- 1Department of Gastroenterology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
- 2Munich Medical Research School, LMU Munich, Munich, Germany
- 3Jiangxi Cardiovascular Research Institute, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi, China
Objective: While hypertension is a well-recognized risk factor for non-alcoholic fatty liver disease (NAFLD), the specific roles of various common blood pressure measurements [diastolic blood pressure (DBP), systolic blood pressure (SBP), pulse pressure (PP), mean arterial pressure (MAP)] in detecting NAFLD and evaluating the associated risk in adults remain unclear.
Methods: A retrospective analysis was conducted on 14,251 adult participants undergoing health screenings in the NAfld in the Gifu Area, Longitudinal Analysis project (NAGALA). Following the Z-transformation of the independent variables, we evaluated the relationships between the four blood pressure indices and NAFLD through multivariable logistic regression models. This analysis documented the odds ratio (OR) and 95% confidence interval (CI) for each standard deviation (SD) increase. Additionally, the effectiveness of these indices in identifying NAFLD was comparatively analyzed using receiver operating characteristic (ROC) curves.
Results: After adequately adjusting for confounders, all blood pressure indices except PP showed a positive correlation with NAFLD. For each SD increment, MAP had the strongest association with NAFLD compared to SBP and DBP. This finding was confirmed in populations without exercise habits, under 60 years of age, with normal blood pressure, and in non-obese groups. Furthermore, based on ROC analysis, MAP was found to have the highest accuracy in identifying NAFLD compared to the other three blood pressure indices.
Conclusion: Among the four blood pressure indices evaluated, MAP demonstrates the greatest efficacy in identifying NAFLD and assessing its associated risk. These findings underscore the potential of MAP as the most promising blood pressure index for screening NAFLD.
Introduction
NAFLD is a prevalent non-communicable disease, primarily characterized by fat accumulation and associated inflammation in the liver (1, 2). Recent global surveys reveal that approximately 30% (around 2.2 billion) of adults worldwide are affected by NAFLD (3), surpassing the total number of individuals with obesity (650 million) and diabetes (529 million) (4, 5). Despite the startling prevalence of NAFLD, the greater concern lies in its potential to cause chronic damage to the liver and extra-hepatic organ systems during its progression (1, 2, 6). Advanced stages of NAFLD can severely affect health and even be life-threatening, leading to a substantial disease burden (6–8). Considering NAFLD's high prevalence and escalating disease burden, alongside limited treatment options (9), prevention emerges as a crucial public health strategy. There is a pressing need for enhanced screening and assessment of modifiable risk factors for NAFLD.
Hypertension, with a global prevalence of 31.1% (10), is a key modifiable risk factor in NAFLD's development and progression (11, 12). Recent meta-analyses have shown that the presence of hypertension significantly increased the risk of NAFLD events by 47% (13), and the latest evidence from Mendelian randomization analysis based on Genome-Wide Association Studies further indicated a causal relationship between hypertension and commonly measured blood pressure parameters SBP, DBP, and NAFLD (14). Additionally, recent observational studies have confirmed that other blood pressure indices, such as PP and MAP, were also positively correlated with NAFLD, where elevated PP and MAP increased the risk of NAFLD (15, 16). In terms of NAFLD reversal, published studies have shown that controlling SBP/DBP below 140/90 mmHg in non-obese hypertensive patients was independently associated with a 40% reduction in NAFLD prevalence (17). Furthermore, clinical practice guidelines for NAFLD management by the European Association for the Study of the Liver/Diabetes/Obesity recommend close monitoring of NAFLD patients with hypertension, as the presence of hypertension leads to a higher risk of NAFLD disease progression (18). Given the current pandemic of hypertension and its significant impact on the development and progression of NAFLD, actively exploring the roles of various blood pressure indices in NAFLD screening are vital. However, there has been no systematic analysis of the different blood pressure indices in assessing or identifying the risk of NAFLD in adults. Therefore, to fill this gap in the field, our current study aimed to analyze and compare the relative importance of the four blood pressure indices SBP, DBP, PP, and MAP in identifying/assessing the risk of NAFLD.
Methods
Data source
In this study, we analyzed data from the NAGALA project dataset spanning from 1994 to 2016. This dataset, collated by Professor Okamura and colleagues, has been made publicly available in the Dryad database (19). In accordance with the Dryad database's terms of use and the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License (CC BY-NC-ND 4.0), we utilized this dataset for secondary creation, duly crediting the source (19).
Study design and population
The NAGALA project is a cross-sectional and longitudinal survey based on a health examination population, aimed at detecting common chronic diseases and their risk factors to promote public health. The detailed design and study outcomes have been published elsewhere (20). Briefly, the NAGALA project, initiated in 1994 and ongoing, recruited adults undergoing health check-ups at the Murakami Memorial Hospital in Gifu, Japan. The project was approved by the hospital's ethics committee, and informed consent for data use was obtained from each participant.
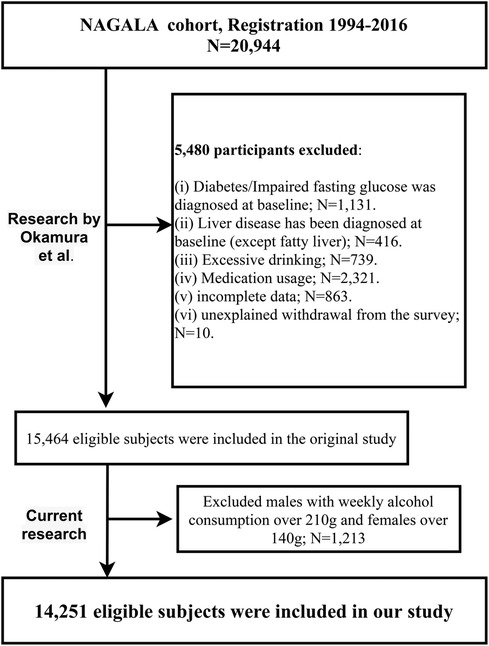
In their initial study, Okamura et al. included 20,944 participants from the NAGALA project (1994–2016), analyzing the role of ectopic fat obesity in diabetes onset. They excluded participants with (i) diagnosed diabetes, alcoholic fatty liver disease, viral hepatitis, or fasting plasma glucose (FPG) ≥6.1 mmol/L at baseline (n = 1,547); (ii) undergoing medication treatment at baseline (n = 2,321); (iii) excessive alcohol consumption (n = 739); (iv) missing baseline data (n = 863); (v) unexplained study withdrawal (n = 10), resulting in a final sample of 15,464 participants for their analysis.
Our current study, a secondary analysis of the NAGALA dataset, aimed to evaluate the relative importance of four blood pressure indices in assessing NAFLD. Building upon Okamura et al.'s research, we further excluded males with weekly alcohol consumption over 210 g and females over 140 g (n = 1,213), in accordance with the safe alcohol consumption limits of NAFLD (21). This resulted in 14,251 participants included in our analysis, as shown in the flowchart in Figure 1. As a secondary analysis, the Jiangxi Provincial People's Hospital ethics committee approved our study; the need for re-signing informed consent was waived due to prior agreements in the original research.
Data collection
In the NAGALA project, all participants were required to fill out baseline questionnaires on demographic data (gender, age), lifestyle habits (smoking/drinking status, exercise habits), and chronic disease history (diabetes, liver diseases). Trained medical staff in a standardized environment measured simple physical parameters [including height, weight, waist circumference (WC)] and biochemical indices using automated biochemistry analyzers. Lifestyle habits were categorized as follows: (i) exercise habit defined as participating in physical activities at least once a week; (ii) drinking status based on weekly consumption in the past month, categorized as <40 g as none or minimal, 40–140 g as light, and 140–210 g as moderate (21, 22); (iii) smoking status defined based on smoking history as non-smoking, past smoking, and current smoking.
Blood pressure measurement is conducted in a quiet environment. After resting for 5 min, participants, assisted by medical personnel, supported both arms at heart level. The cuff is wrapped around the upper arm, adjusted to fit snugly, with the lower edge approximately half an inch above the elbow, and the air tube aligned with the midpoint of the participant's arm. Subsequently, SBP and DBP are recorded as the first and fifth Korotkoff sounds, respectively, using a mercury sphygmomanometer. The process is repeated three times, with a 2-min interval between measurements. The final recorded blood pressure value is the average of the second and third measurements.
Venous blood samples for biochemical analysis were taken after overnight fasting and analyzed in a standard laboratory for high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), triglycerides (TG), hemoglobin A1c (HbA1c), FPG, and concentrations of gamma-glutamyl transferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST).
Calculations
Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared;
PP was calculated as SBP minus DBP (23);
MAP was calculated as one-third of SBP plus two-thirds of DBP (23).
NAFLD diagnosis
NAFLD was diagnosed through abdominal ultrasonography, after excluding other liver diseases and confirming alcohol consumption within safe limits for NAFLD (21). The ultrasound was performed by professional technicians, and gastroenterology experts, blind to the participants' details, diagnosed NAFLD based on criteria such as liver brightness, hepatorenal echo contrast, vascular blurring, and deep attenuation (24).
Statistical analysis
Data in the current study were analyzed using Empower(R) Version 2.0 and R language 4.2.1. Baseline information was described as frequency (%) for categorical data and mean (SD) or median (interquartile range) for continuous data. Marginal structural models were employed to quantify differences in baseline characteristics of the study population (25, 26).
The association between the four blood pressure indices and NAFLD was analyzed using multivariable logistic regression models. Before inclusion in the regression models, the four indices were Z-transformed to eliminate disparities in OR due to different magnitudes of blood pressure values, ensuring comparability of the OR values calculated. Moreover, variance inflation factors were calculated to assess collinearity between the four indices and other covariates (Supplementary Tables S1–S4) (27); with a critical value of 5 for variance inflation factors, collinearity was found between the four indices and weight, WC. In models with NAFLD as the dependent variable, SBP and DBP showed collinearity with other blood pressure indices except themselves (Supplementary Tables S1 and S2); whereas, in models with PP and MAP as independent variables, no collinearity was found between PP and MAP, but they showed collinearity with SBP and DBP (Supplementary Tables S3 and S4). After identifying factors with multicollinearity, three progressively adjusted multivariable models were established. Model I primarily considered demographic data effects, adjusting for gender, age, height, and BMI. Model II further considered lifestyle factors, adjusting for smoking status, drinking status, and exercise habits. Model III, the final model, adjusted for all non-collinear variables, with mutual adjustment of PP and MAP in their respective models.
Several sensitivity analyses were conducted to verify the reliability of the association between the four blood pressure indices and NAFLD. (i) To mitigate the potential influence of exercise on NAFLD (28), we replicated the analysis based on Model III in the population without exercise habits. (ii) As aging is a significant contributor to NAFLD (29), and to reduce this influence, we also conducted the same analysis in participants younger than 60 years. (iii) The analysis was continued in the normotensive population. (iv) Since obesity is a major promoter of NAFLD (1, 2), we further validated the stability of the associations between the four indices and NAFLD in the non-obese population.
Using ROC analysis and Delong's test (30), we further evaluated and compared the ability of the four blood pressure indices to identify NAFLD, calculating the corresponding area under the curve (AUC), optimal threshold, sensitivity, and specificity.
Results
Baseline characteristics
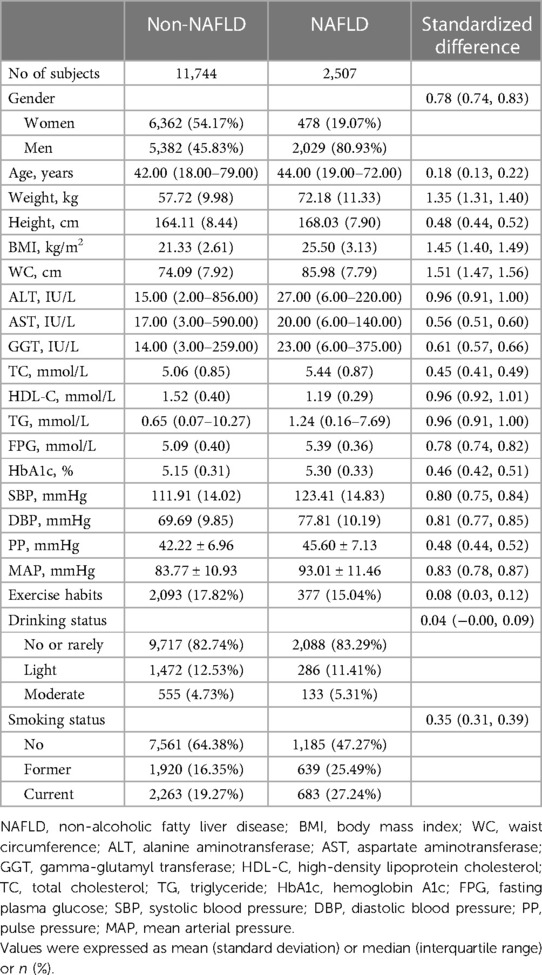
Of the 14,251 participants included in this study, the male-to-female ratio was 1.08, with an average age of 43 years, and the prevalence of NAFLD was 17.59%. We compared the baseline characteristics of the study population grouped based on NAFLD diagnosis (Table 1), and observed significant differences (standardized difference >0.1) in all baseline characteristics except for drinking status and exercise habits. Compared to the non-NAFLD group, participants with NAFLD had relatively higher age, height, weight, BMI, WC, ALT, AST, GGT, TC, TG, FPG, HbA1c, and blood pressure indices. Among these, SBP, DBP, and MAP showed a larger difference (standardized difference value >0.8), while PP showed a relatively smaller difference (standardized difference value = 0.48) (Figure 2). In addition, it should also be noted that there was a large gap in the prevalence of NAFLD between the two groups [Women 478/6,840 (6.99%) vs. Men 2,029/7,411 (27.38%)], and the standardized difference value was further calculated to be 0.56.
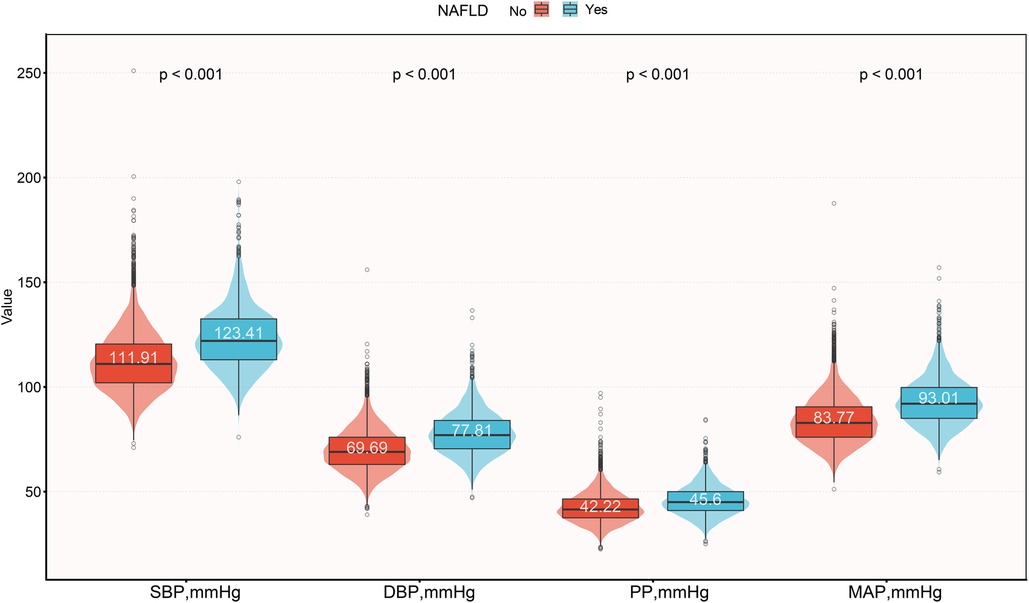
Figure 2. Violin plots show baseline characteristics of the four blood pressure indices in the NAFLD and non-NAFLD groups. NAFLD, non-alcoholic fatty liver disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MAP, mean arterial pressure.
Assessment of the four blood pressure indices in estimating NAFLD risk
Table 2 presents the variations in the associations between the four blood pressure indices and NAFLD in progressively adjusted models. Initially, in the unadjusted model, all four indices were positively correlated with NAFLD; the corresponding OR values per SD increase for SBP, DBP, PP, and MAP were 2.16, 2.21, 1.58, 2.23, respectively, with MAP showing the largest OR value in relation to NAFLD. After adjusting for gender, age, height, and BMI in Model I, the association between all four indices and NAFLD substantially weakened, yet MAP still had the largest OR, while PP had the lowest. The results remained similar after further adjusting for lifestyle factors. Finally, to fully consider the impact of covariates, we adjusted for all non-collinear variables in Model III. The results indicated that compared to the other three blood pressure indices, MAP still had the strongest association with NAFLD (OR per SD increase: 1.20, 95% CI: 1.11–1.30). Additionally, it is worth noting that the association between PP and NAFLD disappeared in the fully adjusted model.
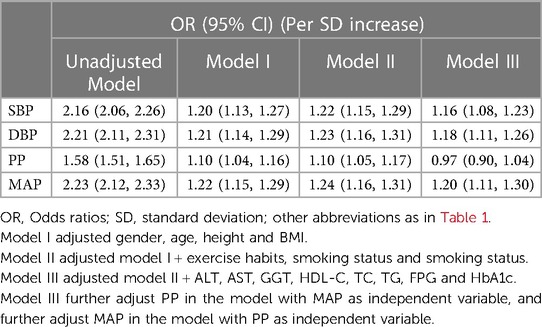
Table 2. Multivariable logistic regression analysis of the correlation between blood pressure index and NAFLD.
Considering that the prevalence of NAFLD was quite different between men and women in baseline characteristic analysis, it is necessary to further evaluate whether there is gender difference in the association between blood pressure indices and NAFLD. We further conducted stratified analyzes by gender and examined whether gender played a modifying role in the association using likelihood ratio tests. The results of these analyses revealed a significant positive correlation between blood pressure indices except PP and NAFLD in both sexes (Supplementary Table S5). In addition, blood pressure indices SBP, DBP, and MAP showed a stronger correlation with NAFLD in men compared to women, but further interactive tests showed that the difference was not statistically significant (All P-interactions >0.05).
Sensitivity analysis
To verify the stability of the association between the four blood pressure indices and NAFLD, we conducted the same analysis in populations with relatively lower NAFLD risk. The results were consistent with the primary analysis across all subgroups, including those without exercise habits, aged <60 years, normotensive, and non-obese. The association between PP and NAFLD disappeared after full adjustment for confounders, while MAP showed a superior ability to assess NAFLD risk compared to SBP and DBP (Table 3).
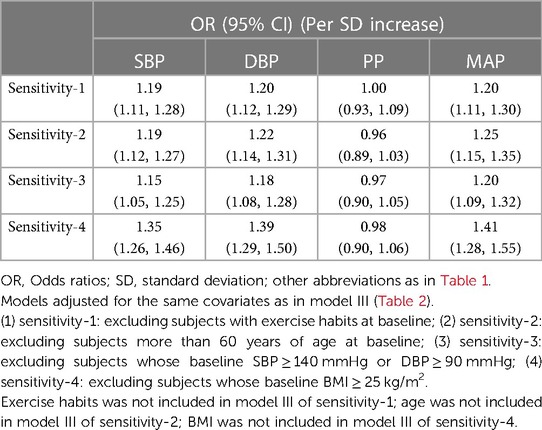
Table 3. Adjusted odds ratios and 95% confidence intervals for NAFLD risk associated with the blood pressure index in different test populations: sensitivity analysis.
Assessment of the four blood pressure indices in identifying NAFLD
ROC curves were plotted to evaluate the ability of the four blood pressure indices to identify NAFLD (Figure 3). The results indicated that MAP had the highest AUC value (0.7258), followed by DBP (0.7210), SBP (0.7196), and finally PP (0.6379) (Table 4). After further comparison using the Delong test, significant statistical differences were found between MAP and SBP, DBP, and PP in identifying NAFLD (All Delong P < 0.05).
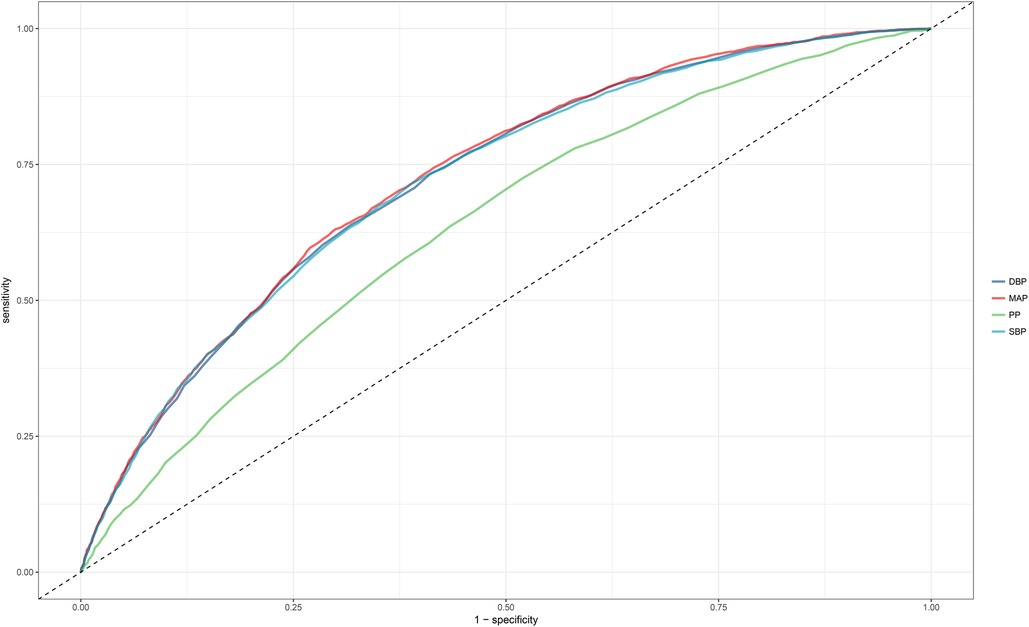
Figure 3. Area under the receiver operating characteristic curve for SBP, DBP, PP, and MAP for identification of NAFLD in the entire population. NAFLD, non-alcoholic fatty liver disease; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MAP, mean arterial pressure.
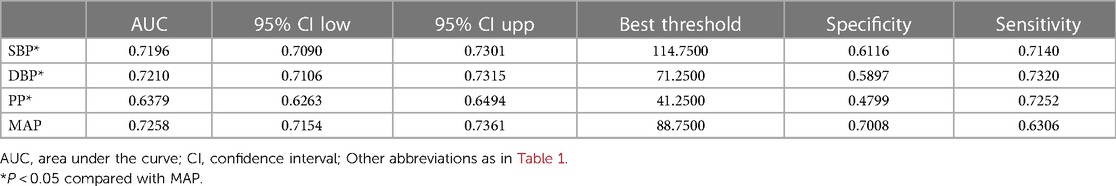
Table 4. The best threshold, sensitivities, specificities, and area under the curve of blood pressure index for the screening of NAFLD in the general population.
Discussion
In this survey analysis based on a health examination population, after adequately adjusting for confounding factors, we found that all blood pressure indices, except PP, were positively correlated with NAFLD. Among these, MAP may be the optimal index for assessing NAFLD risk. Additionally, in terms of identifying NAFLD, MAP was the most accurate compared to SBP, DBP, and PP. These findings emphasized that MAP might be the most promising blood pressure index in screening for NAFLD.
The association between blood pressure indices and NAFLD has been reported in many studies, with published evidence generally supporting a positive correlation between SBP, DBP, PP, MAP, and NAFLD (14–16, 31–34). However, it's noteworthy that most of these studies focused on evaluating the association of individual blood pressure indices with NAFLD and rarely assessed the impact of different indices on NAFLD risk simultaneously. To our knowledge, only one study has investigated the association between multiple blood pressure indices and fatty liver; Patel et al.'s study based on a British birth cohort observed a positive correlation between fatty liver and SBP, DBP, and MAP in the same adolescent cohort, while no significant correlation with PP (35). The findings about PP in Patel et al.'s study are inconsistent with previous results from Zhang et al. based on Mendelian randomization (15), possibly due to the lack of adjustment for confounders in Zhang et al.'s study, masking the true association between PP and NAFLD. In our current study, a positive correlation between PP and NAFLD was observed in the unadjusted and partially adjusted models (Models I and II), but this association disappeared in the fully adjusted model (Model III). Therefore, combining the results of our study with Patel et al.'s (35), we believe that PP has a weak and unstable, non-independent positive correlation with NAFLD. Furthermore, considering the significant differences in magnitude among blood pressure indices, we performed Z-transformation and calculated the OR values of all blood pressure indices per SD increase in relation to NAFLD. Compared to SBP and DBP, MAP had the highest degree of association with NAFLD. This finding was further confirmed in several sensitivity analyses (Table 3); in our study, among the four indices, MAP had the strongest association with NAFLD in populations without exercise habits, non-obese, relatively younger, and normotensive, while the association between PP and NAFLD disappeared after full adjustment for confounders. Compared to Patel et al.'s study (35), our study population consisted of adults, with a larger sample size (14,251 vs. 1,904), and data normalization was conducted for relative comparability of effect sizes. Based on the findings of the association analysis in our current study, MAP might be the best blood pressure index for assessing NAFLD risk.
The gender difference of NAFLD has been widely concerned in recent years. Generally speaking, the prevalence of NAFLD in men is higher than that in women (36–38), and the epidemiological survey based on the Japanese population shows that the prevalence of NAFLD in men is about three times that of women (39, 40), which is highly similar to the results of current research. Compared with women, men usually show more visceral fat deposition, are more susceptible to leptin resistance, lack estrogen receptors, and tend to synthesize fatty acids into fat storage (38). Considering the significant gender difference in the prevalence of NAFLD, in the current study, we further evaluated whether the association between blood pressure indices and NAFLD was modified by gender, and the results were consistent with the main analysis: In both sexes, except for PP, the correlation between blood pressure indices and NAFLD is significant, and the correlation between MAP and NAFLD is the highest in both sexes. However, in further interactive tests, we did not detect a significant gender difference in the correlation between blood pressure indices and NAFLD.
Reports on blood pressure indices in identifying NAFLD are currently quite limited. Most published studies have primarily focused on revealing the association between blood pressure indices and NAFLD (14–16, 31–34), affirming the importance of blood pressure indices in NAFLD risk assessment, even at normal blood pressure levels. After establishing this association, further evaluating the value of blood pressure indices in identifying NAFLD is worth researching and emphasizing. In a recent study by Xu et al., they assessed the value of SBP, DBP, and MAP in identifying NAFLD in a non-obese population, noting that MAP had the highest value in identifying NAFLD compared to SBP and DBP (34). Similarly to Xu et al.'s findings, our study, including both obese and non-obese individuals in a general health examination population, found MAP to have the highest value in identifying NAFLD (AUC: 0.7258). Combining the results of the ROC analysis and the standardized OR values from the association analysis, MAP seems to be the most useful blood pressure index for screening NAFLD in a health examination population.
After establishing that MAP was the most advantageous blood pressure index for screening NAFLD, further estimating its effective clinical threshold is valuable. It's noteworthy that MAP has only recently gained popularity in epidemiological studies, previously being primarily used in critical and perioperative clinical monitoring, due to its importance in reflecting vital organ perfusion (41–43). Although there are no official recommendations for the optimal MAP target in critically ill or perioperative patients, literature reviews suggest that maintaining MAP above 65 mmHg is crucial for patient recovery (44–46). For survivors of myocardial infarction, studies showed that maintaining MAP above 80 was important for improving adverse outcomes (47). Additionally, recent epidemiological surveys have proposed gender-specific MAP thresholds for predicting metabolic syndrome in the elderly at 84 mmHg (male) and 83.3 mmHg (female) (48), a MAP threshold of 92.833 mmHg for predicting diabetes (49), and 88 mmHg (male) and 89 mmHg (female) for predicting non-obese NAFLD (34). In our current study, based on general health examination population data, we found the threshold for identifying NAFLD using MAP to be 88.75 mmHg. Based on these results, we suggest that a MAP threshold of 90 mmHg might be appropriate for assessing various chronic diseases, including NAFLD.
Reflections on the clinical significance of the relationship between blood pressure indices and NAFLD can provide insights for subsequent research and clinical applications. Similar to some past studies investigating the relationship between multiple blood pressure indices and diseases (48, 50–59), our study is the first to identify MAP as the most promising blood pressure index for screening NAFLD. These findings are significant because they (i) fill a knowledge gap in NAFLD; (ii) provide ideas and important references for subsequent clinical studies or mechanistic research related to NAFLD; (iii) offer reliable data and insights for future NAFLD risk modeling studies or model improvement research; (iv) considering the high prevalence of NAFLD, the most important clinical significance of this study lies in its application to NAFLD screening, as blood pressure indices are easily obtainable and self-measurable, making them valuable for healthcare professionals and individuals for daily NAFLD identification/risk assessment.
Our study has some limitations that need to be addressed: (i) NAFLD diagnosis in our study was based on ultrasonography, which might miss some cases with mild hepatic steatosis (60). (ii) The cross-sectional design limits the study's ability to further explore the predictive value of blood pressure indices for NAFLD. (iii) The evidence from our study is applicable to the Japanese population and needs further validation in other populations. (iv) Although we have made comprehensive adjustments with the available data, some unmeasured factors were not included in our analysis, which could lead to some residual confounding (61). (v) Since June 2023, it has gradually become noteworthy that the term NAFLD is being replaced by the new term “Metabolic dysfunction-associated steatotic liver disease (MASLD),” as MASLD is considered to better align with the pathophysiological characteristics of the disease (62–64). Furthermore, the definition of MASLD further emphasizes the significant impact of cardiac metabolic factors on disease diagnosis (62). Given that blood pressure index is one of the most crucial cardiac metabolic factors (56, 65, 66), and multiple blood pressure indices have been established in current research for screening NAFLD, we speculate that blood pressure indices may similarly play a significant role in MASLD screening; however, further research is still needed.
Conclusion
We discovered that, in a general population, SBP, DBP, and MAP all positively correlate with NAFLD, except for PP. After data normalization, MAP showed the strongest association with NAFLD. Furthermore, subsequent ROC analysis revealed that MAP was the most accurate blood pressure index for identifying NAFLD compared to SBP, DBP, and PP. These findings highlight a key point: MAP may be the most important blood pressure index for assessing NAFLD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Jiangxi Provincial People's Hospital ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
CH: Data curation, Formal Analysis, Software, Validation, Writing – original draft. ZY: Formal Analysis, Software, Validation, Writing – original draft. CW: Formal Analysis, Software, Validation, Writing – original draft. GS: Formal Analysis, Validation, Writing – review & editing. JC: Conceptualization, Project administration, Supervision, Writing – review & editing. YZ: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the National Natural Science Foundation of China [No. 81960111 to JC] and the Natural Science Foundation of Jiangxi Province [No. 20232BAB216004 to YZ].
Acknowledgments
We sincerely thank Professor Okamura and his team for their great efforts in data collection and follow-up.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1338156/full#supplementary-material
References
1. Grander C, Grabherr F, Tilg H. Non-alcoholic fatty liver disease: pathophysiological concepts and treatment options. Cardiovasc Res. (2023) 119(9):1787–98. doi: 10.1093/cvr/cvad095
2. Leow WQ, Chan AW, Mendoza PGL, Lo R, Yap K, Kim H. Non-alcoholic fatty liver disease: the pathologist’s perspective. Clin Mol Hepatol. (2023) 29(Suppl):S302–18. doi: 10.3350/cmh.2022.0329
3. Le MH, Le DM, Baez TC, Wu Y, Ito T, Lee EY, et al. Global incidence of non-alcoholic fatty liver disease: a systematic review and meta-analysis of 63 studies and 1,201,807 persons. J Hepatol. (2023) 79(2):287–95. doi: 10.1016/j.jhep.2023.03.040
4. World Health Organization. Obesity and overweight (2018). Available online at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed October 1, 2023).
5. GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global burden of disease study 2021. Lancet. (2023) 402(10397):203–34. doi: 10.1016/S0140-6736(23)01301-6
6. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. (2015) 62(1 Suppl):S47–64. doi: 10.1016/j.jhep.2014.12.012
7. Mantovani A, Scorletti E, Mosca A, Alisi A, Byrne CD, Targher G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metab Clin Exp. (2020) 111S:154170. doi: 10.1016/j.metabol.2020.154170
8. Henry L, Paik J, Younossi ZM. Review article: the epidemiologic burden of non-alcoholic fatty liver disease across the world. Aliment Pharmacol Ther. (2022) 56(6):942–56. doi: 10.1111/apt.17158
9. Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. (2021) 6(7):578–88. doi: 10.1016/S2468-1253(21)00020-0
10. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. (2016) 134(6):441–50. doi: 10.1161/CIRCULATIONAHA.115.018912
11. Golubeva JA, Sheptulina AF, Elkina AY, Liusina EO, Kiselev AR, Drapkina OM. Which comes first, nonalcoholic fatty liver disease or arterial hypertension? Biomedicines. (2023) 11(9):2465. doi: 10.3390/biomedicines11092465
12. Oikonomou D, Georgiopoulos G, Katsi V, Kourek C, Tsioufis C, Alexopoulou A, et al. Non-alcoholic fatty liver disease and hypertension: coprevalent or correlated? Eur J Gastroenterol Hepatol. (2018) 30(9):979–85. doi: 10.1097/MEG.0000000000001191
13. Huang Q, Yu H, Zhong X, Tian Y, Cui Z, Quan Z. Association between hypertension and nonalcoholic fatty liver disease: a cross-sectional and meta-analysis study. J Hum Hypertens. (2023) 37(4):313–20. doi: 10.1038/s41371-022-00686-w
14. Yuan M, He J, Hu X, Yao L, Chen P, Wang Z, et al. Hypertension and NAFLD risk: insights from the NHANES 2017-2018 and Mendelian randomization analyses. Chin Med J (Engl). (2024) 137(4):457–64. doi: 10.1097/CM9.0000000000002753
15. Zhang Z, Li L, Hu Z, Zhou L, Zhang Z, Xiong Y, et al. The causal associations of non-alcoholic fatty liver disease with blood pressure and the mediating effects of cardiometabolic risk factors: a Mendelian randomization study. Nutr Metab Cardiovasc Dis. (2023) 33(11):2151–9. doi: 10.1016/j.numecd.2023.07.010
16. Li X, Yang H, Xie G, Kuang M, Sheng G, Zou Y. Association of mean arterial pressure with non-alcoholic fatty liver disease: results from the NAGALA study. Front Cardiovasc Med. (2023) 10:1266879. doi: 10.3389/fcvm.2023.1266879
17. Aneni EC, Oni ET, Martin SS, Blaha MJ, Agatston AS, Feldman T, et al. Blood pressure is associated with the presence and severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk. J Hypertens. (2015) 33(6):1207–14. doi: 10.1097/HJH.0000000000000532
18. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts. (2016) 9(2):65–90. doi: 10.1159/000443344
19. Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Data from: ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study, Dryad, Dataset (2019). doi: 10.5061/dryad.8q0p192
20. Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes (Lond). (2019) 43(1):139–48. doi: 10.1038/s41366-018-0076-3
21. Choi JH, Sohn W, Cho YK. The effect of moderate alcohol drinking in nonalcoholic fatty liver disease. Clin Mol Hepatol. (2020) 26(4):662–9. doi: 10.3350/cmh.2020.0163
22. Hashimoto Y, Hamaguchi M, Kojima T, Ohshima Y, Ohbora A, Kato T, et al. Modest alcohol consumption reduces the incidence of fatty liver in men: a population-based large-scale cohort study. J Gastroenterol Hepatol. (2015) 30(3):546–52. doi: 10.1111/jgh.12786
23. Kodama S, Horikawa C, Fujihara K, Yoshizawa S, Yachi Y, Tanaka S, et al. Meta-analysis of the quantitative relation between pulse pressure and mean arterial pressure and cardiovascular risk in patients with diabetes mellitus. Am J Cardiol. (2014) 113(6):1058–65. doi: 10.1016/j.amjcard.2013.12.005
24. Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. (2007) 102(12):2708–15. doi: 10.1111/j.1572-0241.2007.01526.x
25. Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. (2003) 14(6):680–6. doi: 10.1097/01.EDE.0000081989.82616.7d
26. Muanda FT, Weir MA, Bathini L, Blake PG, Chauvin K, Dixon SN, et al. Association of baclofen with encephalopathy in patients with chronic kidney disease. JAMA. (2019) 322(20):1987–95. doi: 10.1001/jama.2019.17725
27. Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. (2019) 72(6):558–69. doi: 10.4097/kja.19087
28. Babu AF, Csader S, Lok J, Gómez-Gallego C, Hanhineva K, El-Nezami H, et al. Positive effects of exercise intervention without weight loss and dietary changes in NAFLD-related clinical parameters: a systematic review and meta-analysis. Nutrients. (2021) 13(9):3135. doi: 10.3390/nu13093135
29. Bloomer SA, Moyer ED. Hepatic macrophage accumulation with aging: cause for concern? Am J Physiol Gastrointest Liver Physiol. (2021) 320(4):G496–505. doi: 10.1152/ajpgi.00286.2020
30. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44(3):837–45. doi: 10.2307/2531595
31. Wu SJ, Zou H, Zhu GQ, Wang LR, Zhang Q, Shi KQ, et al. Increased levels of systolic blood pressure within the normal range are associated with significantly elevated risks of nonalcoholic fatty liver disease. Medicine (Baltimore). (2015) 94(19):e842. doi: 10.1097/MD.0000000000000842
32. Mori K, Tanaka M, Hosaka I, Mikami T, Endo K, Hanawa N, et al. Metabolic dysfunction-associated fatty liver disease is associated with an increase in systolic blood pressure over time: linear mixed-effects model analyses. Hypertens Res. (2023) 46(5):1110–21. doi: 10.1038/s41440-023-01179-0
33. Qian LY, Tu JF, Ding YH, Pang J, Che XD, Zou H, et al. Association of blood pressure level with nonalcoholic fatty liver disease in nonhypertensive population: normal is not the new normal. Medicine (Baltimore. (2016) 95(29):e4293. doi: 10.1097/MD.0000000000004293
34. Xu S, Chen L, Hong D, Yang L, Li X, Wang X. Mean arterial pressure is related to incident nonalcoholic fatty liver disease among the nonobese female with normal low-density lipoprotein cholesterol levels: a large cohort study in China. Gastroenterol Res Pract. (2020) 2020:3580840. doi: 10.1155/2020/3580840
35. Patel S, Lawlor DA, Ferreira DL, Hughes AD, Chaturvedi N, Callaway M, et al. The association of nonalcoholic fatty liver disease with central and peripheral blood pressure in adolescence: findings from a cross-sectional study. J Hypertens. (2015) 33(3):546–52; discussion 553. doi: 10.1097/HJH.0000000000000445
36. Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. (2019) 70(4):1457–69. doi: 10.1002/hep.30626
37. Chen XY, Wang C, Huang YZ, Zhang LL. Nonalcoholic fatty liver disease shows significant sex dimorphism. World J Clin Cases. (2022) 10(5):1457–72. doi: 10.12998/wjcc.v10.i5.1457
38. Chang M, Shao Z, Wei W, Shen P, Shen G. Sex-specific prevalence and risk factors of metabolic-associated fatty liver disease among 75,570 individuals in eastern China. Front Endocrinol (Lausanne). (2023) 14:1241169. doi: 10.3389/fendo.2023.1241169
39. Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. (2012) 47(5):586–95. doi: 10.1007/s00535-012-0533-z
40. Sugiyama A, Kurisu A, Ouoba S EB, Ko K, Rakhimov A, et al. Relationship between drinking frequency and fatty liver prevalence or incidence in Japanese undergoing health checkup in 2008–2019. Liver Int. (2021) 41(12):2914–23. doi: 10.1111/liv.15055
41. Safar ME. Pulse pressure in essential hypertension: clinical and therapeutical implications. J Hypertens. (1989) 7(10):769–76. doi: 10.1097/00004872-198910000-00001
42. Wang D, Wang J, Liu J, Qin Y, Lou P, Zhang Y, et al. The role of cumulative mean arterial pressure levels in first stroke events among adults with hypertension: a 10-year prospective cohort study. Clin Epidemiol. (2022) 14:665–76. doi: 10.2147/CLEP.S359284
43. Grillo A, Salvi P, Furlanis G, Baldi C, Rovina M, Salvi L, et al. Mean arterial pressure estimated by brachial pulse wave analysis and comparison with currently used algorithms. J Hypertens. (2020) 38(11):2161–8. doi: 10.1097/HJH.0000000000002564
44. Burstein B, Tabi M, Barsness GW, Bell MR, Kashani K, Jentzer JC. Association between mean arterial pressure during the first 24 h and hospital mortality in patients with cardiogenic shock. Crit Care. (2020) 24(1):513. doi: 10.1186/s13054-020-03217-6
45. Song Q, Li J, Jiang Z. Provisional decision-making for perioperative blood pressure management: a narrative review. Oxid Med Cell Longev. (2022) 2022:5916040. doi: 10.1155/2022/5916040
46. Beloncle F, Radermacher P, Guerin C, Asfar P. Mean arterial pressure target in patients with septic shock. Minerva Anestesiol. (2016) 82(7):777–84.26967829
47. Avanzini F, Alli C, Boccanelli A, Chieffo C, Franzosi MG, Geraci E, et al. High pulse pressure and low mean arterial pressure: two predictors of death after a myocardial infarction. J Hypertens. (2006) 24(12):2377–85. doi: 10.1097/01.hjh.0000251897.40002.bf
48. Hsu CH, Chang JB, Liu IC, Lau SC, Yu SM, Hsieh CH, et al. Mean arterial pressure is better at predicting future metabolic syndrome in the normotensive elderly: a prospective cohort study in Taiwan. Prev Med. (2015) 72:76–82. doi: 10.1016/j.ypmed.2014.12.036
49. Wu Y, Hu H, Cai J, Chen R, Zuo X, Cheng H, et al. Association of mean arterial pressure with 5-year risk of incident diabetes in Chinese adults:a secondary population-based cohort study. BMJ Open. (2022) 12(9):e048194. doi: 10.1136/bmjopen-2020-048194
50. Ren L, Gao Y, Jiang Y, Wang G, Li Q, Gu Y, et al. Association between blood pressure indicators and stroke in aged population: a community-based nested case-control study. Clin Interv Aging. (2021) 16:997–1005. doi: 10.2147/CIA.S304847
51. Salman E, Kadota A, Hisamatsu T, Segawa H, Torii S, Fujiyoshi A, et al. Relationship of four blood pressure indexes to subclinical cerebrovascular diseases assessed by brain MRI in general Japanese men. J Atheroscler Thromb. (2022) 29(2):174–87. doi: 10.5551/jat.58537
52. Hadaegh F, Shafiee G, Hatami M, Azizi F. Systolic and diastolic blood pressure, mean arterial pressure and pulse pressure for prediction of cardiovascular events and mortality in a middle eastern population. Blood Press. (2012) 21(1):12–8. doi: 10.3109/08037051.2011.585808
53. Inoue R, Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, et al. Predicting stroke using 4 ambulatory blood pressure monitoring-derived blood pressure indices: the ohasama study. Hypertension. (2006) 48(5):877–82. doi: 10.1161/01.HYP.0000242285.83728.ee
54. Ishimitsu T, Nakano N, Sudo Y, Akashiba A, Takahashi T, Ohta S, et al. Predictive significance of blood pressure values for the incidence of cardiovascular events in chronic hemodialysis patients. Hypertens Res. (2008) 31(9):1703–9. doi: 10.1291/hypres.31.1703
55. Miura K, Dyer AR, Greenland P, Daviglus ML, Hill M, Liu K, et al. Pulse pressure compared with other blood pressure indexes in the prediction of 25-year cardiovascular and all-cause mortality rates: the Chicago heart association detection project in industry study. Hypertension. (2001) 38(2):232–7. doi: 10.1161/01.hyp.38.2.232
56. Weitzman D, Goldbourt U. The significance of various blood pressure indices for long-term stroke, coronary heart disease, and all-cause mortality in men: the Israeli ischemic heart disease study. Stroke. (2006) 37(2):358–63. doi: 10.1161/01.STR.0000198869.84540.80
57. Oh HJ, Lee S, Lee EK, Lee O, Ha E, Park EM, et al. Association of blood pressure components with mortality and cardiovascular events in prehypertensive individuals: a nationwide population-based cohort study. Ann Med. (2018) 50(5):443–52. doi: 10.1080/07853890.2018.1492146
58. Koh H, Hayashi T, Sato KK, Harita N, Maeda I, Nakamura Y, et al. Blood pressure components and risk for chronic kidney disease in middle-aged Japanese men: the kansai healthcare study. Hypertens Res. (2011) 34(4):536–41. doi: 10.1038/hr.2011.2
59. Rifkin DE, Katz R, Chonchol M, Shlipak MG, Sarnak MJ, Fried LF, et al. Blood pressure components and decline in kidney function in community-living older adults: the cardiovascular health study. Am J Hypertens. (2013) 26(8):1037–44. doi: 10.1093/ajh/hpt067
60. Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. (2011) 54(3):1082–90. doi: 10.1002/hep.24452
61. Black N. Why we need observational studies to evaluate the effectiveness of health care. Br Med J. (1996) 312(7040):1215–8. doi: 10.1136/bmj.312.7040.1215
62. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety delphi consensus statement on new fatty liver disease nomenclature. Ann Hepatol. (2024) 29(1):101133. doi: 10.1016/j.aohep.2023.101133
63. Lekakis V, Papatheodoridis GV. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur J Intern Med. (2024) 122:3–10. doi: 10.1016/j.ejim.2023.11.005
64. Chan WK, Chuah KH, Rajaram RB, Lim LL, Ratnasingam J, Vethakkan SR. Metabolic dysfunction-associated steatotic liver disease (MASLD): a state-of-the-art review. J Obes Metab Syndr. (2023) 32(3):197–213. doi: 10.7570/jomes23052
65. Lawes CM, Bennett DA, Parag V, Woodward M, Whitlock G, Lam TH, et al. Blood pressure indices and cardiovascular disease in the Asia pacific region: a pooled analysis. Hypertension. (2003) 42(1):69–75. doi: 10.1161/01.HYP.0000075083.04415.4B
Keywords: blood pressure indices, NAFLD, evaluating, non-alcoholic fatty liver disease, survey report
Citation: Hu C, Yu Z, Wei C, Sheng G, Chen J and Zou Y (2024) Evaluating the relative importance of different blood pressure indices in screening for NAFLD: a survey report based on a health examination population. Front. Cardiovasc. Med. 11:1338156. doi: 10.3389/fcvm.2024.1338156
Received: 18 November 2023; Accepted: 18 April 2024;
Published: 29 April 2024.
Edited by:
Francesco Spannella, Università Politecnica delle Marche, ItalyReviewed by:
Shengshuai Shan, University of Georgia, United StatesValeria Visco, University of Salerno, Italy
© 2024 Hu, Yu, Wei, Sheng, Chen and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianyong Chen Y2p5YWN5NjlAMTYzLmNvbQ== Yang Zou anh5eHl6eUAxNjMuY29t
 Chong Hu
Chong Hu Ziqi Yu
Ziqi Yu Changli Wei1
Changli Wei1 Guotai Sheng
Guotai Sheng Yang Zou
Yang Zou