- 1Department of Clinical Medicine, Xi’an Medical University, Xi’an, Shannxi, China
- 2Department of the Project of Prevention and Treatment of Respiratory Diseases, Xi’an Medical University, Xi’an, Shannxi, China
- 3Department of Emergency, Hanjiang Hospital Affiliated to Xi'an Medical University, Hanzhong, Shannxi, China
Introduction: There exists a knowledge gap concerning the clinical significance of miRNA-21; therefore, in the present study, we aimed to estimate the diagnostic and prognostic accuracy and sensitivity of miRNA-21 in acute myocardial infarction (AMI) by performing an evidence-based meta-analysis of previous AMI-related clinical studies.
Methods: Chinese and English literature published before April 2024 were searched, and data were reviewed and extracted. After quality appraisal, the STATA 16.0 software was used for the effect size analysis of the various treatments described in the literature.
Results: A total of 14 valid documents were retrieved from 562 studies. The results of the systematic review revealed that for the patients with AMI vs. those without non-AMI, the aggregated odds ratio reached 5.37 (95% confidence interval 3.70–7.04). The general sensitivity and specificity for the circulating miRNA-21 levels in diagnosing AMI were 0.83 and 0.81, respectively.
Discussion: Thus, the meta-analysis of 14 AMI-related clinical trials highlighted that miRNA-21 may serve as a promising biomarker for diagnosing AMI.
Introduction
Coronary artery disease (CAD) is one of the leading fatal clinical threats around the world (1). The prevalence of myocardial infarction is 4.0% in the United States and 3.1% in the UK (2, 3). Diagnosing acute myocardial infarction (AMI) earlier and predicting the severity of myocardial necrosis as early as possible are hot topics in cardiovascular research. Coronary angiography, being the gold standard for the definitive diagnosis of AMI stenosis, requires strict conditions, as it is an invasive procedure. For the early diagnosis of AMI, guidelines advocate the dynamic monitoring of electrocardiograph (ECG) and markers of myocardial necrosis, such as cardiac troponin I (cTnI) and creatine kinase-MB (CK-MB), which are highly precise and specific (1–3).
miRNAs are a highly conserved subset of small noncoding RNAs (4); they play an integral role in suppressing the inflammatory reactivity of atherosclerotic plaques, improving endothelial cell function and cardiac metabolism, alleviating ischaemia, and other pathophysiological activities. Especially, miRNA-21 is considered to be closely related to myocardial injury (4–6).
In ischaemia–reperfusion injury, knockdown of miRNA-21 in mice exacerbates aldosterone-mediated cardiac hypertrophy and injury (7–9). In reperfused myocardium and during post-infarction remodelling, miRNA-21 protects myocytes from ischaemia/reperfusion-induced apoptosis. Moreover, miRNA-21 levels were found to be elevated in tissues post-infarction and miRNA-21 was confirmed to promote collagen fibril secretion by fibroblasts, which not only specifically regulates the repair of vascular injury, but also effectively regulates myocyte metabolic activity, which in turn, affects the prognosis of patients with AMI (10, 11).
Several investigations have revealed that miRNA-21 is expressed at high circulatory levels in CAD patients and that its levels are correlated with the degree of vascular stenosis (12). Meanwhile, the expression of miRNA-21 varies among different types of anginas and myocardial infarction, suggesting that differences in its expression profiles can also predict disease and the stage of disease development. Furthermore, miRNAs are likely to serve as potential therapeutic targets for heart disease (9, 13). Since many randomised controlled trials could not be performed due to diverse differences in disease characteristics, in the present study, we used an evidence-based approach to effectively combine the influence of miRNA-21 expression levels reported in different AMI-based clinical studies and performed a meta-analysis to investigate the accuracy and specificity of miRNA-21 for diagnosing AMI, in comparison with other markers, such as cTnI and CK-MB.
Methods
Selection procedures
In accordance with the PRISMA guidelines (14), Pubmed, Embase, and Web of SCI in English and Chinese National Knowledge Infrastructure (CNKI), Wanfang Database in Chinese were searched from the time of their creation until April 2024. Search-term were as follows: (“circulating”or “blood” or “plasma” or “serum”) and (“micro RNA 21” or “miR-21” or “miRNAs”) and (“AMI” or “coronary artery disease” or “acute myocardial infarction” or “schematic heart disease”).
The inclusion criteria for the literature reviewed here are as follows: a. The selected literature must focus on human studies. b. The selected literature must be related to research regarding the circulating miRNA-21 levels and AMI. c. The selected literature must provide adequate records to enable an estimation of the predictive and metabolic status of miRNA-21 in AMI (1).
The exclusion criteria for the literature reviewed here are as follows: a. The literature that does not provide sufficient or applicable data, including case reports, literature reviews, meetings, correspondence letters, editorials, and conference abstracts. b. The articles that are not written in English or Chinese. c. The literature unrelated to AMI. d. The articles published repeatedly, as well as those with small sample size and incomplete information.
Data extraction and quality-assessment
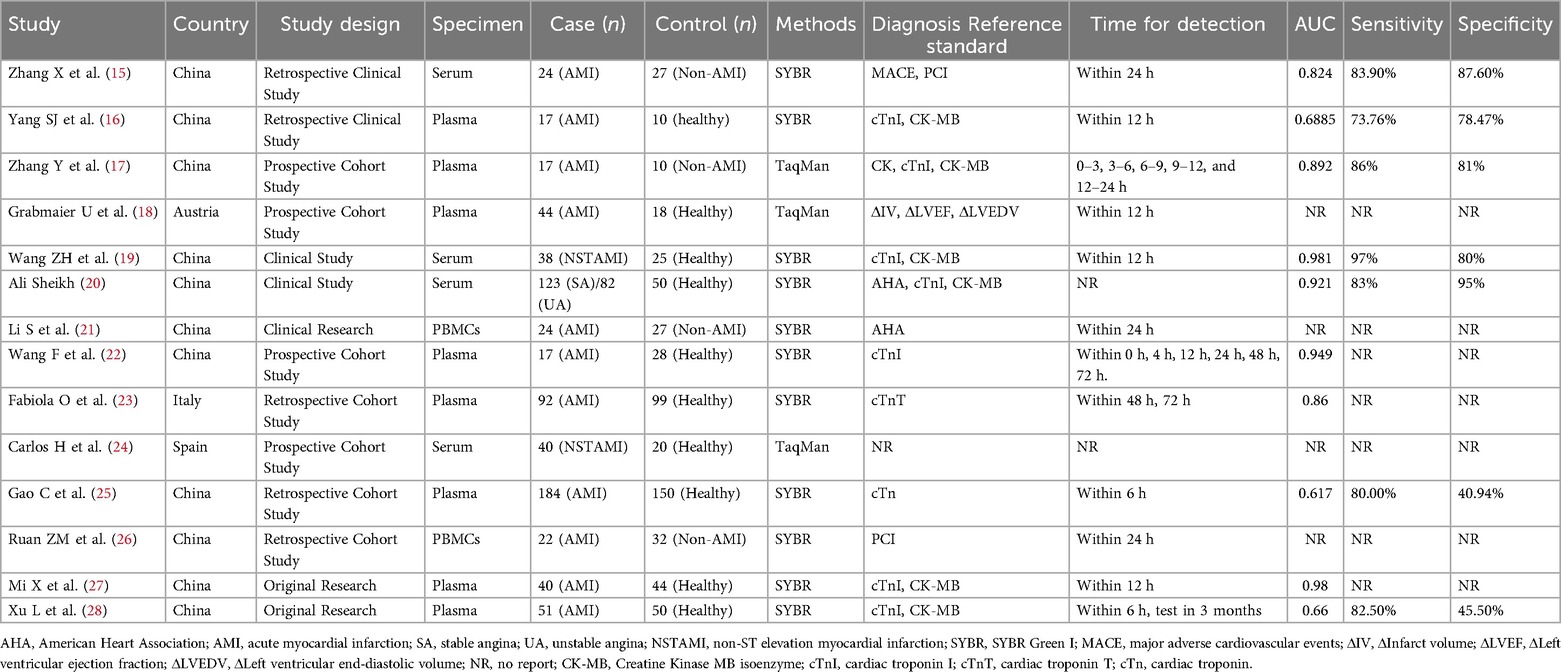
Two researchers reviewed the titles and abstracts of articles eligible for the review in this study and then extracted the relevant data and information independently. When there were disagreements, they were resolved via discussions between the two researchers or with assistance from a third staff member. Based on the previously mentioned inclusion and exclusion criteria, 14 articles (15–28) were identified for a full-text review. The extracted research records derived included information on original sponsor, years and issues, cases numbers, controls, the determination of AMI, the type of specimen and blood sampling time required for diagnosis, the sensitivity and specificity ratings, the AUC values, and the miRNA-21-detection methods. The risk of bias and applicability concerns were checked using QUADAS-2. The process includes the below four critical fields: patient selection, index detection, reference standards, and the process and timeline. Risk of bias was assessed for each domain and applicability was assessed for the first three domains in Revaman 5.3 software. Researches were classified as being at low, high or unclear risk.
Statistical analysis
A meta-analysis summarising the research findings was performed. Meanwhile, more than three papers were scrutinised to forecast the predictive and metabolic significance of miRNA-21 levels for diagnosing AMI. The STATA 16.0 software was utilised to perform the data analyses. To calculate the ensemble the collective odds ratios, along with the 95% confidence interval, via fixed or random-effects models, we used the DerSimonian-Laird (D-L) or Inverse-Variance (I-V) statistical approaches. If the heterogeneity test result is P > 0.10, multiple studies were homogeneous, as fixed effects model of Inverse-Variance (I-V) could be selected. When P < 0.10, inclusive studies were checked for heterogeneity using the D-L method, and analyzed sources of heterogeneity by sub-group. For assessing the accuracy of miRNA-21 in diagnosing AMI, a summary of the sensitivity, specificity, positive likelihood ratio (PLR), and negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were calculated,which indicated the accuracy of microRNAs in the differentiation of AMI and no AMI, were calculated from the TP, FP, FN, and TN. For I2 > 50% or p < 0.05, the bivariate summary receiver operator characteristic (SROC) curve showed a downward trend, after which the AUC could be combined to ascertain the accuracy of miRNA-21 for diagnosing AMI, relative to the controls.
Results
Literature search results
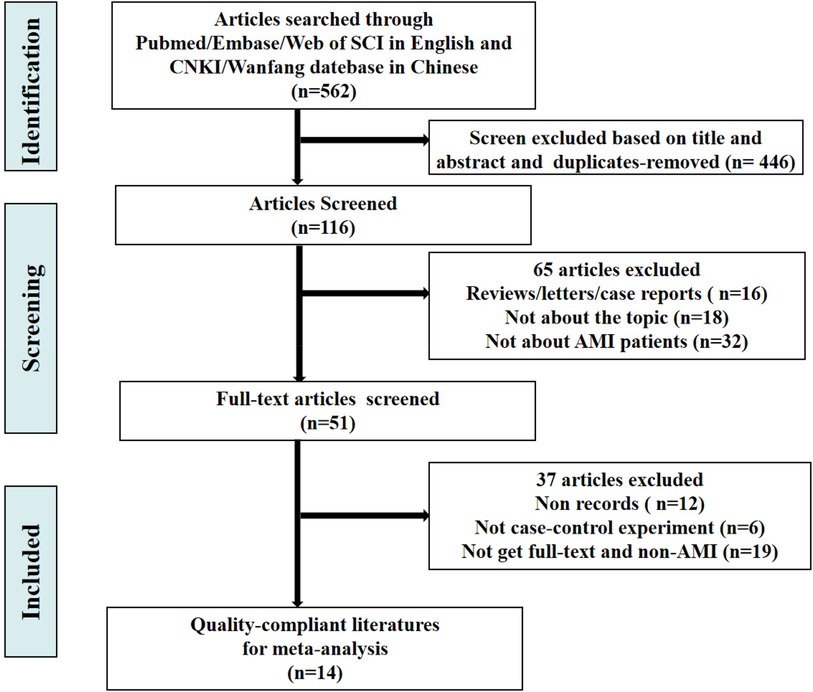
The retrieval process of original literature is presented in Figure 1. Based on the criteria for literature inclusion and exclusion, we got 562 records for miRNA-21, with 165 in duplicates -removed and 281 not for AMI clinical experiments or be without no full test then removed after title and abstract reading (n = 446), 116 articles were screened in full-text in English and Chinese publications regarding miRNA-21. In the end, 14 articles were included (14–25), the basic information of which is shown in Table 1. The risk bias was calculated using the Cochrane bias-risk tool and is presented in Figure 2.
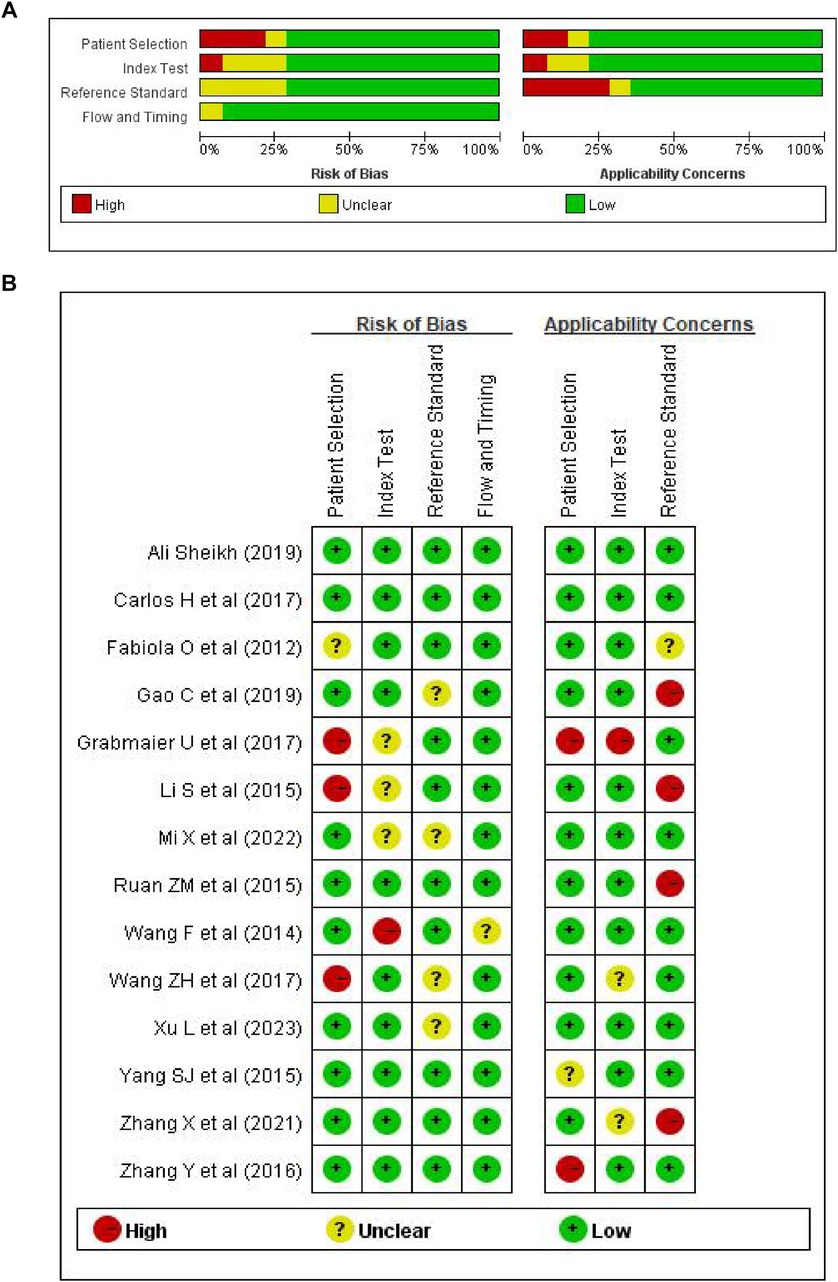
Figure 2. Risk of bias graph. (A) Review authors’ assessment of each risk of bias as percentage of all included studies; (B) risk of bias summary.
There included 1,405 patients in this article, as 815 AMI patients and 590 control groups (494 healthy controls, 96 non-AMI patients). Three kinds of specimen (Serum, Plasma, peripheral blood mononuclear cells-PBMCs) were in inspection in 14 records. MiRNA-21 test methods were SYBR Green for PCR in 11 articles and TaqMan MicroRNA Assays in 3 articles. 11 articles for patients in China and 3 in Austria/Italy/Spain.3 articles in Chinese languages and 11 in English.
The diagnostic accuracy of miRNA-21
Seven studies have detailed the sensitivity and specificity of miRNA-21 in the diagnosis of AMI, among which the paper by Ali Sheikh et al. (2019) categorized the population into Stable Myocardial Infarction (S) and Unstable Myocardial Infarction (US), while the study by Gao et al. (2019) further identified a sub-group of Deceased (D). The accuracy of miRNA-21 for diagnosing AMI was assessed (Figure 3-1). The levels of circulating miRNA-21 demonstrated an overall sensitivity of 0.83 (95% CI, 0.80–0.86) and a specificity of 0.81 (95% CI: 0.66–0.90).
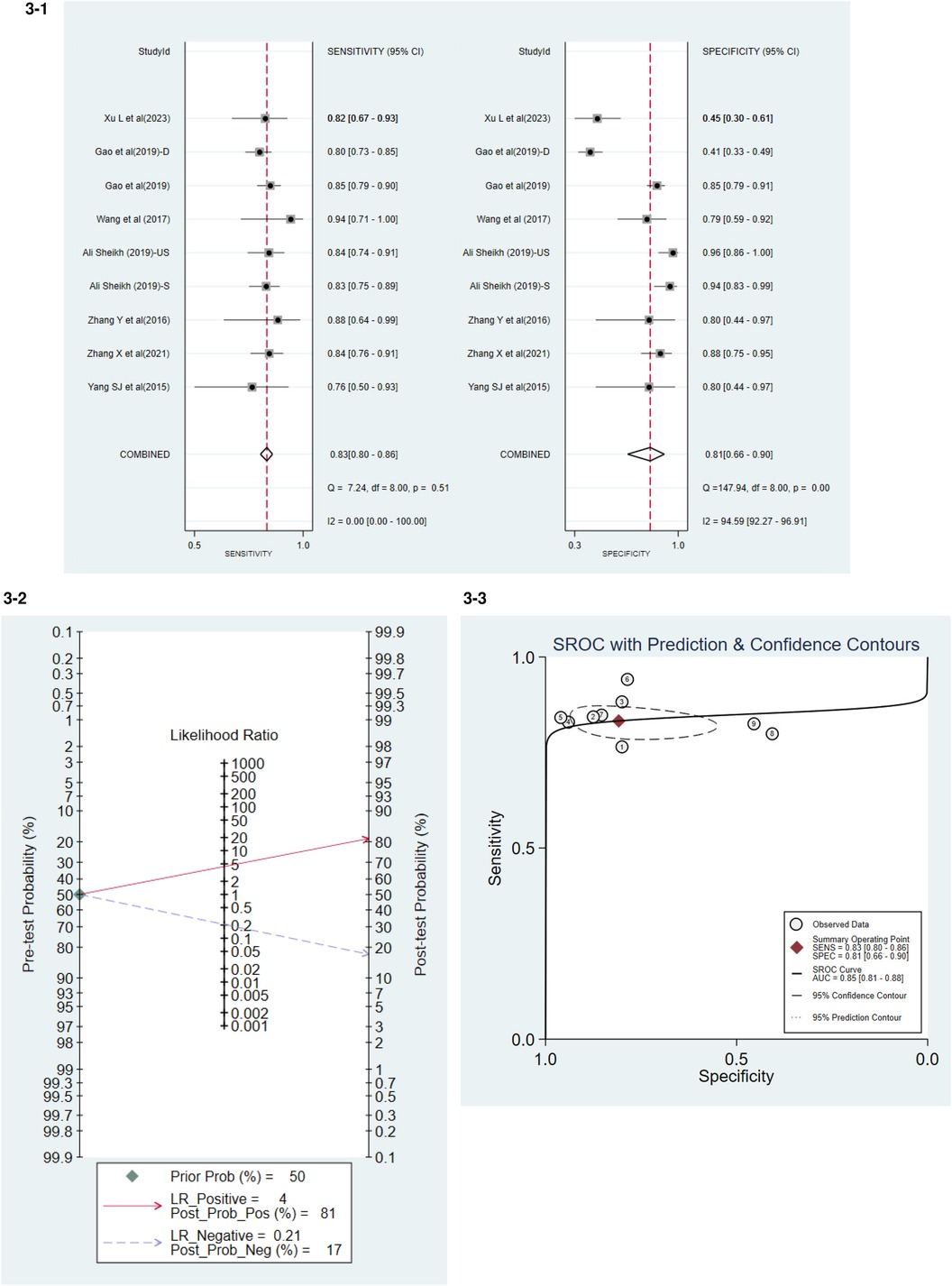
Figure 3. 3-1 Accuracy of miRNA-21 for diagnosing AMI. 3-2 Positive and Negative Likelihood Ratio. 3-3 Summary Receiver Operator Curve (SROC).
The Positive Likelihood Ratio (PLR) was 4, and the Negative Likelihood Ratio (NLR) was 0.21 (Figure 3-2). PLR was the ratio of true positives to false positives. The higher the ratio, the more likely the test result was a true positive if positive. The smaller the ratio of NLR, the more likely the test, if negative, will be true negative.
The combined weighted area under the Summary Receiver Operating Characteristic curve (SROC) was 0.85 (95% CI, 0.81–0.88) (Figure 3-3).
Publication bias
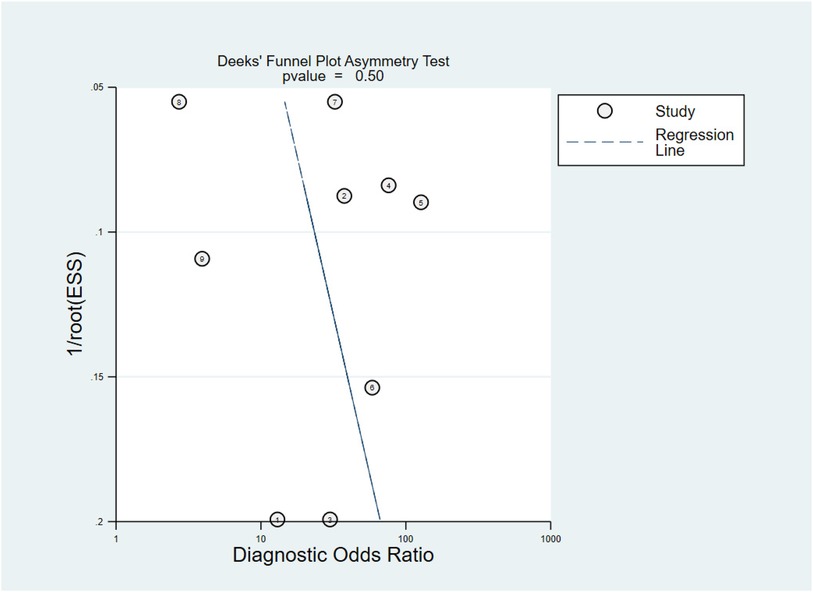
To examine the potential publication bias, Deeks’ Funnel Plot Asymmetry Test was performed. P value < 0.10 were used to judge asymmetry when the number of studies was small. The p-value was found to be 0.50, which implies that the publication bias was moderate (Figure 4).
Summary of findings
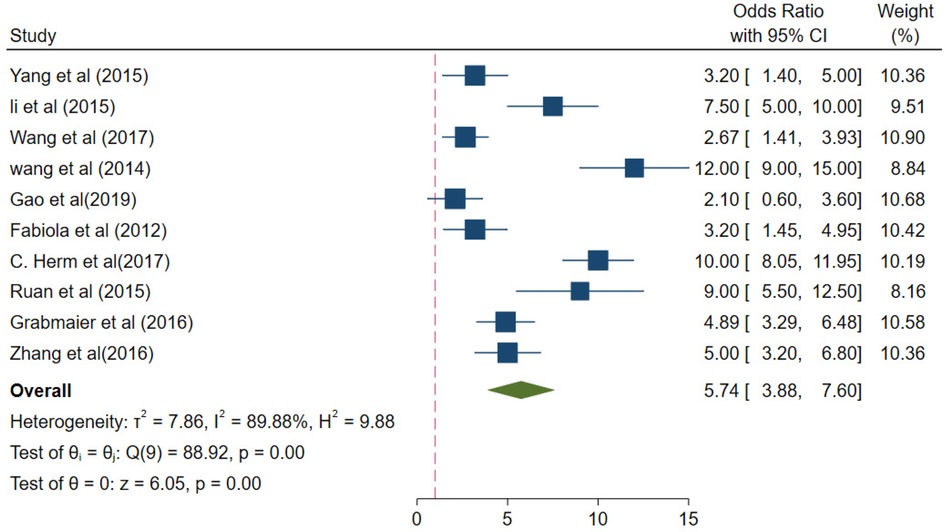
In the patient group, the collective odds ratio (OR) of the miRNA-21 levels was 5.74 (95% CI 3.88–7.60), compared with the control group (Figure 5). The I2 value was 89.88%, and the p-value < 0.01; there was a high degree of heterogeneity in the results from different studies. Therefore, the sources of heterogeneity were investigated through subgroup analysis.
Results of the subgroup meta-analysis
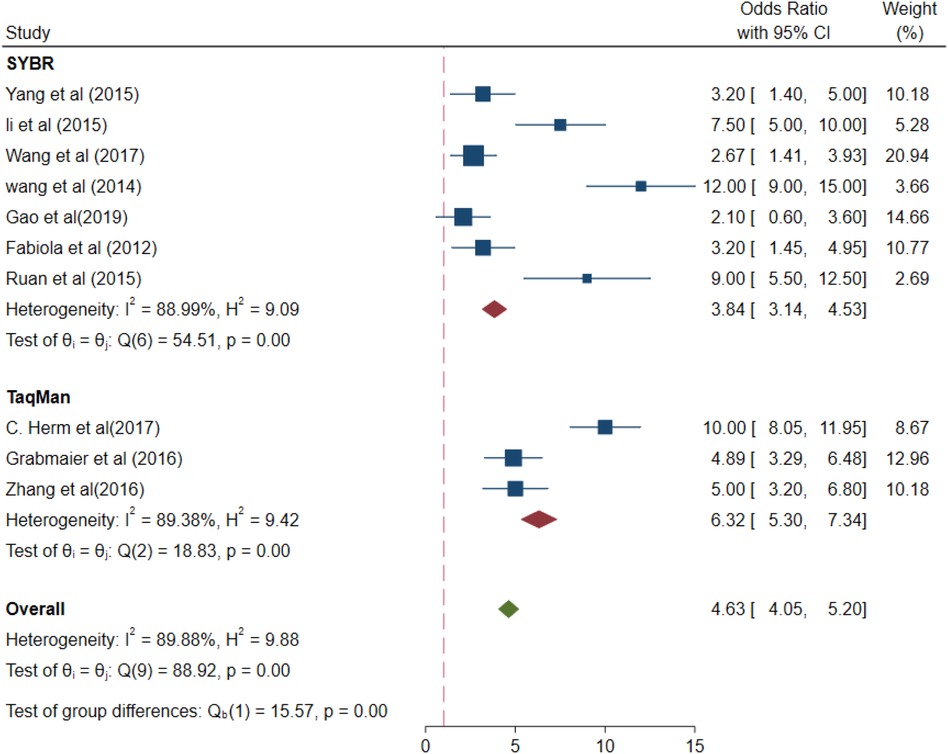
A subgroup meta-analysis was performed based on the outcomes of the meta-regression analysis. The values of different parameters for the miRNA-21 levels measured using different detection methods (SYBR and Taqman assays) were for the SYBR method (OR 3.84, 95% CI 3.14–4.53) and I2 = 88.99% (p < 0.01), with for the Taqman method (OR 6.32, 95% CI 5.30–7.34) and I2 = 89.38% (p < 0.01) (Figure 6). Taqman was preferable to SYBR.
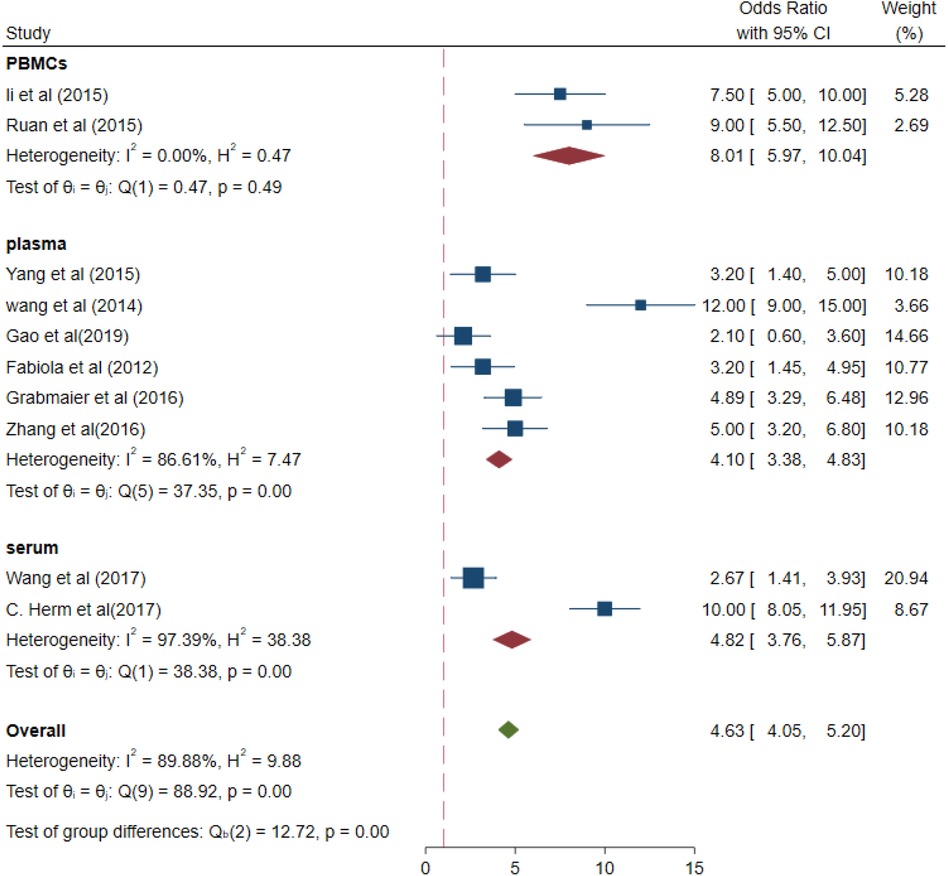
The values of different parameters for the miRNA-21 expression levels in samples were for PBMCs samples (OR 8.01, 95% CI 5.97–10.04), I2 = 0%, p = 0.49 > 0.01; for plasma (OR 4.10, 95% CI 3.38–4.83), I2 = 86.61% (p < 0.01); and for serum samples (OR, 4.82, 95% CI 3.76–5.87), I2 = 97.39% (p < 0.01; Figure 7). Increased- heterogeneity arises from different assays.
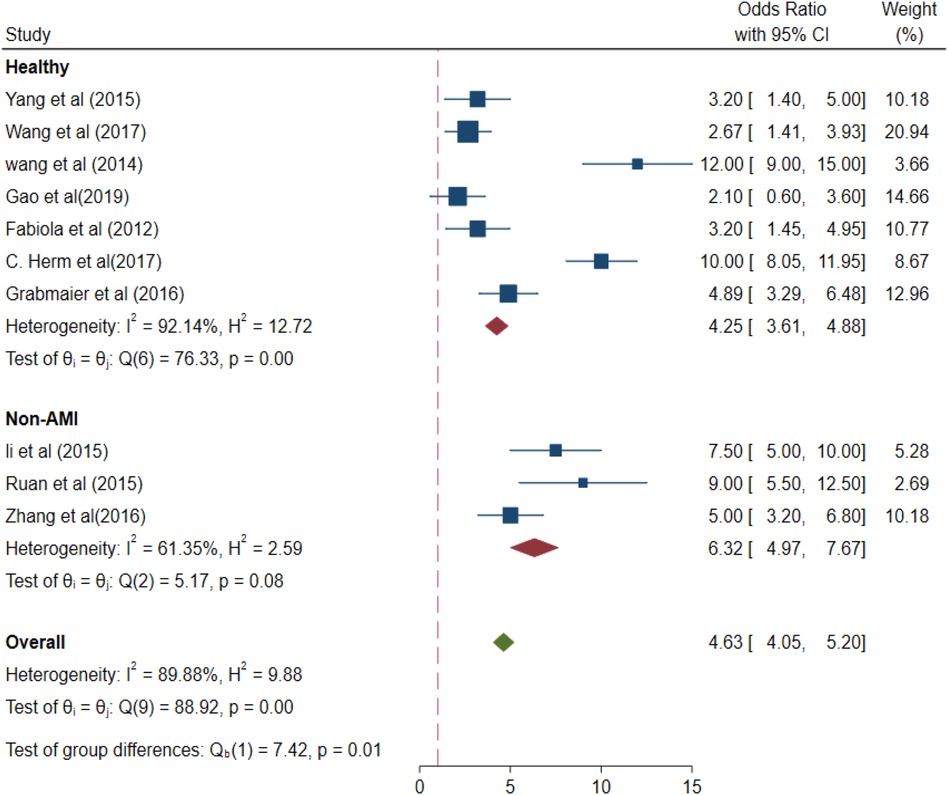
The values of different parameters for the miRNA-21 expression levels in the control group based on the selection differences were as follows: healthy control group (OR 4.25, 95% CI 3.61–4.88) and non-AMI group for control (pooled OR 6.32, 95% CI 4.97–7.67), p < 0.01 (Figure 8).
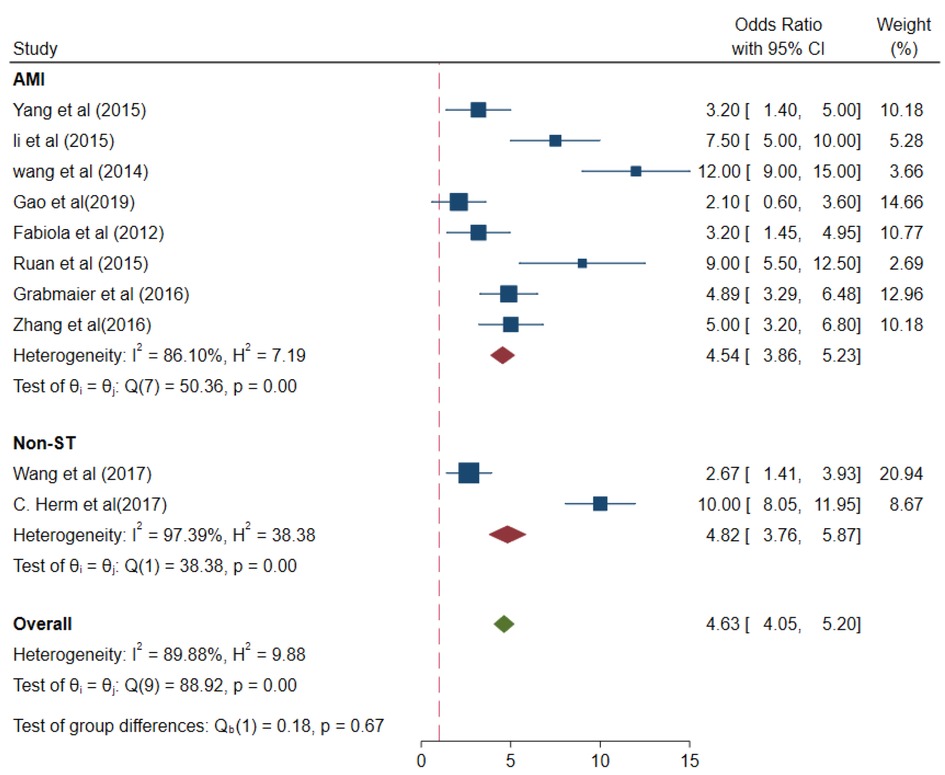
The values of different parameters for the miRNA-21 expression levels in the case group were as follows: AMI cases, (pooled OR 4.54, 95% CI 3.86–5.23); I2 = 86.1%; and p < 0.01 and non-ST-elevation myocardial infarction (NSTEMI) cases, (pooled OR 4.82, 95% CI 3.76–5.87); I2 = 97.39%; and p < 0.01 (Figure 9).
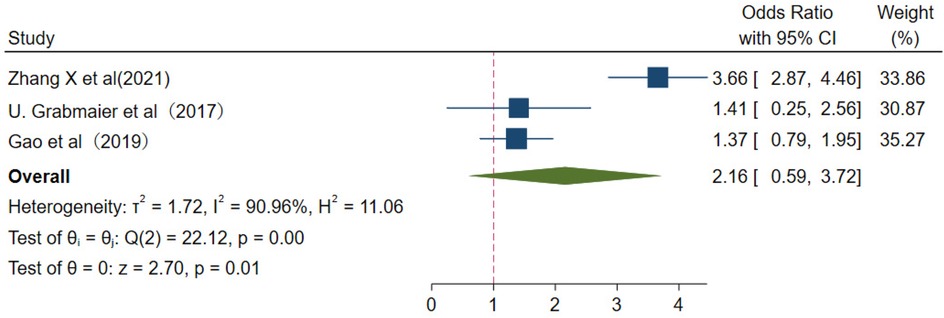
From major adverse cardiovascular events, the values were pooled OR 2.61 (95% CI 0.59–3.72); I2 = 90.96%; and p < 0.01 (Figure 10).
Discussion
Studies have revealed that miRNA-21 has strong sensitivity and specificity as a predictive and diagnostic indicator for AMI. In serum and plasma, the miRNA-21 levels were remarkably elevated, compared with those in the controls. A serious cardiovascular disease, AMI shows a rapid onset and a high risk of mortality (29, 30). Thus, the early diagnosis and treatment of AMI has become an urgent need in recent years.
In the present study, the area under the curve (AUC) showed that miRNA-21 may serve as a reliable diagnostic indicator for AMI. A PLR of 4 implies that a person with AMI is fourth times more likely to have a positive diagnosis than other patients. The NLR indicates that a person with AMI has 21% less chance of diagnosis if miRNA-21 is negative. These results strongly suggest that circulating miRNA-21 is a potential predictor.
As the generally recognized gold standard, circulation cTnI could be found until 3–6 h after AMI, CK-MB could be detected until 6 h when AMI (31). There were constraint in on-time predictive and diagnostic functions. Furthermore, chronic kidney diseases could also be along with cTnI raising-up (32), which desperate to seek for sensitive and highly-accuracy biomarker. MiRNA-21 could be detected within three hours in AMI patients, what's more, it's effective in assessing the degree of myocardial ischemia (31). Combinations with ECG and other biomarkers could be in consistent further studies (31, 32).
Different types of methods for the real-time quantification of miRNA-21 levels, i.e., fluorescence methods such as Taqman probe assay and SYBR Green assay revealed that the miRNA-21 levels were 6.32-fold and 3.84-fold higher, respectively, than those in the healthy controls. miRNAs are a small class of homologous molecules; some small miRNAs may be expressed at very low levels, due to which extremely sensitive and quantitative assays are required for their detection, Taqman probe assay and SYBR Green assay were general and highly sensitivity for miRNA-test in clinical laboratories (30). In plasma, and serum, the miRNA-21 levels were found to be 4.10- and 4.82-fold higher, respectively, than those in the control cases. Conversely, in different types of blood samples from AMI patients, the miRNA-21 levels were reported to be increased.
In the analysis of different subgroups relative to the control group, the miRNA-21 level in the AMI group was 4.25-fold higher than that in the healthy group, while compared with the non-AMI group for control, the miRNA-21 concentration was 6.32-fold higher. Thus, the circulating miRNA-21 level is a good diagnostic indicator for AMI in healthy individuals and those with a history of CAD. With regard to the experimental group, the miRNA-21 levels of unclassified AMI patients were 4.54-fold higher than those in the controls. The miRNA-21 levels in patients with NSTEMI miRNA-21 were 4.82-fold higher than those in the controls, implying that miRNA-21 is a diagnostic indicator that is very closely associated with NSTEMI.
Numerous studies (2, 15, 33, 34), have shown that plasma miRNA-21 levels can effectively assess the severity of ischaemia and show a high predictive accuracy for the prognosis of adverse cardiovascular events at different periods. Zhang X (15) found plasma miRNA-21 levels were higher in AMI patients who developed MACE at 1-year follow-up. In the study of Mi (27), miRNA-21 levels were increased in infarct-related artery total occlusion and or infarct related blood-vessel recanalization patients.Range researches (16, 28) showed it also a good indicator to predict vascular restenosis post-PCI in advance.
The miRNA-21 level has good diagnostic value for analysing AMI patients who received early revascularisation therapy. In addition, miRNA-21 exerts protective effects on ischaemia-reperfused myocardium. Yin (9) performed heat shock pretreatment in a mouse model in vivo, and found that several miRNAs, including miRNA-21, could reduce the infarct size. Weber (10) found that reducing the degree of apoptosis via miRNA-21 over-expression improved endothelial function and slowed down the progression of atherosclerosis. A high level of miR-21 expression may promote inflammatory aggregation, induce apoptosis of cardiomyocytes and worsen the progression of myocardial fibrosis,making it the best target for specific diagnosis and treatment of CVD (35).
As a biomarker in the early stage of AMI, miRNA 21 may be superior to cardiac troponin (cTn) I. miRNA 21 could be detected in the plasma of all patients within 4 h of symptom onset, as cTnI detected in only 85% patients. The ECG parameters were combined with the peripheral blood miR-21 provide a reference for the clinical diagnosis of Acute Myocardial Infarction (18, 28). There is an urgent need for more clinical studies on the diagnostic efficacy of combined tests.
Despite the small bias and strong sensitivity and specificity, this study has a few limitations: (1) there is a gap with regard to the results of evidence-based clinical studies and randomised controlled trials; (2) the time of blood collection was not uniform in various studies; and (3) the inclusion criteria for the diagnosis of AMI in clinical trials were not uniform, since some used percutaneous coronary intervention to determine the degree of stenosis and others used the levels of cardiac markers.
Conclusions and future perspectives
This study underscores that circulating the miRNA-21 level shows great potential possibility to serve as a reliable biomarker for the clinical diagnosis of AMI. In addition to the diagnostic gold standards for AMI diagnosis, i.e., the levels of cTnI, CK-MB, and other cardiac enzymes, to confirm the utility of miRNA-21 as a diagnostic indicator of AMI, we need large scale and rigorous clinical trials to validate the changes in the circulating levels of miRNA-21 in AMI patients and explore the specific biomedical mechanisms underlying these changes. Furthermore, whether the levels of different miRNAs can be combined or form a combined signature for increasing the diagnostic accuracy of AMI is yet to be ascertained.
Author contributions
KW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Writing – original draft. KL: Data curation, Methodology, Writing – review & editing. ZYL: Data curation, Methodology, Writing – review & editing. XZY: Conceptualization, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by Shaanxi Provincial Department of Science and Technology (2021JM-495), With The Project of Prevention and Treatment of Respiratory Diseases (2016HXKF08), and Xi'an Medical funding (2024HXZR07, 2021DXS48, 2016QN15).
Acknowledgments
We are grateful to the Xi'an key laboratory of Innovative and Translational Cancer Early Diagnosis, The Project of Prevention and Treatment of Respiratory Diseases, and Basic and Translational Innovation Team of Nepal Traditional Medicine Antitumor Research for their deep commitment.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. (2005) 352:1685–95. doi: 10.1056/NEJMra043430
2. Sundaram V, Bloom C, Zakeri R, Halcox J, Cohen A, Bowrin K, et al. Temporal trends in the incidence, treatment patterns, and outcomes of coronary artery disease and peripheral artery disease in the UK, 2006–2015. Eur Heart J. (2020) 41:1636–49. doi: 10.1093/eurheartj/ehz880
3. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. (2020) 141:e139–596. doi: 10.1161/CIR.0000000000000757
4. Bauersachs J, Thum T. Biogenesis and regulation of cardiovascular microRNAs. Circ Res. (2011) 109:334–47. doi: 10.1161/CIRCRESAHA.110.228676
5. Syed M, Ball JP, Mathis KW, Hall ME, Ryan MJ, Rothenberg ME, et al. MicroRNA-21 ablation exacerbates aldosterone-mediated cardiac injury, remodeling, and dysfunction. Am J Physiol Endocrinol Metab. (2018) 315:E1154–67. doi: 10.1152/ajpendo.00155.2018
6. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. (2020) 382:1395–407. doi: 10.1056/NEJMoa1915922
7. Zhu H, Fan GC. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc Res. (2012) 94:284–92. doi: 10.1093/cvr/cvr291
8. Ben-Nun D, Buja LM, Fuentes F. Prevention of heart failure with preserved ejection fraction (HFpEF): reexamining microRNA-21 inhibition in the era of oligonucleotide-based therapeutics. Cardiovasc Pathol. (2020) 49:107243. doi: 10.1016/j.carpath.2020.107243
9. Yin C, Salloum FN, Kukreja RC. A novel role of microRNA in late preconditioning: upregulation of endothelial nitric oxide synthase and heat shock protein 70. Circ Res. (2009) 104:572–5. doi: 10.1161/CIRCRESAHA.108.193250
10. Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. (2010) 393:643–8. doi: 10.1016/j.bbrc.2010.02.045
11. Dai B, Wang F, Nie X, Du H, Zhao Y, Yin Z, et al. The cell type-specific functions of miR-21 in cardiovascular diseases. Front Genet. (2020) 11:563166. doi: 10.3389/fgene.2020.563166
12. Sala V, Bergerone S, Gatti S, Gallo S, Ponzetto A, Ponzetto C, et al. MicroRNAs in myocardial ischemia: identifying new targets and tools for treating heart disease. New frontiers for miR-medicine. Cell Mol Life Sci. (2014) 71:1439–52. doi: 10.1007/s00018-013-1504-0
13. Kumar D, Narang R, Sreenivas V, Rastogi V, Bhatia J, Saluja D, et al. Circulatory miR-133b and miR-21 as novel biomarkers in early prediction and diagnosis of coronary artery disease. Genes (Basel). (2020) 11(2):164. doi: 10.3390/genes11020164
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
15. Zhang Xiao ZG, Qin S. Value of plasma microRNA-21 level in prediction of myocardial ischemia degree and evaluation of prognosis in patients with acute mvocardial infarction. J Clin Med Pract. (2021) 25:97–101+106.
16. Yang S-j, Zhang Y, Yin LU. Research on expression of miRNA-21 in the peripheral blood of coronary heart disease and its clinical significance. Chin J Appl Physiol. (2015) 31:127–31. PMID: 26248417.
17. Zhang Y, Liu YJ, Liu T, Zhang H, Yang SJ. Plasma microRNA-21 is a potential diagnostic biomarker of acute myocardial infarction. Eur Rev Med Pharmacol Sci. (2016) 20:323–9. PMID: 26875904.26875904
18. Grabmaier U, Clauss S, Gross L, Klier I, Franz WM, Steinbeck G, et al. Diagnostic and prognostic value of miR-1 and miR-29b on adverse ventricular remodeling after acute myocardial infarction - the SITAGRAMI-miR analysis. Int J Cardiol. (2017) 244:30–6. doi: 10.1016/j.ijcard.2017.06.054
19. Wang ZH, Sun XY, Li CL, Sun YM, Li J, Wang LF, et al. miRNA-21 expression in the serum of elderly patients with acute myocardial infarction. Med Sci Monit. (2017) 23:5728–34. doi: 10.12659/msm.904933
20. Ali Sheikh MS. Diagnostic role of plasma MicroRNA-21 in stable and unstable angina patients and association with aging. Cardiol Res Pract. (2020) 2020:9093151. doi: 10.1155/2020/9093151
21. Li S, Fan Q, He S, Tang T, Liao Y, Xie J. MicroRNA-21 negatively regulates treg cells through a TGF-β1/smad-independent pathway in patients with coronary heart disease. Cell Physiol Biochem. (2015) 37:866–78. doi: 10.1159/000430214
22. Wang F, Long G, Zhao C, Li H, Chaugai S, Wang Y, et al. Atherosclerosis-related circulating miRNAs as novel and sensitive predictors for acute myocardial infarction. PLoS One. (2014) 9:e105734. doi: 10.1371/journal.pone.0105734
23. Olivieri F, Antonicelli R, Lorenzi M, D'Alessandra Y, Lazzarini R, Santini G, et al. Diagnostic potential of circulating miR-499-5p in elderly patients with acute non ST-elevation myocardial infarction. Int J Cardiol. (2013) 167:531–6. doi: 10.1016/j.ijcard.2012.01.075
24. Hermenegildo C, Mompeon A, Januario T, Vidal-Gomez X, Pujol M, Perez-Cremades D, et al. Comparative analysis of mirna expression in serum and plasma of patients with acute myocardial infarction. J Hypertens. (2017) 35:e204. doi: 10.1097/01.hjh.0000523575.94981.9b
25. Gao C, Zhao D, Wang J, Liu P, Xu B. Clinical significance and correlation of microRNA-21 expression and the neutrophil-lymphocyte ratio in patients with acute myocardial infarction. Clinics (Sao Paulo). (2019) 74:e1237. doi: 10.6061/clinics/2019/e1237
26. Zhimin R. Correlationship between microRNA-21 and coronary heart disease. J Clin Cardiol. (2015) 31:50–3.
27. Mi XL, Gao YP, Hao DJ, Zhang ZJ, Xu Z, Li T, et al. Prognostic value of circulating microRNA-21-5p and microRNA-126 in patients with acute myocardial infarction and infarct-related artery total occlusion. Front Cardiovasc Med. (2022) 9:947721. doi: 10.3389/fcvm.2022.947721
28. Xu L, Tian L, Yan Z, Wang J, Xue T, Sun Q. Diagnostic and prognostic value of miR-486-5p, miR-451a, miR-21-5p and monocyte to high-density lipoprotein cholesterol ratio in patients with acute myocardial infarction. Heart Vessels. (2023) 38:318–31. doi: 10.1007/s00380-022-02172-2
29. Zhang L, Zhang Y, Zhao Y, Wang Y, Ding H, Xue S, et al. Circulating miRNAs as biomarkers for early diagnosis of coronary artery disease. Expert Opin Ther Pat. (2018) 28:591–601. doi: 10.1080/13543776.2018.1503650
30. Sheikh MS A, Alduraywish A, Almaeen A, Alruwali M, Alruwaili R, Alomair BM, et al. Therapeutic value of miRNAs in coronary artery disease. Oxid Med Cell Longev. (2021) 2021:8853748. doi: 10.1155/2021/8853748
31. Oerlemans MI, Mosterd A, Dekker MS, de Vrey EA, van Mil A, Pasterkamp G, et al. Early assessment of acute coronary syndromes in the emergency department: the potential diagnostic value of circulating microRNAs. EMBO Mol Med. (2012) 4:1176–85. doi: 10.1002/emmm.201201749
32. Kraus D, von Jeinsen B, Tzikas S, Palapies L, Zeller T, Bickel C, et al. Cardiac troponins for the diagnosis of acute myocardial infarction in chronic kidney disease. J Am Heart Assoc. (2018) 7:e008032. doi: 10.1161/JAHA.117.008032
33. Mompeón A, Ortega-Paz L, Vidal-Gómez X, Costa TJ, Pérez-Cremades D, Garcia-Blas S, et al. Disparate miRNA expression in serum and plasma of patients with acute myocardial infarction: a systematic and paired comparative analysis. Sci Rep. (2020) 10:5373. doi: 10.1038/s41598-020-61507-z
34. Kura B, Kalocayova B, Devaux Y, Bartekova M. Potential clinical implications of miR-1 and miR-21 in heart disease and cardioprotection. Int J Mol Sci. (2020) 21(3):700. doi: 10.3390/ijms21030700
Keywords: AMI, promising biomarker, miRNA-21, meta-analysis, miR-21
Citation: Wang K, Li K, Li Z and Yan X (2024) Circulating miRNA-21 as early potential diagnostic biomarker for acute myocardial infarction: a meta-analysis. Front. Cardiovasc. Med. 11:1330884. doi: 10.3389/fcvm.2024.1330884
Received: 31 October 2023; Accepted: 7 August 2024;
Published: 22 August 2024.
Edited by:
Roberto Galea, University Hospital of Bern, SwitzerlandReviewed by:
Wei Feng Ma, University of Virginia, United StatesLei Lei, First Hospital of Shanxi Medical University, China
Copyright: © 2024 Wang, Li, Li and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xizhang Yan, eWFueGl6aGFuZ0B4aXlpLmVkdS5jbg==
 Ke Wang
Ke Wang Kai Li3
Kai Li3 Zhuoyuan Li
Zhuoyuan Li