- 1Structural Heart Intervention Program, Montreal Heart Institute, Montreal, QC, Canada
- 2Department of Interventional Cardiology, Hospices Civils de Lyon, Lyon, France
- 3Service Médico-Chirurgical: Valvulopathies-Chirurgie Cardiaque-Cardiologie Interventionelle Structurelle, Hôpital Cardiologique de Haut Lévèque, CHU Bordeaux, Bordeaux, France
Transcatheter aortic valve replacement (TAVR) has emerged as a viable treatment for aortic valve disease, including low-risk patients. However, as TAVR usage increases, concerns about long-term durability and the potential for addition interventions have arisen. Transcatheter aortic valve (TAV)-in-TAV procedures have shown promise in selected patients in numerous registries, offering a less morbid alternative to TAVR explantation. In this review, the authors aimed to comprehensively review the experience surrounding TAV-in-TAV, summarize available data, discuss pre-procedural planning, highlight associated challenges, emphasize the importance of coronary obstruction assessment and provide insights into the future of this technique.
Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as a viable alternative to surgery in high-risk (Class I) and intermediate-risk (Class IIa, level of evidence B) patients (1), expanding its indications in recent years (2, 3). Recent data have extended its suitability to low-risk patients, demonstrating favorable and sustained outcomes compared to surgery (4–7). As a result, the number of patients undergoing TAVR has surged significantly (8), and as treatment moves into younger patients, this poses new challenges.
The longevity of patients who undergo TAVR raises concerns about potential valve degeneration and the need for multiple percutaneous interventions over their lifetime. This has given rise to the concept of Transcatheter aortic valve in Transcatheter aortic valve (TAV-in-TAV), introducing several complexities in patient management. Registries and expert consensus efforts have been carried out to assess safety, highlight key considerations, and standardize the pre-procedural work up for these patients.
This paper aims to comprehensively review the currently available data, shedding light on the potentials, challenges, and unmet needs associated with TAV-in-TAV procedures.
THV types and characteristics
Over the recent years, there has been an evolution of percutaneous valves approved for TAVR. Understanding the characteristics of these valves is crucial for comprehending the challenges posed by TAV-in-TAV procedures. Figure 1 summarizes the features of available and commonly used Transcatheter heart valve (THV)s (9). Essential data include design, leaflet position, and valve frame height. Additionally, knowledge of the skirt height and the degree of expansion of each valve is crucial. A comparison of these data with computed tomography (CT) evaluation of the index valve remains the cornerstone of the TAV-in-TAV intervention. For clarity purposes, we will mainly focus on the Edwards Sapien valves and Medtronic Corevalve/Evolut.
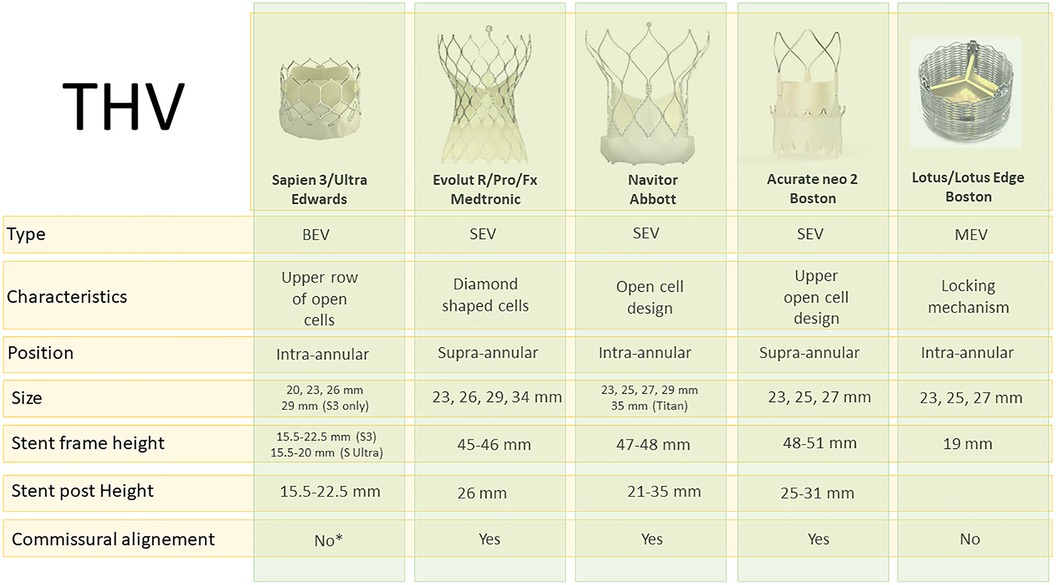
Figure 1. Available THV types and characteristics. *Commissural alignment will be available for Sapien X4. BEV, balloon-expandable valve; MEV, mechanically-expandable valve; SEV, self-expandable valve.
Pushing the limits of transcatheter aortic valves
The short-term safety and efficacy of Transcatheter aortic valve (TAV) replacement have been extensively studied. As younger patients are now being treated, valve durability has become a critical consideration. To standardize the TAVR field, several societies haves established definitions. The European Association of Percutaneous Cardiovascular Interventions (EAPCI) (10) introduced definitions for bioprosthetic valve dysfunction which encompasses structural valve deterioration (SVD), NSVD, thrombosis, and endocarditis. SVD can result from morphologic and intrinsic valve damage or hemodynamic impairment, while bioprosthetic valve failure (BVF) is linked to severe SVD with clinical signs. In 2018, the Valve in Valve International Data (VIVID) group (11) updated the definition of SVD, outlining four distinctive stages: no immediate changes (stage 0), morphological leaflet abnormalities (stage 1), moderate stenosis or regurgitation (stage 2) or severe (stage 3). More recently, the Valve Academic Research Consortium (VARC-3) Committee (12) refined the EAPCI definitions (Table 1).
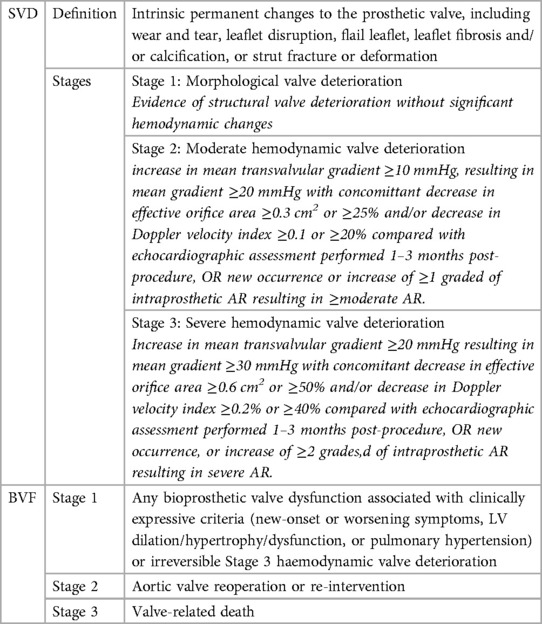
Table 1. SVD and BVF definitions according to the VARC-3 committee (12).
When considering the long-term durability of TAV using the balloon-expandable (BEV) and self-expandable valve (SEV), data is derived from observational registries. Several studies have reported favorable long-term outcomes with low rates of SVD 5–10 years after the procedure (13–17). In the NOTION trial, SVD was lower after TAVR than after surgical aortic valve replacement (SAVR) (13.9% vs. 28.3% respectively), while the risk of BVF was similar at an 8-year follow-up in low-risk patients (16). These results were recently corroborated in the ESC 2023 update, which showed similar risks of all-cause mortality, stroke and myocardial infarction, higher risk of SVD after SAVR (20.2% vs. 37.7% respectively) and similar risk of BVF at 10 years (18). Sathananthan et al. provided a 10-year follow-up of patients with BEV TAVR, demonstrating a low rate of SVD (6.5%), which is very encouraging in a high-risk population (19). Nevertheless, it's worth noting that SVD rates may be underestimated in these series because serial valve imaging is primarily clinically driven, and isolated morphological valve alterations with no hemodynamic impact may occur. Given the increasing use of TAVR in low-risk patients, SVD is expected to persist despite increased experience, systematic planning, and the introduction of newer valve generations.
Surgical explantation or TAV-in-TAV: current perspectives
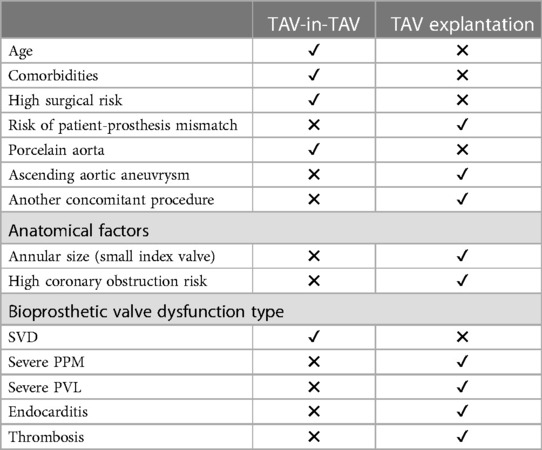
TAV explantation has been considered as an option for patients with BVF after TAVR. Several retrospective studies have investigated the feasibility and outcomes of this procedure. Hirji et al. in 2020 (20), included 227 patients who underwent TAV explantation, among whom intermediate-risk patients were identified. The thirty-day mortality was at 13.2% and the 1-year mortality rate was 22.9%. Reintervention was mainly performed due to BVF (79.3%). However, infectious endocarditis was responsible for 20.7% of cases, potentially contributing to mortality, although this was not conclusive in subgroup analysis. It's worth noting that patients with endocarditis are beyond the scope of this paper, and surgery remains the primary treatment for them. In the Explant-TAVR registry (21), the one-year mortality rate was at 28.5% with 43.1% of patients presenting with endocarditis of the index TAVR. In 2020, Fukuhara et al. (22) presented data on 15 TAV explantation cases. This study highlighted the surgical challenges of this procedure, including neo-endothelization of the TAV in the aortic wall, which may require endarterectomy and aortic root repair, a challenge less prominent in the surgical valve population. This complexity could be exacerbated with newer generation valves featuring taller sealing skirts as well as with tall-frame TAVR devices. It should be noted that the retrospective nature of the study introduces a selection bias, as high-risk patients would be offered repeat TAVR, while operable patients would undergo surgery. It is also worth discussing that in current reports on TAVR explantation, the reported median time from index TAVR to explantation is around 1 year. These cases might differ from those anticipated in the future. A prolonged duration between the index procedure and the explantation may introduce different failures modes and more challenges with regards to the explantation technique.
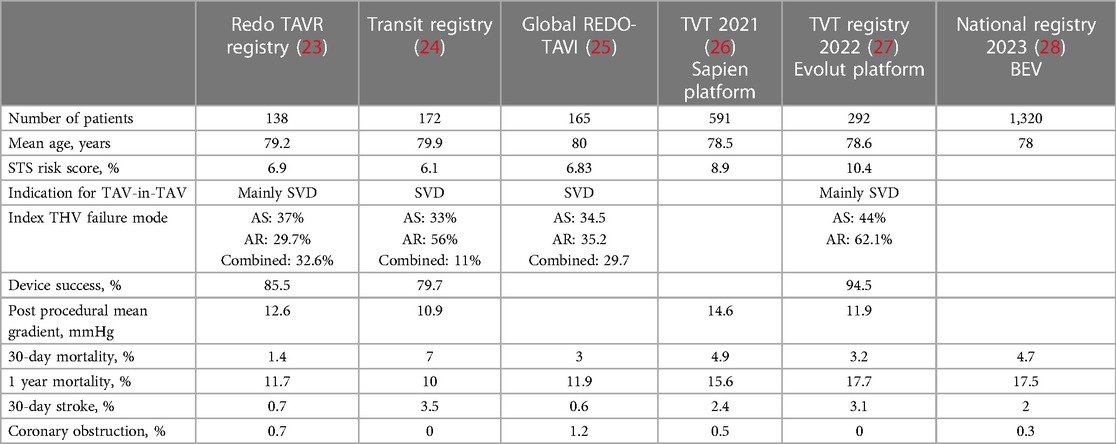
TAV explantation appears feasible in operable patients; however, it is associated with high mortality rates and technical surgical difficulties. As a result, TAV-in-TAV has been explored as an alternative option (Table 2). Several registries have investigated the safety of TAV-in-TAV, demonstrating promising results (Table 3). The RedoTAVR (23) and TRANSIT (24) registries reported favorable early and mid-term outcomes, with low rates of mortality, stroke, and coronary obstruction, along with a high rate of device success. The TVT registry (27) showed similar results using only the SEV valve. Nevertheless, these registries included selected patients and anatomies, and the results may not be representative of the entire population eligible for this intervention. The percentage of patients declined due to complex anatomy with a high risk of coronary occlusion was not specified. Nonetheless, TAV-in-TAV remains a safe intervention in selected patients, particularly when comprehensive CT planning is performed. More recently, Makkar et al. (28) published a national registry including 1,320 TAV-in-TAV procedures using balloon expandable valves (BEV). Through propensity score matching, patients were compared with those undergoing native TAVR, revealing low rates of procedural complications and similar mortality rates between both groups. TAV-in-TAV with BEV was deemed a reasonable treatment for failed TAVR for selected patients. Percy et al. (29) conducted a patient-matched analysis comparing TAV-in-TAV to TAV explantation. Their study included 257 patients with TAV-in-TAV and 130 patients with TAV explantation, with a mean age of 76.9 and 75.1 years respectively. Mortality rates after TAV-in-TAV were 6.2% at 30 days and 21% at 1 year, compared to 12.3% and 20.8%, respectively, after TAV explantation. These preliminary findings suggested a potentially lower short-term mortality rate with TAV-in-TAV but similar mid-term results. However, it is important to note several limitations. Patients selected for TAV explantation are, by definition, operable patients, whereas TAV-in-TAV, especially in its early adoption, was considered as an alternative option. Additionally, the study included patients with an index procedure before 2017. Recent generation devices and the lower-risk patients may also influence the outcome of this comparison.
More recently, the EXPLANTORREDO-TAVR international registry (30), which retrospectively included 181 patients with TAVR-explant and 215 with TAV-in-TAV, confirmed these results of higher mortality at 30 days for TAVR-explant (13.6% vs. 3.4%) and also at 1 year (32.4% vs. 15.4%), with, however, similar rates on landmark analysis after 30 days.
Patient selection remains pivotal in determining the appropriate treatment. Assessment of a patient's surgical risk and careful evaluation through CT imaging are essential before choosing the best management strategy bearing in mind the importance of considering these aspects at the time of the index TAVR. TAV-in-TAV has shown promising results with fewer complications in selected patients. Future studies should focus on long-term outcomes, anatomical considerations, and patients with low surgical risk.
TAV-in-TAV: bridging bench studies to clinical expertise
Numerous studies have assessed the feasibility of TAV-in-TAV. From bench studies to clinical experience, efforts have transitioned towards gaining a deeper understanding of the technical aspects of this procedure, pre-procedural planning and its associated challenges.
Advancements from bench testing
The TAV-in-TAV implantation technique has been subject to numerous studies. Initially, Sathananthan et al. (31) assessed the performance of various THV models (SAPIEN 3, Evolut PRO, ACURATE neo, ALLEGRA, and Portico). Multiple THV implantation depths were tested and are summarized in Figure 2. A Sapien 3 THV was implanted in a Sapien XT, outflow to outflow. The implantation of a SEV was evaluated at 0, −4, and +4 mm from the inflow of the index THV, while a Sapien 3 was implanted in an evolut R at two positions (high and low). The results were encouraging, demonstrating good hydrodynamic performance for all combinations and positions. However, in a combination of a Sapien 3 implantation in an Evolut R in a high position for small valve sizes, additional inflation volume was required to prevent embolization.
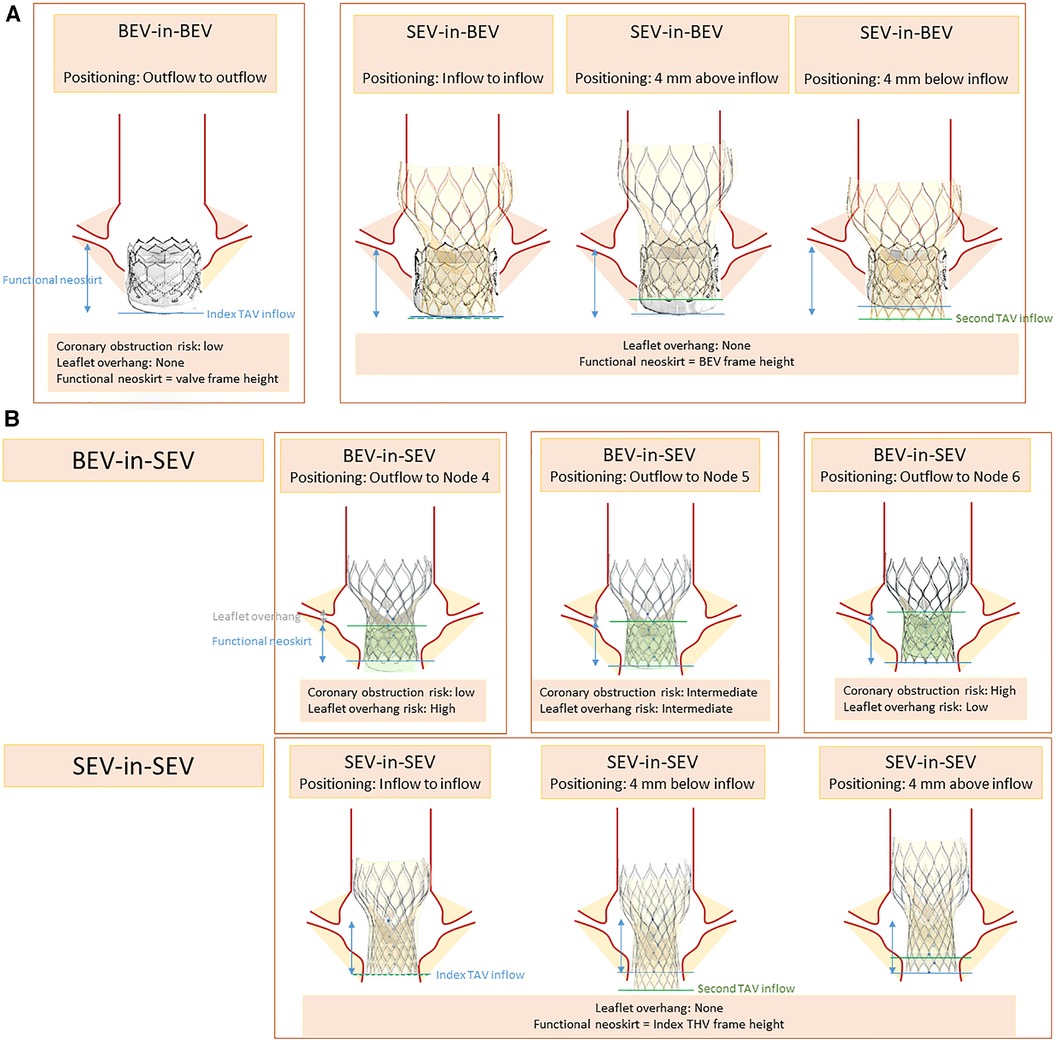
Figure 2. Positioning scenarios and considerations in TAV-in-TAV (A) Sapien index THV (B) Corevalve index THV.
Another important consideration is the depth of THV implantation. The leaflets of the index THV deflect outward against the stent frame, creating a tube graft known as the neoskirt, with the highest point being the top of the trapped leaflets. Akodad et al. (32) studied the depth of a Sapien 3 THV in an Evolut R, by aligning the outflow of the Sapien with nodes 4–6 of the index valve. They found that a lower position reduced the neoskirt of 7.6 mm but resulted in leaflet overhang (ranging from 0% to 94% depending on the THV size). Neoskirt height was lower with short frame valves, which is particularly important when assessing the risk of coronary impairment (33).
Lastly, the phenomenon of leaflets' pinwheeling, characterized by the twisting of the leaflet-free edges due to excessive leaflet redundancy, has been studied. Excessive pinwheeling was observed when implanting a Sapien 3 in a Sapien XT (31). This observation was recently confirmed by Meir et al. (34) who investigated the expansion of TAV-in-TAV using a Sapien 3 (23 mm) in a Sapien XT/3 (23 mm). They found that full valve expansion, which would prevent pinwheeling, was only achieved with pre- and post-dilation. Whether pinwheeling has a long-term impact on valve function remains unknown.
Insights from index-TAV CT evaluations
Tarantini et al. (35) introduced an algorithm assessing the risk of coronary flow compromise following TAV-in-TAV based on CT imaging of the index THV. Drawing comparison from the TAV-in-SAV (surgical aortic valve) experience, the authors defined the risk plane (RP) as the level below which the passage of a catheter would be obstructed after implanting a second valve (Figure 3). They described three primary scenarios: Type 1, where the coronary ostium is above the RP; Type 2a where the ostium is below the RP and the valve to aorta (VTA) distance >2 mm and Type 2B where the VTA < 2 mm). This classification enhances the understanding of TAVR feasibility concerning coronary access and potential impairment. Figure 4 describes a representation of these measurements. In a study by Buzatti et al. (36), 221 CT scans following TAVR were analyzed, revealing a high risk of coronary impairment in 55.6% of cases. Beyond the risk of coronary obstruction, there is also a risk of sinus sequestration, which depends not only on the depth of the index THV but also on the measurements of the native annulus (small annulus or short sinus of Valsalva). Conducting a CT scan before the implantation of the index THV may help address challenges associated with native anatomy. More recently, Grubb et al. (37) examined 204 CT scans of patients identified from the Evolut low-risk trial, using an Evolut as the index THV. They simulated TAV-in-TAV procedures with a Sapien 3 targeting inflow-to-inflow implantation, outflow at nodes 4, 5, and 6, as well as an Evolut inflow to inflow. The results indicated that 20% of patients faced a high risk of compromised coronary flow with a Sapien 3 implanted at node 4, compared to 75% at node 6, and 71% for an evolut implantation. Similarly, coronary access was affected in 71% of the Evolut cases, 20% of Sapien 3 cases at node 4, and 75% at node 6. It is noteworthy that there was no coronary obstruction.
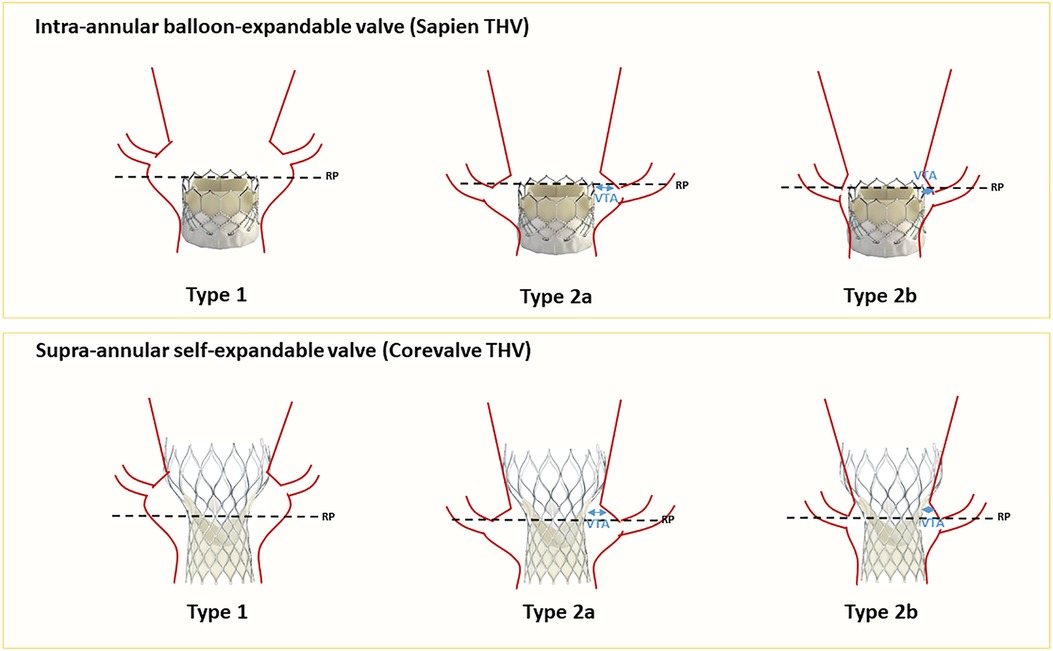
Figure 3. Coronary risk assessment before TAV-in-TAV (for Sapien and Corevalve as index THVs). RP, risk plane; VTA, valve to aorta.
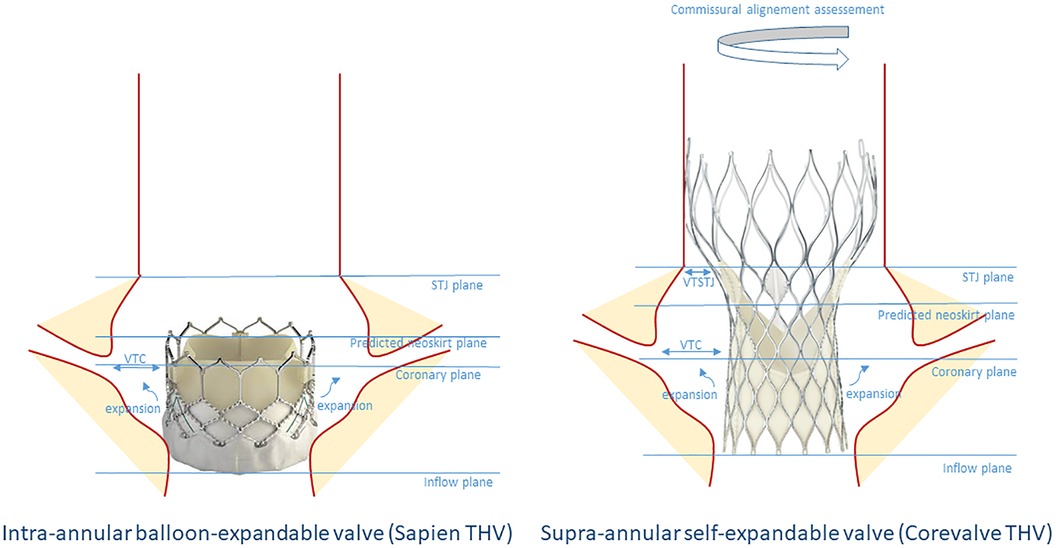
Figure 4. Schematic representation of the criteria to determine the risk of coronary flow compromise before TAV-in-TAV in an index Sapien or Corevalve THV. STJ, sino-tubular junction; VTC, valve to coronary; VTSTJ, valve to sino-tubular junction.
Enhancing TAV-in-TAV insights through CT analysis
The largest morphological study was conducted by De Backer et al. (38) In their research, they examined 45 TAV-in-TAV patients, comprising 20 Evolut-in-Evolut, 10 Sapien-in-Evolut, 10 Sapien-in-Sapien, and 5 Evolut-in-Sapien cases. They assessed variables such as VTA, valve to coronary (VTC), and strut misalignment. Several key observations were made. Firstly, it was noted that when the first THV used is an Evolut, 90% of the coronary arteries originate below the risk plane, compared to only 6.7% when a Sapien is used as the first THV. Secondly, when the coronary arteries originate below the risk plane, the VTA was less than 2 mm in 27% of cases for Evolut-in-Evolut, 24% for Sapien-in-Evolut, 15% for Sapien-in-Sapien, and 14% for Evolut-in-Sapien procedures. Thirdly, in cases of Evolut-in-Evolut procedures, 10% of patients experienced strut misalignment, potentially leading to inaccessible coronary access. This issue can be mitigated through meticulous commissural alignment during both the index and second procedures (39). When considering all these data, it was found that 27% of patients with an Evolut as the index THV and 10% with a Sapien were considered to have impossible coronary access. These findings may support the selection of an intra-annular valve with a low commissural height and wide cells particularly in younger patients.
Insights from clinical practice
Bench studies and investigations based on CT imaging have demonstrated the feasibility of TAV-in-TAV in selected anatomies, contributing significantly to our understanding of this procedure. However, it's important to acknowledge that these data may not fully represent real-world scenarios. The leaflets of the index THV can undergo calcification, potentially affecting the implantation of a second THV. Leaflet overhang, as described when leaflets are not completely trapped between the two valve frames (31), could also impact valve performance, long-term durability, and coronary access. Furthermore, the existing studies often do not encompass the entire patient population, as individuals at risk of coronary obstruction were frequently excluded. Additionally, there is a noticeable absence of large, dedicated studies with robust data, primarily due to the relatively low rates of SVD. Consequently, the medium and long-term outcomes of these patients remain uncertain.
Tarantini et al. (40) published an expert consensus aiming to streamline the TAV-in-TAV procedure, specifically using a BEV. They proposed a tailored step-by-step approach, emphasizing the significance of comprehensive evaluation of native anatomy, commissural alignment of the index THV, the type of SVD, and the risk of coronary obstruction, taking into consideration the reduced space within the sinuses of Valsalva due to valve expansion. In summary, if the index THV is a BEV, they recommend sizing based on the expansion of the initial valve and an outflow-to-outflow positioning. In cases where the index THV is a SEV (such as Evolut), sizing should be based on the inflow and waist of the index THV, accounting for the initial annulus characteristics, and positioning the outflow between nodes 4 and 6, contingent upon the SVD type and coronary risk assessment.
However, it's essential to note that many studies have primarily focused on the Sapien 3 and XT models. Special attention should be given to the Sapien Ultra, which features a higher skirt compared to its predecessors.
Procedural recommendations
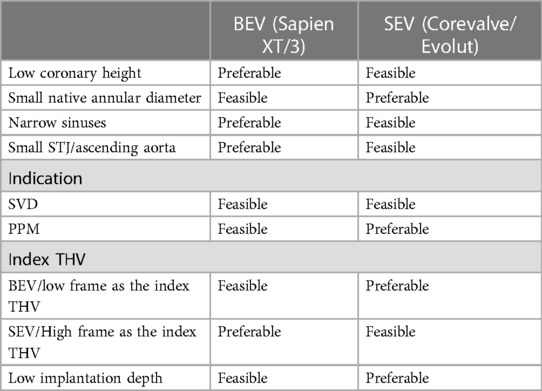
Several elements guide the TAV-in-TAV procedure. In addition to anatomical data obtained from imaging (VTC, VTSTJ and TAV dimensions), precise assessment of the index TAV is of paramount importance. As previously mentioned, factors such as implantation depth, failure mechanism, and commissural alignment are essential to document. It allows to estimate the anticipated height of the neoskirt. Consequently, certain criteria may favor the implantation of a high-frame THV, such as the Corevalve/Evolut, while other criteria may favor a low-frame THV, such as the Sapien valve. For instance, a Sapien BEV THV might be preferred if there is narrow sinus width, small STJ, or short coronary ostium height, to reduce the risk of sinus sequestration. On the other hand, a high risk of patient-prosthesis mismatch (PPM) or an intervention performed due to PPM could indicate a preference for Evolut as the second valve. The data guiding the choice between these options are summarized in Table 4.
The sizing of the second THV depends on both native anatomy and the index THV. Several elements need consideration, and the following recommendations could serve as a rough guide for common practice:
1. BEV-in-BEV: The same size THV should be used to treat index THV failure (A 20 mm Sapien in a 20 mm Sapien, a 23 in a 23 mm Sapien, a 26 in a 26 mm and a 29 in a 29 mm) and is usually positioned with the outflow at the level of the outflow of the index THV. A lower implantation may be considered if there is a risk of coronary obstruction. The expansion of the second THV depends on the expansion of the index THV, bearing in mind the presence of paravalvular leaks and the risk of coronary obstruction. The additional use of a non-compliant balloon for pre and/or post dilation may be considered to achieve full expansion.
2. BEV-in-SEV: The second THV should be sized to the internal dimensions of the index THV. (Roughly, a 20 mm Sapien in a 23 mm Corevalve/Evolut, a 23 mm Sapien in a 26 mm Corevalve/Evolut, a 26 in a 29 mm and 29 mm in a 34 mm). The position of the BEV THV should be tailored according to the risk of coronary obstruction and the mechanism of SVD (Outflow to node 4–6). While expanding the index SEV with a BEV will cause the Corevalve to remodel outwards, resulting in a gain in the effective orifice area and decreasing the chances of embolization, caution should be exercised regarding the risk of coronary flow impairment which should be taken into consideration.
3. SEV-in-BEV: Sizing should involve using a 23 mm Corevalve/Evolut in a 20 mm Sapien, a 26 mm in a 23 mm Sapien, a 29 in a 26 and a 34 in a 29 mm. Attention should be given to the degree of expansion of the index TAV. The inflow of the second TAV is usually positioned at the level of the inflow of the index TAV.
4. SEV-in-SEV: While this scenario ensures no leaflet overhang, the neoskirt is thought to be the highest with the highest percentage risk of coronary obstruction. A same size valve is recommended, positioned inflow to inflow.
Unresolved questions
Safeguarding against coronary obstruction
Coronary obstruction in TAV-in-TAV procedures is exceptional in published registries, primarily due to careful patient selection. Several factors may contribute to this rare occurrence, including the height of the leaflets, reduced neo-sinuses, and tall valve frames. Tang et al. (41) initially developed a classification system to assess the risk of coronary obstruction before TAV-in-TAV based on the diameter of the sino-tubular junction and the height of the sinuses, categorized into types 1–3. In brief, TAV-in-TAV was considered unfeasible and at high risk of obstruction when the valve-to-sinus distance or valve-to-sinus height was less than 2 mm.
In the context of a Valve-in-valve procedures, various techniques have been introduced to mitigate the risk of coronary impairment, such as the chimney technique and BASILICA (bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary obstruction). The chimney technique can be employed in a Valve-in valve setting. However, TAV-in-TAV presents the challenge of placing a stent between the metal layers of the THVs, raising questions about the long-term patency of the stent. The use of the BASILICA technique has recently garnered attention. Damlin et al. (42) published a case report describing a successful BASILICA procedure in a TAV-in-TAV setting using a supra-annular device. Nevertheless, it remains uncertain whether this approach is feasible across all anatomies, valve types and sizes. Despite improvements in commissural alignment techniques (43), particularly with the Evolut FX platform (>90% success rate), complete commissural alignment is not achieved in all cases, potentially affecting the feasibility of the leaflet laceration technique. Khan et al. (44) assessed the in vitro feasibility of BASILICA in TAV-in-TAV and found that the leaflets of the index THV were less likely to split adequately and could not move beyond the frame of the index THV. This challenge is further compounded with new-generation valves (such as Sapien 3 and Evolut), which exhibited narrower splits after BASILICA compared to earlier models. Greenbaum et al. (45) described the balloon-assisted BASILICA which aims to expand the traversal point in the leaflet with the use of an inflated non-compliant balloon. This technique requires further validation in all valve types and generations. The CATHEDRAL procedure is another procedure that has not been described in a TAV-in-TAV setting (46).
Also, the shortcut device is a device dedicated to splitting the index valve leaflets to prevent coronary obstruction, with precise placement and control of the splitting location. It has been extensively tested in bench test (on surgical valves and THV BEV and SEV) and, preclinical studies. The initial data published on 8 patients showed a complete success in preventing coronary obstruction without stroke. Among these patients, there was a Sapien XT and a Sapien 3 valve (47). An update of this serie was performed at TCT 2023 without the addition of TAV-in-TAV patients. It is important to emphasize the future of this technique in a population where the trend is towards an increase in TAV-in-TAV cases compared to valve-in-valve. Once again, the importance of commissural alignment in the implantation of the index valve is crucial. That said, data pertaining to coronary obstruction prevention techniques remain limited, highlighting the need for further research in this area.
Finally, a hybrid approach could be considered in cases with a high risk of coronary obstruction (SURPLUS TAVR) (48). To avoid a replacement of the aortic root and re-implantation of the coronary arteries, a trans-aortic approach can be performed. This involves a sternotomy and a short aortotomy under extracorporeal circulation. The leaflets of the index valve are resected under the direct visual of the surgeon. Thus, the new valve can be implanted according to standard recommendations, ensuring commissural alignment without the risks associated with the neoskirt. This practice is used in our center for selected cases at high risk of coronary obstruction.
Stroke prevention
Despite advancements in TAVR, the rates of stroke have remained stable over the years (49). The risk of stroke following TAV-in-TAV merits discussion. In the study by Hudad et al, the overall stroke rate at 30 days in a large TAVR cohort was 2.3% (49). A meta-analysis comparing stroke rates in TAV-in-SAVR to native TAVR populations showed no significant differences (50). In the TAV-in-TAV population, stroke rates are reported to be approximately 0.5%–3% at 30 days, similar to rates described in TAVR registries. Preventive strategies studied in TAVR populations, such as embolic cerebral protection devices, did not demonstrate an advantage in pre-procedural stroke incidence (51). In the TAV-in-TAV population, the use of these devices is a subject of debate. Studies exploring their utility could be insightful. Certain criteria might guide the operator in identifying the patients that may require the use of these devices including a history of valve thrombosis, extensive calcifications of the index THV leaflets or the necessity of a leaflet modification technique.
Post-procedural care considerations
Standardized anti-thrombotic treatment following TAV-in-TAV procedure has not been established. The introduction of foreign material in the TAV-in-TAV setting may increase the risk of leaflet or valve thrombosis. Furthermore, the presence of neosinuses, particularly in low-frame valves, is being recognized as a potential risk factor for thrombosis (52). Future studies should focus on identifying anatomical factors that may necessitate anticoagulation, considering factors such as valve geometries and flow patterns.
Future perspective
The future of TAV-in-TAV procedures hold great promise as it enters a phase of exciting development. This technique may become the preferred treatment option for patients experiencing THV failure. Figure 5 suggests a treatment algorithm of patients with TAV failure. To ensure its success, there is a pressing need for more robust evidence and guidelines to guide procedure planning. A heightened focus on assessing the long-term durability, structural integrity, and functional performance of these THVs is on the horizon. This may lead to broader indications, especially as comparative studies between TAVR explantation and TAV-in-TAV in low-risk patients are anticipated to provide essential insights for clinicians. Furthermore, addressing concerns related to the risk of coronary obstruction will be essential to expand the indications of TAV-in-TAV procedures. Above all, a patient-centered approach to lifetime management, starting with the first intervention and considering the likelihood of further interventions, will play a pivotal role in shaping the future landscape of TAV-in-TAV procedures.
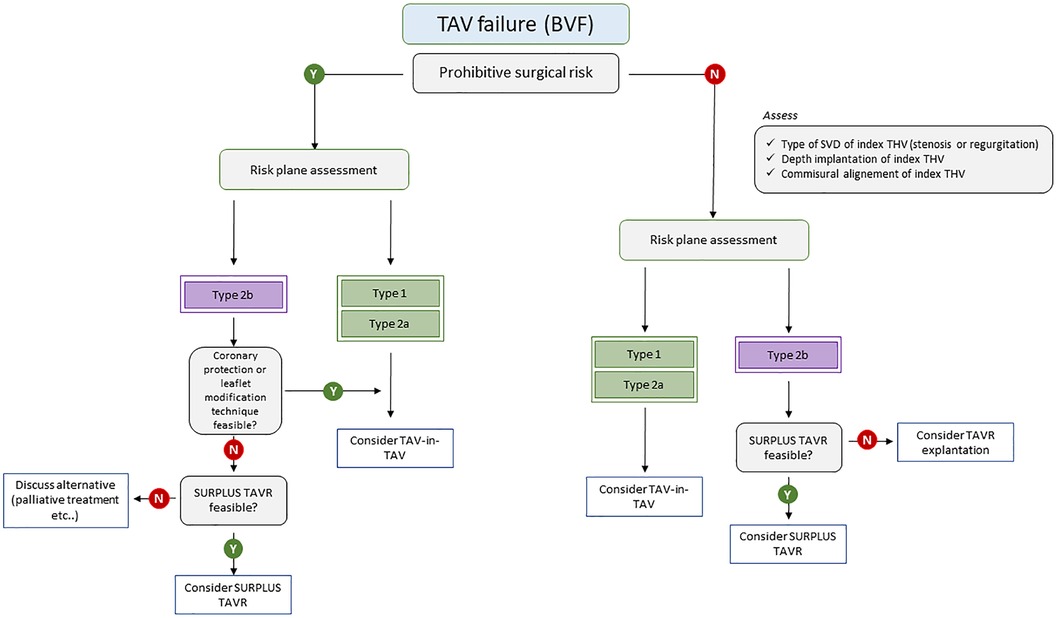
Figure 5. Treatment algorithm of patients with TAV failure. BVF, bioprosthetic valve failure; SVD, structural valve deterioration; TAV, transcatheter aortic valve; THV, transcatheter heart valve.
Conclusion
THV durability appears favorable, making TAV-in-TAV an attractive option for patients with TAV dysfunction due to its lower morbidity when compared to TAV explantation. Careful patient selection is paramount, emphasizing the importance of CT assessment and coronary risk evaluation.
Clinical experience is steadily expanding leading to a better understanding of the intricacies of the procedure. Dedicated studies are eagerly awaited to ensure the successful advancement of this technique.
Author contributions
AH: Writing – original draft, Writing – review & editing. CP: Validation, Writing – original draft. ND: Supervision, Writing – review & editing. QC: Validation, Writing – original draft. RI: Supervision, Writing – review & editing. AA: Supervision, Writing – review & editing. TM: Supervision, Writing – review & editing. WB: Supervision, Validation, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2017) 135(25):e1159–95. doi: 10.1161/CIR.0000000000000503
2. Writing Committee Members, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2021) 77(4):450–500. doi: 10.1016/j.jacc.2020.11.035
3. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43(7):561–632. doi: 10.1093/eurheartj/ehab395
4. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380(18):1695–705. doi: 10.1056/NEJMoa1814052
5. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. (2019) 380(18):1706–15. doi: 10.1056/NEJMoa1816885
6. Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, et al. 3-year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol. (2023) 81(17):1663–74. doi: 10.1016/j.jacc.2023.02.017
7. Leon MB, Mack MJ, Hahn RT, Thourani VH, Makkar R, Kodali SK, et al. Outcomes 2 years after transcatheter aortic valve replacement in patients at low surgical risk. J Am Coll Cardiol. (2021) 77(9):1149–61. doi: 10.1016/j.jacc.2020.12.052
8. Cahill TJ, Chen M, Hayashida K, Latib A, Modine T, Piazza N, et al. Transcatheter aortic valve implantation: current status and future perspectives. Eur Heart J. (2018) 39(28):2625–34. doi: 10.1093/eurheartj/ehy244
9. Leone PP, Scotti A, Ho EC, Assafin M, Doolittle J, Chau M, et al. Prosthesis tailoring for patients undergoing transcatheter aortic valve implantation. J Clin Med. (2023) 12(1):338. doi: 10.3390/jcm12010338
10. Capodanno D, Petronio AS, Prendergast B, Eltchaninoff H, Vahanian A, Modine T, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European association of percutaneous cardiovascular interventions (EAPCI) endorsed by the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2017) 38(45):3382–90. doi: 10.1093/eurheartj/ehx303
11. Dvir D, Bourguignon T, Otto CM, Hahn RT, Rosenhek R, Webb JG, et al. Standardized definition of structural valve degeneration for surgical and transcatheter bioprosthetic aortic valves. Circulation. (2018) 137(4):388–99. doi: 10.1161/CIRCULATIONAHA.117.030729
12. VARC-3 WRITING COMMITTEE:, Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. (2021) 77(21):2717–46. doi: 10.1016/j.jacc.2021.02.038
13. Pibarot P, Ternacle J, Jaber WA, Salaun E, Dahou A, Asch FM, et al. Structural deterioration of transcatheter versus surgical aortic valve bioprostheses in the PARTNER-2 trial. J Am Coll Cardiol. (2020) 76(16):1830–43. doi: 10.1016/j.jacc.2020.08.049
14. Gleason TG, Reardon MJ, Popma JJ, Deeb GM, Yakubov SJ, Lee JS, et al. 5-year outcomes of self-expanding transcatheter versus surgical aortic valve replacement in high-risk patients. J Am Coll Cardiol. (2018) 72(22):2687–96. doi: 10.1016/j.jacc.2018.08.2146
15. Abdel-Wahab M, Landt M, Neumann FJ, Massberg S, Frerker C, Kurz T, et al. 5-year outcomes after TAVR with balloon-expandable versus self-expanding valves: results from the CHOICE randomized clinical trial. JACC Cardiovasc Interv. (2020) 13(9):1071–82. doi: 10.1016/j.jcin.2019.12.026
16. Jørgensen TH, Thyregod HGH, Ihlemann N, Nissen H, Petursson P, Kjeldsen BJ, et al. Eight-year outcomes for patients with aortic valve stenosis at low surgical risk randomized to transcatheter vs. Surgical aortic valve replacement. Eur Heart J. (2021) 42(30):2912–9. doi: 10.1093/eurheartj/ehab375
17. Blackman DJ, Saraf S, MacCarthy PA, Myat A, Anderson SG, Malkin CJ, et al. Long-term durability of transcatheter aortic valve prostheses. J Am Coll Cardiol. (2019) 73(5):537–45. doi: 10.1016/j.jacc.2018.10.078
18. Jørgensen T. NOTION 10 years - The nordic aortic valve intervention trial. Available online at: https://www.pcronline.com/News/Whats-new-on-PCRonline/2023/ESC/NOTION-The-nordic-aortic-valve-intervention-trial (Cited September 4, 2023).
19. Sathananthan J, Lauck S, Polderman J, Yu M, Stephenson A, Sathananthan G, et al. Ten year follow-up of high-risk patients treated during the early experience with transcatheter aortic valve replacement. Catheter Cardiovasc Interv. (2021) 97(3):E431–7. doi: 10.1002/ccd.29124
20. Hirji SA, Percy ED, McGurk S, Malarczyk A, Harloff MT, Yazdchi F, et al. Incidence, characteristics, predictors, and outcomes of surgical explantation after transcatheter aortic valve replacement. J Am Coll Cardiol. (2020) 76(16):1848–59. doi: 10.1016/j.jacc.2020.08.048
21. Bapat VN, Zaid S, Fukuhara S, Saha S, Vitanova K, Kiefer P, et al. Surgical explantation after TAVR failure: mid-term outcomes from the EXPLANT-TAVR international registry. JACC Cardiovasc Interv. (2021) 14(18):1978–91. doi: 10.1016/j.jcin.2021.07.015
22. Fukuhara S, Brescia AA, Shiomi S, Rosati CM, Yang B, Kim KM, et al. Surgical explantation of transcatheter aortic bioprostheses: results and clinical implications. J Thorac Cardiovasc Surg. (2021) 162(2):539–47.e1. doi: 10.1016/j.jtcvs.2019.11.139
23. Landes U, Webb JG, De Backer O, Sondergaard L, Abdel-Wahab M, Crusius L, et al. Repeat transcatheter aortic valve replacement for transcatheter prosthesis dysfunction. J Am Coll Cardiol. (2020) 75(16):1882–93. doi: 10.1016/j.jacc.2020.02.051
24. Testa L, Agnifili M, Van Mieghem NM, Tchétché D, Asgar AW, De Backer O, et al. Transcatheter aortic valve replacement for degenerated transcatheter aortic valves: the TRANSIT international project. Circ Cardiovasc Interv. (2021) 14(6):E010440. doi: 10.1161/CIRCINTERVENTIONS.120.010440
25. Landes U, Sathananthan J, Witberg G, De Backer O, Sondergaard L, Abdel-Wahab M, et al. Transcatheter replacement of transcatheter versus surgically implanted aortic valve bioprostheses. J Am Coll Cardiol. (2021) 77(1):1–14. doi: 10.1016/j.jacc.2020.10.053
26. Makkar RR, Kapadia S, Chakravarty T, Cubeddu R, Mahoney P, Yadav P, et al. Out-comes of repeat TAVR with balloon-expandable SAPIEN 3/ultra valves. tctMD. (2021). Available online at: https://www.tctmd.com/slide/outcomes-repeat-tavr-balloon-expandable-sapien-3ultra-valves
27. Attizzani GF, Dallan LAP, Forrest JK, Reardon MJ, Szeto WY, Liu F, et al. Redo-transcatheter aortic valve replacement with the supra-annular, self-expandable evolut platform: insights from the transcatheter valve therapy registry. Catheter Cardiovasc Interv. (2022) 99(3):869–76. doi: 10.1002/ccd.29941
28. Makkar RR, Kapadia S, Chakravarty T, Cubeddu RJ, Kaneko T, Mahoney P, et al. Outcomes of repeat transcatheter aortic valve replacement with balloon-expandable valves: a registry study. Lancet. (2023) 402(10412):1529–40. doi: 10.1016/S0140-6736(23)01636-7
29. Percy ED, Harloff MT, Hirji S, McGurk S, Yazdchi F, Newell P, et al. Nationally representative repeat transcatheter aortic valve replacement outcomes: report from the centers for medicare and medicaid services. JACC Cardiovasc Interv. (2021) 14(15):1717–26. doi: 10.1016/j.jcin.2021.06.011
30. Tang GHL, Zaid S, Kleiman NS, Goel SS, Fukuhara S, Marin-Cuartas M, et al. Explant vs redo-TAVR after transcatheter valve failure: mid-term outcomes from the EXPLANTORREDO-TAVR international registry. JACC Cardiovasc Interv. (2023) 16(8):927–41. doi: 10.1016/j.jcin.2023.01.376
31. Sathananthan J, Fraser R, Landes U, Rich C, Sellers S, Leipsic J, et al. Repeat transcatheter aortic valve implantation and implications for transcatheter heart valve performance: insights from bench testing. EuroIntervention. (2021) 17(10):856–64. doi: 10.4244/EIJ-D-20-00697
32. Akodad M, Sellers S, Landes U, Meier D, Tang GHL, Gada H, et al. Balloon-expandable valve for treatment of evolut valve failure: implications on neoskirt height and leaflet overhang. JACC Cardiovasc Interv. (2022) 15(4):368–77. doi: 10.1016/j.jcin.2021.12.021
33. Akodad M, Sellers S, Gulsin GS, Tzimas G, Landes U, Chatfield AG, et al. Leaflet and neoskirt height in transcatheter heart valves: implications for repeat procedures and coronary access. JACC Cardiovasc Interv. (2021) 14(20):2298–300. doi: 10.1016/j.jcin.2021.07.034
34. Meier D, Landes U, Sondergaard L, De Backer O, Lutter G, Puehler T, et al. Redo-TAVI with SAPIEN 3 in SAPIEN XT or SAPIEN 3 - impact of pre- and post-dilatation on final THV expansion. EuroIntervention. (2023) 19(9):757–65. doi: 10.4244/EIJ-D-23-00308
35. Tarantini G, Fabris T, Fovino LN. TAVR-in-TAVR and coronary access: importance of preprocedural planning. EuroIntervention. (2020) 16(2):E129–32. doi: 10.4244/EIJ-D-19-01094
36. Buzzatti N, Montorfano M, Romano V, De Backer O, Soendergaard L, Rosseel L, et al. A computed tomography study of coronary access and coronary obstruction after redo transcatheter aortic valve implantation. EuroIntervention. (2020) 16(12):E1005–13. doi: 10.4244/EIJ-D-20-00475
37. Grubb KJ, Shekiladze N, Spencer J, Perdoncin E, Tang GHL, Xie J, et al. Feasibility of redo-TAVI in self-expanding evolut valves: a CT analysis from the evolut low risk trial substudy. EuroIntervention. (2023) 19(4):e330–9. doi: 10.4244/EIJ-D-22-01125
38. De Backer O, Landes U, Fuchs A, Yoon SH, Mathiassen ON, Sedaghat A, et al. Coronary access after TAVR-in-TAVR as evaluated by multidetector computed tomography. JACC Cardiovasc Interv. (2020) 13(21):2528–38. doi: 10.1016/j.jcin.2020.06.016
39. Tarantini G, Nai Fovino L, Scotti A, Massussi M, Cardaioli F, Rodinò G, et al. Coronary access after transcatheter aortic valve replacement with commissural alignment: the ALIGN-ACCESS study. Circ Cardiovasc Interv. (2022) 15(2):E011045. doi: 10.1161/CIRCINTERVENTIONS.121.011045
40. Tarantini G, Delgado V, de Backer O, Sathananthan J, Treede H, Saia F, et al. Redo-transcatheter aortic valve implantation using the SAPIEN 3/ultra transcatheter heart valves-expert consensus on procedural planning and techniques. Am J Cardiol. (2023) 192:228–44. doi: 10.1016/j.amjcard.2023.01.010
41. Tang GHL, Zaid S, Gupta E, Ahmad H, Khan A, Kovacic JC, et al. Feasibility of repeat TAVR after SAPIEN 3 TAVR: a novel classification scheme and pilot angiographic study. JACC Cardiovasc Interv. (2019) 12(13):1290–2. doi: 10.1016/j.jcin.2019.02.020
42. Damlin A, Meduri C, Manouras A, Verouhis D, Linder R, Rück A, et al. BASILICA procedure prior to valve-in-valve TAVR in a supra-annular TAV prosthesis. JACC Case Reports. (2023) 11:101777. doi: 10.1016/j.jaccas.2023.101777
43. Tang GHL, Sengupta A, Alexis SL, Zaid S, Leipsic JA, Blanke P, et al. Conventional versus modified delivery system technique in commissural alignment from the evolut low-risk CT substudy. Catheter Cardiovasc Interv. (2022) 99(3):924–31. doi: 10.1002/ccd.29973
44. Khan JM, Bruce CG, Babaliaros VC, Greenbaum AB, Rogers T, Lederman RJ. TAVR roulette: caution regarding BASILICA laceration for TAVR-in-TAVR. JACC Cardiovasc Interv. (2020) 13(6):787–9. doi: 10.1016/j.jcin.2019.10.010
45. Greenbaum AB, Kamioka N, Vavalle JP, Lisko JC, Gleason PT, Paone G, et al. Balloon-assisted BASILICA to facilitate redo TAVR. JACC Cardiovasc Interv. (2021) 14(5):578–80. doi: 10.1016/j.jcin.2020.10.056
46. Babaliaros VC, Gleason PT, Xie JX, Khan JM, Bruce CG, Byku I, et al. Toward transcatheter leaflet removal with the CATHEDRAL procedure: CATHeter electrosurgical debulking and RemovAL. JACC Cardiovasc Interv. (2022) 15(16):1678–80. doi: 10.1016/j.jcin.2022.05.038
47. Dvir D, Leon MB, Abdel-Wahab M, Unbehaun A, Kodali S, Tchetche D, et al. First-in-human dedicated leaflet splitting device for prevention of coronary obstruction in transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2023) 16(1):94–102. doi: 10.1016/j.jcin.2022.10.050
48. Pirelli L, Basman CL, Brinster DR, Wang D, Patel N, Scheinerman SJ, et al. Surgical resection of prosthetic valve leaflets under direct vision (SURPLUS) for redo TAVR. JACC Cardiovasc Interv. (2021) 14(9):1036–7. doi: 10.1016/j.jcin.2021.02.026
49. Huded CP, Tuzcu EM, Krishnaswamy A, Mick SL, Kleiman NS, Svensson LG, et al. Association between transcatheter aortic valve replacement and early postprocedural stroke. JAMA. (2019) 321(23):2306–15. doi: 10.1001/jama.2019.7525
50. Macherey S, Meertens M, Mauri V, Frerker C, Adam M, Baldus S, et al. Meta-analysis of stroke and mortality rates in patients undergoing valve-in-valve transcatheter aortic valve replacement. J Am Heart Assoc. (2021) 10(6):e019512. doi: 10.1161/JAHA.120.019512
51. Kapadia SR, Makkar R, Leon M, Abdel-Wahab M, Waggoner T, Massberg S, et al. Cerebral embolic protection during transcatheter aortic-valve replacement. N Engl J Med. (2022) 387(14):1253–63. doi: 10.1056/NEJMoa2204961
Keywords: coronary obstruction, structural valve deterioration, TAV-in-TAV, TAVR, CT planning
Citation: Hayek A, Prieur C, Dürrleman N, Chatelain Q, Ibrahim R, Asgar A, Modine T and Ben Ali W (2024) Clinical considerations and challenges in TAV-in-TAV procedures. Front. Cardiovasc. Med. 11:1334871. doi: 10.3389/fcvm.2024.1334871
Received: 7 November 2023; Accepted: 24 January 2024;
Published: 19 February 2024.
Edited by:
Maurizio Taramasso, University Hospital Zürich, SwitzerlandReviewed by:
Jonathan Curio, University Hospital of Cologne, GermanyPier Pasquale Leone, Albert Einstein College of Medicine, United States
© 2024 Hayek, Prieur, Dürrleman, Chatelain, Ibrahim, Asgar, Modine and Ben Ali. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Walid Ben Ali ZHIud2FsaWRiZW5hbGlAZ21haWwuY29t
Abbreviations BEV, balloon-expandable valve; BVF, bioprosthetic valve failure; CT, computed tomography; SAV, surgical aortic valve; SAVR, surgical aortic valve replacement; SEV, self-expandable valve; SVD, structural valve deterioration; TAV-in-TAV, transcatheter aortic valve in transcatheter aortic valve; TAVR, transcatheter aortic valve replacement; THV, transcatheter heart valve; VTA, valve to aorta; VTC, valve to coronary.
 Ahmad Hayek
Ahmad Hayek Cyril Prieur2
Cyril Prieur2 Anita Asgar
Anita Asgar Thomas Modine
Thomas Modine Walid Ben Ali
Walid Ben Ali