
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 18 March 2024
Sec. Cardiovascular Epidemiology and Prevention
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1342686
Background: Iron deficiency (ID) is the most common nutritional deficiency, with little research on its prevalence and long-term outcomes in the general population and those with heart failure (HF). Both the relationships between dietary iron and ID, as well as dietary folate and ID, are understudied.
Methods: We used data from the National Health and Nutrition Examination Survey from 1999 to 2002 to investigate the prevalence, prognosis, and relationship between dietary and ID defined by different criteria in the general population (n = 6,660) and those with HF (n = 182).
Results: There was no significant difference in the prevalence of ID between HF patients and the general population after propensity score matching. Transferrin saturation (TSAT) <20% was associated with higher 5-year all-cause mortality (HR: 3.49, CI: 1.40–8.72, P = 0.007), while ferritin <30 ng/ml was associated with higher 10-year (HR: 2.70, CI: 1.10–6.67, P = 0.031) and 15-year all-cause mortality (HR: 2.64, CI: 1.40–5.00, P = 0.003) in HF patients. Higher dietary total folate but dietary iron reduced the risk of ID (defined as ferritin <100 ng/ml) in HF patients (OR: 0.80; 95% CI: 0.65–1.00; P = 0.047).
Conclusions: The prevalence of ID was identical in HF and non-HF individuals. Ferritin <30 ng/ml was associated with long-term outcomes whereas TSAT <20% was associated with short-term prognosis in both the general population and HF patients. A diet rich in folate might have the potential for prevention and treatment of ID in HF patients.
Iron deficiency (ID) with or without anemia is a common disease worldwide that is associated with impaired exercise capacity and mortality (1, 2). The prevalence of ID in individuals with heart failure (HF) ranges from 37% to 75.3%, while in the general population, it varies from 16.4% to 64.3% (3–6). These prevalence rates are contingent upon the particular definition of ID employed and the specific demographic under investigation. Nevertheless, literature directly comparing the incidence of ID between these two cohorts is notably lacking.
ID has been defined in various ways (as defined by heart failure guideline criteria: ferritin <100 ng/ml or, when 100–299 ng/ml, a transferrin saturation (TSAT) <20%; ferritin <15 ng/ml; ferritin <30 ng/ml; ferritin <100 ng/ml; TSAT <20%; or serum iron <13 μmol/L) (1, 14), prior research has primarily examined the association between isolated or a few definitions of ID and mortality. Moreover, research efforts have predominantly concentrated on the link between ID and short-term prognosis, leaving a gap in our understanding of its impact on long-term prognostic outcomes. In instances where certain definitions of ID are subject to scrutiny (7, 11, 15), a thorough assessment of the relationship between widely accepted definitions of ID and both short-term and long-term prognoses is imperative.
In certain research studies, a notable and statistically significant positive association between ferritin levels and iron intake was evident (16, 17). Inadequate iron consumption emerged as a noteworthy predictor for maintaining serum ferritin levels below 30 ng/ml, though not for achieving ferritin concentrations <100 ng/ml (16). The accuracy of these findings necessitates validation within a more expansive and diverse population sample. Additionally, it is imperative to determine their applicability, especially among individuals affected by HF. Moreover, folate, a vital determinant of hematological status, is advocated for concurrent use with iron to expedite the resolution of ID anemia, particularly during pregnancy (18, 19). However, the association between dietary folate and ID remains uncertain both within the general population and among individuals with HF.
We aimed to investigated the prevalence of ID defined by all the aforementioned definitions in both the general population and HF individuals. Besides, the association of ID with long-term outcomes was explored. We finally studied how dietary iron and total folate are linked to ID to find potential ways for ID prevention and treatment.
The publicly available data were collected from the 1999–2002 cycle of the National Health and Nutrition Examination Survey (NHANES), which is a program of studies designed to assess the health and nutritional status of adults and children in the United States. Each year, the survey assesses a sample of approximately 5,000 individuals from counties across the country, with 15 counties visited annually. NHANES is a significant program under the auspices of the National Center for Health Statistics (NCHS), which is a division of the Centers for Disease Control and Prevention (CDC), and is responsible for generating critical health and vital statistics for the country (20). Participants who had complete information on demographics, medical history (congestive heart failure, coronary heart disease, stroke, cancer, hypertension, diabetes and hospitalization), specific laboratory measurements (NT-proBNP, hemoglobin, serum iron, ferritin, transferrin saturation, creatinine, alanine aminotransferase, aspartate aminotransferase, C-reactive protein, segmented neutrophils percent and glycohemoglobin), dietary iron and total folate and corresponding sample weights were included. Of the 6,660 participants, 182 had HF. The survey protocol was approved by the NCHC (National Center for Health Statistics) Ethics Review Board and all participants provided informed written consent (21). All methods were carried out in accordance with Declaration of Helsinki.
The glomerular filtration rate was estimated by the CKD-EPI equation (22). In this study, the third quartile of the percentage of neutrophils in the general population was 64.6%. Based on this value, both the general population and HF patients were divided into high and low neutrophil percentage groups. In the present study, the median daily intake of iron and total folate in the general population were 13.42 mg and 345 mcg, respectively. Based on these values, the population was divided into high and low intake groups. Anemia was diagnosed in males with hemoglobin levels below 13 g/dl and females with hemoglobin levels below 12 g/dl. Participants with a ratio of family income to poverty less than 1.3 were classified as low-income group, while those with a ratio greater than or equal to 1.3 were classified as middle-to-high income group. According to the Physical Activity Questionnaire which was based on the Global Physical Activity Questionnaire and included questions related to daily activities, leisure time activities, and sedentary activities, the classification of cardiac function was divided into two groups: NYHA I/II and NYHA III/IV.
The in-person interview for participants was conducted in a private room in the NHANES mobile examination center in the United States to collect dietary data during the 24-h period prior to the interview. Some of the NHANES 1999–2000 dietary interview data were collected by a telephone dietary interview. In 2001, dietary intake data were collected using the NHANES computer-assisted dietary interview system, which was also used to collect dietary data for the 1999–2000 collection period. In 2002, data were collected using USDA's dietary data collection instrument, the Automated Multiple Pass Method. The sample weights Weighted Dietary Recall 4-Year (WTDR4YR) which were constructed by taking the Mobile Examination Center sample weights (WTMEC2YR) and further adjusting for the additional non-response and the differential allocation by day of the week for the dietary intake data collection were applied to produce national, representative estimates.
The National Center for Health Statistics has linked data collected from several NCHS population surveys with death certificate records from the National Death Index. The public-use LMF provide mortality follow-up data from the date of survey participation through December 31, 2019 (23).
The statistical analysis was performed using the R programming language (version 4.1.3). Continuous variables were presented as Median (IQR). Categorical variables were presented as n (unweighted) (%). To compare the baseline characteristics, the Wilcoxon rank-sum test for complex survey samples and the chi-squared test with Rao & Scott's second-order correction were used. Propensity score matching (PSM) was utilized to eliminate bias and control for demographics (age, sex, race, income and BMI) using the “MatchIt” package. The weighted Cox proportional hazards models and weighted logistic regression models were performed using the “survey” package. Variables with P ≤ 0.1 in univariable models were included in the multivariable models. Significance was defined as two-sided probability values less than 0.05.
During the period of 1999–2002, the NHANES database documented data from 21,004 individuals. However, only 6,663 individuals had complete baseline information, and an additional 3 individuals did not have survival data. Among the remaining 6,660 individuals, 182 self-reported that they had congestive heart failure (Figure 1).
For both HF patients and non-HF individuals, there were no significant differences in their iron indices (serum ferritin (99 vs. 85 ng/ml, P = 0.2), serum iron (14 vs. 15 μmol/L, P = 0.088) and TSAT (21% vs. 24%, P = 0.12) (Table 1). Besides, after adjusting for age, gender, income, and BMI using propensity score matching, there was no significant difference in the incidence of ID (as defined by all the aforementioned definitions) between individuals with HF and those without (Supplementary Table S1).
Among the cohort of 6,660 individuals, 373 decease cases were recorded after a 5-year follow-up, followed by 899 mortalities at the 10-year mark, and 1,416 mortalities at the 15-year follow-up period.
In general population recorded in NHANES 1999–2002, after adjusting for confounding factors, there was no significant association between ID, as defined by various methods, and five-year all-cause mortality in the general population (Supplementary Table S2). However, subgroup analysis revealed that in the general population aged 45 years and above, TSAT <20% was associated with an increased risk of all-cause mortality at 5 years (HR: 1.40; 95% CI: 1.05–1.86; P = 0.021), as well as ferritin <30 ng/ml (HR: 1.85; 95% CI: 1.18–2.89; P = 0.007) (Supplementary Table S3).
In adjusted models, only ferritin <30 ng/ml was significantly associated with 10-year all-cause mortality (HR: 1.35; 95% CI: 1.03–1.77; P = 0.030, Supplementary Table S4), particularly among individuals aged 45 years and above (HR: 1.47; 95% CI: 1.14–1.89; P = 0.003, Supplementary Table S3). Alternative definitions for ID were found to be inadequate in predicting 10-year all-cause mortality (Supplementary Tables S3, S4).
In Univariable analysis, ID defined by criteria in guideline for heart failure (12) (HR: 0.69; 95% CI: 0.61–0.78; P < 0.001), ferritin <300 ng/ml (HR: 0.62; 95% CI: 0.49–0.79; P < 0.001), ferritin <100 ng/ml (HR: 0.66; 95% CI: 0.57–0.76; P < 0.001), ferritin <30 ng/ml (HR: 0.48; 95% CI: 0.38–0.60; P < 0.001), ferritin <15 ng/ml (HR: 0.42; 95% CI: 0.30–0.59; P < 0.001) were significantly associated with the 15-year all-cause mortality while serum iron (≤10 μmol/L or ≤13 μmol/L) and TSAT <20% were not (Supplementary Table S5). After adjusting for confounding factors, ferritin levels, iron levels or TSAT was not associated with 15-year all-cause mortality (Supplementary Tables S3, S5).
Within the group of 182 patients diagnosed with HF, 46 fatalities were recorded during the 5-year follow-up, followed by 94 mortalities at the 10-year follow-up, and 126 mortalities at the 15-year follow-up.
In unadjusted analyses, ID defined by serum iron levels (≤10 μmol/L: HR: 3.32; 95% CI: 1.83–6.03; P < 0.001; ≤13 μmol/L: HR: 3.08; 95% CI: 1.64–5.77; P < 0.001), TSAT <20% (HR: 3.96; 95% CI: 1.93–8.12; P < 0.001) and ferritin <30 ng/ml (HR: 2.73; 95% CI: 1.24–6.02; P = 0.013) were associated with higher 5-year all-cause mortality (Table 2). In adjusted models, TSAT <20% (HR: 3.49; 95% CI: 1.40–8.72; P = 0.007) and ferritin <300 ng/ml (HR: 4.79; 95% CI: 1.43–16.0; P = 0.011) were significantly associated with higher 5-year all-cause mortality. ID defined by heart failure guideline criteria (HR: 2.52; 95% CI: 0.92–6.90; P = 0.073) or iron ≤10 μmol/L (HR: 2.17; 95% CI: 0.97–4.83; P = 0.059) seemed to associated with a higher mortality, but not significant (Table 2).
Ferritin <30 ng/ml (10-year: HR: 2.83; 95% CI: 1.67–4.80; P < 0.001, Table 3, and 15-year: HR: 2.45; 95% CI: 1.40–4.30; P = 0.002, Supplementary Table S6) significantly associated with 10-year and 15-year all-cause mortality, even after adjusting confounding factors (10-year HR: 2.70; 95% CI: 1.10–6.67; P = 0.031, Table 3, and 15-year: HR: 2.64; 95% CI: 1.40–5.00; P = 0.003, Supplementary Table S6).
In the general population, the median daily intake of iron is 13.42 mg, while the median daily intake of total folate is 345 mcg. Based on the median intake, the entire population was categorized into high and low intake groups.
Through univariable logistic regression analysis, a correlation was found between a higher intake of dietary iron and a decreased risk of ID. However, when gender was included in the multivariable regression analysis, no significant relationship was observed between dietary iron intake and ID (Supplementary Table S7).
Except when ID was defined as ferritin <300 ng/ml, ferritin <15 ng/ml, or iron <13 μmol/L, univariable regression analysis showed a significant relationship between dietary total folate and ID. Despite this, when including gender in the multivariable regression analysis, there was no significant association between the dietary intake of total folate and any of the ID definitions (Supplementary Table S7).
The analysis of HF patients revealed no significant relationship between dietary iron intake and ID in univariable logistic regression analysis. However, in multivariable logistic regression analysis, a higher intake of iron seemed to be associated with ferritin <15 ng/ml (OR: 1.08; 95% CI: 1.00–1.16; P = 0.050) (Supplementary Table S8).
In HF patients, the relationship between total folate intake and ID was significant when ID was defined as ferritin <30 ng/ml. A higher intake of total folate was associated with a higher risk of ID, as shown by both univariable (OR: 1.19; 95% CI: 1.03–1.38; P = 0.023) and multivariable regression analyses (excluding gender: OR: 1.21; 95% CI: 1.04–1.41; P = 0.015, and including gender: OR: 1.21; 95% CI: 1.05–1.39; P = 0.009) (Supplementary Table S8).
Following the exclusion of HF patients with ferritin <30 ng/ml, a re-analysis of the remaining subjects revealed a potential association between higher levels of dietary total folate intake and the lower risk of ID, as defined by ferritin <100 ng/ml (univariable: OR: 0.78; 95% CI: 0.63–0.96; P = 0.022, multivariable (excluding gender): OR: 0.80; 95% CI: 0.65–0.99; P = 0.042 and multivariable (including gender): OR: 0.80; 95% CI: 0.65–1.00; P = 0.047) (Table 4).
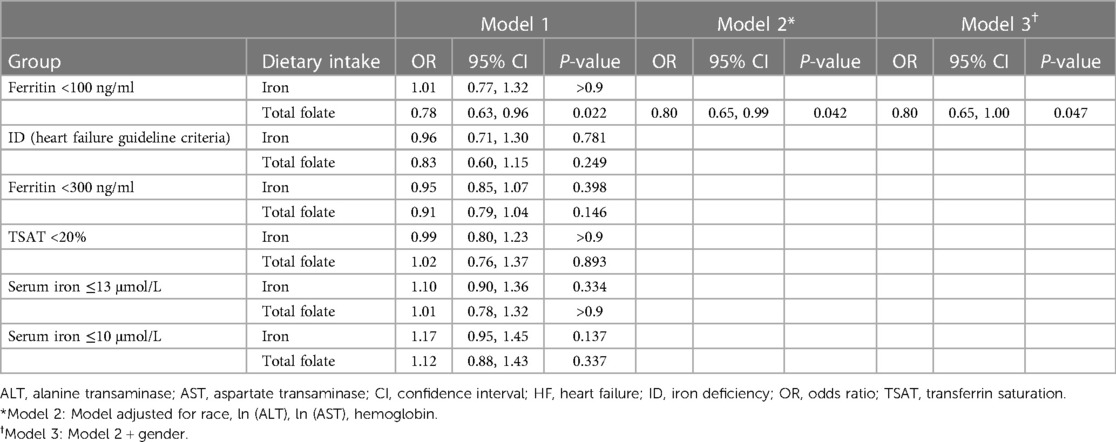
Table 4. Relationship of dietary intake to iron deficiency in HF patients with ferritin ≥30 ng/ml (logistic regression model).
In the present investigation, we substantiated that the occurrence of ID was consistent between individuals in the general population and those diagnosed with HF. Moreover, a ferritin level below 30 ng/ml was linked to a higher risk of all-cause mortality over 10 and 15 years in HF patients, and similarly, it was associated with a 10-year all-cause mortality in the general population. Furthermore, a reduced dietary intake of total folate emerged as a significant predictive factor for maintaining serum ferritin levels below 100 ng/ml specifically in HF patients.
From the data of NHANES III, the prevalence of ID (defined by heart failure guideline criteria) in 574 participants (male: 50.2%) with self-reported HF was 61.3% (5). In a prospective observational study of 546 patients (male: 88%) with stable systolic chronic HF, the prevalence of ID (defined by heart failure guideline criteria) was 37% in the entire population of systolic chronic HF patients (4). In a survey of 832 hospitalized patients with decompensated chronic HF, the prevalence of ID (defined by heart failure guideline criteria) was found to be 68.6% in male and 75.3% in female (3). In three European population-based cohorts (male: 45.2%), 60% of individuals had ferritin levels below 100 ng/ml, 16.4% had ferritin levels below 30 ng/ml, and 64.3% met the heart failure guideline criteria for ID (6). In the present study, the prevalence of ID defined by guideline criteria in HF patients (male: 52%) was 66%. It was noteworthy that the prevalence of ID was comparable in the non-HF population (Table 1, Supplementary Table S1), which contradicted our previous assumptions. A large proportion of HF patients had impaired nutritional status (24), which may affect iron metabolism. Besides, the proportion of anemia in HF patients is relatively high. These factors make us unconsciously believe that the proportion of ID in HF patients is relatively high. Alternatively, it was also hypothesized that HF patients would demonstrate a lower incidence of ID due to the pro-inflammatory state associated with HF, which could lead to elevated levels of ferritin (25, 26). In terms of disease prevalence, the existing diagnostic criteria for ID are ineffective in distinguishing between individuals with HF and those without.
In line with the findings reported by Masini et al. (7), our study revealed a significant association between TSAT <20% and all-cause mortality over a five-year period in HF patients, whereas levels of ferritin did not demonstrate a significant association (Table 2). However, our findings demonstrated a significant association between low levels of ferritin (<30 ng/ml) and an elevated risk of long-term all-cause mortality in both HF patients and the general population. Hence, TSAT emerges as a predictor of short-term all-cause mortality in HF patients, whereas ferritin demonstrates greater suitability for predicting long-term all-cause mortality, possibly due to the inherent fluctuations of TSAT and ferritin levels in the human body. In patients with HF, 55.5% of patients with iron ≤13 μmol/L at baseline remained ID after 1 year. Applying TSAT <20% as the definition of ID, 58.2% of patients remained ID after 1 year. When applying the heart failure guideline criteria, this proportion rose to 79.4% (27). Of the 537 patients with baseline ID (TSAT <20%), 53% (284 individuals) still had ID after 6 months. Meanwhile, 20% (128 individuals) of the 451 patients without baseline ID developed ID by the 6-month follow-up (15). Another possible reason is that the definitions of ID mentioned above were overly inclusive, encompassing a substantial proportion of individuals who did not truly exhibit ID. Therefore, the observed changes in iron status may have been only normal fluctuations within the normal range. A randomized, placebo-controlled, double-blind trial (IronIC) found that heart transplant recipients with ID defined by heart failure guideline criteria had no improvement in peak oxygen consumption 6 months after intravenous iron treatment while the peak oxygen consumption was significantly improved in patients with ferritin <30 ng/ml at baseline (4.4 ml/kg/min, 95% confidence interval 0.01–8.72, P = 0.0495) (28). Hence, a diagnostic threshold of ferritin <30 ng/ml may be more appropriate, as it is associated with the prognosis of both HF and non-HF individuals. Nevertheless, additional research and validation are warranted to investigate the stability of iron status in individuals presenting ferritin levels below this threshold.
In the present study, it is recommended that when the ferritin level is <30 ng/ml or the TSAT level is <20%, treatment should be considered for the general population aged 45 years and above. For patients with HF, intravenous iron replacement therapy should be considered when the ferritin level is <30 ng/ml (15%) or the TSAT level is <20% (45%). In our study, a ferritin level <300 ng/ml is also associated with all-cause mortality in patients with HF over a period of five years, therefore, a ferritin level >300 ng/ml may be used as a treatment target, and revious research has demonstrated the feasibility of achieving a ferritin level >400 ng/ml through intravenous iron replacement therapy (8).
Iron from dietary sources exists in two primary forms: haem iron and non-haem iron. Haem iron is absorbed from the gastrointestinal tract with notably higher efficiency (29). Notably, the consumption of haem iron, rather than total iron intake, exhibits a significant correlation with ferritin levels (16, 17). Extensive research has demonstrated the enhanced effectiveness of a combination of folic acid and iron, as opposed to iron supplementation alone, in addressing ID (18, 19). In the present study, after adjusting for confounding factors such as gender, there was no significant relationship between dietary iron intake and ID. It is not surprising that there is no significant association between dietary iron intake and ID when oral iron therapy failed to achieve the desired effect (30). However, for HF patients, after adjusting for covariates such as anemia, gender and anemia-related treatment, a significant correlation was observed between higher total folate intake and an increased risk of ferritin <30 ng/ml. That might be explained by the tendency of HF patients with low levels of ferritin to receive recommendations or to spontaneously increase their consumption of iron and folate-rich foods. After excluding these severe ID patients (ferritin <30 ng/ml), it is promising that an increase in the consumption of folate-rich foods seems to contribute to a reduction in the incidence of ID (ferritin <100 ng/ml, OR: 0.80; 95% CI: 0.65–1.00; P = 0.047). Given the suboptimal outcomes observed in the treatment of ID in HF patients using oral iron supplementation, oral folic acid may represent a promising avenue for intervention (30). However, it is imperative to elucidate the underlying mechanism through which increased folate intake mitigates iron deficiency, without considering iron intake, warranting further exploration.
This study has several limitations. Firstly, out of the total 21,004 participants, only a subset underwent testing for ferritin and other related measures, and not all selected individuals were able to comply with the scheduled appointments. However, NHANES provided sample weights to correct for unequal sampling probabilities and non-participation of selected individuals, ensuring that the final results were representative of the national population. Secondly, heart failure status in the NHANES database relied on self-reported participant responses through questionnaires, potentially introducing self-report bias. Meanwhile, there was a dearth of heart failure classification data. Thirdly, the cohort of patients with HF was relatively small, which presented a significant numerical disparity compared to the non-HF group and raised notable concerns. Fourthly, while this study analyzed neutrophil percentage and C-reactive protein as confounding factors, it's important to recognize that the complex interplay between ID, inflammation, ferritin, and iron metabolism could potentially influence the research conclusions. In addition, the paradoxical correlation between total folate intake and severe iron deficiency (ferritin <30 ng/ml) in the present study requires further investigation, as our assumption lacks supporting evidence. Furthermore, the results of this study need to be validated in further prospective research with larger sample sizes. Dynamic monitoring of iron status and dietary data should be conducted, and there was also a lack of measurement data for soluble transferrin receptor.
The prevalence of iron deficiency was identical in heart failure and non-heart failure individuals. Ferritin <30 ng/ml was associated with the long-term outcome of both the general and heart failure populations, while transferrin saturation (TSAT) <20% was associated with the 5-year mortality of heart failure patients and the relatively healthy population aged 45 years and above. A diet rich in folate might have the potential for prevention and treatment of iron deficiency in heart failure patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the NCHC (National Center for Health Statistics) Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
HS: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. QW: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. WH: Data curation, Investigation, Writing – original draft. CC: Data curation, Writing – original draft. TW: Data curation, Writing – original draft. JZ: Conceptualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Natural Science Foundation of China (82270331) and Qingdao Key Clinical Specialty Elite Discipline (QDZDZK-2022008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1342686/full#supplementary-material
1. Klip IT, Comin-Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J. (2013) 165(4):575–82.e3. doi: 10.1016/j.ahj.2013.01.017
2. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. J Card Fail. (2011) 17(11):899–906. doi: 10.1016/j.cardfail.2011.08.003
3. Cohen-Solal A, Damy T, Terbah M, Kerebel S, Baguet JP, Hanon O, et al. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur J Heart Fail. (2014) 16(9):984–91. doi: 10.1002/ejhf.139
4. Jankowska EA, Rozentryt P, Witkowska A, Nowak J, Hartmann O, Ponikowska B, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J. (2010) 31(15):1872–80. doi: 10.1093/eurheartj/ehq158
5. Parikh A, Natarajan S, Lipsitz SR, Katz SD. Iron deficiency in community-dwelling US adults with self-reported heart failure in the national health and nutrition examination survey III: prevalence and associations with anemia and inflammation. Circ Heart Fail. (2011) 4(5):599–606. doi: 10.1161/circheartfailure.111.960906
6. Schrage B, Rübsamen N, Ojeda FM, Thorand B, Peters A, Koenig W, et al. Association of iron deficiency with incident cardiovascular diseases and mortality in the general population. ESC Heart Fail. (2021) 8(6):4584–92. doi: 10.1002/ehf2.13589
7. Masini G, Graham FJ, Pellicori P, Cleland JGF, Cuthbert JJ, Kazmi S, et al. Criteria for iron deficiency in patients with heart failure. J Am Coll Cardiol. (2022) 79(4):341–51. doi: 10.1016/j.jacc.2021.11.039
8. Kalra PR, Cleland JGF, Petrie MC, Thomson EA, Kalra PA, Squire IB, et al. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): an investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial. Lancet. (2022) 400(10369):2199–209. doi: 10.1016/s0140-6736(22)02083-9
9. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. (2009) 361(25):2436–48. doi: 10.1056/NEJMoa0908355
10. WHO Guidelines Approved by the Guidelines Review Committee. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations. Geneva: World Health Organization (2020).
11. Grote Beverborg N, Klip IT, Meijers WC, Voors AA, Vegter EL, van der Wal HH, et al. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail. (2018) 11(2):e004519. doi: 10.1161/circheartfailure.117.004519
12. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. (2022) 24(1):4–131. doi: 10.1002/ejhf.2333
13. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Am Coll Cardiol. (2017) 70(6):776–803. doi: 10.1016/j.jacc.2017.04.025
14. Philip KEJ, Sadaka AS, Polkey MI, Hopkinson NS, Steptoe A, Fancourt D. The prevalence and associated mortality of non-anaemic iron deficiency in older adults: a 14 years observational cohort study. Br J Haematol. (2020) 189(3):566–72. doi: 10.1111/bjh.16409
15. Fitzsimons S, Yeo TJ, Ling LH, Sim D, Leong KTG, Yeo PSD, et al. Impact of change in iron status over time on clinical outcomes in heart failure according to ejection fraction phenotype. ESC Heart Fail. (2021) 8(6):4572–83. doi: 10.1002/ehf2.13617
16. Lavriša Ž, Hristov H, Hribar M, Koroušić Seljak B, Gregorič M, Blaznik U, et al. Dietary iron intake and biomarkers of iron Status in slovenian population: results of SI.menu/nutrihealth study. Nutrients. (2022) 14(23):5144. doi: 10.3390/nu14235144
17. Young I, Parker HM, Rangan A, Prvan T, Cook RL, Donges CE, et al. Association between haem and non-haem iron intake and Serum ferritin in healthy young women. Nutrients. (2018) 10(1):81. doi: 10.3390/nu10010081
18. Bumrungpert A, Pavadhgul P, Piromsawasdi T, Mozafari MR. Efficacy and safety of ferrous bisglycinate and folinic acid in the control of iron deficiency in pregnant women: a randomized, controlled trial. Nutrients. (2022) 14(3):452. doi: 10.3390/nu14030452
19. Juarez-Vazquez J, Bonizzoni E, Scotti A. Iron plus folate is more effective than iron alone in the treatment of iron deficiency anaemia in pregnancy: a randomised, double blind clinical trial. BJOG Int J Obstet Gynaecol. (2002) 109(9):1009–14. doi: 10.1111/j.1471-0528.2002.01378.x
20. National Center for Health Statistics. NHANES Questionnaires, Datasets, and Related Documentation. Available online at: https://wwwn.cdc.gov/Nchs/Nhanes/ (accessed December 20, 2022).
21. National Center for Health Statistics. NCHS Ethics Review Board (ERB) Approval. Available online at: https://www.cdc.gov/nchs/nhanes/irba98.htm (accessed June 17, 2023).
22. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
23. National Center for Health Statistics. 2019 Public-Use Linked Mortality Files. Available online at: https://www.cdc.gov/nchs/data-linkage/mortality-public.htm (accessed December 20, 2022).
24. Kałużna-Oleksy M, Krysztofiak H, Sawczak F, Kukfisz A, Szczechla M, Soloch A, et al. Sex differences in the nutritional status and its association with long-term prognosis in patients with heart failure with reduced ejection fraction: a prospective cohort study. Eur J Cardiovasc Nurs. (2024):zvad105. doi: 10.1093/eurjcn/zvad105. [Epub ahead of print].
25. von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail. (2019) 7(1):36–46. doi: 10.1016/j.jchf.2018.07.015
26. Cabrera CC, Ekström M, Linde C, Persson H, Hage C, Eriksson MJ, et al. Increased iron absorption in patients with chronic heart failure and iron deficiency. J Card Fail. (2020) 26(5):440–3. doi: 10.1016/j.cardfail.2020.03.004
27. Graham FJ, Masini G, Pellicori P, Cleland JGF, Greenlaw N, Friday J, et al. Natural history and prognostic significance of iron deficiency and anaemia in ambulatory patients with chronic heart failure. Eur J Heart Fail. (2022) 24(5):807–17. doi: 10.1002/ejhf.2251
28. Brautaset Englund KV, Østby CM, Rolid K, Gude E, Andreassen AK, Gullestad L, et al. Intravenous iron supplement for iron deficiency in cardiac transplant recipients (IronIC): a randomized clinical trial. J Heart Lung Transplant. (2021) 40(5):359–67. doi: 10.1016/j.healun.2021.01.1390
29. Diego Quintaes K, Barberá R, Cilla A. Iron bioavailability in iron-fortified cereal foods: the contribution of in vitro studies. Crit Rev Food Sci Nutr. (2017) 57(10):2028–41. doi: 10.1080/10408398.2013.866543
30. Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, et al. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA. (2017) 317(19):1958–66. doi: 10.1001/jama.2017.5427
Keywords: iron deficiency, heart failure, ferritin, transferrin saturation, long-term prognosis, folate
Citation: Sun H, Wang Q, Han W, Chen C, Wang T and Zhong J (2024) Iron deficiency: prevalence, mortality risk, and dietary relationships in general and heart failure populations. Front. Cardiovasc. Med. 11:1342686. doi: 10.3389/fcvm.2024.1342686
Received: 27 November 2023; Accepted: 28 February 2024;
Published: 18 March 2024.
Edited by:
Vassilios Vassilikos, Aristotle University of Thessaloniki, GreeceReviewed by:
Michał Czapla, Wroclaw Medical University, Poland© 2024 Sun, Wang, Han, Chen, Wang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingquan Zhong MTk4NzYyMDAwNzc4QGVtYWlsLnNkdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.