
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 22 April 2024
Sec. Cardiovascular Surgery
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1335407
Background: Currently, the bipolar radiofrequency ablation forceps manufactured by AtriCure are the main instrument for surgical ablation in patients with rheumatic heart disease (RHD) concomitant with atrial fibrillation (AF). The bipolar radiofrequency ablation forceps by Med-Zenith has a greater advantage in price compared with AtriCure. However, few studies have been reported on the comparison of their clinical efficacy. The aim of this study is to compare the short-term clinical efficacy of the two ablation forceps for RHD concomitant with AF.
Methods: Clinical data of 167 patients with RHD concomitant with AF admitted to the Department of Cardiac Major Vascular Surgery, Affiliated Hospital of North Sichuan Medical College, were retrospectively analyzed, and the restoration efficacy of sinus rhythm (SR) and cardiac function after surgery were compared with two ablation forceps.
Results: The end-systolic diameter of the right atrium and the end-systolic diameter of the left atrium in the patients of both groups at each postoperative time point decreased compared with that of the preoperative period (P < 0.05), and the left ventricular ejection fraction started to improve significantly at 6 months after surgery compared with that of the preoperative period (P < 0.05). There was no difference between the two groups of patients in the comparison of the aforementioned indicators at different points in time (P > 0.05). At 12 months postoperatively, the SR maintenance rate in using the ablation forceps by Med-Zenith (73.3%) was lower than that for AtriCure (86.4%) and the cumulative recurrence rate of AF in using the Med-Zenith ablation forceps was greater than that for AtriCure.
Conclusions: The two bipolar radiofrequency ablation forceps compared in the study are safe and effective in treating patients of RHD concomitant with AF, and the ablation forceps by AtriCure may be more effective in restoring SR in the short term.
Atrial fibrillation (AF) has been the subject of focused research on cardiac arrhythmias for a long time, and is one of the most common arrhythmias in clinical practice today; especially when combined with rheumatic heart disease (RHD), the risk of stroke is tripled, causing a high rate of disability and death (1–3). The Cox-Maze III procedure was once considered the gold standard for surgical treatment of AF, with the recurrence rate of AF of less than 10% at long-term postoperative follow-up (4). After decades of development, the radiofrequency-based Cox-Maze IV procedure has gradually become the mainstream of AF surgical ablation (5). As the Cox-Maze IV concomitant with a rheumatic valve procedure has matured, the ablation devices used in the procedure are no longer limited to the AtriCure bipolar radiofrequency ablation forceps as the Med-Zenith bipolar radiofrequency ablation forceps have also gradually begun to be used in the clinic (6). Compared with the radiofrequency ablation forceps by AtriCure, those by Med-Zenith undoubtedly have more advantages in terms of price, but there are few reports in the literature on their comparative clinical efficacy. This study focuses on the short-term efficacy of the two bipolar radiofrequency ablation forceps for RHD concomitant with AF, and further evaluates the clinical value and application prospect of the ablation forceps by Med-Zenith, with the aim of providing different choices of intraoperative ablation devices and reducing the economic burden of patients.
From September 2018 to December 2021, about 212 patients with RHD concomitant with AF underwent the rheumatic valve concomitant with Cox-Maze IV procedure in the Department of Cardiovascular Surgery, Affiliated Hospital of North Sichuan Medical College. The final selection of 167 patients was made strictly in accordance with the following inclusion exclusion criteria for this study.
The selected patients were divided into two groups, with 81 cases in the control group using the ablation forceps Isolator Synergy OLL2, by AtriCure, USA, and 86 cases in the observation group using the ablation forceps MZ-RFK by Med-Zenith, China.
(1) Meeting the diagnostic criteria of RHD concomitant with AF in the 2017 HRS/EHRA/ECAS Guidelines (7); (2) age ≥ 18 years; (3) intraoperative use of either of the two bipolar radiofrequency ablation forceps (Med-Zenith or AtriCure).
(1) Combination of other cardiac diseases, such as infective endocarditis; (2) end-systolic diameter of the left atrium (LAESD) ≥ 70 mm; (3) previous ablation of AF or rheumatic valve procedure; (4) combination of hepatic and renal failure (requiring dialysis treatment); (5) stroke within the last 6 months and acute myocardial infarction within the last 6 weeks; (6) combination of other cardiovascular surgeries during the same period, such as coronary artery bypass grafting, ascending aortic replacement, or plasty; and (7) irregular intake of medication after the operation and significant review data missing (including in-hospital death).
All the selected patients were operated by the same group of doctors. After general anesthesia, the chest of each patient was opened by a median sternal incision, heparinization was routinely performed at 3 mg/kg, the pericardium was incised in an inverted T-shape, the extracorporeal circulation was routinely established by the ascending aorta—upper and lower vena cava veins, and after the circulation was cooled down, the aorta was blocked, and radiofrequency ablation was performed at the root of the right auricle: the right atrium was incised obliquely, and radiofrequency ablation was performed successively at the right atrial incision to the superior vena cava line, the right atrial incision to the inferior vena cava line, the right atrial incision to the right auricle, the right atrial incision to the tricuspid annulus, and the right atrial incision to the coronary sinus line (Figure 1). The atrial septum was incised, and radiofrequency ablation was performed in the lines from the right superior pulmonary vein to the left superior pulmonary vein, the right inferior pulmonary vein to the left inferior pulmonary vein, the right inferior pulmonary vein to the medial posterior mitral annulus, the left inferior pulmonary vein to the left auricle, the left superior pulmonary vein to the left auricle, and the left superior and inferior pulmonary veins (Figure 2). Finally, ablation was performed at the root of the left auricle, the left auricle was ligated or excised, the ligament of Marshall was cut off, and the valve surgery was performed after the completion of ablation; the patient was routinely placed with temporary epicardial pacing wires during the operation, and a temporary pacemaker was connected after the operation (ablation of each pathway was performed four times until the wall was transmuted, and the radiofrequency ablation pen was added to deal with the isthmus lesion).
(1) Postoperatively, amiodarone 450 mg + 5% dextrose 45 ml was routinely pumped intravenously (2 ml/h, the speed was adjusted according to the heart rate), and it was changed to oral amiodarone after extubation of the tracheal tube, the first 200 mg t.i.d. × 7 days, the dosage was reduced to 200 mg b.i.d. × 7 days, and the last oral amiodarone (200 mg q.d.) was discontinued until 3 months after the operation (the drug was discontinued when the heart rate was <60 beats/min and the QTc was > 500 ms); postoperatively, long-term anticoagulant of warfarin was given, and no other antiarrhythmic drugs (AADs) were generally added during the treatment period, and the long-term oral angiotensin-converting enzyme inhibitors (ACEIs) were administered after discharge from the hospital.
(2) All patients will have regular postoperative follow-ups in our outpatient clinic for at least 1 year: Rechecking 12-lead or 24-h ambulatory electrocardiogram (ECG) at the time of discharge from the hospital, and at 3/6/9/12 months postoperatively; and cardiac ultrasound at the time of discharge from the hospital, and at 6/12 months postoperatively (note: discharged patients should be informed that if they are unable to come to the hospital for follow-up examination or experience palpitations after the operation, they can undergo the relevant examinations nearby and inform their physicians of the results at the next follow-up examination).
Any atrial arrhythmia greater than 30 s, including atrial fibrillation, atrial flutter, and atrial tachycardia, detected by 12-lead or 24-h ambulatory ECG, without the use of AADs (AF occurring within 3 months after surgery was not included as a recurrent AF event in this study).
All statistical analyses were performed with SPSS software (version 26.0; SPSS Inc., Chicago, IL, USA). The missing values were handled by the K-Nearest Neighbors algorithm. Categorical variables are expressed as frequencies and percentages, and continuous variables are expressed as the mean ± standard deviation. Median and interquartile range was used for non-normally distributed data.
(1) Two independent samples t test was used for comparisons between the groups for continuous variables, and Wilcoxon test was used for non-normally distributed data.
(2) Comparisons between groups for categorical variables were performed using the χ2 test (chi-square Pearson test for minimum expected counts T ≥ 5, chi-square continuity correction for 1 ≤ T < 5, and chi-square Fisher's exact method for T < 1).
(3) Repeated-measures continuous variables were analyzed by repeated-measures ANOVA, and two-by-two comparisons at different time points were performed using the Least-Significant Difference (LSD)-t test.
(4) The variables from univariate analyses (P < 0.05) were jointly included in multivariate binary logistic regression, and the results were expressed using corrected odds ratio (OR) [95% confidence interval (CI)].
(5) The Kaplan–Meier survival analysis describes the cumulative recurrence rate of AF at 1 year postoperatively, and the results were subjected to the Log-Rank test.
As provided in Table 1, a total of 110 mitral valve replacements, 8 aortic valve replacements, 43 aortic combined valve mitral valve replacements, and 6 mitral valve repairs were performed in the two groups of patients; 165 cases of tricuspid valvuloplasty were performed during the same period. The control group and the observation group differed only in terms of previous coronary artery disease, preoperative oral warfarin, number of valves, and lactate level (P < 0.05), and there was no statistical difference between the two groups in terms of cardiac function class, LAESD, ablation time, duration and type of AF, etc. (P > 0.05).
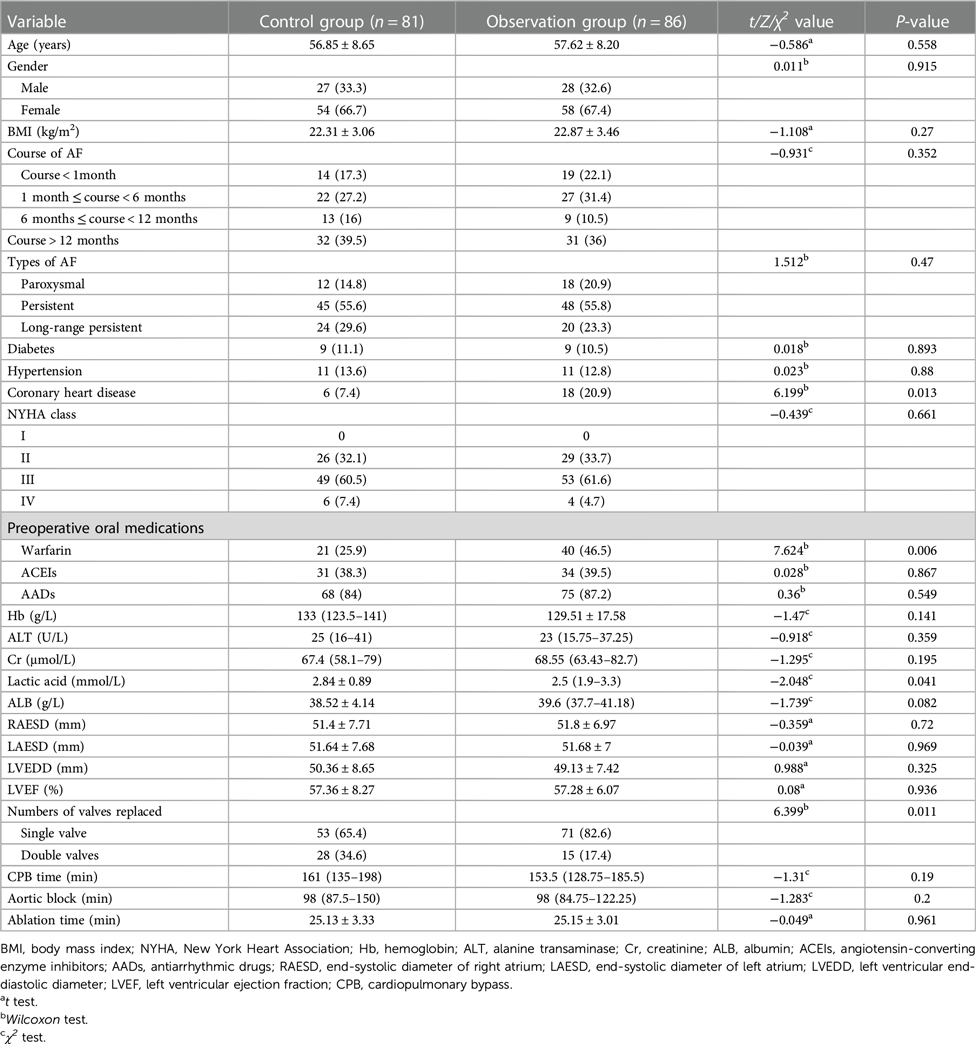
Table 1. Comparison of baseline data between the two groups [ ± s, n (%), M(P25, P15) ± s, n (%), M(P25, P15)].
To exclude the interference of related confounding factors, we included postoperative AF recurrence as the dependent variable, and included the aforementioned factors (P < 0.05) together with the grouping variable (different ablation forceps) in the binary logistic regression model for multifactorial analysis. As provided in Table 2, after correcting for the aforementioned influences, the grouping variable remained (OR = 2.345, 95% CI: 1.117–4.923, P = 0.024) an independent influence on postoperative AF recurrence.
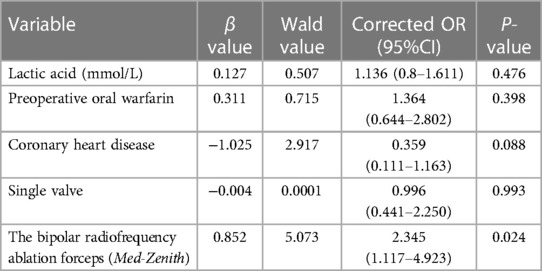
Table 2. Multifactorial Logistic Regression Analysis of AF recurrence after the rheumatic valve procedure concomitant with Cox-Maze IV procedure.
We analyzed the end-systolic diameter of the right atrium (RAESD), LAESD, and left ventricular ejection fraction (LVEF) of the two groups at four time points: preoperatively, at discharge, at 6 months postoperatively, and at 12 months postoperatively by repeated-measures ANOVA, and performed simple effects analysis (two-by-two comparison), as presented in Table 3 and Figure 3.
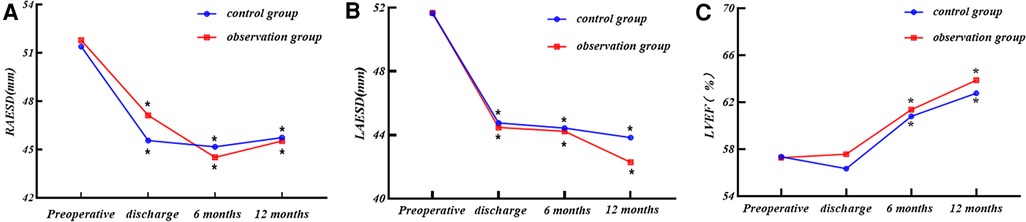
Figure 3. (A) RAESD, (B) LAESD, and (C) LVEF changes at each time point for the two groups. Comparison within group: compared with preoperative period. *P < 0.05.
(1) There was no statistical difference in the RAESD of the two groups of patients at each time point (P > 0.05), nor was there any statistical difference in the trend of RAESD of the two groups at each time point (P > 0.05).
(2) The RAESD of the two groups showed a decreasing trend with the increase of time (P < 0.05).
(3) The RAESD decreased in both groups at all time points after surgery compared with the preoperative period (P < 0.05).
(1) There was no statistical difference in the LAESD of the two groups of patients at each time point (P > 0.05), nor was there any statistical difference in the trend of the LAESD of the two groups at each time point (P > 0.05).
(2) The LAESD of the two groups showed a decreasing trend with the increase of time (P < 0.05).
(3) The LAESD decreased in both groups at all time points after surgery compared with the preoperative period (P < 0.05).
(1) There was no statistical difference in LVEF of the two groups of patients at each time point (P > 0.05), nor was there any statistical difference in the trend of LVEF of the two groups at each time point (P > 0.05).
(2) The LVEF of the two groups showed the upward trend with the increase of time (P < 0.05).
(3) There was no significant difference in postoperative LVEF of the two groups at the time of discharge compared with the preoperative period (P > 0.05), and it started to improve significantly at 6 months postoperatively compared with the preoperative period (P < 0.05).
The sinus rhythm (SR) maintenance rate of the two groups showed a slow decreasing trend with the increase of time, and there was no significant difference in the first postoperative day, at the time of discharge, and at 3/6/9 months postoperatively (P > 0.05); the maintenance rate of SR of the control group (86.4%) was higher than that of the observation group (73.3%) at 12 months postoperatively, and the difference was statistically significant (P < 0.05), as shown in Figure 4.
The Kaplan–Meier survival analysis was performed with postoperative AF recurrence as the outcome end point event, with the independent variable being the grouping variable (different ablation forceps), and the time until the end point event was the time to AF recurrence (3 months ≤ time ≤ 12 months). The results revealed 31 cases of AF recurrence in the observation group and 17 cases of AF recurrence in the control group at 1 year after surgery. The cumulative recurrence rate of AF at 1 year after surgery in the observation group was higher than that in the control group (Log-Rank: P = 0.035), as shown in Figure 5.
Atrial fibrillation, a common clinical arrhythmia, has been shown to be associated with the progression and worsening of heart failure, with the incidence of heart failure in patients with persistent and long-standing persistent AF being approximately 40%–55% (8, 9). In addition, the higher disability and mortality rates due to stroke in AF are a major global problem, and in particular, the risk of stroke is tripled in combination with RHD (1). Rhythm-control-based pharmacotherapy and catheter ablation were earlier proposed for the treatment of AF with the aim of preventing stroke, controlling heart rate, reducing symptoms, and improving the cardiac function and quality of life of patients (10–12). Unfortunately, drug therapy generally has serious side effects, and the stable maintenance of SR after catheter ablation often requires multiple ablations, both of which have not significantly improved patients’ left heart function and quality of life (13). Currently, the radiofrequency-based Cox-Maze IV procedure has replaced the original “cut-and-sew technique” as the main treatment for surgical ablation of AF, and the corresponding surgical ablation devices have also been developed rapidly (14). After decades of turnover, in the current domestic market the bipolar radiofrequency ablation forceps (MZ-RFK) manufactured by Med-Zenith are more frequently used in clinical application. Compared with the unipolar radiofrequency ablation device (unipolar linear ablation pen), the bipolar radiofrequency ablation forceps undoubtedly have a greater advantage. They have two parallel jaws, a curved upper jaw and a lower jaw, which can realize full and continuous contact between the electrode and the tissue when clamping the lesion tissue, creating a continuous ablation pathway and determining whether the tissue has reached complete wall permeability through the change of electrical conductivity. In addition, the parallel clamp design can avoid local energy concentration and reduce the damage to some low-impedance tissues (esophagus) (15).
Since the introduction of the first bipolar radiofrequency ablation forceps by AtriCure in 2000, after decades of development, the bipolar radiofrequency ablation forceps Isolator Synergy OLL2 have become the mainstay of surgical ablation applications for atrial fibrillation worldwide and are FDA approved surgical instruments for the surgical treatment of AF (16, 17). They provide radio frequency energy, and the two sets of electrodes ablate alternately, forming a columnar ablation line in the center without gaps, while parallel clamping closure, deformation, and pressure are consistent to ensure that ablation achieves galvanic isolation (18). The bipolar radiofrequency ablation clamp MZ-RFK independently developed by Med-Zenith Medical Devices, which was established in 2005 in China, together with its radiofrequency ablation generator MZ-RFG, can dynamically monitor 50 times/s of impedance and temperature changes when ablating the target tissue, and achieve precise wall penetration with the minimum effective power output (19). Relevant experimental data showed that bipolar radiofrequency ablation forceps heated the tissue with radiofrequency energy at 70–80°C for about 1 min to produce an ablation radius of 3–6 mm in depth, which is sufficient to achieve the required transmural effect for cardiac conduction block (20, 21). At present, it is not possible to say which of the two ablation forceps is superior in terms of wall penetration integrity, as there is no clear evidence that the created ablation trails completely block electrical conduction, and the surgeon's skill and left atrial size may affect wall penetration, which can only be assessed indirectly on the basis of the appropriate parameters. For further confirmation, perhaps a complete animal experimental design is needed to explore the tissue permeability of the two ablation forceps. It has been shown that the two ablation forceps with similar parallel clamping devices (embedded electrodes) can create similar ablation pathways and require roughly equivalent ablation times, which was confirmed by our results (22). The difference in the ablation time between the two groups was not significant, both taking an average of 25 min.
Although there is no direct evidence of a difference in the clinical efficacy of the two ablation clamps today, the present study found that the rate of SR maintenance at 12 months after the procedure was lower in the observation group (73.3%) than in the control group (86.4%). It has been reported in the literature that compared with the Med-Zenith ablation forceps, the two groups of bipolar electrodes of the AtriCure ablation forceps can transmit radiofrequency alternately at 264 cycles/s, forming a cross-electrode network to avoid deep tissue fissures leading to failure of ablation; the two groups of bipolar electrodes work in an alternating manner, leaving an intermittent period of electrocoagulation, so that there will not be a case of overheating of the ablated tissues that will cause the ablated tissues to become charred and deformed under continuous work, and the alternating emission can allow the impedance to rise slowly to reduce the loss of the radiofrequency energy transmitted to the deep tissues to make the tissues more likely to achieve a full permeability of the wall (23). We further found by the Kaplan–Meier survival analysis that the cumulative incidence of AF in the observation group was greater than that in the control group at 1 year post procedure (P < 0.05), suggesting that the AtriCure bipolar radiofrequency ablation forceps were indeed more effective in restoring and maintaining SR in the short term compared with the Med-Zenith ablation forceps. In addition, there was no significant difference in the rate of SR maintenance in the early postoperative period between the two groups in this study, which could not be separated from the role of oral amiodarone in the early postoperative period. Relevant studies have confirmed that amiodarone, as a Class III AADs, can significantly reduce the recurrence of AF in the early postoperative period (≤3 months) after surgical ablation of AF, but whether there is an effect on recurrence in the distant postoperative period has not yet been clearly confirmed (24, 25). The mechanisms by which amiodarone reduces early recurrence after ablation of AF include: (1) inhibition of autoregulation in the sinus node and atrioventricular junction area, slowing atrioventricular node and atrioventricular bypass conduction; (2) prolongation of the myocardial tissue action potential and the effective period of inactivity to eliminate atrioventricular refractoriness and to reverse the electrical remodeling of AF. We also found that the SR maintenance rate tended to decrease slowly with time in both groups, and a number of studies have also found that the rate of postoperative AF recurrence increases progressively with time. The cause of long-term recurrence may be the fading of scar trails isolating pulmonary veins leads to restoration of electrical conduction, ectopic origins of the triggering foci, and alterations of cardiomyocyte stroma (26, 27).
The results of repeated-measures ANOVA in this study showed that there were statistically significant differences in LAESD and RAESD of the two groups at each postoperative time point compared with the preoperative time point; LVEF significantly began to improve at 6 months postoperatively (P < 0.05); there were no statistically significant differences in intergroup comparisons between the two groups for LAESD, RAESD, and LVEF at each time point, and trends in the aforementioned indicators also did not differ between the two groups. This indicates that regardless of the kind of ablation forceps used, cardiac function improved in both groups of patients treated with the rheumatic valve procedure concomitant with Cox-Maze IV procedure, and it was a slow and long-term process. The relevant literature has shown that the rheumatic valve procedure can correct organic valve pathology, restore patients’ hemodynamics, reduce cardiac load, and significantly improve cardiac function (28, 29). It has been found that the Cox-Maze procedure can help restore the contractile function of the left atrium, reduce the load on the left atrium, avoid further dilatation of the left atrium, and improve the patient's cardiac function while reversing SR (30). This study also found that the postoperative LVEF of both groups did not improve much at the time of discharge from the hospital compared with the preoperative period, and it has been shown that the early postoperative LVEF after the rheumatic valve procedure concomitant with Cox-Maze IV procedure does not improve significantly compared with the preoperative period, and even decreases 10%, which may be a result of the combination of several factors: (1) preoperative valve disease leads to long-term overload of the left ventricle, myocardial persistent damage, and contractile function being severely impaired; (2) early postoperative cardiomyocyte edema, which affects myocardial fibers to regulate themselves abnormally, the myocardial contractile force is not fully restored to reach the optimal initial length, and left ventricular ejection volume may even be reduced (31). Pericardial tamponade and cardiac perforation rupture did not occur during the perioperative period in either group of patients in this study. Two cases of Degree III atrioventricular block were seen in the observation group and one case in the control group, both of which underwent permanent pacemaker implantation, which is not much different from the results of previous studies (32).
Nowadays, surgical ablation of AF is developing in the direction of being non-beating and minimally invasive. Mei's minimally invasive ablation technique created by Prof. Meiju in China utilizes TV thoracoscopy to ablate AF in a non-beating heart through a self-designed ablation line, which improves the success rate of the operation and reduces the occurrence of postoperative complications (33). Recently, given the close collaboration between cardiac surgeons and electrophysiologists, a one-stop sequential ablation strategy based on surgical/catheter ablation has been introduced with good results in patients with persistent AF. A related study demonstrated that this hybrid ablation model combines the advantages of intracardiac catheter ablation and epicardial surgical ablation, and to some extent can overcome the shortcomings of a single ablation technique (34). The rapid development of AF ablation technology has also led to innovations in ablation devices. Recently, the Three-dimensional Electronic Anatomical Marking System (CARTO®3 Version7) has been developed by Johnson & Johnson to quickly and accurately perform endocardial and epicardial marking to construct a three-dimensional model of the heart through a new marking catheter (OCTARAY™), as well as marking intracardiac electrical signal conduction, locating the foldback loop, and determining the target range of ablation (35). It is believed that in the future, a variety of advanced ablation devices will appear one by one, bringing the treatment of AF into a new era.
The present study was conducted to evaluate the clinical value and application prospect of the ablation forceps by Med-Zenith, and to provide some clinical basis for the selection of intraoperative ablation forceps for patients with RHD concomitant with AF in developing countries, but there are limitations in this study. This is a retrospective study, the baseline data of the two groups still have differences in a few factors; only 1-year postoperative examination data of the patients were collected, the number of included cases was small, and the postoperative ECG only recorded the results of a few time points, which can roughly assess the short-term efficacy of the two types of ablation forceps, while the medium-term and even long-term efficacy need to be further investigated.
In patients with RHD concomitant with AF who undergo the rheumatic valve concomitant with Cox-Maze IV procedure, the bipolar radiofrequency ablation forceps by AtriCure are indeed more effective in restoring SR in the short term. However, to a certain extent, the bipolar radiofrequency ablation forceps by Med-Zenith can be used to achieve similar results and reduce the financial burden on the patient.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
This study does not involve identifiable human body images or personal privacy of participating patients, and is designed as a retrospective study. Therefore, formal informed consent from patients is not required to participate in this study. In addition, the study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Affiliated Hospital of North Sichuan Medical College (2022ER313-1 & 2022.08.12).
NZ: Data curation, Formal Analysis, Methodology, Software, Validation, Writing – original draft. MH: Conceptualization, Data curation, Investigation, Writing – review & editing. BM: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. YL: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. Y-lL: Methodology, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This research was funded by the “Applied Basic Research Program of Sichuan Provincial Science and Technology Department (2021YJ0208)” and the “Sichuan Provincial Department of Science and Technology Key R&D Program Special (2022YFS0365)”.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1335407/full#supplementary-material
1. Kim WK, Kim HJ, Kim JB, Jung SH, Choo SJ, Chung CH, et al. Clinical outcomes in 1731 patients undergoing mitral valve surgery for rheumatic valve disease. Heart. (2018) 104:841–8. doi: 10.1136/heartjnl-2017-312249
2. Liu J, Wang Y, Guo W, Cheng Y, Zhang S, Wu B, et al. Temporal trends of atrial fibrillation and/or rheumatic heart disease-related ischemic stroke, and anticoagulant use in Chinese population: an 8-year study. Int J Cardiol. (2021) 322:258–64. doi: 10.1016/j.ijcard.2020.08.046
3. Noubiap JJ, Nyaga UF, Ndoadoumgue AL, Nkeck JR, Ngouo A, Bigna JJ. Meta-analysis of the incidence, prevalence, and correlates of atrial fibrillation in rheumatic heart disease. Glob Heart. (2020) 15:38. doi: 10.5334/gh.807
4. Ruaengsri C, Schill MR, Khiabani AJ, Schuessler RB, Melby SJ, Damiano RJ. The Cox-maze IV procedure in its second decade: still the gold standard? Eur J Cardiothorac Surg. (2018) 53:i19–25. doi: 10.1093/ejcts/ezx326
5. Meng Y, Zhang Y, Liu P, Zhu C, Lu T, Hu E, et al. Clinical efficacy and safety of Cox-maze IV procedure for atrial fibrillation in patients with hypertrophic obstructive cardiomyopathy. Front Cardiovasc Med. (2021) 8:720950. doi: 10.3389/fcvm.2021.720950
6. Khiabani AJ, MacGregor RM, Bakir NH, Manghelli JL, Sinn LA, Maniar HS, et al. The long-term outcomes and durability of the Cox-Maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg. (2022) 163:629–41. doi: 10.1016/j.jtcvs.2020.04.100
7. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. (2018) 20:e1–160. doi: 10.1093/europace/eux274
8. Brachmann J, Sohns C, Andresen D, Siebels J, Sehner S, Boersma L, et al. Atrial fibrillation burden and clinical outcomes in heart failure: the CASTLE-AF trial. JACC Clin Electrophysiol. (2021) 7:594–603. doi: 10.1016/j.jacep.2020.11.021
9. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. (2016) 133:484–92. doi: 10.1161/CIRCULATIONAHA.115.018614
10. Jamil S, Batool S, Ehsan US, Aschalew YN, Zahra T, Maheshwari L, et al. Comparison of interrupted and uninterrupted anticoagulation therapy for patients with atrial fibrillation undergoing catheter ablation: a meta-analysis. Cureus. (2022) 14:e30742. doi: 10.7759/cureus.30742
11. Jin Y, Wang HS, Han JS, Zhang J, Zhang YJ, Xin FR, et al. Recovery of atrial contractile function after cut-and-sew maze for long-standing persistent valvular atrial fibrillation. Int J Cardiol. (2021) 324:84–9. doi: 10.1016/j.ijcard.2020.09.010
12. Lu S, Du X, Yang X, Jia Z, Li J, Xia S, et al. Physical activity and atrial tachyarrhythmia recurrence in atrial fibrillation patients after catheter ablation. Pacing Clin Electrophysiol. (2020) 43:922–9. doi: 10.1111/pace.14006
13. Karthikeyan G, Connolly SJ, Ntsekhe M, Benz A, Rangarajan S, Lewis G, et al. The INVICTUS rheumatic heart disease research program: rationale, design and baseline characteristics of a randomized trial of rivaroxaban compared to vitamin K antagonists in rheumatic valvular disease and atrial fibrillation. Am Heart J. (2020) 225:69–77. doi: 10.1016/j.ahj.2020.03.018
14. Jiang Z, Song L, Liang C, Zhang H, Tan H, Sun Y, et al. Machine learning-based analysis of risk factors for atrial fibrillation recurrence after Cox-Maze IV procedure in patients with atrial fibrillation and chronic valvular disease: a retrospective cohort study with a control group. Front Cardiovasc Med. (2023) 10:1140670. doi: 10.3389/fcvm.2023.1140670
15. Matteucci F, Maesen B, De Asmundis C, Bidar E, Parise G, Maessen JG, et al. Comparison between biparietal bipolar and uniparietal bipolar radio frequency ablation techniques in a simultaneous procedural setting. J Interv Card Electrophysiol. (2021) 61:567–75. doi: 10.1007/s10840-020-00852-5
16. Philpott JM, Zemlin CW, Cox JL, Stirling M, Mack M, Hooker RL, et al. The ABLATE trial: safety and efficacy of Cox Maze-IV using a bipolar radiofrequency ablation system. Ann Thorac Surg. (2015) 100:1541–6; discussion 1547–8. doi: 10.1016/j.athoracsur.2015.07.006
17. Gillinov AM, McCarthy PM. Atricure bipolar radiofrequency clamp for intraoperative ablation of atrial fibrillation. Ann Thorac Surg. (2002) 74:2165–8; discussion 2168. doi: 10.1016/S0003-4975(02)04484-3
18. Jiang Q, Liu SZ, Jiang L, Huang KL, Guo J, Hu SS. Comparison of two radiofrequency ablation devices for atrial fibrillation concomitant with a rheumatic valve procedure. Chin Med J. (2019) 132:1414–9. doi: 10.1097/CM9.0000000000000276
19. Zhang K, Chen X. Safety and medium- and long-term efficacy of treating atrial fibrillation with surgical radiofrequency ablation during concomitant mitral valve surgery. Sichuan Da Xue Xue Bao Yi Xue Ban. (2021) 52:1022–7. doi: 10.12182/20211160503
20. Matteucci F, Maesen B, De Asmundis C, Bidar E, Micali L, Parise G, et al. Biparietal bidirectional bipolar radiofrequency in hybrid cardiac ablation: an in vitro evaluation. Interact Cardiovasc Thorac Surg. (2021) 33:34–42. doi: 10.1093/icvts/ivab047
21. Voeller RK, Zierer A, Schuessler RB, Damiano RJ. Performance of a novel dual-electrode bipolar radiofrequency ablation device: a chronic porcine study. Innovations. (2011) 6:17–22. doi: 10.1097/IMI.0b013e31820bc57f
22. Varzaly JA, Chapman D, Lau DH, Edwards S, Louise J, Edwards J, et al. Contact force and ablation assessment of surgical bipolar radiofrequency clamps in the treatment of atrial fibrillation. Interact Cardiovasc Thorac Surg. (2019) 28:85–93. doi: 10.1093/icvts/ivy191
23. Zhu X, Li Q, Li Y, Wu Z. Analysis of bipolar radiofrequency ablation in treatment of atrial fibrillation associated with rheumatic heart disease. PLoS One. (2016) 11:e151248. doi: 10.1371/journal.pone.0151248
24. Ad N, Holmes SD, Shuman DJ, Pritchard G, Miller CE. Amiodarone after surgical ablation for atrial fibrillation: is it really necessary? A prospective randomized controlled trial. J Thorac Cardiovasc Surg. (2016) 151:798–803. doi: 10.1016/j.jtcvs.2015.07.034
25. Maniar H, Novak E. Postoperative medication management after surgical ablation: clarifying the role of amiodarone therapy. J Thorac Cardiovasc Surg. (2016) 151:804–5. doi: 10.1016/j.jtcvs.2015.10.098
26. Lin ZQ, Luo ZR, Li QZ, Chen LW, Lin F. Efficacy, safety, and long-term survival of concomitant valve replacement and bipolar radiofrequency ablation in patients aged 70 years and older: a comparative study with propensity score matching from a single-centre. J Cardiothorac Surg. (2020) 15:291. doi: 10.1186/s13019-020-01322-9
27. Musharbash FN, Schill MR, Sinn LA, Schuessler RB, Maniar HS, Moon MR, et al. Performance of the Cox-maze IV procedure is associated with improved long-term survival in patients with atrial fibrillation undergoing cardiac surgery. J Thorac Cardiovasc Surg. (2018) 155:159–70. doi: 10.1016/j.jtcvs.2017.09.095
28. Vervoort D, Antunes MJ, Pezzella AT. Rheumatic heart disease: the role of global cardiac surgery. J Card Surg. (2021) 36:2857–64. doi: 10.1111/jocs.15597
29. Peters F, Karthikeyan G, Abrams J, Muhwava L, Zuhlke L. Rheumatic heart disease: current status of diagnosis and therapy. Cardiovasc Diagn Ther. (2020) 10:305–15. doi: 10.21037/cdt.2019.10.07
30. Brennan AP, Martin W, Adams H, Yii M. Impact of the Cox-Maze IV procedure on left atrial mechanical function. Heart Lung Circ. (2019) 28:1835–40. doi: 10.1016/j.hlc.2018.11.008
31. Lam CS, Rienstra M, Tay WT, Liu LC, Hummel YM, van der Meer P, et al. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail. (2017) 5:92–8. doi: 10.1016/j.jchf.2016.10.005
32. Saini A, Hu YL, Kasirajan V, Han FT, Khan MZ, Wolfe L, et al. Long-term outcomes of minimally invasive surgical ablation for atrial fibrillation: a single-center experience. Heart Rhythm. (2017) 14:1281–8. doi: 10.1016/j.hrthm.2017.04.029
33. Ma N, Ding S, Zeng L, Tan C, Li S, Liu X, et al. Immediate electrophysiological characteristics following modified thoracoscopic ablation via unilateral approach for non-valvular atrial fibrillation. Heart Vessels. (2021) 36:874–81. doi: 10.1007/s00380-020-01760-4
34. Osmancik P, Herman D, Kacer P, Rizov V, Vesela J, Rakova R, et al. The efficacy and safety of hybrid ablations for atrial fibrillation. JACC Clin Electrophysiol. (2021) 7:1519–29. doi: 10.1016/j.jacep.2021.04.013
Keywords: bipolar radiofrequency ablation forceps, rheumatic heart disease, atrial fibrillation, Cox-Maze procedure, short-term efficacy
Citation: Zhang N, Hou M, Mei B, Liu Y and Lai Y-l (2024) Comparison of short-term efficacy of two bipolar radiofrequency ablation forceps for rheumatic heart disease concomitant with atrial fibrillation. Front. Cardiovasc. Med. 11:1335407. doi: 10.3389/fcvm.2024.1335407
Received: 8 November 2023; Accepted: 1 April 2024;
Published: 22 April 2024.
Edited by:
Antonio Miceli, Istituto Clinico Sant'Ambrogio, ItalyReviewed by:
Sung Il Im, Kosin University, Republic of Korea© 2024 Zhang, Hou, Mei, Liu and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-long Lai bGFpeWluZ2xvbmcyMDAwQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.