemmasani S, Desai R. Marijuana use among young adults (18–44 years of age) and risk of stroke: a behavioral risk factor surveillance system survey analysis. Stroke. (2020) 51(1):308–10. doi: 10.1161/STROKEAHA.119.027828
147. Burton TM, Bushnell CD. Reversible cerebral vasoconstriction syndrome: a diagnostic imaging review. Stroke. (2019) 50(8):2253–8. doi: 10.1161/STROKEAHA.119.024416
148. Adams M, Earnhardt J, Dewey W, Harris L. Vasoconstrictor actions of delta8-and delta9-tetrahydrocannabinol in the rat. J Pharmacol Exp Ther. (1976) 196(3):649–56.4606
149. Herning RI, Better WE, Tate K, Cadet JL. Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology. (2005) 64(3):488–93. doi: 10.1212/01.WNL.0000150882.69371.DD
150. Filbey FM, Aslan S, Lu H, Peng S-L. Residual effects of THC via novel measures of brain perfusion and metabolism in a large group of chronic cannabis users. Neuropsychopharmacology. (2018) 43(4):700–7. doi: 10.1038/npp.2017.44
151. Behrouz R, Birnbaum L, Grandhi R, Johnson J, Misra V, Palacio S, et al. Cannabis use and outcomes in patients with aneurysmal subarachnoid hemorrhage. Stroke. (2016) 47(5):1371–3. doi: 10.1161/STROKEAHA.116.013099
152. Brust J. Spice, pot, and stroke response. Neurology. (2014) 83(8):772. doi: 10.1212/01.wnl.0000437310.96160.fa
153. Catapano JS, Rumalla K, Srinivasan VM, Labib MA, Nguyen CL, Rutledge C, et al. Cannabis use and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke. (2022) 53(2):e42–e3. doi: 10.1161/STROKEAHA.121.035650
154. Auger N, Paradis G, Low N, Ayoub A, He S, Potter BJ. Cannabis use disorder and the future risk of cardiovascular disease in parous women: a longitudinal cohort study. BMC Med. (2020) 18:1–9. doi: 10.1186/s12916-020-01804-6
155. Malhotra K, Rumalla K, Mittal MK. Association and clinical outcomes of marijuana in patients with intracerebral hemorrhage. J Stroke Cerebrovasc Dis. (2018) 27(12):3479–86. doi: 10.1016/j.jstrokecerebrovasdis.2018.08.011
156. Stoelting RK, Martz RC, Gartner J, Creasser C, Brown DJ, Forney RB. Effects of Delta–9–tetrahydrocannabinol on halothane MAC in dogs. Anesthesiology. (1973) 38:521–4. doi: 10.1097/00000542-197306000-00002
157. Frizza J, Chesher G, Jackson D, Malor R, Starmer G. The effect of Δ 9, cannabidiol, and cannabinol on the anaesthesia induced by various anaesthetic agents in mice. Psychopharmacology. (1977) 55(1):103–7. doi: 10.1007/BF00432824
158. Malor R, Jackson D, Chesher G. The effect of Δ9-tetrahydrocannabinol, cannabidiol and cannabinol on ether anaesthesia in mice. J Pharm Pharmacol. (1975) 27(8):610–2. doi: 10.1111/j.2042-7158.1975.tb09516.x
159. Schuster J, Ates M, Brune K, Gühring H. The cannabinoids R (−)-7-hydroxy-delta-6-tetra-hydrocannabinol-dimethylheptyl (HU-210), 2-O-arachidonoylglycerylether (HU-310) and arachidonyl-2-chloroethylamide (ACEA) increase isoflurane provoked sleep duration by activation of cannabinoids 1 (CB1)-receptors in mice. Neurosci Lett. (2002) 326(3):196–200. doi: 10.1016/S0304-3940(02)00302-6
160. Patel S, Wohlfeil ER, Rademacher DJ, Carrier EJ, Perry LJ, Kundu A, et al. The general anesthetic propofol increases brain N-arachidonylethanolamine (anandamide) content and inhibits fatty acid amide hydrolase. Br J Pharmacol. (2003) 139(5):1005–13. doi: 10.1038/sj.bjp.0705334
161. Brand P-A, Paris A, Bein B, Meybohm P, Scholz J, Ohnesorge H, et al. Propofol sedation is reduced by δ9-tetrahydrocannabinol in mice. Anesth Analg. (2008) 107(1):102–6. doi: 10.1213/ane.0b013e318173287a
162. Meybohm P, Brand P-A, Renhof F, Scholz J, Tonner PH. Interaction of the general anaesthetic propofol and selective agonists for cannabinoid-receptor type 1 and 2. Anesthesiology. (2005) 103:A679. doi: 10.1097/00000542-200510000-00003
163. Ruesch D, Brand P, Schneider H, Hoecker J, Wulf H. Potentiation of GABA-A receptors by propofol is inhibited by Delta9-tetrahydrocannabinol: 7AP5-3. European Journal of Anaesthesiology (EJA). (2008) 25:102. doi: 10.1097/00003643-200805001-00323
164. Kimura T, Takaya M, Usami N, Watanabe K, Yamamoto I. Δ 9-tetrahydrocannabinol, a major marijuana component, enhances the anesthetic effect of pentobarbital through the CB 1 receptor. Forensic Toxicol. (2019) 37(1):207–14. doi: 10.1007/s11419-018-0457-2
165. Uršič M, Babič A, Vake T, Snoj T. The impact of cannabidiol on the induction of isoflurane anesthesia and recovery in wistar rats. Cannabis Cannabinoid Res. (2021) 7(3):289–93. doi: 10.1089/can.2021.0014
166. Thakkar H, Mahindajit A, Taylor D, Roberts L, Cooke J. Conscious sedation for transoesophageal echocardiography in cannabis users. Heart, Lung and Circ. (2017) 26:S254. doi: 10.1016/j.hlc.2017.06.484
167. Symons I. Cannabis smoking and anaesthesia. Anaesthesia. (2002) 57(11):1142–3. doi: 10.1046/j.1365-2044.2002.288312.x
168. Richtig G, Bosse G, Arlt F, von Heymann C. Cannabis consumption before surgery may be associated with increased tolerance of anesthetic drugs: a case report. Int J Case Rep Images. (2015) 6(7):436–9. doi: 10.5348/ijcri-201573-CR-10534
169. Flisberg P, Paech M, Shah T, Ledowski T, Kurowski I, Parsons R. Induction dose of propofol in patients using cannabis. Eur J Anaesthesiol. (2009) 26(3):192–5. doi: 10.1097/EJA.0b013e328319be59
170. Ibera C, Shalom B, Saifi F, Shruder J, Davidson E. Effects of cannabis extract premedication on anesthetic depth. Harefuah. (2018) 157(3):162–6.29582946
171. King DD, Stewart CSA, Collins-Yoder MA, Price MLL MASM. Anesthesia for patients who self-report cannabis (marijuana) use before esophagogastroduodenoscopy: a retrospective review. AANA J. (2021) 89(3):205–12.34042571
172. Alexander JC, Joshi GP. A review of the anesthetic implications of marijuana use. Proc (Bayl Univ Med Cent). (2019) 32(3):364–71. doi: 10.1080/08998280.2019.160303431384188
173. Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. (2004) 74(11):1317–24. doi: 10.1016/j.lfs.2003.09.038
174. Twardowski MA, Link MM, Twardowski NM. Effects of cannabis use on sedation requirements for endoscopic procedures. J Am Osteopath Assoc. (2019) 119(5):307–11. doi: 10.7556/jaoa.2019.052
175. Holmen IC, Beach JP, Kaizer AM, Gumidyala R. The association between preoperative cannabis use and intraoperative inhaled anesthetic consumption: a retrospective study. J Clin Anesth. (2020) 67:109980. doi: 10.1016/j.jclinane.2020.109980
176. Davidson EM, Raz N, Eyal AM. Anesthetic considerations in medical cannabis patients. Curr Opin Anaesthesiol. (2020) 33(6):832–40. doi: 10.1097/ACO.0000000000000932
177. Cohen K, Weizman A, Weinstein A. Modulatory effects of cannabinoids on brain neurotransmission. Eur J Neurosci. (2019) 50(3):2322–45. doi: 10.1111/ejn.14407
178. Bakas T, Van Nieuwenhuijzen P, Devenish S, McGregor I, Arnold J, Chebib M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol Res. (2017) 119:358–70. doi: 10.1016/j.phrs.2017.02.022
179. Zendulka O, Dovrtelová G, Nosková K, Turjap M, Sulcová A, Hanus L, et al. Cannabinoids and cytochrome P450 interactions. Curr Drug Metab. (2016) 17(3):206–26. doi: 10.2174/1389200217666151210142051
180. Imasogie N, Rose RV, Wilson A. High quantities: evaluating the association between cannabis use and propofol anesthesia during endoscopy. PLos One. (2021) 16(3):e0248062. doi: 10.1371/journal.pone.0248062
181. McAfee J, Boehnke KF, Moser SM, Brummett CM, Waljee JF, Bonar EE. Perioperative cannabis use: a longitudinal study of associated clinical characteristics and surgical outcomes. Reg Anesth Pain Med. (2021) 46(2):137–44. doi: 10.1136/rapm-2020-101812
182. Jennings JM, Angerame MR, Eschen CL, Phocas AJ, Dennis DA. Cannabis use does not affect outcomes after total knee arthroplasty. J Arthroplasty. (2019) 34(8):1667–9. doi: 10.1016/j.arth.2019.04.015
183. Chiu AK, Fuller SI, Agarwal AR, Cuero K, Kreulen T, Srikumaran U. Effect of Cannabis Use Disorder on Total Shoulder Arthroplasty Outcomes. (2022).
184. Goel A, McGuinness B, Jivraj NK, Wijeysundera DN, Mittleman MA, Bateman BT, et al. Cannabis use disorder and perioperative outcomes in major elective surgeries: a retrospective cohort analysis. Anesthesiology. (2020) 132(4):625–35. doi: 10.1097/ALN.0000000000003067
185. Zhang BH, Saud H, Sengupta N, Chen M, Bakshi D, Richardson L, et al. Effect of preoperative cannabis use on perioperative outcomes: a retrospective cohort study. Reg Anesth Pain Med. (2021) 46(8):650–5. doi: 10.1136/rapm-2021-102479
186. Anderson SR, Wimalawansa SM, Markov NP, Fox JP. Cannabis abuse or dependence and post-operative outcomes after appendectomy and cholecystectomy. J Surg Res. (2020) 255:233–9. doi: 10.1016/j.jss.2020.05.009
187. Shah NV, Lavian JD, Moattari CR, Eldib H, Beyer GA, Mai DH, et al. The impact of isolated baseline Cannabis use on outcomes following thoracolumbar spinal fusion: a propensity score-matched analysis. Iowa Orthop J. (2022) 42(1):57.35821925
188. Echeverria-Villalobos M, Todeschini AB, Stoicea N, Fiorda-Diaz J, Weaver T, Bergese SD. Perioperative care of cannabis users: a comprehensive review of pharmacological and anesthetic considerations. J Clin Anesth. (2019) 57:41–9. doi: 10.1016/j.jclinane.2019.03.011
189. Huson HB, Granados TM, Rasko Y. Surgical considerations of marijuana use in elective procedures. Heliyon. (2018) 4(9):e00779. doi: 10.1016/j.heliyon.2018.e00779
190. Shah S, Schwenk ES, Sondekoppam RV, Clarke H, Zakowski M, Rzasa-Lynn RS, et al. ASRA pain medicine consensus guidelines on the management of the perioperative patient on cannabis and cannabinoids. Reg Anesth Pain Med. (2023) 48(3):97–117. doi: 10.1136/rapm-2022-104013
191. Cuttler C, LaFrance EM, Stueber A. Acute effects of high-potency cannabis flower and cannabis concentrates on everyday life memory and decision making. Sci Rep. (2021) 11(1):1–13. doi: 10.1038/s41598-021-93198-5
192. ElSohly MA, Chandra S, Radwan M, Majumdar CG, Church JC. A comprehensive review of cannabis potency in the United States in the last decade. Biol Psychiatry Cogn Neurosci Neuroimaging. (2021) 6(6):603–6. doi: 10.1016/j.bpsc.2020.12.016
193. Bonn-Miller MO, Loflin MJ, Thomas BF, Marcu JP, Hyke T, Vandrey R. Labeling accuracy of cannabidiol extracts sold online. JAMA. (2017) 318(17):1708–9. doi: 10.1001/jama.2017.11909
194. Ladha KS, McLaren-Blades A, Goel A, Buys MJ, Farquhar-Smith P, Haroutounian S, et al. Perioperative pain and addiction interdisciplinary network (PAIN): consensus recommendations for perioperative management of cannabis and cannabinoid-based medicine users by a modified delphi process. Br J Anaesth. (2021) 126(1):304–18. doi: 10.1016/j.bja.2020.09.026
196. Ashton CH. Adverse effects of cannabis and cannabinoids. Br J Anaesth. (1999) 83(4):637–49. doi: 10.1093/bja/83.4.637
197. Musshoff F, Madea B. Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther Drug Monit. (2006) 28(2):155–63. doi: 10.1097/01.ftd.0000197091.07807.22
198. Martel ML, Klein LR, Miner JR, Cole JB, Nystrom PC, Holm KM, et al. A brief assessment of capacity to consent instrument in acutely intoxicated emergency department patients. Am J Emerg Med. (2018) 36(1):18–23. doi: 10.1016/j.ajem.2017.06.043
199. Cahalan MK, Weiskopf RB, Eger EI, Yasuda N, Ionescu P, Rampil IJ, et al. Hemodynamic effects of desflurane/nitrous oxide anesthesia in volunteers. Anesth Analg. (1991) 73(2):157–64. doi: 10.1213/00000539-199108000-00008
200. Brioni JD, Varughese S, Ahmed R, Bein B. A clinical review of inhalation anesthesia with sevoflurane: from early research to emerging topics. J Anesth. (2017) 31(5):764–78. doi: 10.1007/s00540-017-2375-6
201. Akata T, Warltier DC. General anesthetics and vascular smooth muscle: direct actions of general anesthetics on cellular mechanisms regulating vascular tone. J Am Soc Anesth. (2007) 106(2):365–91. doi: 10.1097/00000542-200702000-00026
202. Johnson S, Domino EF. Some cardiovascular effects of marihuana smoking in normal volunteers. Clin Pharmacol Ther. (1971) 12(5):762–8. doi: 10.1002/cpt1971125762
203. Alshaarawy O, Elbaz HA. Cannabis use and blood pressure levels: united States national health and nutrition examination survey, 2005–2012. J Hypertens. (2016) 34(8):1507. doi: 10.1097/HJH.0000000000000990
204. Hernandez M, Birnbach DJ, Van Zundert AA. Anesthetic management of the illicit-substance-using patient. Current Opinion in Anesthesiology. (2005) 18(3):315–24. doi: 10.1097/01.aco.0000169241.21680.0b
205. Ladha KS, Manoo V, Virji A-F, Hanlon JG, Mclaren-Blades A, Goel A, et al. The impact of perioperative cannabis use: a narrative scoping review. Cannabis and Cannabinoid Research. (2019) 4(4):219–30. doi: 10.1089/can.2019.0054
206. Cossu AE, Latham LB, Hardacker DM, Jea AH. Pharmacologic chaos: severe hypotension from interactions of anesthetics, marijuana, amphetamines, and paroxetine. J Clin Anesth. (2019) 55:17. doi: 10.1016/j.jclinane.2018.12.040
207. McGuinness B, Goel A, Elias F, Rapanos T, Mittleman MA, Ladha KS. Cannabis use disorder and perioperative outcomes in vascular surgery. J Vasc Surg. (2021) 73(4):1376–87.e3. doi: 10.1016/j.jvs.2020.07.094
208. Vakharia RM, Mannino A, Salem HS, Roche MW, Wong CHJ, Mont MA. The association between cannabis use disorder and the outcome following primary total hip arthroplasty: analysis of a nationwide administrative claims database. Bone Joint J. (2021) 103(7 Supple B):111–5. doi: 10.1302/0301-620X.103B7.BJJ-2020-2424.R1
209. Jain S, Cloud GW, Gordon AM, Lam AW, Vakharia RM, Saleh A, et al. Cannabis use disorder is associated with Longer in-hospital lengths of stay, higher rates of medical complications, and costs of care following primary 1-to 2-level lumbar fusion. Global Spine J. (2022) 14(1):67–73. doi: 10.1177/21925682221093965
210. Chiu RG, Fuentes AM, Patil SN, Chiu R, McGuire LS, Mehta AI. Cannabis abuse and perioperative complications after treatment of intracranial aneurysms: a nationwide analysis. World Neurosurg. (2022) 158:e184–e95. doi: 10.1016/j.wneu.2021.10.156
Keywords: cannabis, cannabinoids, cardiovascular effects, perioperative cardiovascular outcomes, postoperative cardiovascular outcomes
Citation: Echeverria-Villalobos M, Guevara Y, Mitchell J, Ryskamp D, Conner J, Bush M, Periel L, Uribe A and Weaver TE (2024) Potential perioperative cardiovascular outcomes in cannabis/cannabinoid users. A call for caution. Front. Cardiovasc. Med. 11:1343549. doi: 10.3389/fcvm.2024.1343549
Received: 1 December 2023; Accepted: 15 May 2024;
Published: 21 June 2024.
Edited by:
Maria Monsalve, Autonomous University of Madrid, SpainReviewed by:
Jahan Marcu, University of the Sciences, United StatesBruno Miguel Fonseca, University of Porto, Portugal
© 2024 Echeverria-Villalobos, Guevara, Mitchell, Ryskamp, Conner, Bush, Periel, Uribe and Weaver. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Echeverria-Villalobos, bWFyY28uZWNoZXZlcnJpYXZpbGxhbG9ib3NAb3N1bWMuZWR1
 Marco Echeverria-Villalobos
Marco Echeverria-Villalobos Yosira Guevara2
Yosira Guevara2 Justin Mitchell
Justin Mitchell Margo Bush
Margo Bush Luis Periel
Luis Periel Alberto Uribe
Alberto Uribe Tristan E. Weaver
Tristan E. Weaver