
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 13 May 2024
Sec. Cardiovascular Epidemiology and Prevention
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1339094
Objective: To investigate the causal role of venous thrombolism mediating sodium-glucose cotransporter 2 (SGLT2) inhibition in death due to cardiac causes using Mendelian randomization (MR).
Methods: A two-sample two-step MR was used to determine (1) the causal effects of SGLT2 inhibition on death due to cardiac causes; (2) the causal effects of venous thrombolism on death due to cardiac causes; and (3) the mediation effects of venous thrombolism. Genetic proxies for SGLT2 inhibition were identified as variants in the SLC5A2 gene that were associated with both levels of gene expression and hemoglobin A1c. Additionally, employing MR to investigate the causal association between SGLT2 inhibition and cardiac arrest as well as coronary heart disease (CHD).
Results: SGLT2 inhibition was associated with a lower risk of death due to cardiac causes (odds ratio [OR] = 0.983, [95% CI = 0.972, 0.993], P = 0.0016). Venous thrombolism was associated with death due to cardiac causes ([OR] = 1.031, [95% CI = 1.005, 1.057], P = 0.0199). Mediation analysis showed evidence of indirect effect of SGLT2 inhibition on death due to cardiac causes through venous thrombolism [β = −0.0015, (95% CI = −0.0032 −0.0002), P = 0.042], with a mediated proportion of 8.9% (95% CI = 1.2%, 18.7%) of the total. Furthermore, SGLT2 inhibition was linked to a lower risk of cardiac arrest ([OR] = 0.097, [95% CI = 0.013, 0.742], P = 0.025). SGLT2 inhibition was linked to a lower risk of CHD ([OR] = 0.957, [95% CI = 0.932, 0.982], P = 0.0009).
Conclusions: Our study identified the causal roles of SGLT2 inhibition in venous thrombolism. SGLT2 inhibition may influence death due to cardiac causes through venous thrombolism. Additionally, SGLT2 inhibition was associated with reduced risk of cardiac arrest and CHD.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as dapagliflozin, and canagliflozin, are antidiabetic medications that function by impeding the glucose reuptake in the renal tubule, as well, as facilitating the secretion of glucose through the urinary system (1). Extensive research unequivocally has confirmed the substantial therapeutic effects of SGLT2 inhibitors in mitigating cardiovascular disorders, addressing heart failure, and enhancing renal function (2). Furthermore, growing findings indicated the potentiality of reducing the hazard of cardiac-related death, but a causal relationship between SGLT2 inhibition and cardiac-related death has not been reported. Cardiac arrest and coronary heart disease (CHD) stand as the predominant contributors to mortality due to cardiovascular diseases. The causal link between SGLT2 inhibition and both cardiac arrest and CHD remains uncertain.
Venous thrombolism is a common complication of cardiac events including heart failure (3), atrial fibrillation (4), and myocardial infarction (5). A study conducted a significant association between venous thrombosis and increased mortality rates in patients undergoing cardiac surgery (6). However, nowadays, whether SGLT2 inhibition could reduce the risk of venous thrombosis is unclear.
In light of this, a Mendelian randomization (MR) study was required to explore whether SGLT2 inhibition was significantly linked to the risk of cardiac events-induced death via the mediation of venous thrombosis at a genetic level. Furthermore, we investigated the causal relationship between SGLT2 inhibitors and cardiac arrest as well as CHD.
MR represents a robust methodology that employs genetic variants linked to the exposure serving as instruments to explore the potential cause-effect connections between exposure and outcome (7). MR could emulate randomized controlled trials by allocating genetic variants randomly during conception, thereby reducing the likelihood of bias.
The present study utilized an MR design (Figure 1). To establish the accuracy of potential cause-effect connections, three fundamental assumptions must be complied with in MR studies (7): genetic variants should (1) be powerfully linked to the exposure, (2) be independent of any potential confounding factors, (3) exert impact on outcome solely through exposure.
We first performed a two-sample MR to assess the relationship between SGLT2 inhibition on death due to cardiac causes. Then, we employed a two-step MR approach to ascertain the potential causal involvement of venous thrombolism in mediating the pathway between SGLT2 inhibition and death due to cardiac causes. Finally, we utilized two-sample MR to investigate the causal relationships between SGLT2 inhibition and the incidence of cardiac arrest and CHD.
The systematic identification of genetic variants acting as representatives for SGLT2 inhibition was conducted through a four-step process. (1) Choose genetic variants linked to SLC5A2 mRNA employing data from Genotype-Tissue Expression (GTEx) (8) and eQTLGen Consortium (9). (2) Evaluate the correlation between genetic variants selected with hemoglobin A1c (HbA1c) level, which serve as marker of the glucose-lowering effect resulting from SGLT2 inhibition and identify variants displaying regional-scale connection with HbA1c utilizing data deriving from a non-diabetic subset of independent individuals of European descent in the UK Biobank (n = 344,182) (P = 1 × 10−4). (3) Employ the genetic colocalization method to ascertain whether SLC5A2 and HbA1c share a common cause-effect variant. Colocalization, as a statistical genetic method, utilizes a Bayesian model to assess a posteriori probability of a variant having shared cause-effect on two traits: SLC5A2 expression and HbA1c level in circulation within the genomic locus of SLC5A2. The possibility of colocalization exceeding 70% between the gene expression of SLC5A2 and the levels of HbA1 was an indicative proof of colocalization, denoted as “colocalized”. Conversely, “not colocalized” was used to describe the remaining gene-disease associations. (4) Perform a standard clumping process (whereby variants with a correlation greater than 0.8 were eliminated as they exhibited excessively high correlations among one another). Then, the statistical power of each genetic variant selected was estimated using F statistics. Eventually, six single nucleotide polymorphisms (SNPs) forcefully linked to SGLT2 inhibition through HbA1c were identified as the instruments of the exposure (10) (Supplementary Table S1).
Summary data for venous thrombolism was retrieved from a genome-wide association study (GWAS) (https://gwas.mrcieu.ac.uk/), (ebi-a-GCST90038607) containing 12,240 venous thrombolism cases and 472,358 controls of European descent with 9,587,836 SNPs. Genetic variants demonstrating significant associations with venous thrombolism (P < 5 × 10−8) were identified and subsequently considered as potential genetic predictors for venous thrombolism. We then performed a clumping procedure to exclude genetic variants that displayed significant intercorrelation (with a correlation coefficient <0.001) among themselves. Finally, thirteen SNPs connected with venous thrombolism were designated as genetic instruments of venous thrombolism. Then, we estimated the strength of thirteen variants using F statistics. An F-statistic threshold of less than 10 was employed to classify an instrumental variable (IV) as weak, and the weak IVs were excluded from our study. In addition, we applied the PhenoScanner web (http://www.phenoscanner.medschl.cam.ac.uk/) to analyze the selected genetic predictors and removed the SNPs related to potential confounding factors (Low-density lipoprotein, coronary artery disease) for cardiac events related to death. Finally, seven genetic variants vigorously connected with venous thrombolism were regarded as valid IVs (Supplementary Table S2).
A GWAS data (ukb-d-I9_K_CARDIAC) was used as the summary association statistics of death due to cardiac causes, involving a European descent population consisting of 1,597 cases and 359,597 controls. Mortality resulting from cardiac disease is the primary outcome, with cardiac arrest and CHD investigated as secondary outcomes. Cardiac arrest data was sourced from the Finngen, incorporating 2,471 cases and 210,652 controls. The CHD data originated from the UK Biobank, incorporating 361,194 individuals of European descent.
First, we assessed the impact of SGLT2 inhibition on death due to cardiac causes. We ran an MR study using five approaches: inverse variance weighted (IVW), MR-Egger, weighted median, simple mode, and weighted mode (11–13). The IVW approach is widely regarded as the most accurate method to assess causality, particularly in cases where there is no indication of directional pleiotropy in the findings (P for MR-Egger intercept P > 0.05). Due to its robustness, the IVW method is frequently employed as the chief analytical approach in the field of causal evaluation (14). The MR-Egger method possesses the capability to detect and account for directional pleiotropy. Yet, it demonstrated an inadequate statistical power (15). For the utilization of the weighted median approach to estimate cause-effect, a minimum criterion of considering 50% of SNPs was deemed reliable IVs (12). The simple mode represents a model-based evaluation method that provides robustness against pleiotropy (16). The weighted mode is susceptible to challenges in selecting an appropriate bandwidth for mode estimation (17). We verified the consistency of the causal associations using all five approaches collectively and established their statistical significance by employing a significance threshold of P < 0.05 (18, 19).
In our study, the causal connection of SGLT2 inhibition on venous thrombolism was evaluated. The six SNPs imitating SGLT2 inhibition were regarded as the exposure, and the venous thrombolism was utilized as an outcome. We used five approaches to assess the cause-effect of SGLT2 inhibition on venous thrombolism. Among them, IVW was considered as our main approach (β1).
In addition, we evaluated the causal relationship between venous thrombolism on death due to cardiac causes. The seven variants robustly related to venous thrombolism were regarded as IVs for the exposure, and death due to the cardiac cause was selected as the outcome. We used five approaches to assess the effect of venous thrombolism on death because of cardiac causes. Amongst them, IVW was our main approach (β2).
Lastly, we used the product of coefficients method as a primary approach to assessing the indirect impact of SGLT2 inhibition on death due to cardiac cause via venous thrombolism (β1 × β2). Hence, the proportion of the impact mediated by venous thrombolism was calculated as the ratio between the mediated effect and the overall effect.
To ensure the accuracy of our MR results, we conducted a comprehensive set of other MR examinations, consisting of sensitivity, heterogeneity, and pleiotropy assessment. These additional investigations were aimed at addressing possible biases arising from variations and polymorphisms among individual SNPs. To detect potential heterogeneity in MR analysis, we used Cochran's Q test, a recognized statistical method (20). Subsequently, we employed the “leave-one-out” approach for assessing the cause-effect genetically of exceptional SNPs and determining whether the non-inclusion of these SNPs had any impact on the MR results. This approach assisted us in evaluating the sensitivity of our findings to the presence or absence of specific SNPs in the analysis (21). To measure potential directional pleiotropy, we examined the intercept coefficient in the MR-Egger's regression analysis. A significant deviation of the intercept coefficient from zero (p < 0.05) would indicate the presence of directional pleiotropy, whereby the genetic variants have effects on the outcome that are independent of the exposure variable (11). Additionally, to assess for horizontal pleiotropy, and identify, and account for potential outliers, we employed the MR-PRESSO approach (19).
For a more comprehensive understanding of the mechanisms underlying the association between SGLT2 inhibition and venous thrombosis, we carried out additional investigations by leveraging protein correlation data sourced from the STRING database. To construct a protein-protein interaction (PPI) network, we curated proteins from two distinct sources: (1) gene SLC5A2, which is associated with SGLT2, (2) 117 proteins associated with venous thrombolism from the Disgenet website, (3) first neighbors of SLC5A2 from 117 proteins. The protein network was constructed using the stringApp plugin within the Cytoscape platform.
The IVW approach was employed as the chief analysis method in this study. SGLT2 inhibition was found to be connected with decreased hazard of mortality attributed to cardiac causes [OR = 0.983, (95% CI = 0.972, 0.993), P = 0.0016], and the weighted median approach yielded additional evidence to substantiate this finding [OR = 0.982, (95% CI = 0.969, 0.996), P = 0.0127]. Elaborate assessments can be found in Supplementary Table S3, accompanied by visual representations such as forest plots and scatter plots (Figures 2A, 3A). Moreover, the heterogeneity analysis utilizing Cochran's Q test for the IVW method demonstrated that the Q statistics and corresponding p-values did not exhibit statistical significance (P = 0.98), which suggested no proof of heterogeneity for the conclusion of SGLT2 inhibition on death due to cardiac causes. The MR-Egger regression analysis provided evidence that our findings remained unaffected by any potential pleiotropy (intercept P = 0.497), and the MR-PRESSO method indicated that our results were not affected by pleiotropy (Global Test P = 0.976). The leave-one-out plot, depicted in Figure 4A, illustrated that eliminating an individual SNP from the set of genetic variants had a minimal impact on the overall outcome.
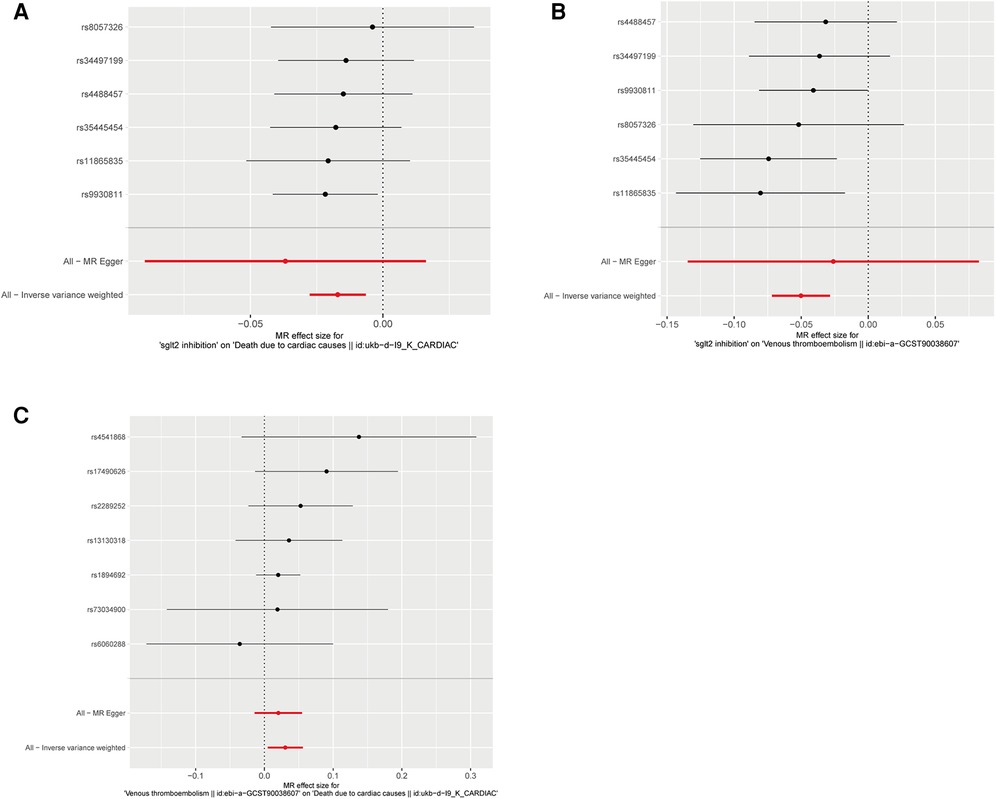
Figure 2. Forest plots of Mendelian randomization analyses in our study. (A) Forest plot of Mendelian randomization analyses of the causal effects of SGLT2 Inhibition on death due to cardiac causes; (B) Forest plot of Mendelian randomization analyses of the causal effects of SGLT2 inhibition on venous thrombolism; (C) Forest plot of venous thrombolism on the risk for death due to cardiac causes.
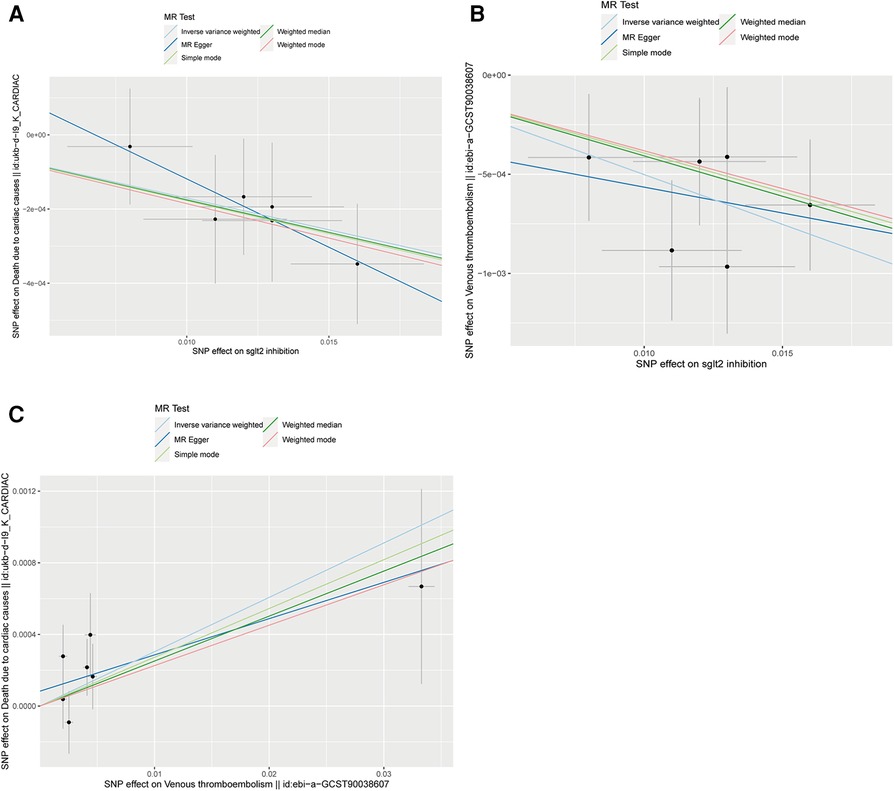
Figure 3. Scatter plots of Mendelian randomization analyses in our study. (A) Scatter plot of Mendelian randomization analyses of the causal effects of SGLT2 Inhibition on death due to cardiac causes; (B) Scatter plot of Mendelian randomization analyses of the causal effects of SGLT2 inhibition on venous thrombolism; (C) Scatter plot of venous thrombolism on the risk for death due to cardiac causes.
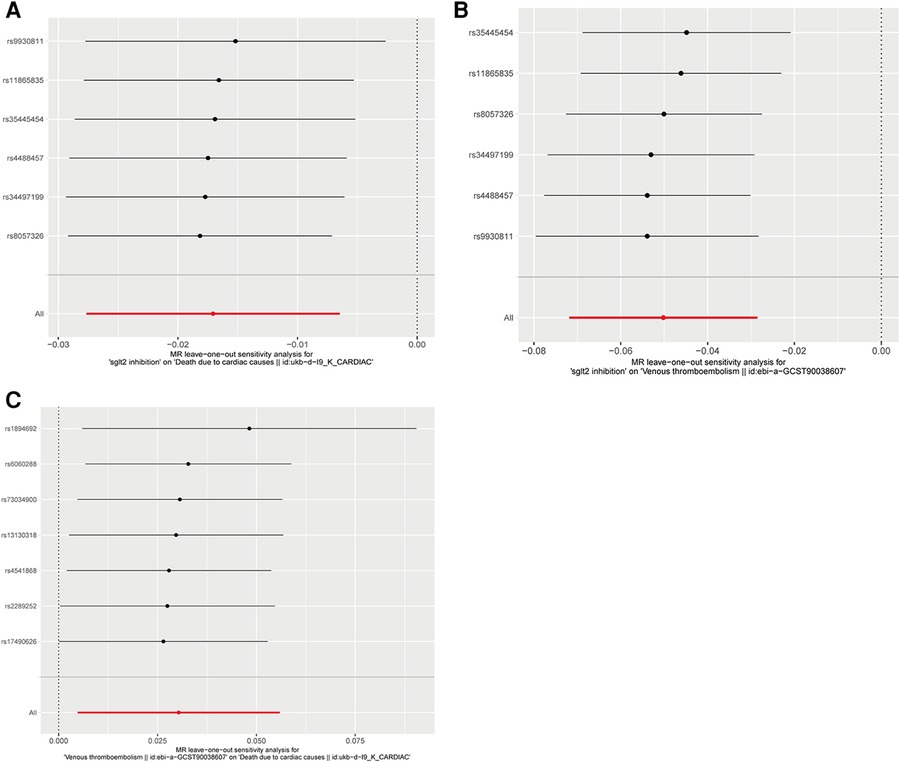
Figure 4. Leave-one-out plots of Mendelian randomization analyses in our study. (A) Leave-one-out plot of Mendelian randomization analyses of the causal effects of SGLT2 Inhibition on death due to cardiac causes; (B) Leave-one-out plot of Mendelian randomization analyses of the causal effects of SGLT2 inhibition on venous thrombolism; (C) Leave-one-out plot of venous thrombolism on the risk for death due to cardiac causes.
In this study, the cause-effect of SGLT2 inhibition on venous thrombolism was estimated. SGLT2 inhibition demonstrated a significant association with a decreased risk of venous thrombolism [OR = 0.951, (95% CI = 0.931, 0.972), P = 0.0000057]. Elaborate assessments can be found in Supplementary Table S4, accompanied by visual representations such as forest plots and scatter plots (Figures 2B, 3B). The heterogeneity analysis utilizing Cochran's Q test for the IVW method demonstrated that the Q statistics and corresponding p-values did not exhibit statistical significance (P = 0.750), which suggested no proof of heterogeneity for the conclusion of SGLT2 inhibition on venous thrombolism. The MR-Egger regression analysis provided evidence that our findings remained unaffected by any potential pleiotropy (intercept P = 0.680), and the MR-PRESSO method indicated that our results were not affected by pleiotropy (Global Test P = 0.798). The leave-one-out plot, depicted in Figure 4B, illustrated that eliminating an individual SNP from the set of genetic variants had a minimal impact on the overall outcome.
Then, we evaluated the effect of venous thrombolism on the risk of death due to cardiac causes. Venous thrombolism was linked to a higher mortality rate due to cardiac causes [OR = 1.031, (95% CI = 1.005, 1.057), P = 0.0199]. Elaborate assessments can be found in Supplementary Table S5, accompanied by visual representations such as forest plots and scatter plots (Figures 2C, 3C). The heterogeneity analysis utilizing Cochran's Q test for the IVW method demonstrated that the Q statistics and corresponding p-values did not exhibit statistical significance (P = 0.612), which suggested no proof of heterogeneity for the conclusion of venous thrombolism on death due to cardiac causes. The MR-Egger regression analysis provided evidence that our findings remained unaffected by any potential pleiotropy (intercept P = 0.432), and the MR-PRESSO method indicated that our results were not affected by pleiotropy (Global Test P = 0.626). The leave-one-out plot, depicted in Figure 4C, illustrated that eliminating an individual SNP from the set of genetic variants had a minimal impact on the overall outcome.
We detected a mediated impact of SGLT2 inhibition on death due to cardiac causes through venous thrombolism [β = −0.0015, (95% CI = −0.0032 −0.0002), P = 0.042], with a mediated proportion of 8.9% (95% CI = 1.2%, 18.7%) of the total effect (Table 1).
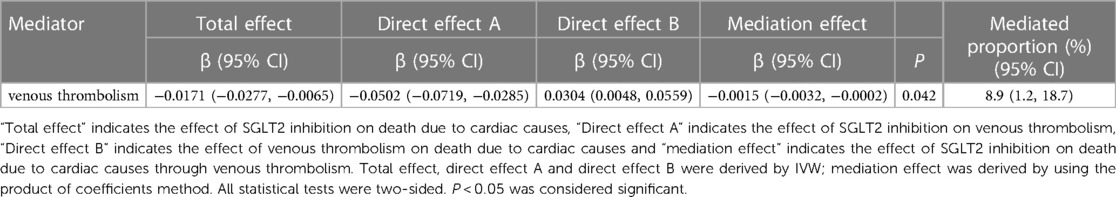
Table 1. The mediation effect of SGLT2 inhibition on death due to cardiac cause through venous thrombolism.
We performed protein-protein interaction analysis between SLC5A2 and the obtained proteins associated with venous thrombolism from the Disgenet website and identified eight proteins that primarily interact with SLC5A2, including interleukin 1 beta (IL1B), major histocompatibility complex, class I, B (HLA-B), angiotensinogen (AGT), aquaporin 2 (AQP2), erythropoietin (EPO), angiotensin I converting enzyme (ACE), C-C motif chemokine ligand 2 (CCL2), leptin (LEP) (Figure 5).
MR results demonstrated that SGLT2 inhibition was negatively associated with cardiac arrest [OR = 0.097, (95% CI = 0.013, 0.742), P = 0.025, IVW]. Elaborate assessments can be found in Supplementary Table S6, accompanied by visual representations such as forest plots and scatter plots (Supplementary Figures S1A,B). Moreover, the heterogeneity analysis utilizing Cochran's Q test for the IVW method demonstrated that the Q statistics and corresponding p-values did not exhibit statistical significance (P = 0.917), which suggested no proof of heterogeneity for the conclusion of SGLT2 inhibition on cardiac arrest. The MR-Egger regression analysis provided evidence that our findings remained unaffected by any potential pleiotropy (intercept P = 0.615), and the MR-PRESSO method indicated that our results were not affected by pleiotropy (Global Test P = 0.929). The leave-one-out plot, depicted in Supplementary Figure S1C, illustrated that eliminating an individual SNP from the set of genetic variants had a minimal impact on the overall outcome.
This study showed that SGLT2 inhibition was negatively linked to CHD [OR = 0.957, (95% CI = 0.932, 0.982), P = 0.0009, IVW]. Elaborate assessments can be found in Supplementary Table S7, accompanied by visual representations such as forest plots and scatter plots (Supplementary Figures S1D,E). Moreover, the heterogeneity analysis utilizing Cochran's Q test for the IVW method demonstrated that the Q statistics and corresponding p-values did not exhibit statistical significance (P = 0.650), which suggested no proof of heterogeneity for the conclusion of SGLT2 inhibition on CHD. The MR-Egger regression analysis provided evidence that our findings remained unaffected by any potential pleiotropy (intercept P = 0.763), and the MR-PRESSO method indicated that our results were not affected by pleiotropy (Global Test P = 0.682). The leave-one-out plot, depicted in Supplementary Figure S1F, illustrated that eliminating an individual SNP from the set of genetic variants had a minimal impact on the overall outcome.
In this study, we investigated and established the causal effects of SGLT2 inhibition on both venous thrombolism and death due to cardiac causes. Additionally, we estimated the causal impact of venous thrombolism on death due to cardiac causes (Figure 6A). Moreover, the mediation MR study demonstrated the potential causal influence of SGLT2 inhibition on death due to cardiac causes via venous thrombolism. We also suggested that venous thrombolism was genetically linked to a raised hazard of mortality due to cardiac causes. Furthermore, our findings also demonstrate a negative correlation between SGLT2 inhibition and cardiac arrest as well as CHD (Figure 6B).
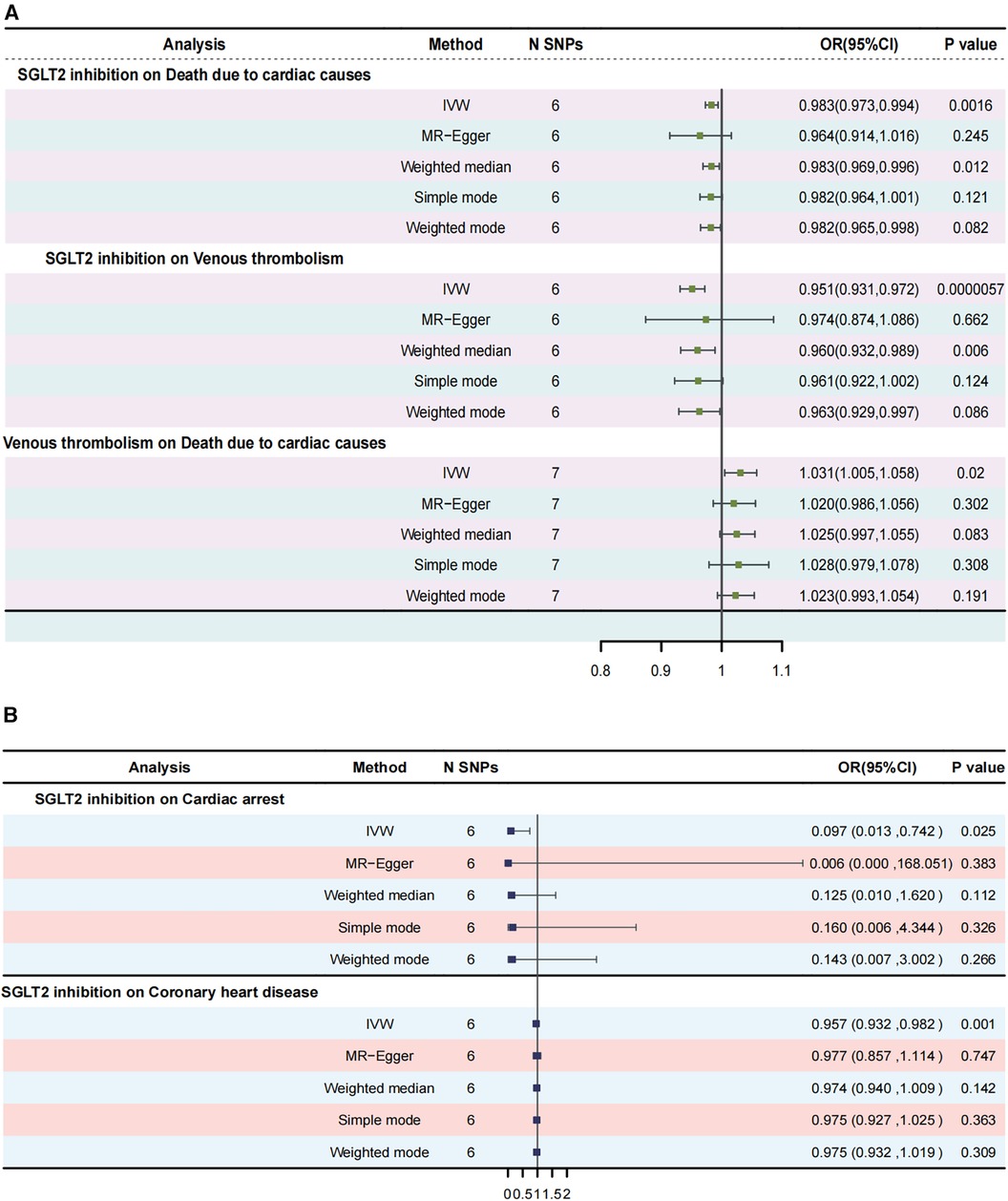
Figure 6. Summary of Mendelian randomization analyses in our study. (A) Mendelian randomization analyses among SGLT2 inhibition, venous thrombolism as well as death due to cardiac causes; (B) Mendelian randomization analyses for SGLT2 inhibition on cardiac arrest and CHD. CHD, coronary heart disease.
A meta-study has reported that among individuals diagnosed with heart failure characterized by reduced ejection fraction, the associations remained consistent irrespective of their diabetic condition, SGLT2 inhibition could reduce the combined risk of cardiovascular death or hospitalisation (22). Another system review and meta-analysis demonstrated that SGLT2 inhibition effectively mitigated the hazard of cardiac mortality, heart failure, and other cardiac events (23). A clinical study showed SGLT2 inhibition was also related to a significantly lower risk of cardiac arrest and CHD (24, 25). In our study, we have shown the causal effects of SGLT2 inhibition on death due to cardiac causes, cardiac arrest as well as CHD. However, limited research has been conducted to investigate the underlying mechanisms responsible for the impact of SGLT2 inhibition on death attributed to cardiac causes.
Previous studies have shown that the amelioration of cardiovascular outcomes associated with empagliflozin, an SGLT2 inhibitor, is not solely reliant on glycemic control (26). This implies the existence of alternative mechanisms and pathways through which the effects are mediated.
Venous thromboembolism is the third main contributor to cardiovascular death in the world (27). In this MR analysis, it has been demonstrated that venous thrombolism is a genetic risk for cardiac events related to death. The potential mechanism is related to vascular endothelial injury, hypercoagulability, and abnormal bloodstream dynamics. Vascular endothelial injury is the important initiation of venous thrombosis. Blood flow disturbances may encompass hypercoagulability of the blood as well as abnormal coagulation states within the vessel wall. Heart diseases often lead to vascular endothelial damage (28), platelet activation, a hypercoagulable state (29), and blood stasis (30).
In addition, up to now, the existing research on the effect of SGLT2 inhibition on venous thromboembolism is in conflict. A pilot study showed that SGLT2 Inhibitors reduced platelet activation and thrombus formation (31). However, another study indicated that the administration of SGLT2 inhibitors has been linked to elevated levels of hematocrit, leading to augmented blood viscosity (32), and, consequently, elevating the potential risk of thromboembolic events (33). Yet, according to a cohort study, the use of SGLT2-targeted medicine in individuals diagnosed with type 2 diabetes was not found to be correlated with an increased incidence rate of venous thromboembolism (34). Additionally, a systematic review and meta-analysis also demonstrated no influence of SGLT2 inhibitors on the incidence of venous thromboembolism in diabetic patients (35). Conflict results may be related to the limitations of different studies. Most studies did not fully adjust for confounding factors and the definition of venous thromboembolism varied across trials. Therefore, we used MR analysis to explore the causal relationship between SGLT2 inhibition and venous thrombolism. We found that SGLT2 inhibition could reduce the risk of venous thrombolism. Further, our mediation MR analysis suggested venous thrombolism mediated the effects of SGLT2 inhibition on death due to cardiac causes with an indirect component of 8.9%. However, the potential mechanism of SGLT2 inhibitors on venous thrombolism remains unclear. We obtained proteins related to venous thrombolism from the Digenet website and selected the proteins which were the first neighbors of SLC5A2. As Figure 5, IL1B, HLA-B, AGT, AQP2, EPO, ACE, CCL2, LEP were related to SLC5A2. IL1B and CCL2 are linked to inflammation. AGT, EPO, LEP, and ACE participate in regulating platelet activity and coagulation process (36, 37). Inflammation could promote platelet hyperreactivity (38) and upregulate pathological thrombosis (39). Therefore, SGLT2 inhibition may inhibit vein thrombolism by regulating inflammation, platelet activation, and the coagulation process.
However, in our study, there is an apparent limitation. The odds ratios of our MR results are quite mild. The potential reasons are following. Firstly, the number of instrumental SNPs used is associated with the OR value to some extent; however, in this research, only six SNPs were employed as proxies for the exposure. Secondly, the OR value is also related to the proportion of cases and controls within outcome data, and in our study, the numbers of cases in the outcome datasets are relatively small. This could potentially lead to mild OR estimates in our investigation. Therefore, the putative link between venous thrombolism-mediated effects of SGLT2 inhibitors and their contribution to cardiovascular mortality requires additional empirical validation through dedicated experiments.
Genetically, SGLT2 inhibition could reduce the mortality rates due to cardiac events via venous thrombolism. The comprehensive mechanism may relate to the effects of SGLT2 inhibition on inflammation, platelet activation, and the coagulation process. Addtionally, SGLT2 inhibition could reduce the risks of cardiac arrest and CHD.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
LS: Writing – original draft, Writing – review & editing. XW: Writing – review & editing. JL: Writing – review & editing. LT: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1339094/full#supplementary-material
Supplementary Figure S1
MR analyses plots for SGLT2 inhibition on cardiac arrest and CHD. (A) Forest plot of Mendelian randomization analyses of the causal effects of SGLT2 Inhibition on cardiac arrest; (B) Scatter plot of Mendelian randomization analyses of the causal effects of SGLT2 inhibition on cardiac arrest; (C) Leave-one-out plot of SGLT2 Inhibition on cardiac arrest; (D) Forest plot of Mendelian randomization analyses of the causal effects of SGLT2 Inhibition on CHD; (E) Scatter plot of Mendelian randomization analyses of the causal effects of SGLT2 inhibition on CHD; (F) Leave-one-out plot of SGLT2 Inhibition on CHD. CHD, coronary heart disease.
MR, Mendelian randomization; OR, odds ratio; SGLT2, sodium-glucose cotransporter 2; GTEx, genotype-tissue expression; HbA1c, hemoglobin A1c; SNPs, single nucleotide polymorphisms; IVs, instrumental variables; IVW, inverse variance weighted; PPI, protein-protein interaction; IL1B, interleukin 1 beta; HLA-B, major histocompatibility complex, class I, B; AGT, angiotensinogen; AQP2, aquaporin 2; EPO, erythropoietin; ACE, angiotensin I converting enzyme; CCL2, C-C motif chemokine ligand 2; LEP, leptin; CHD, coronary heart disease.
1. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. (2016) 134:752–72. doi: 10.1161/circulationaha.116.021887
2. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. (2021) 384:129–39. doi: 10.1056/NEJMoa2030186
3. Wilsbacher L, McNally EM. Genetics of cardiac developmental disorders: cardiomyocyte proliferation and growth and relevance to heart failure. Annu Rev Pathol. (2016) 11:395–419. doi: 10.1146/annurev-pathol-012615-044336
4. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet (London, England). (2009) 373:155–66. doi: 10.1016/s0140-6736(09)60040-4
5. Sejrup JK, Tøndel BG, Morelli VM, Løchen ML, Njølstad I, Mathiesen EB, et al. Joint effect of myocardial infarction and obesity on the risk of venous thromboembolism: the tromsø study. J Thromb Haemostasis. (2022) 20:2342–9. doi: 10.1111/jth.15812
6. Khoury H, Lyons R, Sanaiha Y, Rudasill S, Shemin RJ, Benharash P. Deep venous thrombosis and pulmonary embolism in cardiac surgical patients. Ann Thorac Surg. (2020) 109:1804–10. doi: 10.1016/j.athoracsur.2019.09.055
7. Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. Jama. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
8. GTEx Consortium. The GTEx consortium atlas of genetic regulatory effects across human tissues. Science (New York, N.Y.). (2020) 369:1318–30. doi: 10.1126/science.aaz1776
9. Võsa U, Claringbould A, Westra HJ, Bonder MJ, Deelen P, Zeng B, et al. Large-scale cis- and trans-eQTL analyses identify thousands of genetic loci and polygenic scores that regulate blood gene expression. Nat Genet. (2021) 53:1300–10. doi: 10.1038/s41588-021-00913-z
10. Xu M, Zheng J, Hou T, Lin H, Wang T, Wang S, et al. SGLT2 inhibition, choline metabolites, and cardiometabolic diseases: a mediation Mendelian randomization study. Diabetes Care. (2022) 45:2718–28. doi: 10.2337/dc22-0323
11. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
12. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
13. Xu J, Zhang S, Tian Y, Si H, Zeng Y, Wu Y, et al. Genetic causal association between iron status and osteoarthritis: a two-sample Mendelian randomization. Nutrients. (2022) 14(18):3683. doi: 10.3390/nu14183683
14. Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. (2017) 14:577–90. doi: 10.1038/nrcardio.2017.78
15. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
16. Milne RL, Kuchenbaecker KB, Michailidou K, Beesley J, Kar S, Lindström S, et al. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat Genet. (2017) 49:1767–78. doi: 10.1038/ng.3785
17. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
18. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology (Cambridge, Mass.). (2017) 28:30–42. doi: 10.1097/ede.0000000000000559
19. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
20. Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. (2015) 34:2926–40. doi: 10.1002/sim.6522
21. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. eLife. (2018) 7:e34408. doi: 10.7554/eLife.34408
22. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-reduced and DAPA-HF trials. Lancet (London, England). (2020) 396:819–29. doi: 10.1016/s0140-6736(20)31824-9
23. Toyama T, Neuen BL, Jun M, Ohkuma T, Neal B, Jardine MJ, et al. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and meta-analysis. Diabetes Obes Metab. (2019) 21:1237–50. doi: 10.1111/dom.13648
24. Fawzy AM, Rivera-Caravaca JM, Underhill P, Fauchier L, Lip GYH. Incident heart failure, arrhythmias and cardiovascular outcomes with sodium-glucose cotransporter 2 (SGLT2) inhibitor use in patients with diabetes: insights from a global federated electronic medical record database. Diabetes Obes Metab. (2023) 25:602–10. doi: 10.1111/dom.14854
25. Wong CKH, Lau KTK, Tang EHM, Lee CH, Lee CYY, Woo YC, et al. Cardiovascular benefits of SGLT2 inhibitors in type 2 diabetes, interaction with metformin and role of erythrocytosis: a self-controlled case series study. Cardiovasc Diabetol. (2022) 21:92. doi: 10.1186/s12933-022-01520-w
26. Inzucchi SE, Kosiborod M, Fitchett D, Wanner C, Hehnke U, Kaspers S, et al. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation. (2018) 138:1904–7. doi: 10.1161/circulationaha.118.035759
27. Wolberg AS, Rosendaal FR, Weitz JI, Jaffer IH, Agnelli G, Baglin T, et al. Venous thrombosis. Nature Rev Dis Primers. (2015) 1:15006. doi: 10.1038/nrdp.2015.6
28. Seeger JP, Benda NM, Riksen NP, van Dijk AP, Bellersen L, Hopman MT, et al. Heart failure is associated with exaggerated endothelial ischaemia-reperfusion injury and attenuated effect of ischaemic preconditioning. Eur J Prev Cardiol. (2016) 23:33–40. doi: 10.1177/2047487314558377
29. Lin AY, Dinatolo E, Metra M, Sbolli M, Dasseni N, Butler J, et al. Thromboembolism in heart failure patients in sinus rhythm: epidemiology, pathophysiology, clinical trials, and future direction. JACC Heart Fail. (2021) 9:243–53. doi: 10.1016/j.jchf.2021.01.009
30. McCullough PA. Practical experience using galectin-3 in heart failure. Clin Chem Lab Med. (2014) 52:1425–31. doi: 10.1515/cclm-2014-0278
31. Pignatelli P, Baratta F, Buzzetti R, D'Amico A, Castellani V, Bartimoccia S, et al. The sodium-glucose co-transporter-2 (SGLT2) inhibitors reduce platelet activation and thrombus formation by lowering NOX2-related oxidative stress: a pilot study. Antioxidants (Basel, Switzerland). (2022) 11(10):1878. doi: 10.3390/antiox11101878
32. Kohler S, Zeller C, Iliev H, Kaspers S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I-III clinical trials. Adv Ther. (2017) 34:1707–26. doi: 10.1007/s12325-017-0573-0
33. Braekkan SK, Mathiesen EB, Njølstad I, Wilsgaard T, Hansen JB. Hematocrit and risk of venous thromboembolism in a general population. The tromso study. Haematologica. (2010) 95:270–5. doi: 10.3324/haematol.2009.008417
34. Schmedt N, Enders D, Walker J, Garbe E, Douros A. Sodium-glucose co-transporter 2 inhibitors and the risk of venous thromboembolism in patients with type 2 diabetes: a cohort study. Am J Med. (2021) 134:606–13.e606. doi: 10.1016/j.amjmed.2020.10.046
35. Wang A, Yang K, Wang T, Zhang N, Tang H, Feng X. Effects of sodium-glucose cotransporter 2 inhibitors on risk of venous thromboembolism in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. (2020) 36:e3174. doi: 10.1002/dmrr.3174
37. Moreno PR, Sanz J, Fuster V. Atherosclerosis. Curr Mol Med. (2006) 6:437–8. doi: 10.2174/156652406778018662
38. Davizon-Castillo P, McMahon B, Aguila S, Bark D, Ashworth K, Allawzi A, et al. TNF-α-driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood. (2019) 134:727–40. doi: 10.1182/blood.2019000200
Keywords: SGLT2 inhibition, venous thrombolism, mortality rates, cardiac events, Mendelian randomization
Citation: Shi L, Wei X, Luo J and Tu L (2024) SGLT2 inhibition, venous thrombolism, and death due to cardiac causes: a mediation Mendelian randomization study. Front. Cardiovasc. Med. 11:1339094. doi: 10.3389/fcvm.2024.1339094
Received: 16 November 2023; Accepted: 1 May 2024;
Published: 13 May 2024.
Edited by:
Sebhat Erqou, Brown University, United StatesReviewed by:
Lingfeng Luo, Stanford University, United States© 2024 Shi, Wei, Luo and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Tu, bGluZ3R1QHRqaC50am11LmVkdS5jbg==
Jinlan Luo, amlubGFubHVvQGh1c3QuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.