- 1Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Department of Cardiology, The Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
Objectives: This study focused on the association between visceral adiposity index (VAI) and the prevalence of hypertension in a nationally representative population of American adults.
Methods: The study obtained data from the National Health and Nutrition Examination Survey (NHANES) database from 2003–2018 for a large-scale study. This study incorporated participants ≥18 years of age. Multivariate logistic regression modelling and smoothed curve fitting were applied to investigate the existence of a correlation between VAI and hypertension prevalence. Subgroups were analyzed to confirm the stationarity of the association between VAI and hypertension prevalence. In addition, an interaction test was conducted in this study.
Results: In completely adapted sequential models, the risk of hypertension prevalence in the overall population increased 0.17-fold with each 1-unit increase in VAI [odds ratio (OR) = 1.17; 95% confidence interval (CI) 1.12–1.22]. In the wholly adapted categorical model, there was a 0.95-fold increased risk of hypertension in the population of VAI quartile 4 (Q4) vs. VAI quartile 1 (Q1) (OR = 1.95; 95% CI 1.62–2.35). These results indicate that VAI was strongly related to the occurrence of hypertension, and smoothed curve-fitting analysis showed nonlinearity. Adjustment for covariates revealed no apparent interactions in the subgroup analyses, and results were stable across subgroups.
Conclusion: This cross-sectional study suggests a nonlinear and positive correlation between elevated VAI and the adult risk of developing hypertension in U.S. adults.
1 Introduction
Hypertension is a common chronic disease with high prevalence and is one of the most prevalent risk elements for cardiovascular disease (CVD) (1, 2). According to epidemiologic statistics, there are approximately 1 billion hypertensive patients worldwide, and it is projected that the population of hypertensive patients will increase to 1.292 billion by 2025 (3, 4). In the United States, it was found that the prevalence of hypertension in adults is about 32%–46% (5). Among those aged 60 years and above, the percentage of women suffering from hypertension is about 75% (6). The global incidence of hypertension has been gradually increasing in recent years, and the age of hypertension has been decreasing. Hypertension is the disease that causes the most significant number of deaths in the world and causes severe damage to the heart, brain, and kidneys. At the same time, hypertension itself and its complications cause suffering to patients and a substantial financial burden to families. The prevention, treatment, and management of hypertension has become an urgent issue in the field of public health and medical care.
Risk considerations for hypertension include high sodium and low potassium intake in the diet, smoking and use of alcohol in lifestyle behaviours, psychological stress, sleep disorders, obesity and overweight (7). Hypertension due to obesity and overweight has become a hot issue widely studied by scientists, and its prevalence has increased significantly in the United States and worldwide. Among the many complications of obesity, hypertension is the most common and significant complication, accounting for approximately 70% of the obese population (8). Measures of obesity include body mass index (BMI), waist circumference (WC), and hip circumference (9, 10). However, there are limitations in clinical application, such as BMI is incapable of evaluating body fat percentage, and WC is incapable of distinguishing between visceral adipose tissue and abdominal subcutaneous adipose tissue (11). The visceral adiposity index (VAI) has been proposed to address this issue. VAI is an indicator of visceral adiposity related to cardiometabolic status and has been shown to correlate with visceral adipose tissue area and volume independently of subcutaneous adipose tissue (12). Hence, VAI, which has been introduced as a surrogate indicator of adipose tissue function, could more directly predict the progression and risk of cardiovascular disease (13, 14). Numerous studies have found VAI to be positively associated with insulin resistance (IR), kidney stones, heart failure, and depression (15–18). In addition, epidemiologic studies exploring the relationship between VAI and the risk of hypertension have shown that VAI is positively linked to the risk of hypertension in the Chinese population (19) and that there is a nonlinear positive association between VAI and cardiovascular disease (20). In contrast, other studies have found no correlation with gender (21). There are no extensive cross-sectional studies on the association between VAI and the risk of hypertension in the United States.
Therefore, the purpose of the current investigation is to detect the potential link between VAI and the prevalence of hypertension in U.S. adults through the data collected by NHANES between 2003 and 2018 to provide a scientific basis for the prevention, diagnosis, treatment, and of hypertension as well as to provide strong evidence for the results of previous studies.
2 Materials and methods
2.1 Study population
NHANES is an extensive cross-sectional survey designed to assess the health and nutritional status of adults and children in the United States. Our data are all obtainable from the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm), all participants signed informed consent forms, and the project was endorsed by the National Center for Health Statistics (NCHS) Ethics Review Board. We analyzed data from the last eight cycles (2003–2018). A total of 80,312 subjects were enrolled in 8 consecutive NHANES investigative cycles between 2003 and 2018, including 14,569 participants. The reasons for excluding other participants were as follows: 1. 32,549 aged <18 years; 2.18 missing information on hypertension; 3. 28,076 missing information on the VAI index; and 4. 5,100 missing information on covariates (Figure 1).
2.2 Definition of VAI
NHAENS blood sample collection and measurements are performed according to standardized protocols from the Centers for Disease Control and Prevention (CDC). A dedicated staff is available to organize the information, specimen testing, and analysis.VAI assesses visceral fat using anthropometric data (BMI, WC) and biochemical indicators (TG, HDL-C). The formula was calculated as follows: VAI = [WC/(39.68 + 1.88 × BMI)] × (TG/1.03) × (1.31/HDL) for men, and VAI = [WC/(36.58 + 1.89 × BMI)] × (TG/0.81) × (1.52/HDL) for women. BMI = body weight (kg)/height (m2), WC units are cm, TG and HDL units are mmol/L.
2.3 Definition of hypertension
NHANES organized experienced investigators to record participants' blood pressure based on the American Heart Association guidelines (22). The specific blood pressure measurements are all available on the official NHANES website. In the resting state for at least 5 min, we performed three consecutive blood pressure measurements (each at least 1 min apart) to obtain their average values. Hypertension was defined as (i) self-reported hypertension, (ii) current use of antihypertensive medication, and (iii) mean systolic blood pressure (SBP) ≥ 140 mmHg and/or mean diastolic blood pressure (DBP) ≥ 90 mmHg.
2.4 Covariates
Based on the methodology of previous studies, we selected variables such as demographic data, socioeconomic status, lifestyle, physical examination, laboratory tests, and personal history as potential confounders to estimate the relation between VAI and hypertension. Covariates comprised age, gender, ethnicity, education, marital status, household poverty-to-income ratio (PIR), smoking, alcohol consumption, BMI, WC, total cholesterol (TC), high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides (TG), fasting blood glucose (FPG), hemoglobin A1c (HbA1c), and estimated glomerular filtration rate (eGFR), diabetes mellitus(DM), coronary heart disease(CHD), antihypertensive medications, and antiHyperlipidemic medications. Participants were categorized into five racial groups: Mexican American, non-Hispanic white, non-Hispanic black, other Hispanic, and other races. Age (18–34, 35–54, 55–74, ≥75), sex (male/female), level of education (less than 9th grade, 9th–11th grade, high school graduation, some college graduation, college and above), marital status (married, widowed, divorced, separated, unmarried, living with a partner), PIR (<1.3, 1.3–3.5, ≥3.5), and cigarettes (previous, current, non-smoking), alcohol consumption (previous, light/moderate, heavy, non-drinking). Diabetes mellitus (yes/no), coronary heart disease (yes/no), antihypertensive drugs (yes/no), antihyperlipidemic drugs (yes/no).
2.5 Statistical analysis
All data in this study were statistically analyzed by applying R language (version 4.3.1). Continuous variables were represented as mean ± standard deviation, and categorical variables were represented as frequency (percentage). Differences between categorical variables were analyzed using the chi-square test, while continuous variables meeting normal distribution were tested using the weighted Student's t-test; otherwise, the Mann-Whitney u-test was used. We quadruple-categorized the VAI. Multivariate logistic regression models were applied to investigate the relevance of VAI to hypertension after adjusting for potential confounders. The degree of correlation was represented as OR and 95% CI. Three models were developed: Model 1 was not adjusted for confounders; age, gender, and ethnicity were adjusted in Model 2, and Model 3 further adjusted for level of education, marital status, PIR, tobacco use, alcohol use, eGFR, DM, CHD, antihypertensive drugs, and antihyperlipidemic drugs. Smoothed curve fitting was then applied to examine the existence of a nonlinear relationship between VAI and hypertension. Ultimately, subgroup analyses of the confounders listed in the baseline table (age, sex, race, education, marriage, eGFR, smoking, alcohol use, DM, and CHD) were performed using hierarchical logistic regression modelling for the presence of interactions. The threshold for a statistically significant difference was set at P < 0.05.
3 Results
3.1 Baseline characteristics
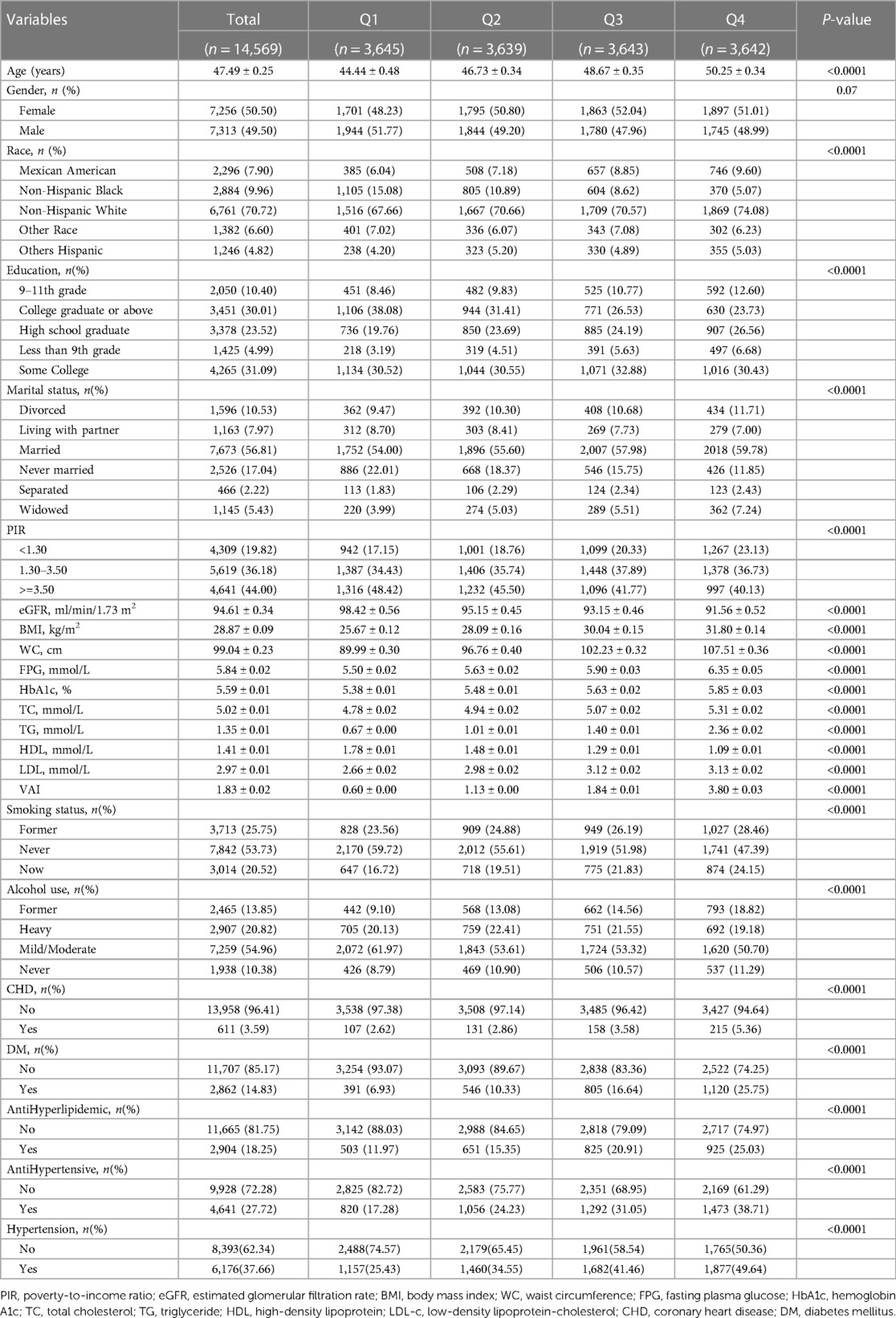
The characteristics of all participants are shown in Table 1. A total of 14,569 (Figure 1) participants ≥18 years of age were included in this research. Hypertension was present in 37.66% of the participants, and the weighted mean age was 47.49 ± 0.25 years. Among them, 49.50% were males, and 50.50% were females. Patients at higher risk for hypertension tended to be female, ≥50 years of age, non-Hispanic white, married, less educated, lower household PIR, higher BMI and WC, higher lipid and glucose levels, and higher alcohol and tobacco use. In addition, patients at higher risk for hypertension were more likely to have diabetes and coronary heart disease. They were divided into four groups (Q1-Q4) according to the VAI quartiles. The differences in age, race, level of education, marital status, PIR, smoking, alcohol consumption, BMI, WC, FPG, HbA1c, TC, TG, LDL, HDL, eGFR, DM, CHD, antihypertensive medication, and antihyperlipidemic medication among the four groups of patients were statistically significant (P < 0.05).
3.2 Multifactorial logistic regression analysis
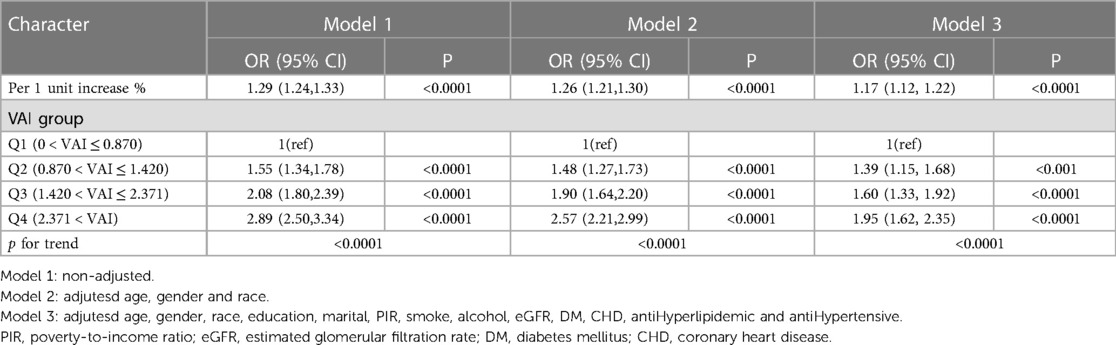
Table 2 shows the relationship between VAI and hypertension incidence. Three models were constructed: model 1, model 2, and model 3. VAI was used as a continuous variable, and in model 1 (Unadjusted model), the prevalence of hypertension increased by 29% with each unit increase in VAI, with an effect value OR and 95% CI of 1.29 (1.24, 1.33), respectively. Model 2 adjusted for age, gender, and ethnicity and had an effect value OR and 95% CI of 1.26 (1.21, 1.30), respectively. Model 3 continued to adjust for education, marriage, PIR, smoking, alcohol consumption, eGFR, DM, CHD, antihypertensive medication, and antihyperlipidemic medication based on model 2, with an effect value OR and 95% CI of 1.17 (1.12, 1.22), respectively. VAI was changed from a continuous variable to a categorical variable and categorized into four levels based on quartiles of VAI. Using Q1 as a control, the prevalence of VAI and hypertension exhibited a monotonically increasing trend in all models (P < 0.0001). In conclusion, VAI is positively linked to the development of hypertension.
3.3 Curve fitting analysis
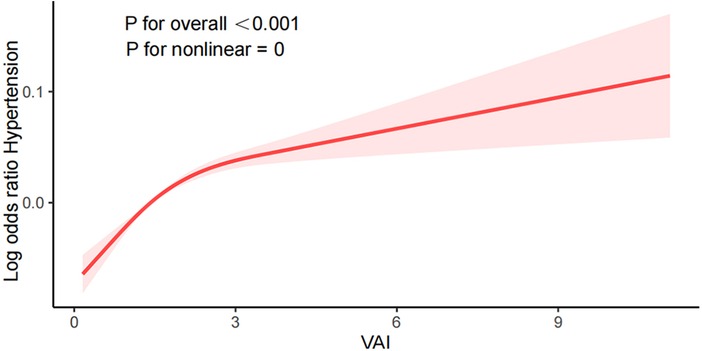
Smooth curve fitting plots, like those shown in Figure 2, visualize the connection between VAI and the prevalence of hypertension. The nonlinear association between VAI and hypertension(P for nonlinear <0.001) was observed after adjusting for confounders such as age, gender, ethnicity, level of education, marital status, PIR, smoking, alcohol consumption, eGFR, DM, CHD, antihypertensive drugs, and antihyperlipidemic drugs. The risk of hypertension showed a nonlinear positive increasing trend with increasing VAI, and the trend was steeper with larger VAI.
3.4 Subgroup analysis
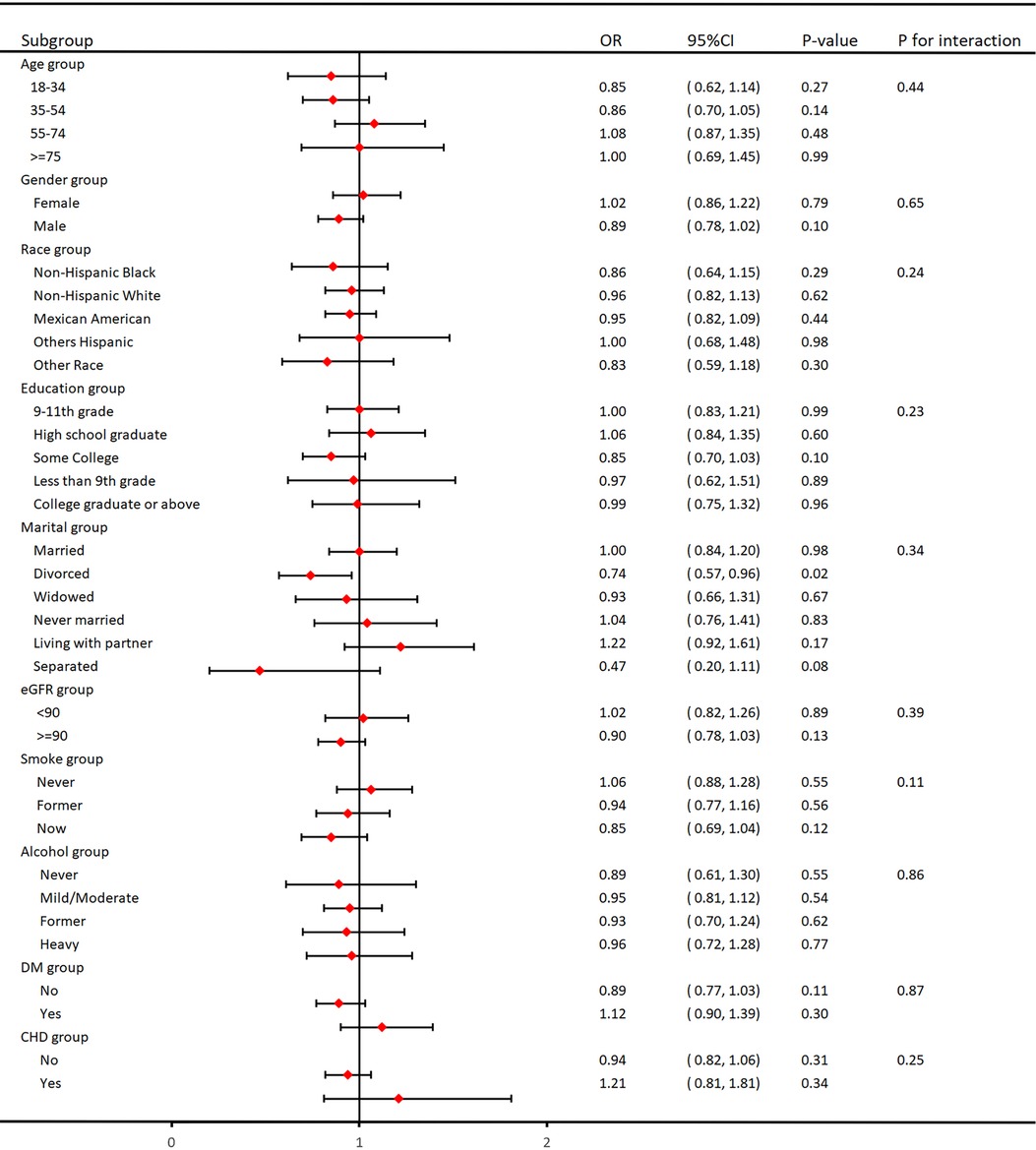
As shown in Figure 3, we performed subgroup analyses to validate the relationship between VAI and hypertension. It is worth noting that there was no interaction between age, gender, ethnicity, education, marital status, tobacco use, alcohol use, eGFR, DM, and CHD in the subgroups, and they were highly stable.
4 Discussion
In this large cross-sectional study, we included 14,569 participants aged ≥18 in the United States to evaluate the link between VAI and hypertension. The outcome shows that in Table 1, the associations between VAI and hypertension were not affected by confounders such as age, race, education, marital relationship, and PIR. Upon adjustment for potential covariates, we discovered a positive relation between VAI and hypertension, suggesting that VAI is a risk factor for the development of hypertension. The greater the level of VAI, The greater the risk of developing hypertension. We performed smoothed curve fitting and found that this positive correlation was nonlinear (P for nonlinear <0.001), with a steeper elevation trend with higher VAI values. In addition, subgroup analyses were performed to reveal potential relationships through these data. The subgroups were non-interacting and stable.
The prevalence of overweight and obesity in the population is continuing to rise in the United States and globally, inevitably creating a major epidemic (23, 24). According to epidemiologic statistics, approximately 2.1 billion people worldwide will suffer from the disease by 2030 (25). Obesity is also a significant risk factor for cardiovascular disease, coronary heart disease, type 2 diabetes, heart failure, and hypertension. Although hypertension has been studied in detail, the pathogenesis of obesity is currently unknown. However, it is related to adipose tissue. Adipose tissue dysfunction activates the renin-angiotensin-aldosterone system, which increases blood pressure. First, subcutaneous adipose tissue in the abdomen of obese individuals secretes large amounts of angiotensin II (AngII) (26); second, angiotensinogen overexpression in adipose tissue increases blood pressure (27); and third, adipocytes depend on AngII for aldosterone production (28). Fourth, adipose tissue-derived mineralized corticotropin-releasing factor stimulates aldosterone release in adrenocortical cells (29). Hypertension has also been found to be associated with a variety of hormones secreted in adipose tissue (30). Leptin is an adipocyte-derived hormone, and obesity leads to elevated leptin levels, which stimulates increased sympathetic nerve activity (31), leading to increased heart rate and blood pressure. Resistin is significantly increased during obesity (32), plays a role in insulin resistance (33), and induces hypertension by inducing angiotensinogen (34). Lipocalin secretion is reduced in obese patients (35), leading to a decrease in endothelial nitric oxide synthase and prostaglandin I 2 synthase, which allows for diminished vasodilatation and exhibits elevated blood pressure (36). Therefore, excess adipose tissue may cause hormonal, inflammatory, and endothelial alterations resulting in events such as increased insulin resistance, increased sympathetic activity, activation of the renin-angiotensin-aldosterone system, endothelial dysfunction, and renal sodium reabsorption, which ultimately lead to elevated blood pressure (37).
VAI is a reliable and comprehensive index for assessing visceral fat (11). Visceral fat is measured using abdominal CT or magnetic resonance imaging (MRI). Although these methods are more accurate, they are costly and inefficient, with fewer clinical applications and side effects (17).VAI is calculated from the anthropometric indexes BMI and WC and the biochemical indexes TG and HDL, which are simple to operate and have easily accessible and safe data.VAI is associated with abdominal aortic calcification, cardiovascular disease, metabolic syndrome, and stroke and has been studied epidemiologically (38–41). The present research indicated a nonlinear positive correlation between VAI and the prevalence of hypertension, which may be related to inflammation, insulin resistance and adipocytokine production. Inflammation plays a crucial role in hypertension, and many studies have shown that hypertensive patients have elevated levels of systemic inflammation, and inhibition of inflammation reduces hypertension, which helps to predict the risk of hypertension through inflammation (42). When obesity after excessive calorie intake increases the association between adipose tissue and inflammation, inflammatory expression and systemic inflammation levels increase, allowing vasoconstriction, increased endothelial adhesion, sympathetic excitation and the development of hypertension (43, 44). Visceral adipocytes produce adipocytokines, including leptin, resistin, lipocalin, and inflammatory cytokines, which increase IR (15). Excess adipose tissue can increase IR by promoting inflammation through increased levels of resistin or tumour necrosis factor-α. Adipose tissue macrophages activate inflammatory signalling pathways within neighbouring insulin-targeting cells (adipocytes), releasing inflammatory factors directly involved in the development of IR (45).IR increases the sympathetic nervous system by enhancing angiotensin II (AngII) and aldosterone activity, and oxidative stress leads to elevated blood pressure (46). Therefore, it is advisable to eat a light diet, reduce the intake of high-fat diets, control body weight and blood glucose, enhance exercise, and regularly monitor fat content, blood glucose, and lipid levels to prevent hypertension.
This study has a number of strengths: (i) these are the first cross-sectional investigations to evaluate the link between VAI and hypertension in a widespread American population. (ii) The sample selection and sample size are representative and adequate, and are worthy of replication. (iii) We reviewed related studies and adjusted for the interference of confounders to make the results more accurate. In addition, this study has some limitations: (i) due to the long period, the complicated calculation of VAI, and the fact that the assessment may be subject to anthropometric indicator errors and biochemical indicator inaccuracies. (ii) We could not analyze special populations Because the observation was U.S. adults, excluding minors. (iii) Although confounding factors affecting hypertension were adjusted for in the study, other influencing factors may exist.
5 Conclusion
In summary, after adjusting for potential confounders, this cross-sectional study based on eight cycles of NHANE (2003–2018) data showed a nonlinear positive association between VAI and hypertension in US adults. Thus, VAI may serve as a specific marker to identify hypertension risk at an early period in the adult population in order to reduce its prevalence. In the future, more randomized controlled trials or prospective studies are needed to confirm this finding.Secondly, VAI will be extended to basic experiments to explore its pathogenesis with related diseases. Finally, we will promote VAI in the clinic to provide convenience for patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics (NCHS) Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HZ: Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing. TL: Formal Analysis, Methodology, Software, Writing – review & editing. JL: Methodology, Software, Writing – review & editing, Funding acquisition. DZ: Formal analysis, Resources, Software, Visualization, Writing – review & editing. JY: Conceptualization, Data curation, Methodology, Writing – review & editing. XZ: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the National Natural Science Foundation of China (No. 82374385) and the Shandong Province “Taishan Scholar Youth Expert” Construction Project Funds (No. tsqn202211352)
Acknowledgments
The author thanks the staff and the participants of the NHANES study for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Parati G, Lackland DT, Campbell NRC, Owolabi MO, Bavuma C, Mamoun Beheiry H, et al. How to improve awareness, treatment, and control of hypertension in Africa, and how to reduce its consequences: a call to action from the world hypertension league. Hypertension. (2022) 79:1949–61. doi: 10.1161/HYPERTENSIONAHA.121.18884
2. Di Palo KE, Barone NJ. Hypertension and heart failure: prevention, targets, and treatment. Cardiol Clin. (2022) 40:237–44. doi: 10.1016/j.ccl.2021.12.011
3. Hengel FE, Sommer C, Wenzel U. Arterial hypertension. Dtsch Med Wochenschr. (2022) 147:414–28. doi: 10.1055/a-1577-8663
4. Chen S, Cheng W. Relationship between lipid profiles and hypertension: a cross-sectional study of 62,957 Chinese adult males. Front Public Health. (2022) 10:895499. doi: 10.3389/fpubh.2022.895499
5. Wu M, Si J, Liu Y, Kang L, Xu B. Association between composite dietary antioxidant index and hypertension: insights from NHANES. Clin Exp Hypertens. (2023) 45(1):2233712. doi: 10.1080/10641963.2023.2233712
6. Yu J, Li J, Li M, Wang L, Xu X, Li M. Association between serum Klotho concentration and hypertension in postmenopausal women, a cross-sectional study from NHANES 2013–2016. BMC Geriatr. (2023) 23(1):466. doi: 10.1186/s12877-023-04191-8
7. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16(4):223–37. doi: 10.1038/s41581-019-0244-2
8. Chrysant SG. Pathophysiology and treatment of obesity-related hypertension. J Clin Hypertens (Greenwich). (2019) 21(5):555–9. doi: 10.1111/jch.13518
9. Gnatiuc L, Alegre-Díaz J, Halsey J, Herrington WG, López-Cervantes M, Lewington S, et al. Adiposity and blood pressure in 110,000 Mexican adults. Hypertension. (2017) 69:608–14. doi: 10.1161/HYPERTENSIONAHA.116.08791
10. de Oliveira CM, Ulbrich AZ, Neves FS, Dias FAL, Horimoto ARVR, Krieger JE, et al. Association between anthropometric indicators of adiposity and hypertension in a Brazilian population: baependi heart study. PLoS One. (2017) 12:e185225. doi: 10.1371/journal.pone.0185225
11. Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. (2010) 33:920–2. doi: 10.2337/dc09-1825
12. Xue Y, Shen Q, Li C, Dai Z, He T. The visceral adipose index in relation to incidence of hypertension in Chinese adults: china health and nutrition survey (CHNS). Nutrients. (2020) 12(3):805. doi: 10.3390/nu12030805
13. Ferreira FG, Juvanhol LL, da Silva DCG, Longo GZ. Visceral adiposity index is a better predictor of unhealthy metabolic phenotype than traditional adiposity measures: results from a population-based study. Public Health Nutr. (2019) 22:1545–54. doi: 10.1017/S136898001800335X
14. Kouli GM, Panagiotakos DB, Kyrou I, Georgousopoulou EN, Chrysohoou C, Tsigos C, et al. Visceral adiposity index and 10-year cardiovascular disease incidence: the ATTICA study. Nutr Metab Cardiovasc Dis. (2017) 27:881–9. doi: 10.1016/j.numecd.2017.06.015
15. Jiang K, Luan H, Pu X, Wang M, Yin J, Gong R. Association between visceral adiposity index and insulin resistance: a cross-sectional study based on US adults. Front Endocrinol (Lausanne). (2022) 13:921067. doi: 10.3389/fendo.2022.921067
16. Wang J, Yang Z, Bai Y, Yin S, Cui J, Xiao Y, et al. Association between visceral adiposity index and kidney stones in American adults: a cross-sectional analysis of NHANES 2007–2018. Front Nutr. (2022) 9:994669. doi: 10.3389/fnut.2022.994669
17. Zhang X, Sun Y, Li Y, Wang C, Wang Y, Dong M, et al. Association between visceral adiposity index and heart failure: a cross-sectional study. Clin Cardiol. (2023) 46(3):310–9. doi: 10.1002/clc.23976
18. Lei J, Luo Y, Xie Y, Wang X. Visceral adiposity index is a measure of the likelihood of developing depression among adults in the United States. Front Psychol. (2022) 13:772556. doi: 10.3389/fpsyg.2022.772556
19. Fan Y, He D, Liu S, Qiao Y, Gao H, Xin L. Association between visceral adipose index and risk of hypertension in a middle-aged and elderly Chinese population. Nutr Metab Cardiovasc Dis. (2021) 31(8):2358–65. doi: 10.1016/j.numecd.2021.04.024
20. Zhang Y, He Q, Zhang W, Xiong Y, Shen S, Yang J, et al. Non-linear associations between visceral adiposity index and cardiovascular and cerebrovascular diseases: results from the NHANES (1999–2018). Front Cardiovasc Med. (2022) 9:908020. doi: 10.3389/fcvm.2022.908020
21. Leite NN, Cota BC, Gotine AREM, Rocha DMUP, Pereira PF, Hermsdorff HHM. Visceral adiposity index is positively associated with blood pressure: a systematic review. Obes Res Clin Pract. (2021) 15(6):546–56. doi: 10.1016/j.orcp.2021.10.001
22. Isselbacher EM, Preventza O, Hamilton Black J 3rd, Augoustides JG, Beck AW, Bolen MA, et al. 2022 Acc/Aha guideline for the diagnosis and management of aortic disease: a report of the American heart association/American college of cardiology joint committee on clinical practice guidelines. Circulation. (2022) 146(24):e334–482. doi: 10.1161/CIR.0000000000001106
23. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. (2014) 384:766–81. doi: 10.1016/S0140-6736(14)60460-8
24. Qasim A, Turcotte M, de Souza RJ, Samaan MC, Champredon D, Dushoff J, et al. On the origin of obesity: identifying the biological, environmental and cultural drivers of ge- netic risk among human populations. Obesity. (2018) 19:121–49. doi: 10.1111/obr.12625
25. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. American heart association statistics committee and stroke statistics subcommittee. heart disease and stroke statistics-2017 update: a report from the American heart association. Circulation. (2017) 135:e146–603. doi: 10.1161/CIR.0000000000000485
26. Harte A, McTernan P, Chetty R, Coppack S, Katz J, Smith S, et al. Insulin-mediated upregulation of the renin angiotensin system in human subcutaneous adipocytes is reduced by rosiglitazone. Circulation. (2005) 111:1954–61. doi: 10.1161/01.CIR.0000161954.17870.5D
27. Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose reninangiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. (2004) 287:R943–9. doi: 10.1152/ajpregu.00265.2004
28. Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. (2012) 59(5):1069–78. doi: 10.1161/HYPERTENSIONAHA.111.190223
29. Schütten MT, Houben AJ, de Leeuw PW, Stehouwer CD. The link between adipose tissue renin-angiotensin-aldosterone system signaling and obesity-associated hypertension. Physiology (Bethesda). (2017) 32(3):197–209. doi: 10.1152/physiol.00037.2016
30. Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, adipose tissue and vascular dysfunction. Circ Res. (2021) 128(7):951–68. doi: 10.1161/CIRCRESAHA.121.318093
31. Mark AL, Correia M, Morgan DA, Shaffer RA, Haynes WG. State-of-the-art-lecture: obesity-induced hypertension: new concepts from the emerging biology of obesity. Hypertension. (1999) 33(1 Pt 2):537–41. doi: 10.1161/01.hyp.33.1.537
32. Park SY, Kim KH, Seo KW, Bae JU, Kim YH, Lee SJ, et al. Resistin derived from diabetic perivascular adipose tissue up-regulates vascular expression of osteopontin via the AP-1 signalling pathway. J Pathol. (2014) 232(1):87–97. doi: 10.1002/path.4286
33. Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. (2001) 409(6818):307–12. doi: 10.1038/35053000
34. Jiang Y, Lu L, Hu Y, Li Q, An C, Yu X, et al. Resistin induces hypertension and insulin resistance in mice via a TLR4-dependent pathway. Sci Rep. (2016) 6:22193. doi: 10.1038/srep22193
35. Reneau J, Goldblatt M, Gould J, Kindel T, Kastenmeier A, Higgins R, et al. Effect of adiposity on tissue-specific adiponectin secretion. PLoS One. (2018) 13(6):e0198889. doi: 10.1371/journal.pone.0198889
36. Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. (2006) 47(6):1108–16. doi: 10.1161/01.HYP.0000222368.43759
37. Seravalle G, Grassi G. Obesity and hypertension. Pharmacol Res. (2017) 122:1–7. doi: 10.1016/j.phrs.2017.05.013
38. Zhang Z, Shi D, Zhang Q, Wang S, Liu K, Meng Q, et al. Visceral adiposity index (VAI), a powerful predictor of incident hypertension in prehypertensives. Intern Emerg Med. (2018) 13(4):509–16. doi: 10.1007/s11739-018-1836-8
39. Qin Z, Jiang L, Sun J, Geng J, Chen S, Yang Q, et al. Higher visceral adiposity index is associated with increased likelihood of abdominal aortic calcification. Clinics (Sao Paulo). (2022) 77:100114. doi: 10.1016/j.clinsp.2022.100114
40. Li Y, Zheng R, Li S, Cai R, Ni F, Zheng H, et al. Association between four anthropometric indexes and metabolic syndrome in US adults. Front Endocrinol (Lausanne). (2022) 13:889785. doi: 10.3389/fendo.2022.889785
41. Chen Q, Zhang Z, Luo N, Qi Y. Elevated visceral adiposity index is associated with increased stroke prevalence and earlier age at first stroke onset: based on a national cross-sectional study. Front Endocrinol (Lausanne). (2023) 13:1086936. doi: 10.3389/fendo.2022.1086936
42. Zhou N, Xie ZP, Liu Q, Xu Y, Dai SC, Lu J, et al. The dietary inflammatory index and its association with the prevalence of hypertension: a cross-sectional study. Front Immunol. (2023) 13:1097228. doi: 10.3389/fimmu.2022.1097228
43. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. (2021) 320(3):C375–91. doi: 10.1152/ajpcell.00379.2020
44. Xiao L, Harrison DG. Inflammation in hypertension. Can J Cardiol. (2020) 36(5):635–47. doi: 10.1016/j.cjca.2020.01.013
45. Fu M, Yang L, Wang H, Chen Y, Chen X, Hu Q, et al. Research progress into adipose tissue macrophages and insulin resistance. Physiol Res. (2023) 72(3):287–99. doi: 10.33549/physiolres.935046
Keywords: VAI, hypertension, NHANES, cross-sectional study, association
Citation: Zhou H, Li T, Li J, Zheng D, Yang J and Zhuang X (2024) Association of visceral adiposity index with hypertension (NHANES 2003–2018). Front. Cardiovasc. Med. 11:1341229. doi: 10.3389/fcvm.2024.1341229
Received: 15 December 2023; Accepted: 26 April 2024;
Published: 9 May 2024.
Edited by:
Elena Succurro, University of Magna Graecia, ItalyReviewed by:
Dafeng Liu, Public Health and Clinical Center of Chengdu, ChinaMatina Kleftaki, Harokopio University, Greece
© 2024 Zhou, Li, Li, Zheng, Yang and Zhuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Yang, c3VtdXpoZXlhbmdqaWVAMTYzLmNvbQ==
Xin Zhuang, emh1YW5neGluMTk3MkAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Haoran Zhou
Haoran Zhou Tianshu Li1,†
Tianshu Li1,† Jie Yang
Jie Yang Xin Zhuang
Xin Zhuang