- 1Department of Cardiology, Fujian Heart Medical Center, Fujian Institute of Coronary Heart Disease, Fujian Medical University Union Hospital, Fuzhou, China
- 2Department of Epidemiology, School of Public Health, Fujian Medical University, Fuzhou, China
Background: Vasovagal syncope (VVS) is a prevalent medical condition with a lack of efficient methods for its detection.
Aim: This study aimed to explore an objective clinical indicator in diagnosing VVS.
Methods: The retrospective analysis involved clinical data of 243 syncope patients from 1 June 2020 to 31 July 2023. Among them, 108 patients had a negative result in the tilt test (TTT), while the remaining 135 patients had a positive result in the TTT. Relevant statistical methods were utilized to examine the correlation between VVS and different indicators of heart rate variability.
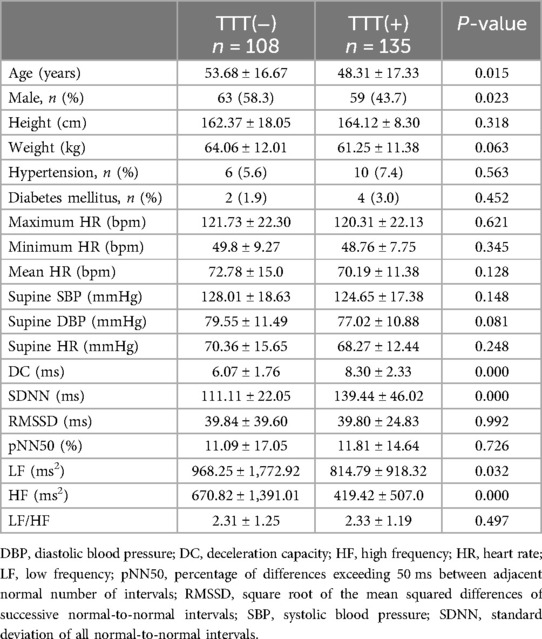
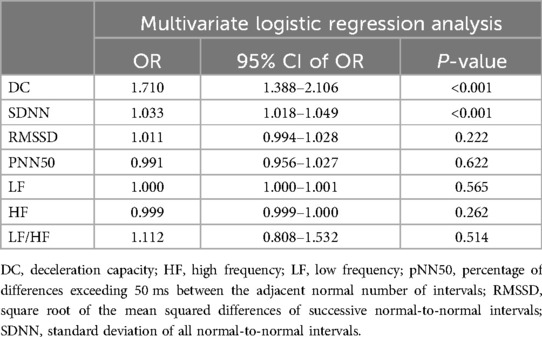
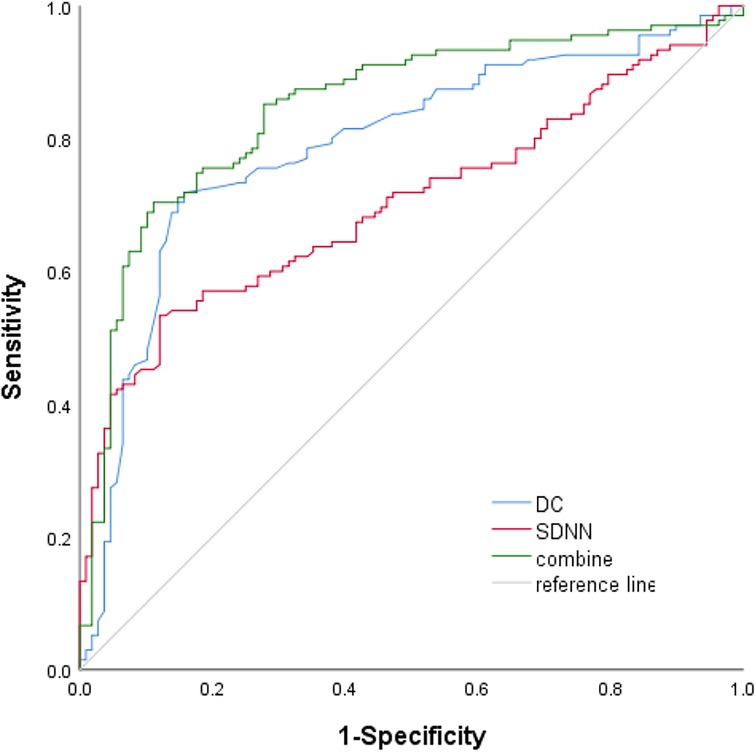
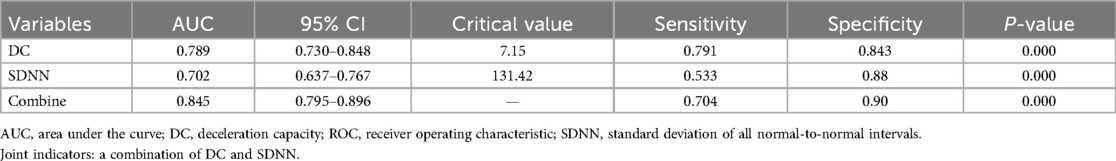
Results: After screening, 354 patients being considered for VVS were evaluated, resulting in a final sample size of 243. Sex, age, deceleration capacity (DC), and standard deviation of all normal-to-normal intervals (SDNNs) were the variables that showed statistical significance between the TTT(−) group and the TTT(+) group. Independent risk factors identified by multivariate logistic regression were DC [odds ratio (OR) 1.710, 95% confidence interval (CI) 1.388–2.106, P < 0.001] and SDNN (OR 1.033, 95% CI 1.018–1.049, P < 0.001). Comparing the groups, receiver operating characteristic analysis revealed a notable distinction in both DC and SDNN [the respective areas under the curve were 0.789 (95% CI 0.730–0.848) and 0.702 (95% CI 0.637–0.767); the cutoff values were 7.15 and 131.42; P < 0.001, respectively].
Conclusion: In summary, DC can function as an impartial and easily accessible clinical marker for differentiating VVS. A value exceeding 7.15 ms might suggest a higher likelihood of syncope.
1 Introduction
Vasovagal syncope (VVS) is the predominant reason for fainting in individuals of all age groups (1–4), characterized by an abrupt decline in blood pressure (BP) and/or heart rate (HR). It is distinguished by rapid onset, brief duration, and natural full recovery (5). Due to the high prevalence and lack of efficient medical treatments for VVS (6), numerous patients experience significant physical and psychological distress, resulting in a diminished quality of life (2, 7–10).
The complex pathophysiological mechanisms responsible for vasovagal syncope are still not completely understood. An imbalance in the parasympathetic and sympathetic nerves could have a substantial impact (11–13). Cardioinhibition occurs due to the heightened stress on the parasympathetic nerve of the heart, resulting in bradycardia, asystole, and conduction blockage. Conversely, vasodilation is caused by the inadequate tension of the sympathetic nerve in the blood vessels (14, 15). Several studies have supported the association between VVS and dysfunction of the parasympathetic nervous system (14–21).
In addition to the patient’s medical background and usual clinical symptoms, an objective measure of the performance of the pneumogastric nerve is also a crucial factor in diagnosing VVS. Previous research has yielded inconsistent findings regarding VVS, despite the conventional assessment of heart rate variability (HRV) for analyzing cardiac autonomic function (22, 23). After the initial clinical evaluation, the tilt test (TTT) remains the most valuable diagnostic examination for individuals with suspected reflex syncope (24–26). Vagal modulation has been characterized using the novel measure of heart rate deceleration capacity (DC) (16, 27–30). A decrease in cardiac direct current indicates a decline in the vagal tone of the cardiac autonomic function. Hence, it appears that DC exhibits a higher diagnostic efficacy in individuals with VVS (3, 20, 31). The objective of this research was to determine the involvement of DC and other measures of fundamental autonomic nervous system (ANS) activity in forecasting VVS.
2 Materials and methods
2.1 Patient recruitment
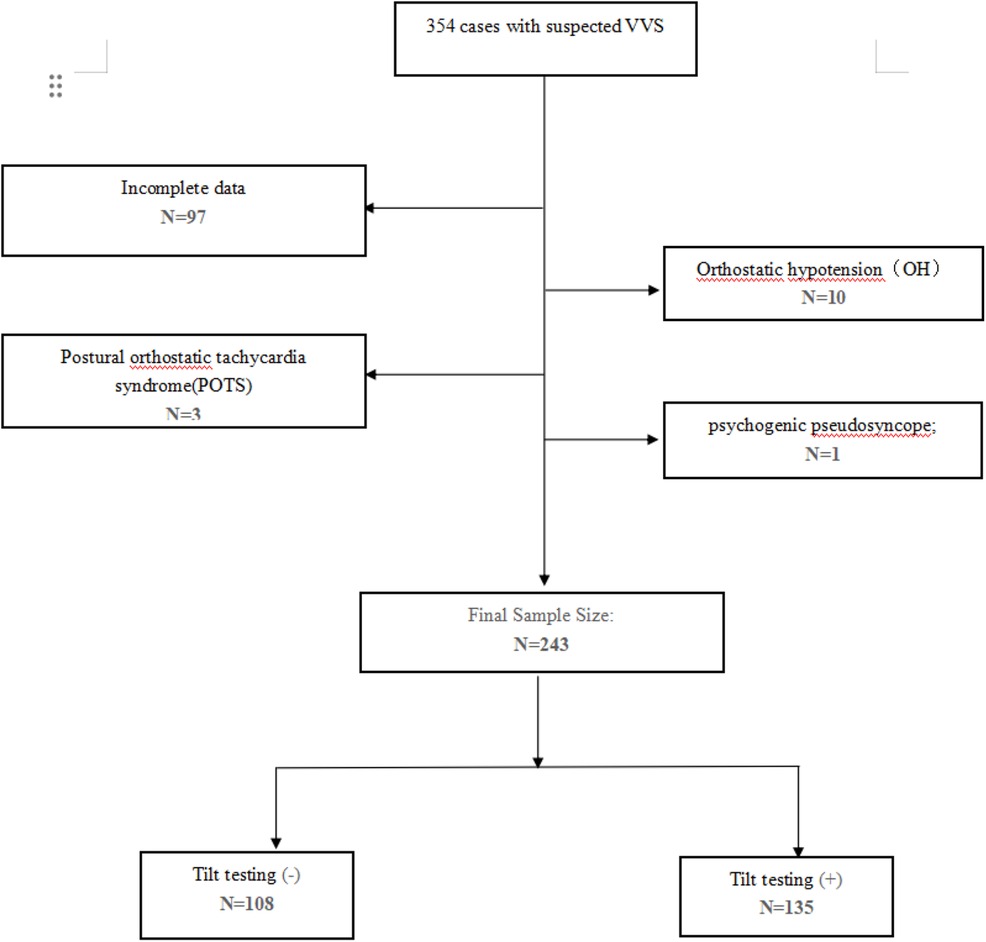
The institutional research ethics committee of Fujian Medical University Union Hospital approved this retrospective study conducted at a single center. Between June 2020 and May 2023, this hospital gathered clinical information from 354 individuals diagnosed with suspected VVS. VVS was considered if syncope was triggered by fear, pain, or standing up and was accompanied by the usual progressive prodrome (pallor, perspiration, and/or queasiness) (5). The exclusion criteria included (a) heart rhythm abnormalities (paroxysmal supraventricular tachycardia, ventricular tachycardia, atrial fibrillation, Mobitz type Ⅱ second-degree or third-degree atrioventricular block, arrhythmias caused by medication); (b) severe heart or cardiopulmonary conditions (coronary heart disease, cardiac valve diseases, hypertrophic obstructive cardiomyopathy, New York Heart Association class Ⅲ or Ⅳ heart failure, pulmonary hypertension, pulmonary embolism); (c) cerebrovascular disorders (stroke, severe neurological disorders, seizures); (d) syncope caused by medication (antidiabetic drugs, antipsychotics, vasodilators); and (e) conditions affecting the autonomic nervous system (diabetes mellitus, diseases related to the nervous system). The exclusion of patients with orthostatic hypotension was based on the criteria of a minimum decrease of 20 mmHg in systolic blood pressure or 10 mmHg in diastolic blood pressure within the initial 3 min of TTT, as it had been previously demonstrated to impact HRV in a prior study (32). The study included 243 patients who were clinically suspected of having VVS and were screened to rule out any related conditions. These patients underwent echocardiograms, general and biochemical examinations, 24-h Holter recordings, and TTTs after admission.
2.2 Tilt test
All TTTs followed the same protocol (33). TTTs were conducted in a softly illuminated chamber following a minimum of 6 h of fasting on an electrically powered table equipped with a footboard. Electrocardiography (ECG) and BP were continuously monitored during the test. The procedure included two stages. Initially, the individuals were inclined at a 70° angle for 30 min (passive stage), followed by an additional 20 min with sublingual administration of 0.25 mg of nitroglycerin (provocative stage) in case the initial stage yielded negative results. TTT was considered positive only if syncope occurred during the testing (5). As per the classification of the Vasovagal Syncope International Study (VASIS) (34), positive findings were categorized into different types. Type 1 (mixed) involved a simultaneous decrease in heart rate and blood pressure, with the heart rate remaining above 40 bpm. Type 2a (cardioinhibition without asystole) indicated a heart rate below 40/min without asystole lasting more than 3 s. Type 2b (cardioinhibition with asystole) referred to a decreased heart rate accompanied by asystole lasting more than 3 s. Type 3 (vasodepressor) was characterized by a rapid drop in blood pressure during syncope, without a decrease in heart rate exceeding 10/min from the baseline. Negative evaluations were assigned to tests with different outcomes.
2.3 Holter recording
A minimum of 20 h was spent obtaining 12-channel 24-h Holter data. The digitized recordings were automatically processed using dedicated software (Cardio Care H1200, Nalong Health Technology Co, LTD, Xiamen, China) to determine DC and HRV.
2.4 Deceleration capacity
The calculation of DC is based on the phase-rectified signal averaging (PRSA) algorithm, which uses referenced heartbeat intervals (20, 31, 35). Anchors are defined as heartbeat intervals that are longer than the previous interval. To prevent errors caused by artifacts, R-R interval prolongations greater than 5% are excluded. Segments of equal dimensions surrounding the anchors are chosen and aligned with the anchors. Next, the X signals in the aligned segments are averaged. The quantification of DC is determined by the following equation: DC = 1/4 (X0 + X1 − X−1 − X−2), where X0 and X1 represent the mean values of the anchor points and the subsequent R-R intervals and X−1 and X−2 denote the averages of the two R-R intervals preceding the anchor points. Daytime DC and nighttime DC were computed for the full 24-h period spanning from 8:00 to 23:00 and from 23:00 to 8:00, respectively.
2.5 Heart rate variability
HRV was assessed using five time-domain indexes (36): (a) SDNN, which measures the standard deviation of all normal-to-normal intervals; (b) RMSSD, which calculates the square root of the mean squared differences of successive normal-to-normal intervals; (c) pNN50, which determines the proportion of adjacent R-R intervals differing by more than 50 ms in the 24-h recording; and (d) the frequency domain indices of HRV including the low-frequency (LF) and high-frequency (HF) spectral components, along with the LF/HF ratio.
3 Statistical analysis
The data were examined using IBM SPSS version 27.0 in Somers, NY, USA. With this sample size, it was possible to achieve a statistical power of 80% to evaluate a notable distinction (with a margin of error of 0.05). Data normality was assessed using the Kolmogorov–Smirnov test. The normal distribution was followed by all continuous variables, which were expressed as mean ± standard deviation (SD) and examined using an independent-samples t-test. The chi-square test and Fisher's exact test were used to compare the case proportion when the expected frequency was less than 5. Multivariate logistic regression analysis was used to investigate the potential link between risk factors and VVS. The discriminatory ability was assessed using the area under the receiver operating characteristic (ROC) curve and its corresponding 95% confidence interval (CI). A two-sided probability value of less than 0.05 was chosen as the threshold for statistical significance.
4 Results
4.1 Patient characteristics
In summary, 354 patients diagnosed with suspected VVS underwent TTT, performed from 1 June 2020 to 31 July 2023 in Fujian Medical University Union Hospital. After removing individuals with orthostatic hypotension (OH), postural orthostatic tachycardia syndrome (POTS), psychogenic pseudosyncope, and incomplete or unavailable data, 243 patients were divided into two groups based on their TTT outcomes (Figure 1). In the TTT(−) group, 108 patients exhibited increased height, reduced weight, lower rates of hypertension and diabetes mellitus, and higher average maximum HR, minimum HR, mean HR, supine SBP, supine DBP, and supine HR. However, no statistical significance was observed in these measurements. In addition, both groups noted a similarity in RMSSD, pNN50, and LF/HF (P > 0.05 for all). According to the independent-samples t-test, the TTT(−) group reported significantly lower values of DC (6.07 ± 1.76 vs. 8.30 ± 2.33, P < 0.05) and SDNN (111.11 ± 22.05 vs. 139.44 ± 46.02, P < 0.05) and seemingly higher LF (968.25 ± 1,772.92 vs. 814.79 ± 918.32, P = 0.032) and HF (670.82 ± 1,391.01 vs. 419.42 ± 507.0, P < 0.05) than the TTT(+) group. Meanwhile, the TTT(−) group showed an older age (53.68 ± 16.67 vs. 48.31 ± 17.33, P = 0.015) and a greater proportion of male patients (58.3 vs. 43.7, p = 0.023) (Table 1).
4.2 Logistic regression and ROC curve analyses
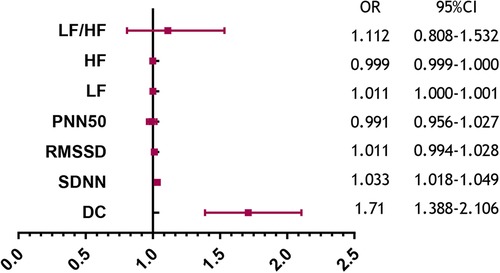
Table 2 summarizes the indexes (DC, SDNN, RMSSD, pNN50, LF, HF, and LF/HF) from Table 1 with a significance level of P < 0.05. Multivariate logistic regression analysis revealed that VVS was independently associated with DC [odds ratio (OR) 1.710, 95% CI 1.388–2.106, P < 0.001] and SDNN (OR 1.033, 95% CI 1.018–1.049, P < 0.001). The forest plot (Figure 2) displayed the associations between different variables and VVS, highlighting DC as the factor with the strongest correlation. Figure 3 displays the ROC curve analysis, which helped identify the critical value of continuous variables (DC and SDNN) for detecting patients with VVS. Comprehensive optimization results of sensitivity and specificity were used as the basis for selecting the optimal cutoff point criterion. A clear distinction was noted in DC and SDNN, with corresponding areas under the curve of 0.789 (95% CI 0.730–0.848) and 0.702 (95% CI 0.637–0.767) (P < 0.001 for both). According to the ROC curves, the occurrence of VVS was more probable when DC was greater than 7.15 ms or SDNN surpassed 131.42 ms. Table 3 displays all the data related to the ROC curve.
5 Discussion
Fainting is a prevalent and harmless condition, with a cumulative incidence of at least 35% throughout a person's lifetime and a high likelihood of recurring after the first occurrence (37). Among these fainting cases, approximately 60% are classified as VVS (3, 4), resulting from a combination of various central and peripheral mechanisms (5, 38). As previously stated, VVS is closely linked to dysfunction of both the parasympathetic and sympathetic systems, leading to withdrawal symptoms. To clarify further, triggers that are understood to cause VVS likely lead to vasodilation, resulting in a decrease in the return of blood to the heart and a reduction in the amount of blood in the heart before contraction, as well as a potential decrease in resistance in the outer parts of the body. The low blood pressure linked to VVS could be caused by a lack of narrowing in the small arteries, constriction in the veins, or both (6). Conversely, irregular heart rhythms in VVSs are mainly controlled by the parasympathetic system through the vagal nerve. The heightened activity of the cardiac parasympathetic nerve results in cardioinhibition, resulting in bradycardia, asystole, and conduction blockage (14, 15).
VVS patients believe that heightened parasympathetic activity has a greater role in triggering syncope events (16). HRV refers to the capacity of the heart rate to fluctuate during a specific timeframe and is impacted by the ANS. In recent years, the risk stratification of patients with various diseases has significantly improved due to advancements in parameters related to cardiac autonomic function, measured by HRV (39). A reduced HRV, indicating dysfunction of the cardiac autonomic system, has been linked to an elevated risk of death in various conditions like a heart attack (23, 40, 41), aortic stenosis (42), blood poisoning (43, 44), neurological and psychiatric disorders, blood disorders (45), and even in cancer patients (46, 47). Hence, 24-h Holter monitoring of HRV has emerged as a reliable, non-invasive method for evaluating the ANS, gaining popularity for its practicality and efficiency (48, 49). Nevertheless, prior research has presented contradictory findings regarding VVS (22, 23). Several factors are likely responsible for the inconsistent findings. Analyzing HRV makes it challenging to distinguish the initial impact of vagal and sympathetic modulators on the heart. Furthermore, the absence of a standardized methodology hinders HRV assessments, as the parameters vary depending on factors such as age, gender, physical fitness, sleep quality, and medication use (35, 48, 50).
By analyzing the advantages and disadvantages of HRV, we tried to find some parameters that differed from traditional HRV parameters. Table 1 presents a comparison between DC and SDNN, RMSSD, pNN50, LF, HF, and other commonly used HRV indicators. The findings revealed that the negative group exhibited significantly lower levels of DC and SDNN than the positive group. Moreover, DC displayed a stronger correlation with this disparity than SDNN, with an odds ratio of 1.710 vs. 1.033. This indicated that DC is more effective than conventional HRV in assessing autonomic nervous system function, and this capability seemed to be measurable. Simultaneously, TTT is regarded as a valuable diagnostic instrument for VVS despite its various constraints. The TTT does not meet the desired levels of sensitivity and specificity (51–53). In the present investigation, TTT yielded a positive result in only 55.56% of the individuals who met the clinical diagnostic criteria for VVS. In addition, certain individuals may experience discomfort, particularly during a positive test (3, 20, 31, 54). DC, with its high sensitivity and specificity, addresses the limitations of traditional methods like TTT and HRV while also providing two additional advantages. First, DC allows for the extraction and quantification of the deceleration-induced modulations of the heart rate, enabling a quantitative assessment of vagal tone in patients with VVS. Some studies have shown that DC is consistently modified in patients with VVS (16). Furthermore, the DC algorithm can identify recurring elements of the autonomic regulation process while removing non-recurring elements like disruptive artifacts or irregular heart rhythms. This leads to more consistent and trustworthy assessments in patients with VVS (16). Finally, a recent study indicates that DC seems to have a more robust predictive significance when diagnosing VVS in patients who have a negative TTT response (16).
6 Conclusions
The present study found a strong correlation between the cardiac DC index and VVS in patients. A DC value greater than 7.15 ms suggests a considerably higher likelihood of vasovagal syncope.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board (IRB) of Fujian Medical University Union Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YG: Formal Analysis, Methodology, Writing – original draft. TL: Formal Analysis, Methodology, Writing – original draft. NL: Data curation, Writing – original draft. HL: Conceptualization, Formal Analysis, Funding acquisition, Project administration, Writing – review & editing, Writing – original draft.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was conducted with support from the Fujian Provincial Health Technology Project (No. 2021TG008), the Joint Funds for the Innovation of Science and Technology, Fujian Province (No. 2020Y9069), Fujian Provincial Health Technology Project (No. 2021QNA021) and Fujian Provincial Natural Science Foundation of China (Project No. 2023J01632).
Acknowledgments
The writers extend their appreciation to the healthcare and technological personnel of the cardiac unit and ECG facility who took part in this initiative.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DC, deceleration capacity; ROC, receiver operating characteristic; SDNN, standard deviation of all normal-to-normal intervals; VVS, vasovagal syncope.
References
1. da Silva RM. Syncope: epidemiology, etiology, and prognosis. Front Physiol. (2014) 5:471. doi: 10.3389/fphys.2014.00471
2. Vandenberk B, Lei LY, Ballantyne B, Vickers D, Liang Z, Sheldon RS, et al. Cardioneuroablation for vasovagal syncope: a systematic review and meta-analysis. Heart Rhythm. (2022) 19(11):1804–12. doi: 10.1016/j.hrthm.2022.06.017
3. Sheldon RS, Grubb BP 2nd, Olshansky B, Shen WK, Calkins H, Brignole M, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. (2015) 12(6):e41–63. doi: 10.1016/j.hrthm.2015.03.029
4. Shen WK, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZD, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. (2017) 70(5):e39–110. doi: 10.1016/j.jacc.2017.03.003
5. Brignole M, Moya A, de Lange FJ, Deharo , Elliott PM, Fanciulli A, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J. (2018) 39(21):1883–948. doi: 10.1093/eurheartj/ehy037
6. Lei LY, Raj SR, Sheldon RS. Midodrine for the prevention of vasovagal syncope: a systematic review and meta-analysis. Europace. (2022) 24(7):1171–8. doi: 10.1093/europace/euab323
7. Barón-Esquivias G, Cayuela A, Gómez S, Aguilera A, Campos A, Fernández M, et al. Quality of life in patients with vasovagal syncope. Clinical parameters influence. Med Clin. (2003) 121(7):245–9. doi: 10.1016/s0025-7753(03)75188-4
8. Ng J, Sheldon RS, Ritchie D, Raj V, Raj SR. Reduced quality of life and greater psychological distress in vasovagal syncope patients compared to healthy individuals. Pace. (2018) 42(2):180–8. doi: 10.1111/pace.13559
9. Jorge JG, Pournazari P, Raj SR, Maxey C, Sheldon RS. Frequency of injuries associated with syncope in the prevention of syncope trials. Europace. (2020) 22(12):1896–903. doi: 10.1093/europace/euaa246
10. Jorge JG, Raj SR, Teixeira PS, Teixeira JAC, Sheldon RS. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. (2021) 23(7):1092–9. doi: 10.1093/europace/euab041
11. Mosqueda-Garcia R, Furlan R, Tank J, Fernandez-Violante R. The elusive pathophysiology of neurally mediated syncope. Circulation. (2000) 102(23):2898–906. doi: 10.1161/01.cir.102.23.2898
12. Adkisson WO, Benditt DG. Pathophysiology of reflex syncope: a review. J Cardiovasc Electrophysiol. (2017) 28(9):1088–97. doi: 10.1111/jce.13266
13. van Dijk JG, van Rossum IA, Thijs RD. The pathophysiology of vasovagal syncope: novel insights. Auton Neurosci. (2021) 236:102899. doi: 10.1016/j.autneu.2021.102899
14. Kawano H, Okada R, Yano K. Histological study on the distribution of autonomic nerves in the human heart. Heart Vessels. (2003) 18(1):32–9. doi: 10.1007/s003800300005
15. Wieling W, Jardine DL, de Lange FJ, Brignole M, Nielsen HB, Stewart J, et al. Cardiac output and vasodilation in the vasovagal response: an analysis of the classic papers. Heart Rhythm. (2015) 13(3):798–805. doi: 10.1016/j.hrthm.2015.11.023
16. Zheng L, Sun W, Liu S, Liang E, Du Z, Guo J, et al. The diagnostic value of cardiac deceleration capacity in vasovagal syncope. Circ Arrhythm Electrophysiol. (2020) 13(12):e008659. doi: 10.1161/CIRCEP.120.008659
17. Lee SH, Yang JH, Yim HR, Park J, Park SJ, Park KM, et al. Hemodynamic parameters and baroreflex sensitivity during head-up tilt test in patients with neurally mediated syncope. Pace. (2017) 40(12):1454–61. doi: 10.1111/pace.13217
18. Yao Y, Shi R, Wong T, Zheng L, Chen W, Yang L, et al. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: an early experience in humans. Circ Arrhythm Electrophysiol. (2012) 5(2):279–86. doi: 10.1161/CIRCEP.111.966465
19. Sun W, Zheng L, Qiao Y, Shi R, Hou B, Wu L, et al. Catheter ablation as a treatment for vasovagal syncope: long-term outcome of endocardial autonomic modification of the left atrium. J Am Heart Assoc. (2016) 5(7):e003471. doi: 10.1161/JAHA.116.003471
20. Scanavacca M, Hachul D, Pisani C, Sosa E. Selective vagal denervation of the sinus and atrioventricular nodes, guided by vagal reflexes induced by high frequency stimulation, to treat refractory neurally mediated syncope. J Cardiovasc Electrophysiol. (2008) 20(5):558–63. doi: 10.1111/j.1540-8167.2008.01385.x
21. Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. (2009) 30(21):2631–71. doi: 10.1093/eurheartj/ehp298
22. Onishi Y, Minoura Y, Chiba Y, Onuki T, Ito H, Adachi T, et al. Daily dysfunction of autonomic regulation based on ambulatory blood pressure monitoring in patients with neurally mediated reflex syncope. Pace. (2015) 38(8):997–1004. doi: 10.1111/pace.12661
23. Bauer A, Kantelhardt JW, Barthel P, Schneider R, Mäkikallio T, Ulm K, et al. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet. (2006) 367(9523):1674–81. doi: 10.1016/S0140-6736(06)68735-7
24. Sutton R, Fedorowski A, Olshansky B, Gert van Dijk J, Abe H, Brignole M, et al. Tilt testing remains a valuable asset. Eur Heart J. (2021) 42(17):1654–60. doi: 10.1093/eurheartj/ehab084
25. Glockler A, Cismaru G, Istratoaie S, Gusetu G, Zdrenghea D, Pop D, et al. What is the optimal duration of the TILT after administration of 0.4 mg nitroglycerin spray? Medicine. (2020) 99(11):e19510. doi: 10.1097/MD.0000000000019510
26. Rivasi G, Torabi P, Secco G, Ungar A, Sutton R, Brignole M, et al. Age-related tilt test responses in patients with suspected reflex syncope. Europace. (2021) 23(7):1100–5. doi: 10.1093/europace/euab024
27. Pitzalis M, Parati G, Massari F, Guida P, Di Rienzo M, Rizzon B, et al. Enhanced reflex response to baroreceptor deactivation in subjects with tilt-induced syncope. J Am Coll Cardiol. (2003) 41(7):1167–73. doi: 10.1016/s0735-1097(03)00050-0
28. Mizera L, Rath D, Schoellmann A, Petersen-Uribe A, Avdiu A, Zdanyte M, et al. Deceleration capacity is associated with acute respiratory distress syndrome in COVID-19. Heart Lung. (2021) 50(6):914–8. doi: 10.1016/j.hrtlng.2021.07.016
29. Carricarte Naranjo C, Marras C, Visanji NP, Cornforth DJ, Sanchez-Rodriguez L, Schüle B, et al. Short-term deceleration capacity of heart rate: a sensitive marker of cardiac autonomic dysfunction in idiopathic Parkinson’s disease. Clin Auton Res. (2021) 31(6):729–36. doi: 10.1007/s10286-021-00815-4
30. Duckheim M, Gaebler M, Mizera L, Schreieck J, Poli S, Ziemann U, et al. Deceleration capacity for rapid risk stratification in patients suffering from acute ischemic stroke: a prospective exploratory pilot study. Medicine. (2021) 100(13):e25333. doi: 10.1097/MD.0000000000025333
31. Bauer A, Deisenhofer I, Schneider R, Zrenner B, Barthel P, Karch M, et al. Effects of circumferential or segmental pulmonary vein ablation for paroxysmal atrial fibrillation on cardiac autonomic function. Heart Rhythm. (2006) 3(12):1428–35. doi: 10.1016/j.hrthm.2006.08.025
32. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The consensus committee of the American Autonomic Society and the American Academy of Neurology. Neurology. (1996) 46(5):1470. doi: 10.1212/wnl.46.5.1470
33. Kurbaan AS, Bowker TJ, Wijesekera N, Franzén AC, Heaven D, Itty S, et al. Age and hemodynamic responses to tilt testing in those with syncope of unknown origin. J Am Coll Cardiol. (2003) 41(6):1004–7. doi: 10.1016/s0735-1097(02)02967-4
34. Brignole M, Menozzi C, Del Rosso A, Costa S, Gaggioli G, Bottoni N, et al. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal syncope international study. Europace. (2000) 2(1):66–76. doi: 10.1053/eupc.1999.0064
35. Pencina MJ, D'Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. (2010) 30(1):11–21. doi: 10.1002/sim.4085
36. Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. (1996) 17(3):354–81. doi: 10.1093/oxfordjournals.eurheartj.a014868
37. Ganzeboom KS, Mairuhu G, Reitsma JB, Linzer M, Wieling W, van Dijk N. Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35-60 years. J Cardiovasc Electrophysiol. (2006) 17(11):1172–6. doi: 10.1111/j.1540-8167.2006.00595.x
38. Gampa A, Upadhyay GA. Treatment of neurocardiogenic syncope: from conservative to cutting-edge. J Innov Card Rhythm Manag. (2018) 9(7):3221–31. doi: 10.19102/icrm.2018.090702
39. Boehm K, Duckheim M, Mizera L, Groga-Bada P, Malek N, Kreth F, et al. Heart rate variability for rapid risk stratification of emergency patients with malignant disease. Support Care Cancer. (2018) 26(9):3289–96. doi: 10.1007/s00520-018-4144-y
40. Harris PR, Stein PK, Fung GL, Drew BJ. Heart rate variability measured early in patients with evolving acute coronary syndrome and 1-year outcomes of rehospitalization and mortality. Vasc Health Risk Man. (2014) 10:451–64. doi: 10.2147/VHRM.S57524
41. Ong ME, Goh K, Fook-Chong S, Haaland B, Wai KL, Koh ZX, et al. Heart rate variability risk score for prediction of acute cardiac complications in ED patients with chest pain. Am J Emerg Med. (2013) 31(8):1201–7. doi: 10.1016/j.ajem.2013.05.005
42. Zuern CS, Rizas KD, Eick C, Vogtt MI, Bigalke B, Gawaz M, et al. Severe autonomic failure as a predictor of mortality in aortic valve stenosis. Int J Cardiol. (2014) 176(3):782–7. doi: 10.1016/j.ijcard.2014.07.088
43. Ahmad S, Ramsay T, Huebsch L, Flanagan S, McDiarmid S, Batkin I, et al. Continuous multi-parameter heart rate variability analysis heralds onset of sepsis in adults. PLoS One. (2009) 4(8):e6642. doi: 10.1371/journal.pone.0006642
44. Chen WL, Chen JH, Huang CC, Kuo CD, Huang CI, Lee LS. Heart rate variability measures as predictors of in-hospital mortality in ED patients with sepsis. Am J Emerg Med. (2008) 26(4):395–401. doi: 10.1016/j.ajem.2007.06.016
45. Poreba M, Poreba R, Gac P, Usnarska-Zubkiewicz L, Pilecki W, Piotrowicz E, et al. Heart rate variability and heart rate turbulence in patients with hematologic malignancies subjected to high-dose chemotherapy in the course of hematopoietic stem cell transplantation. Ann Noninvasive Electrocardiol. (2013) 19(2):157–65. doi: 10.1111/anec.12108
46. Zhou X, Ma Z, Zhang L, Zhou S, Wang J, Wang B, et al. Heart rate variability in the prediction of survival in patients with cancer: a systematic review and meta-analysis. J Psychosom Res. (2016) 89:20–5. doi: 10.1016/j.jpsychores.2016.08.004
47. Arab C, Dias DP, Barbosa RT, Carvalho TD, Valenti VE, Crocetta TB, et al. Heart rate variability measure in breast cancer patients and survivors: a systematic review. Psychoneuroendocrinology. (2016) 68:57–68. doi: 10.1016/j.psyneuen.2016.02.018
48. Malik M, Bigger JT, Camm AJ, Kleiger RE, Malliani A, Moss AJ, et al. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. (1996) 17(3):354–81. doi: 10.1093/oxfordjournals.eurheartj.a014868
49. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:258. doi: 10.3389/fpubh.2017.00258
50. Shaffer F, McCraty R, Zerr CL. A healthy heart is not a metronome: an integrative review of the heart’s anatomy and heart rate variability. Front Psychol. (2014) 5:1040. doi: 10.3389/fpsyg.2014.01040
51. Petix NR, Del Rosso A, Furlan R, Guarnaccia V, Zipoli A. Nitrate-potentiated head-up tilt testing (HUT) has a low diagnostic yield in patients with likely vasovagal syncope. Pace. (2013) 37(2):164–72. doi: 10.1111/pace.12235
52. Ungar A, Sgobino P, Russo V, Vitale E, Sutton R, Melissano D, et al. Diagnosis of neurally mediated syncope at initial evaluation and with tilt table testing compared with that revealed by prolonged ECG monitoring. An analysis from the third international study on syncope of uncertain etiology (ISSUE-3). Heart. (2013) 99(24):1825–31. doi: 10.1136/heartjnl-2013-304399
53. Ruiz GA, Madoery C, Arnaldo F, Menéndez C, Tentori MC. Frequency-domain analysis of heart rate variability during positive and negative head-up tilt test: importance of age. Pace. (2000) 23(3):325–32. doi: 10.1111/j.1540-8159.2000.tb06757.x
Keywords: DC, HRV, SDNN, TTT, VVS
Citation: Guo Y, Lin T, Lin N and Lin H (2024) Effectiveness analysis of deceleration capacity and traditional heart rate variability in diagnosing vasovagal syncope. Front. Cardiovasc. Med. 11:1333684. doi: 10.3389/fcvm.2024.1333684
Received: 5 November 2023; Accepted: 13 August 2024;
Published: 3 September 2024.
Edited by:
Pietro Enea Lazzerini, University of Siena, ItalyReviewed by:
Emanuela Teresina Locati, IRCCS San Donato Polyclinic, ItalyBratislav Kircanski, University of Belgrade, Serbia
Copyright: © 2024 Guo, Lin, Lin and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huizhong Lin, bGluaHVpemhvbmdiYmNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yongzhe Guo1,†
Yongzhe Guo1,† Huizhong Lin
Huizhong Lin